Effects of Cold Atmospheric Plasma Pre-Treatment of Titanium on the Biological Activity of Primary Human Gingival Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Fluorescence Microscopy
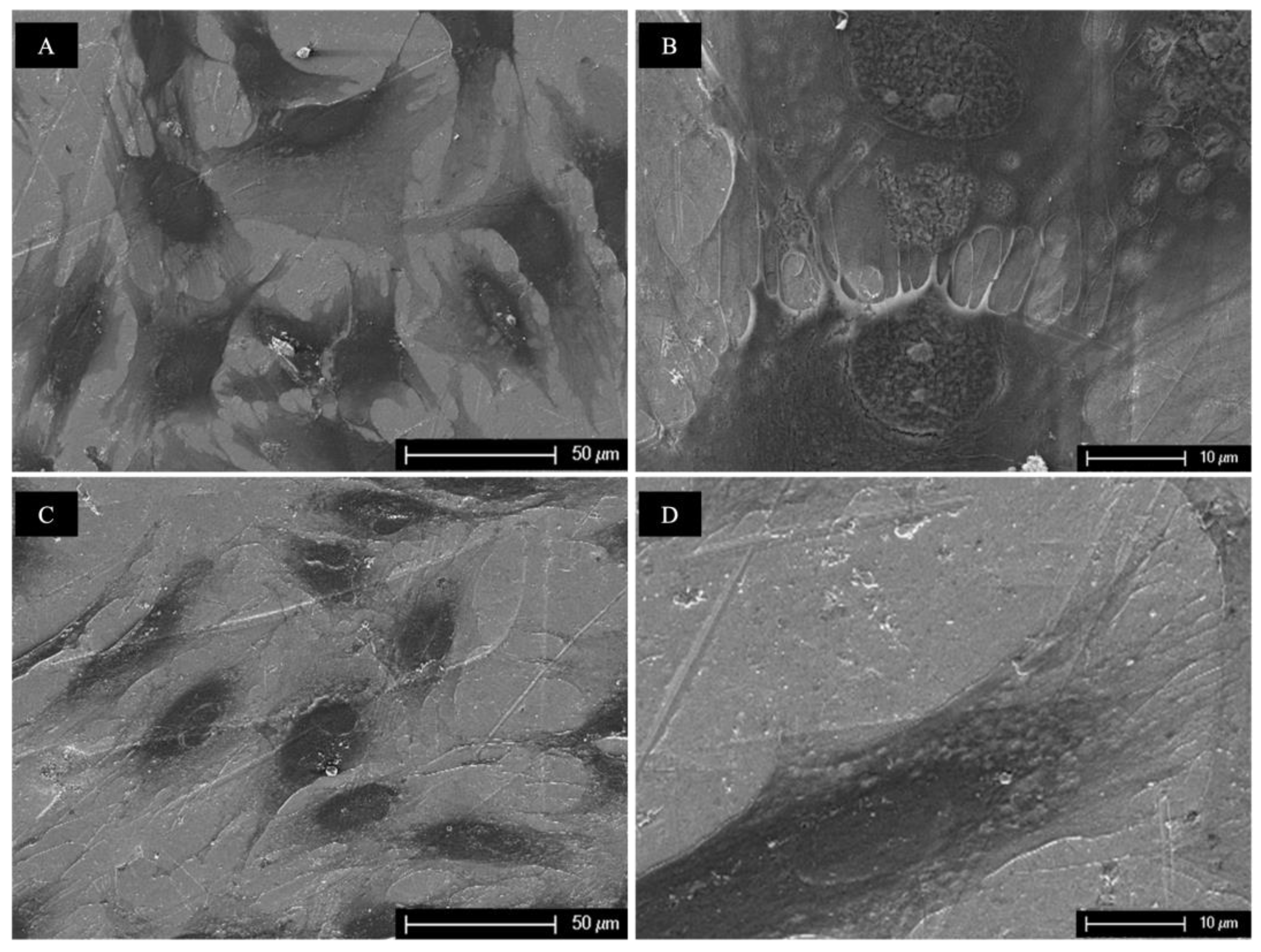
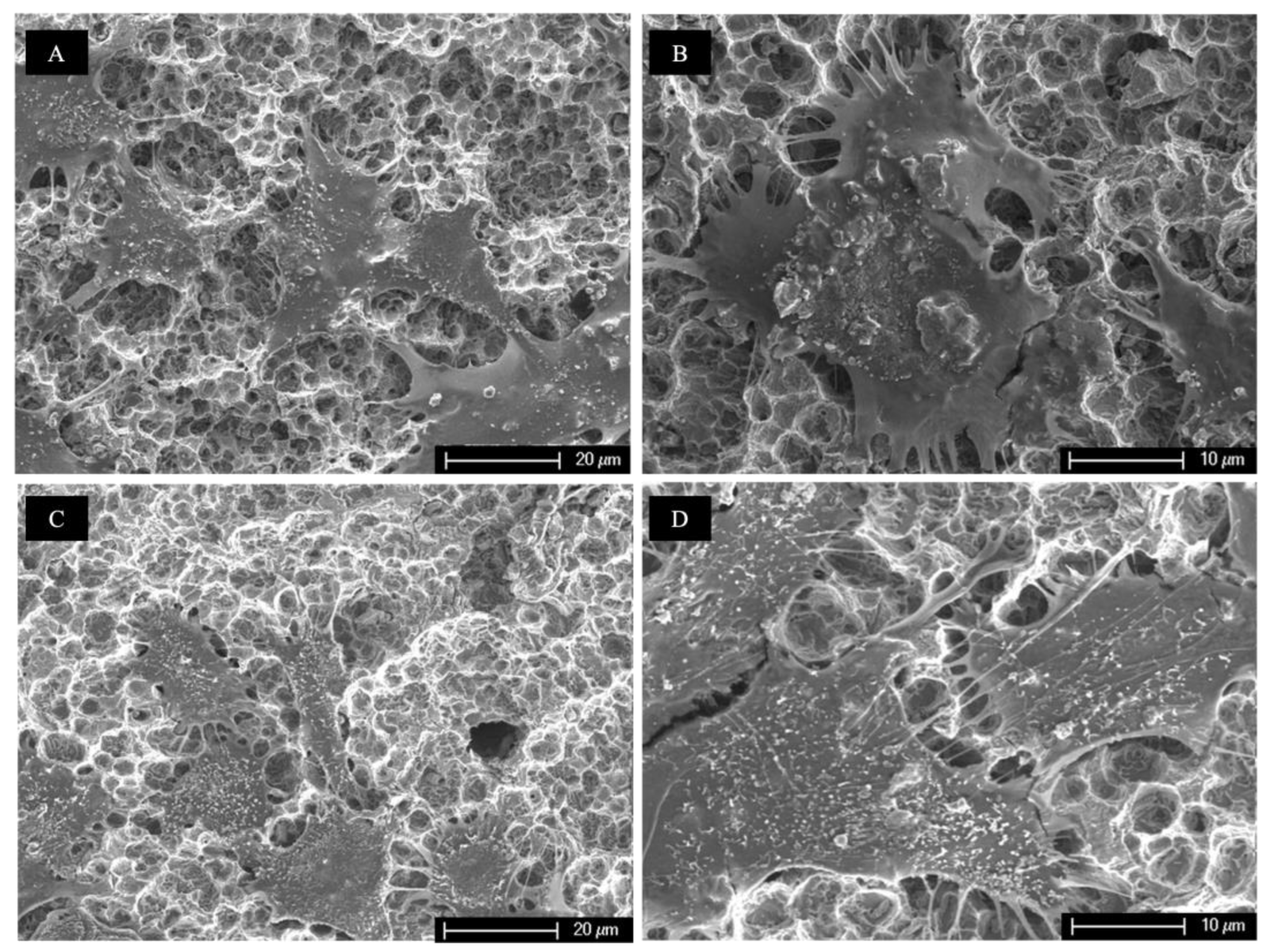
3.2. Scanning Electron Microscopy
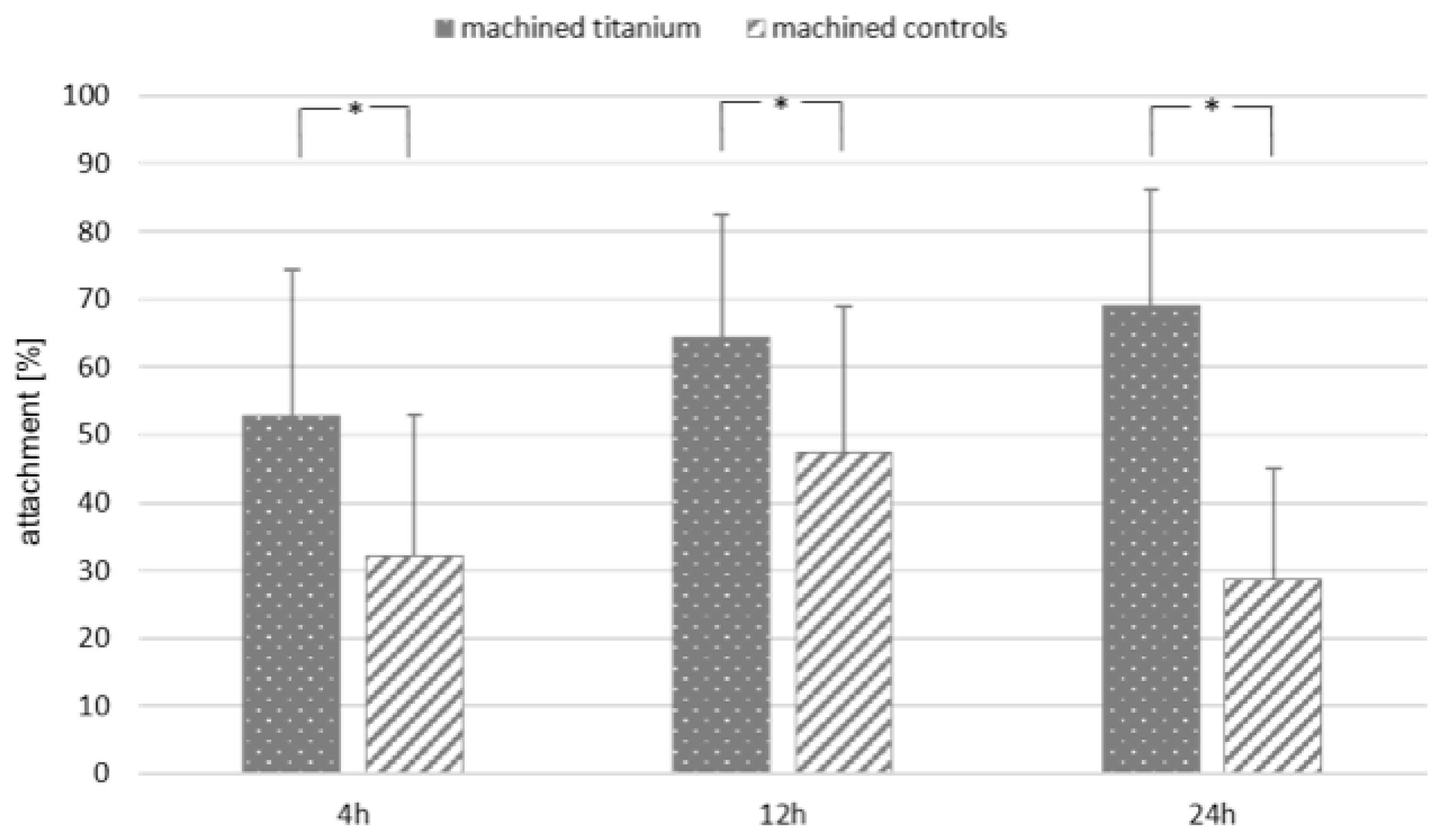
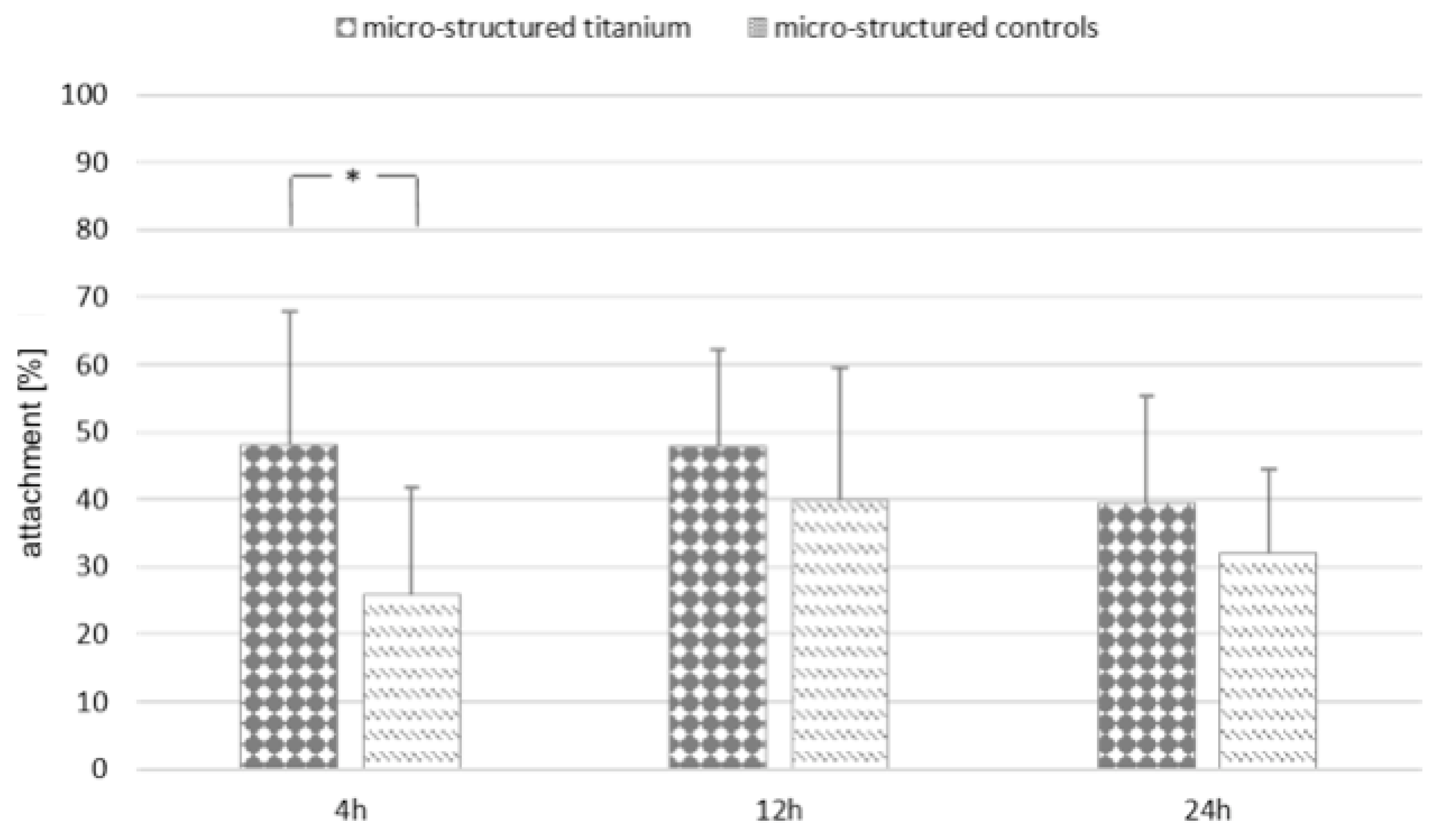
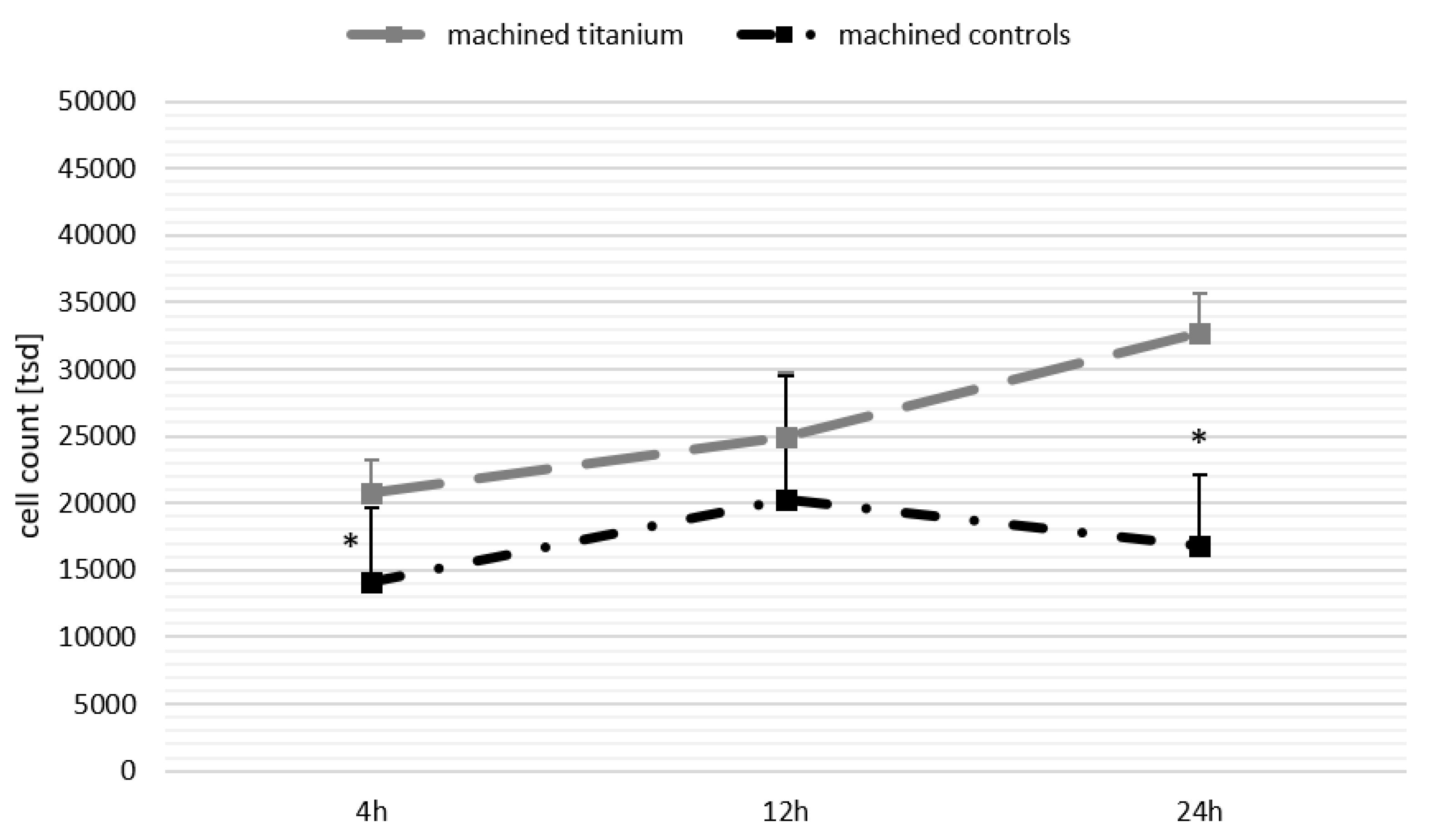
3.3. Biological Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A

Appendix B
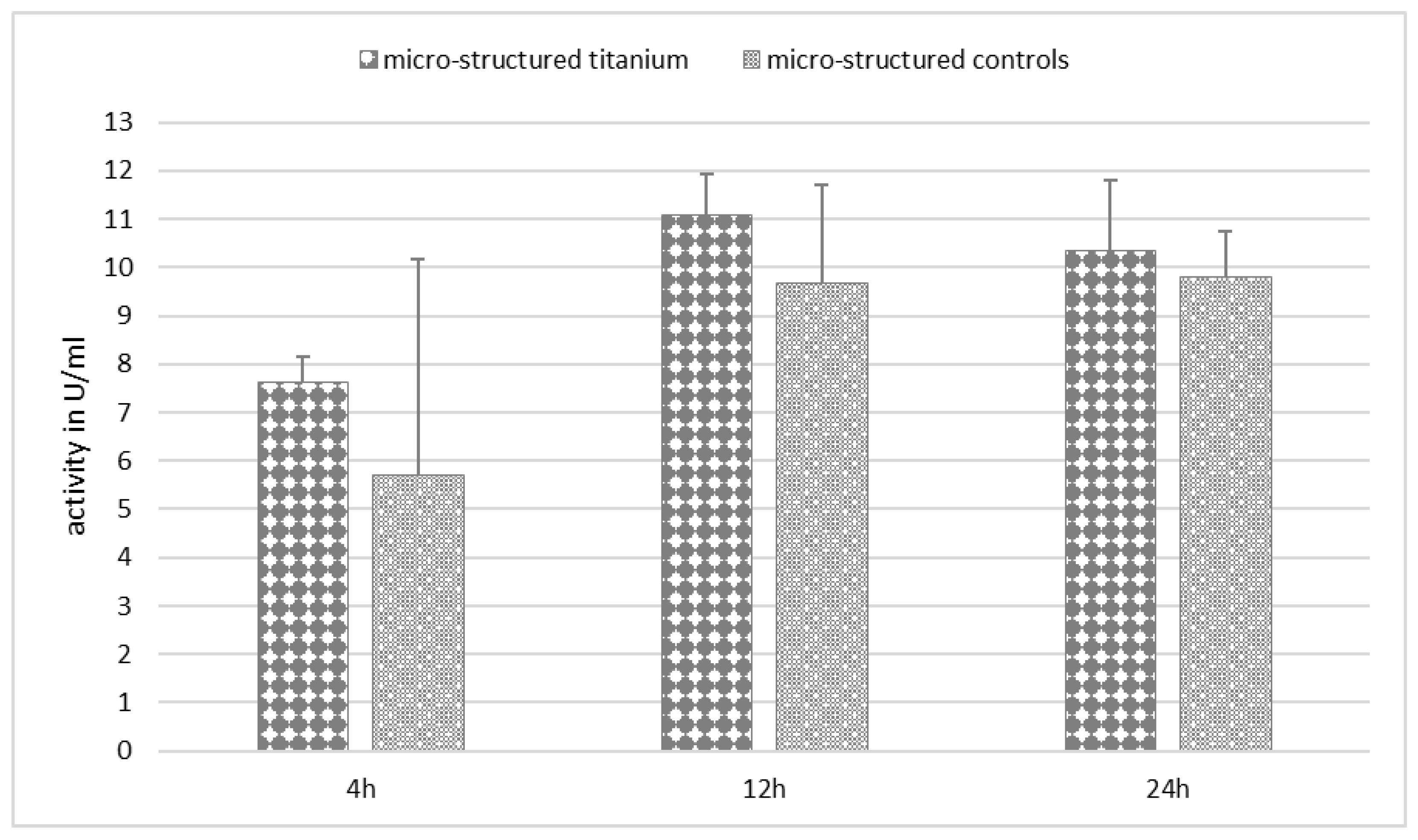
Appendix C
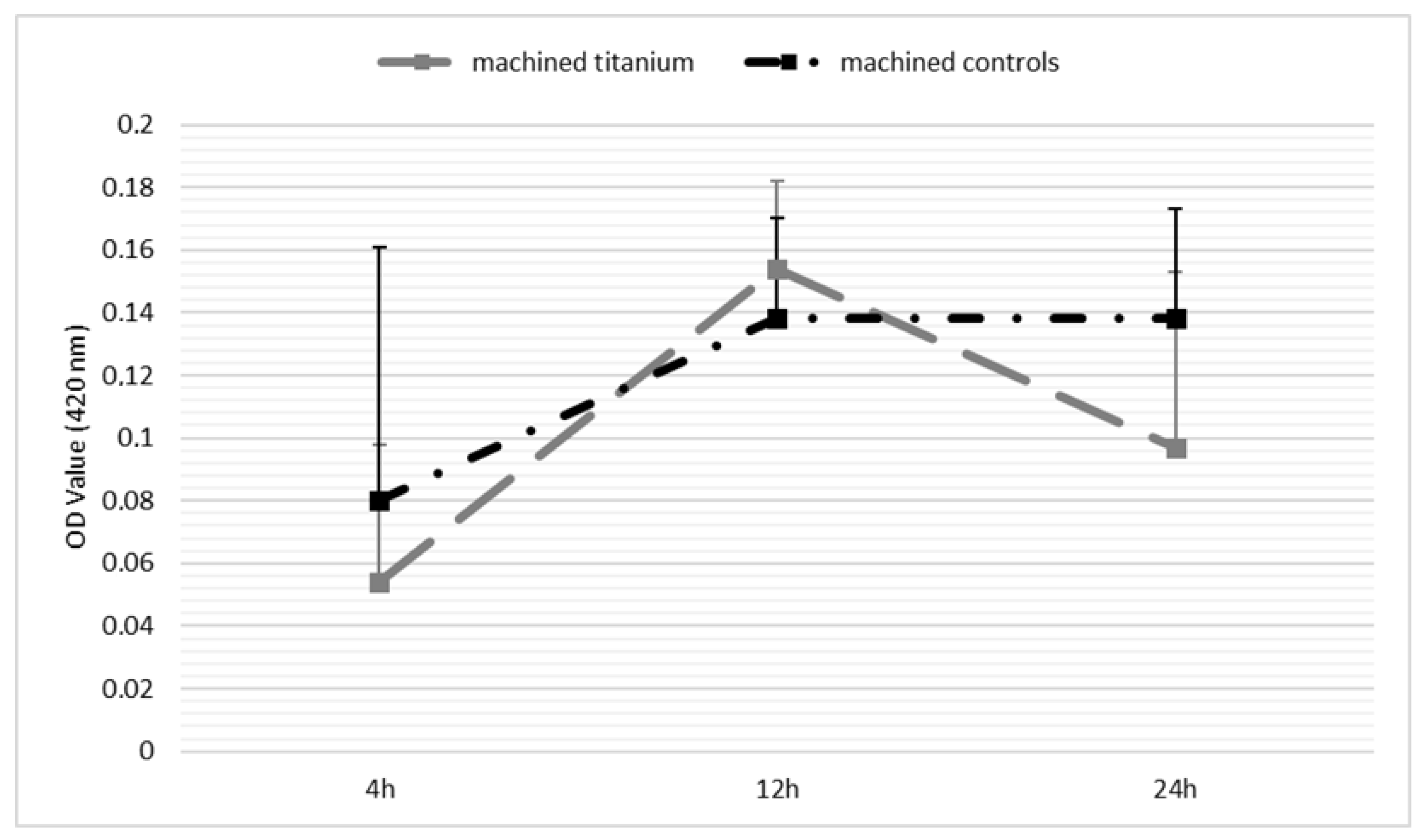
Appendix D
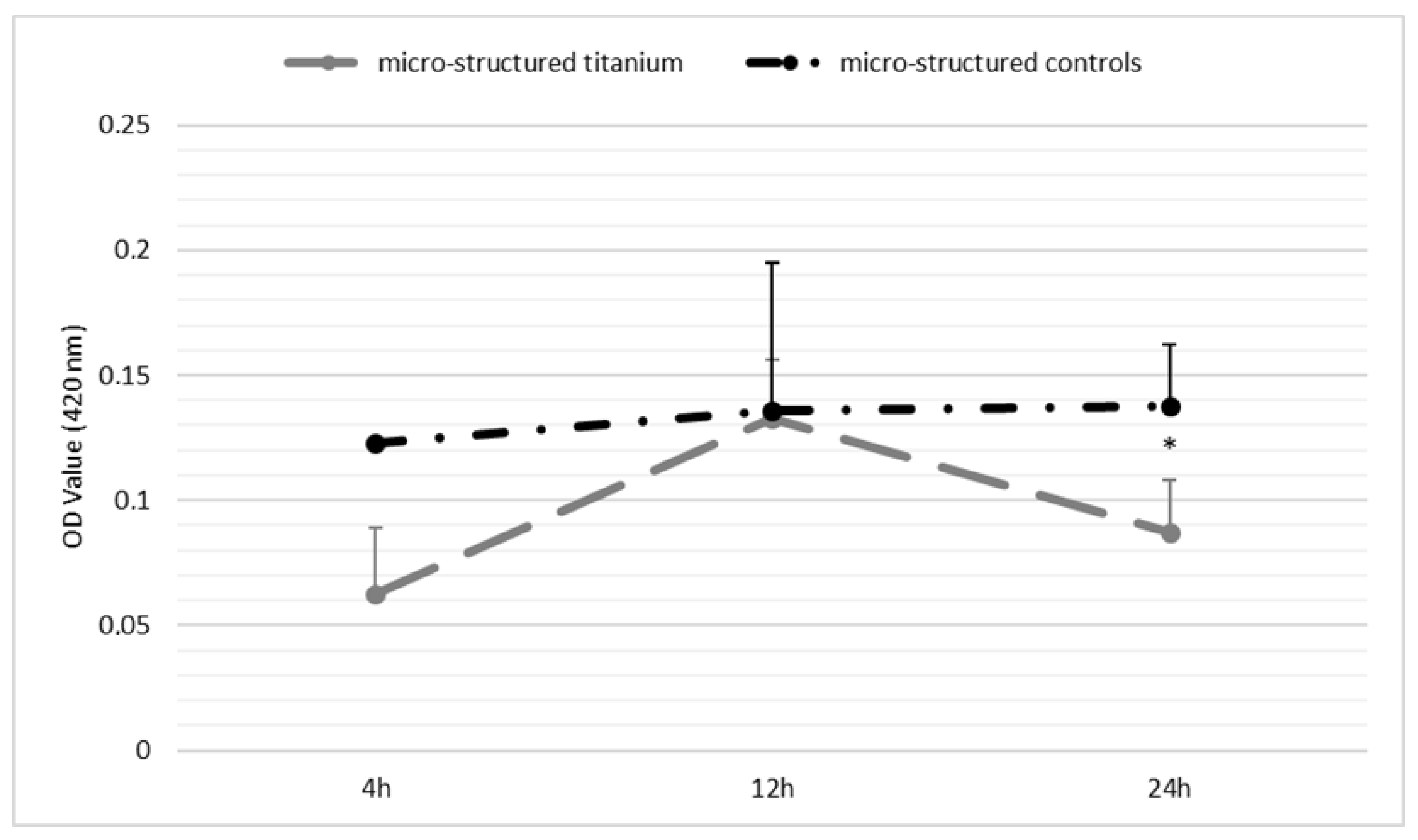
Appendix E
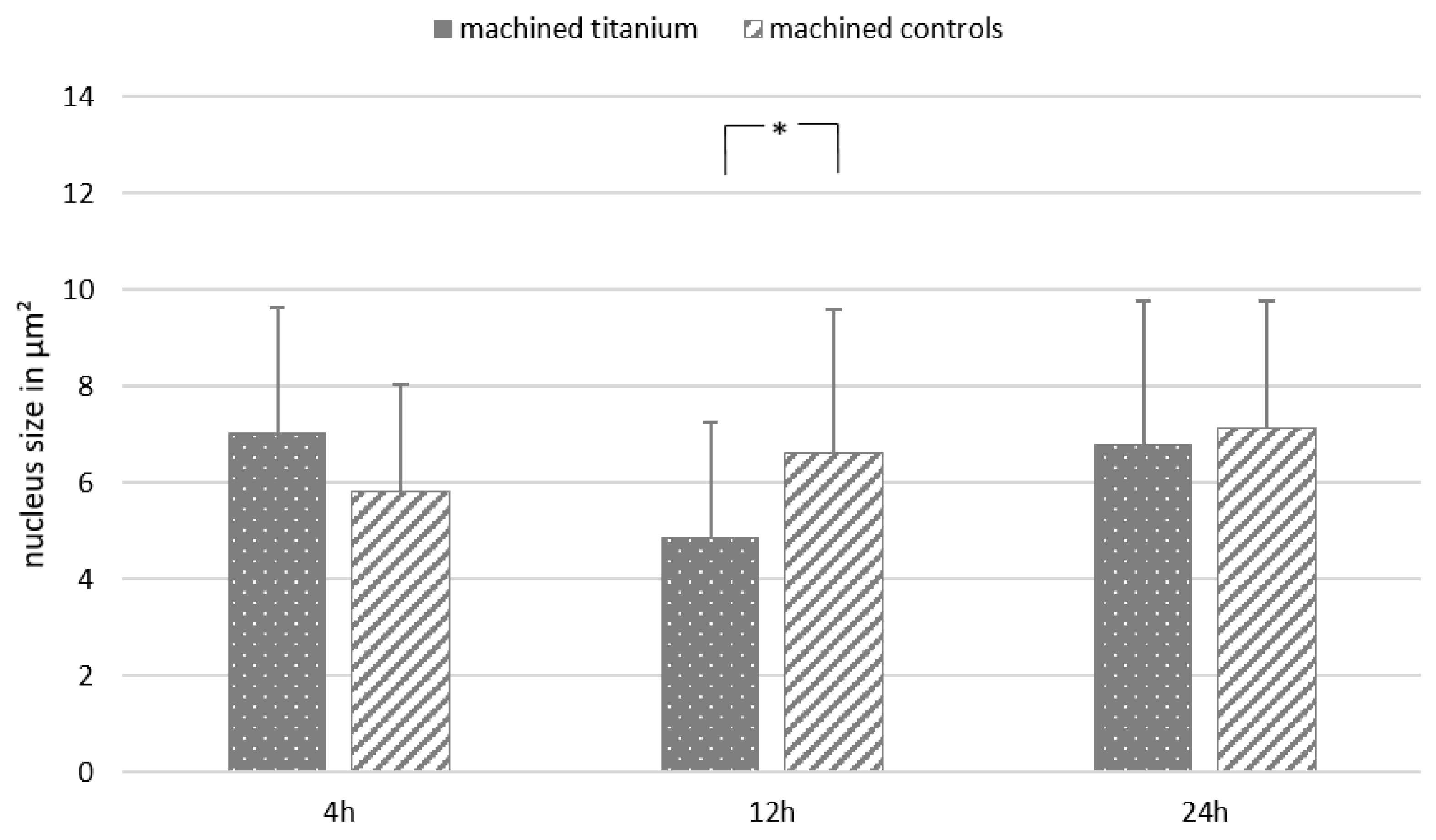
Appendix F

References
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral. Maxillofac. Implant. 2009, 24, 616–626. [Google Scholar]
- Sartoretto, S.C.; Alves, A.T.N.N.; Resende, R.F.B.; Calasans-Maia, J.; Granjeiro, J.M.; Calasans-Maia, M.D. Early osseointegration driven by the surface chemistry and wettability of dental implants. J. Appl. Oral Sci. 2015, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, A.; Parisi, L.; Tatti, R.; Lorenzi, A.; Verucchi, R.; Manfredi, E.; Lumetti, S.; Macaluso, G.M. Thermal-induced hydrophilicity enhancement of titanium dental implant surfaces. J. Oral Sci. 2020, 62, 217–221. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yao, Y.; Tang, W.; Han, D.; Zhang, L.; Zhao, K.; Wang, S.; Meng, Y. Design of dental implants at materials level: An overview. J. Biomed. Mater. Res. Part A 2020, 108, 1634–1661. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 1634–1661. [Google Scholar] [CrossRef]
- Kawase, T.; Tanaka, T.; Minbu, H.; Kamiya, M.; Oda, M.; Hara, T. An atmospheric-pressure plasma-treated titanium surface potentially supports initial cell adhesion, growth, and differentiation of cultured human prenatal-derived osteoblastic cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Alborova, A.; Humme, D.; Patzelt, A.; Kramer, A.; Weltmann, K.-D.; Hartmann, B.; Ottomann, C.; Fluhr, J.W.; et al. Risk assessment of the application of a plasma jet in dermatology. J. Biomed. Opt. 2009, 14, 1289–1296. [Google Scholar] [CrossRef]
- Liebmann, J.; Scherer, J.; Bibinov, N.; Rajasekaran, P.; Kovacs, R.; Gesche, R.; Awakowicz, P.; Kolb-Bachofen, V. Biological effects of nitric oxide generated by an atmospheric pressure gas-plasma on human skin cells. Nitric Oxide 2011, 24, 8–16. [Google Scholar] [CrossRef]
- Matthes, R.; Bekeschus, S.; Bender, C.; Koban, I.; Hübner, N.O.; Kramer, A. Pilot-study on the influence of carrier gas and plasma application (open resp. delimited) modifications on physical plasma and its antimicrobial effect against Pseudomonas aeruginosa and Staphylococcus aureus. GMS Krankenhhyg Interdiszip 2012, 7, Doc02. [Google Scholar] [CrossRef] [PubMed]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a risk factor in implant treatment. Periodontol. 2000 2019, 81, 29–40. [Google Scholar] [CrossRef]
- Pohler, O.E. Unalloyed titanium for implants in bone surgery. Injury 2000, 31, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ptasińska, S.; Bahnev, B.; Stypczyńska, A.; Bowden, M.; Mason, N.J.; Braithwaite, N.S.J. DNA strand scission induced by a non-thermal atmospheric pressure plasma jet. Phys. Chem. Chem. Phys. 2010, 12, 7779–7781. [Google Scholar] [CrossRef]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Kamionka, J.; Matthes, R.; Holtfreter, B.; Pink, C.; Schlüter, R.; Von Woedtke, T.; Kocher, T.; Jablonowski, L. Efficiency of cold atmospheric plasma, cleaning powders and their combination for biofilm removal on two different titanium implant surfaces. Clin. Oral Investig. 2022, 26, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Flörke, C.; Janning, J.; Hinrichs, C.; Behrens, E.; Liedtke, K.R.; Sen, S.; Christofzik, D.; Wiltfang, J.; Gülses, A. In-vitro assessment of the efficiency of cold atmospheric plasma on decontamination of titanium dental implants. Int. J. Implant. Dent. 2022, 8, 12. [Google Scholar] [CrossRef]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef]
- Hui, W.L.; Perrotti, V.; Iaculli, F.; Piattelli, A.; Quaranta, A. The Emerging Role of Cold Atmospheric Plasma in Implantology: A Review of the Literature. Nanomaterials 2020, 10, 1505. [Google Scholar] [CrossRef]
- Wagner, G.; Eggers, B.; Duddeck, D.; Kramer, F.J.; Bourauel, C.; Jepsen, S.; Deschner, J.; Nokhbehsaim, M. Influence of cold atmospheric plasma on dental implant materials—An in vitro analysis. Clin. Oral Investig. 2022, 26, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.M.; Oh, T.J.; Shotwell, J.L.; Misch, C.E.; Wang, H.-L. Significance of Keratinized Mucosa in Maintenance of Dental Implants With Different Surfaces. J. Periodontol. 2006, 77, 1410–1420. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Legeay, G.; Gaillard, C.; Layrolle, P. Radio frequency plasma treatments on titanium for enhancement of bioactivity. Acta Biomater. 2008, 4, 1953–1962. [Google Scholar] [CrossRef]
- Yoshinari, M.; Wei, J.; Matsuzaka, K.; Inoue, T. Effect of Cold Plasma-Surface Modification on Surface Wettability and Initial Cell Attachment. World Acad. Sci. Eng. Technol. 2009, 58, 171–175. [Google Scholar] [CrossRef]
- Bürgers, R.; Gerlach, T.; Hahnel, S.; Schwarz, F.; Handel, G.; Gosau, M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin. Oral Implant. Res. 2010, 21, 156–164. [Google Scholar] [CrossRef]
- Evert, K.; Kocher, T.; Schindler, A.; Müller, M.; Müller, K.; Pink, C.; Holtfreter, B.; Schmidt, A.; Dombrowski, F.; Schubert, A.; et al. Repeated exposure of the oral mucosa over 12 months with cold plasma is not carcinogenic in mice. Sci. Rep. 2021, 11, 20672. [Google Scholar] [CrossRef]
- Lehmann, A.; Pietag, F.; Arnold, T. Human health risk evaluation of a microwave-driven atmospheric plasma jet as medical device. Clin. Plasma Med. 2017, 7, 16–23. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P.; Vörös, J.; Wieland, M.; Ruiz-Taylor, L.; Textor, M.; Brunette, D.M. Characterization of titanium surfaces. In Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer: Berlin/Heidelberg, Germany, 2001; pp. 87–144. [Google Scholar]
- Jeong, W.S.; Kwon, J.S.; Choi, E.H.; Kim, K.M. The Effects of Non-Thermal Atmospheric Pressure Plasma treated Titanium Surface on Behaviors of Oral Soft Tissue Cells. Sci. Rep. 2018, 8, 15963. [Google Scholar] [CrossRef] [PubMed]
- González-Blanco, C.; Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.; Torres-Lagares, D.; Gutiérrez-Pérez, J.L. Human Osteoblast Cell Behaviour on Titanium Discs Treated with Argon Plasma. Materials 2019, 12, 1735. [Google Scholar] [CrossRef] [PubMed]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; van Rienen, U.; Iglič, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar] [CrossRef]
- Gongadze, E.; Van Rienen, U.; Iglič, A. Generalized stern models of the electric double layer considering the spatial variation of permittvity and finite size of ions in saturation regime. Cell. Mol. Biol. Lett. 2011, 16, 576–594. [Google Scholar] [CrossRef]
- Calazans Neto, J.V.; Kreve, S.; Valente, M.; Reis, A.C.D. Protein absorption on titanium surfaces treated with a high-power laser: A systematic review. J. Prosthet. Dent. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rupf, S.; Idlibi, A.; Marrawi, F.; Hannig, M.; Schubert, A.; von Müller, L.; Spitzer, W.; Holtmann, H.; Lehmann, A.; Rueppell, A.; et al. Removing Biofilms from Microstructured Titanium Ex Vivo: A Novel Approach Using Atmospheric Plasma Technology. PLoS ONE 2011, 6, e25893. [Google Scholar] [CrossRef] [PubMed]
- Matthes, R.; Jablonowski, L.; Pitchika, V.; Holtfreter, B.; Eberhard, C.; Seifert, L.; Gerling, T.; Vilardell Scholten, L.; Schlüter, R.; Kocher, T. Efficiency of biofilm removal by combination of water jet and cold plasma: An in-vitro study. BMC Oral Health 2022, 22, 157. [Google Scholar] [CrossRef]
- Idlibi, A.N.; Al-Marrawi, F.; Hannig, M.; Lehmann, A.; Rueppell, A.; Schindler, A.; Jentsch, H.; Rupf, S. Destruction of oral biofilms formed in situ on machined titanium (Ti) surfaces by cold atmospheric plasma. Biofouling 2013, 29, 369–379. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gund, M.P.; Naim, J.; Lehmann, A.; Hannig, M.; Linsenmann, C.; Schindler, A.; Rupf, S. Effects of Cold Atmospheric Plasma Pre-Treatment of Titanium on the Biological Activity of Primary Human Gingival Fibroblasts. Biomedicines 2023, 11, 1185. https://doi.org/10.3390/biomedicines11041185
Gund MP, Naim J, Lehmann A, Hannig M, Linsenmann C, Schindler A, Rupf S. Effects of Cold Atmospheric Plasma Pre-Treatment of Titanium on the Biological Activity of Primary Human Gingival Fibroblasts. Biomedicines. 2023; 11(4):1185. https://doi.org/10.3390/biomedicines11041185
Chicago/Turabian StyleGund, Madline P., Jusef Naim, Antje Lehmann, Matthias Hannig, Constanze Linsenmann, Axel Schindler, and Stefan Rupf. 2023. "Effects of Cold Atmospheric Plasma Pre-Treatment of Titanium on the Biological Activity of Primary Human Gingival Fibroblasts" Biomedicines 11, no. 4: 1185. https://doi.org/10.3390/biomedicines11041185
APA StyleGund, M. P., Naim, J., Lehmann, A., Hannig, M., Linsenmann, C., Schindler, A., & Rupf, S. (2023). Effects of Cold Atmospheric Plasma Pre-Treatment of Titanium on the Biological Activity of Primary Human Gingival Fibroblasts. Biomedicines, 11(4), 1185. https://doi.org/10.3390/biomedicines11041185





