Microneurotrophin BNN27 Reduces Astrogliosis and Increases Density of Neurons and Implanted Neural Stem Cell-Derived Cells after Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Scaffold Fabrication
2.3. Primary Neural Stem Cell Isolation and Culture
2.4. Spinal Cord Injury Animal Model
2.5. Horizontal Ladder Walking Assay
2.6. Histological Evaluation
2.7. Statistical Analysis
3. Results
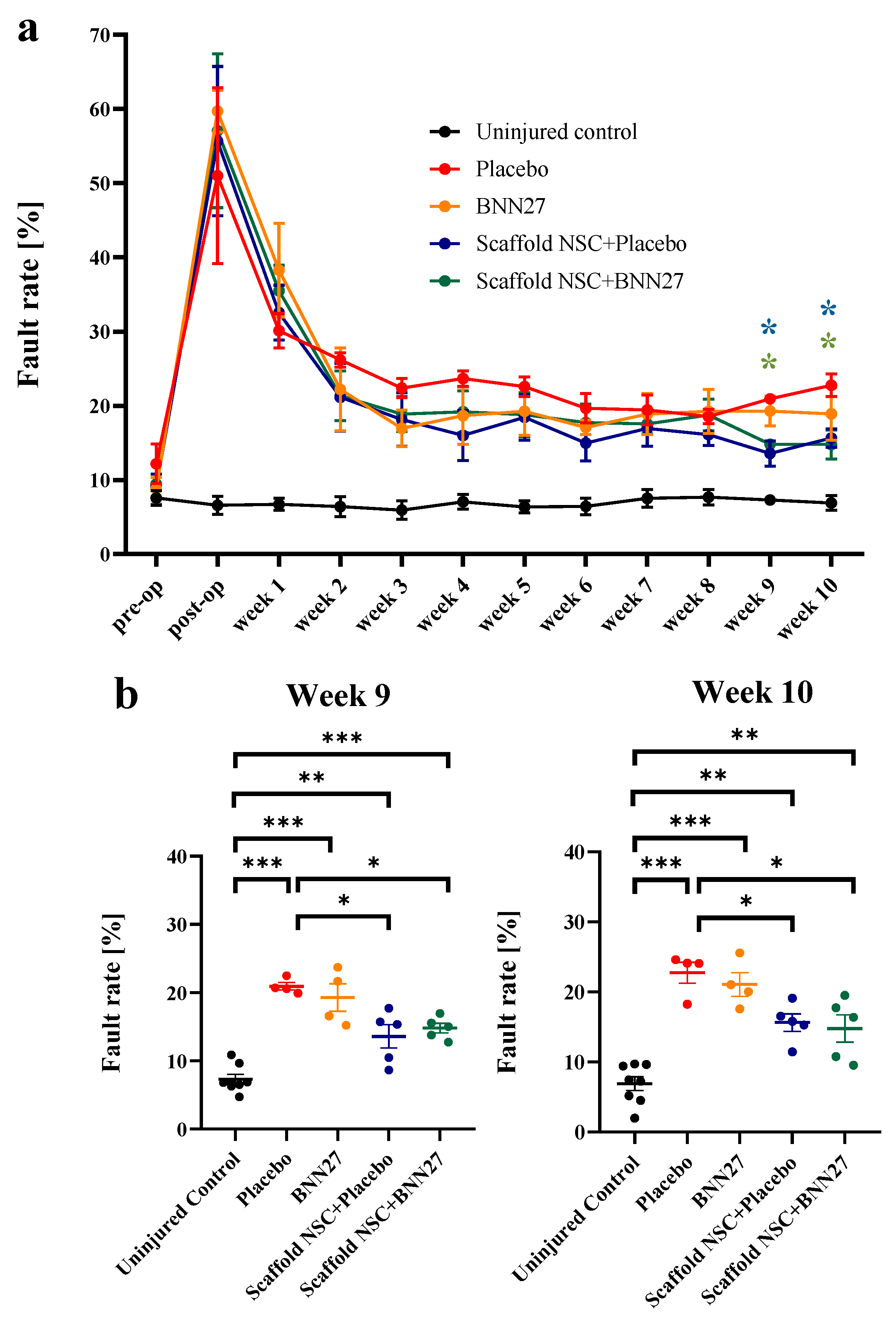
3.1. BNN27 and NSC Graft Treatment Effects on Locomotion Recovery after SCI
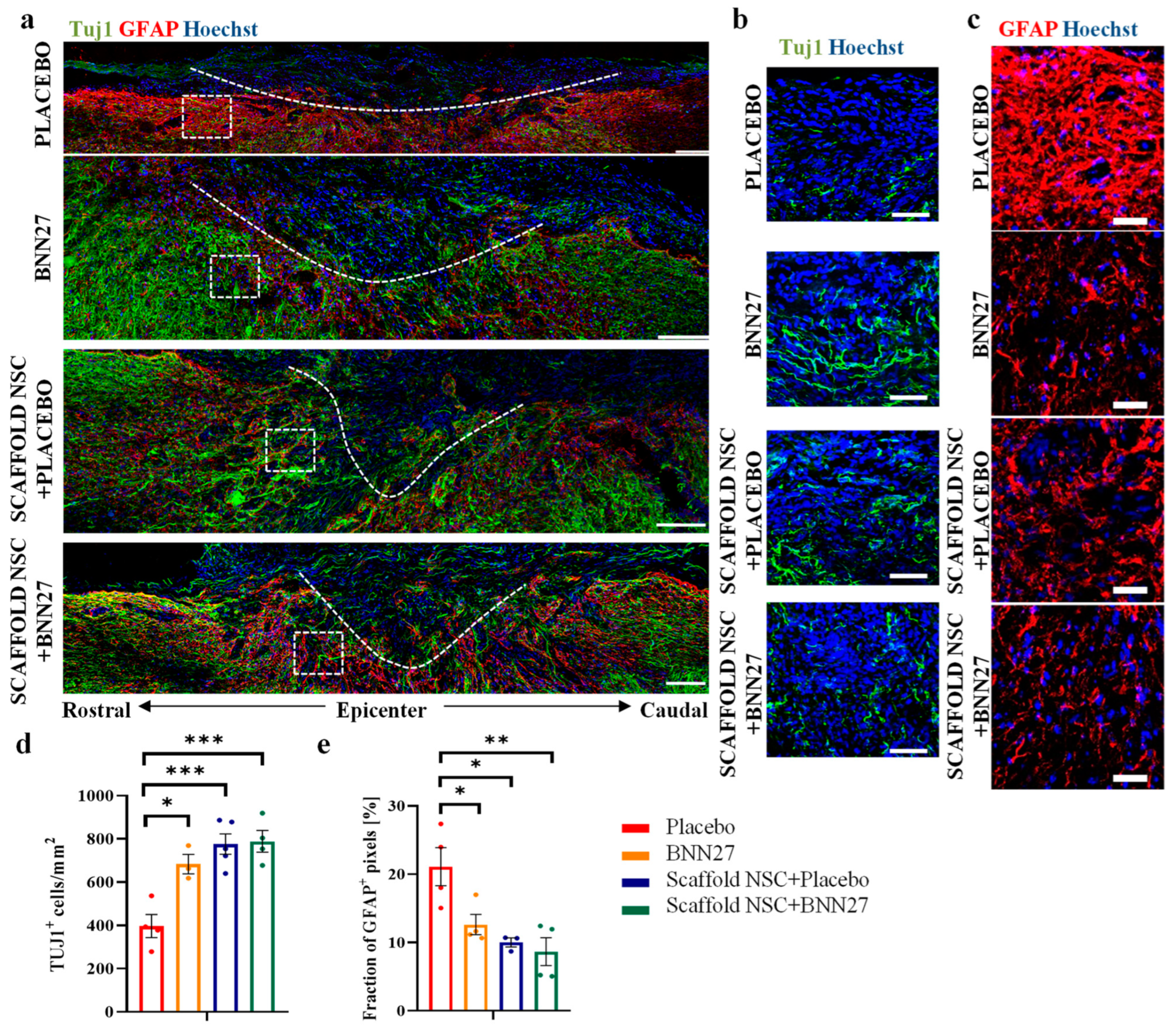
3.2. BNN27 and NSC Graft Treatment Effects on Neuron Presence and Astrogliosis at the Lesion Site
3.3. Treatment Effects on Synapse Formation, Axonal Elongation and Angiogenesis at the Lesion Site
3.4. BNN27 Effects on Implanted NSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zimmermann, R.; Alves, Y.V.; Sperling, L.E.; Pranke, P. Nanotechnology for the Treatment of Spinal Cord Injury. Tissue Eng. Part B Rev. 2021, 27, 353–365. [Google Scholar] [CrossRef]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Gomes, E.D.; Silva, N.A. Combinatorial therapies for spinal cord injury: Strategies to induce regeneration. Neural Regen. Res. 2019, 14, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, K.; Lu, P.; Nguyen, K.; Lee-Kubli, C.; Kumamaru, H.; Yao, L.; Knackert, J.; Poplawski, G.; Dulin, J.N.; Strobl, H.; et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 2016, 22, 479–487. [Google Scholar] [CrossRef]
- Lu, P.; Gomes-Leal, W.; Anil, S.; Dobkins, G.; Huie, J.R.; Ferguson, A.; Graham, L.; Tuszynski, M. Origins of Neural Progenitor Cell-Derived Axons Projecting Caudally after Spinal Cord Injury. Stem Cell Rep. 2019, 13, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950.e6. [Google Scholar] [CrossRef] [PubMed]
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 12. [Google Scholar] [CrossRef]
- Widenfalk, J.; Lundströmer, K.; Jubran, M.; Brené, S.; Olson, L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci. 2001, 21, 3457–3475. [Google Scholar] [CrossRef]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
- Krenz, N.R.; Weaver, L.C. Nerve Growth Factor in Glia and Inflammatory Cells of the Injured Rat Spinal Cord. J. Neurochem. 2001, 74, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Davis-López De Carrizosa, M.A.; Morado-Díaz, C.J.; Morcuende, S.; De La Cruz, R.R.; Pastor, Á.M. Nerve growth factor regulates the firing patterns and synaptic composition of motoneurons. J. Neurosci. 2010, 30, 8308–8319. [Google Scholar] [CrossRef]
- Aloe, L.; Bianchi, P.; De Bellis, A.; Soligo, M.; Rocco, M.L. Intranasal nerve growth factor bypasses the blood-brain barrier and affects spinal cord neurons in spinal cord injury. Neural Regen. Res. 2014, 9, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Grill, R.; Blesch, A.; Tuszynski, M. Robust Growth of Chronically Injured Spinal Cord Axons Induced by Grafts of Genetically Modified NGF-Secreting Cells. Exp. Neurol. 1997, 148, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Murai, K.; Blesch, A.; Grill, R.; Miller, I. Functional Characterization of Ngf-Secreting Cell Grafts to the Acutely Injured Spinal Cord. Cell Transplant. 1997, 6, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, F.; Kong, X.; Yang, J.; Chen, H.; Deng, L.; Cheng, Y.; Ye, L.; Zhu, S.; Zhang, X.; et al. Nerve growth factor improves functional recovery by inhibiting endoplasmic reticulum stress-induced neuronal apoptosis in rats with spinal cord injury. J. Transl. Med. 2014, 12, 130. [Google Scholar] [CrossRef]
- Song, Z.; Wang, Z.; Shen, J.; Xu, S.; Hu, Z. Nerve growth factor delivery by ultrasound-mediated nanobubble destruction as a treatment for acute spinal cord injury in rats. Int. J. Nanomed. 2017, 12, 1717–1729. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xiang, Z.; Ying, Y.; Huang, Z.; Tu, Y.; Chen, M.; Ye, J.; Dou, H.; Sheng, S.; Li, X.; et al. Nerve growth factor (NGF) with hypoxia response elements loaded by adeno-associated virus (AAV) combined with neural stem cells improve the spinal cord injury recovery. Cell Death Discov. 2021, 7, 301. [Google Scholar] [CrossRef]
- Wang, L.; Gu, S.; Gan, J.; Tian, Y.; Zhang, F.; Zhao, H.; Lei, D. Neural Stem Cells Overexpressing Nerve Growth Factor Improve Functional Recovery in Rats Following Spinal Cord Injury via Modulating Microenvironment and Enhancing Endogenous Neurogenesis. Front. Cell. Neurosci. 2021, 15, 773375. [Google Scholar] [CrossRef]
- Faustino, C.; Rijo, P.; Reis, C.P. Nanotechnological strategies for nerve growth factor delivery: Therapeutic implications in Alzheimer’s disease. Pharmacol. Res. 2017, 120, 68–87. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Peterson, D.A.; Ray, J.; Baird, A.; Nakahara, Y.; Gages, F.H. Fibroblasts genetically modified to produce Nerve Growth Factor Induce Robust Neuritic Ingrowth after Grafting to the Spinal Cord. Exp. Neurol. 1994, 126, 1–14. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Gabriel, K.; Gage, F.H.; Suhr, S.; Meyer, S.; Rosetti, A. Nerve growth factor delivery by gene transfer induces dif-ferential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp. Neurol. 1996, 137, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-Q.; Kong, X.-H.; Liu, Y.; Ban, D.-X.; Ning, G.-Z.; Chen, J.-T.; Guo, S.-F.; Wang, P. Regeneration of spinal cord with cell and gene therapy. Orthop Surg. 2009, 1, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Gravanis, A.; Pediaditakis, I.; Charalampopoulos, I. Synthetic microneurotrophins in therapeutics of neurodegeneration. Oncotarget 2017, 8, 9005–9006. [Google Scholar] [CrossRef]
- Pediaditakis, I.; Efstathopoulos, P.; Prousis, K.C.; Zervou, M.; Arévalo, J.C.; Alexaki, V.I.; Nikoletopoulou, V.; Karagianni, E.; Potamitis, C.; Tavernarakis, N.; et al. Selective and differential interactions of BNN27, a novel C17-spiroepoxy steroid derivative, with TrkA receptors, regulating neuronal survival and differentiation. Neuropharmacology 2016, 111, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Pediaditakis, I.; Kourgiantaki, A.; Prousis, K.C.; Potamitis, C.; Xanthopoulos, K.P.; Zervou, M.; Calogeropoulou, T.; Charalampopoulos, I.; Gravanis, A. BNN27, a 17-Spiroepoxy Steroid Derivative, Interacts With and Activates p75 Neurotrophin Receptor, Rescuing Cerebellar Granule Neurons from Apoptosis. Front. Pharmacol. 2016, 7, 512. [Google Scholar] [CrossRef]
- Ibán-Arias, R.; Lisa, S.; Mastrodimou, N.; Kokona, D.; Koulakis, E.; Iordanidou, P.; Kouvarakis, A.; Fothiadaki, M.; Papadogkonaki, S.; Sotiriou, A.; et al. The Synthetic Microneurotrophin BNN27 Affects Retinal Function in Rats With Streptozotocin-Induced Diabetes. Diabetes 2017, 67, 321–333. [Google Scholar] [CrossRef]
- Ibán-Arias, R.; Lisa, S.; Poulaki, S.; Mastrodimou, N.; Charalampopoulos, I.; Gravanis, A.; Thermos, K. Effect of topical administration of the microneurotrophin BNN27 in the diabetic rat retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, G.; Charalampopoulos, I.; Gravanis, A.; Karagogeos, D. The novel synthetic microneurotrophin BNN27 protects mature oligodendrocytes against cuprizone-induced death, through the NGF receptor TrkA. Glia 2017, 65, 1376–1394. [Google Scholar] [CrossRef]
- The Jackson Laboratory. C57BL/6J. Available online: https://www.jax.org/strain/000664 (accessed on 18 November 2022).
- Mouse Genome Informatics. 129X1/SvJ. Available online: http://www.informatics.jax.org/strain/MGI:3044210 (accessed on 18 November 2022).
- The Jackson Laboratory. B6.C-Tg(CMV-cre)1Cgn/Je. Available online: https://www.jax.org/strain/0060543044210 (accessed on 18 November 2022).
- Harrison, M.; O’Brien, A.; Adams, L.; Cowin, G.; Ruitenberg, M.J.; Sengul, G.; Watson, C. Vertebral landmarks for the identification of spinal cord segments in the mouse. Neuroimage 2013, 68, 22–29. [Google Scholar] [CrossRef]
- Farr, T.D.; Liu, L.; Colwell, K.L.; Whishaw, I.Q.; Metz, G.A. Bilateral alteration in stepping pattern after unilateral motor cortex injury: A new test strategy for analysis of skilled limb movements in neurological mouse models. J. Neurosci. Methods 2006, 153, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Savvaki, M.; Kafetzis, G.; Kaplanis, S.; Ktena, N.; Theodorakis, K.; Karagogeos, D. Neuronal, but not glial, Contactin 2 negatively regulates axon regeneration in the injured adult optic nerve. Eur. J. Neurosci. 2021, 53, 1705–1721. [Google Scholar] [CrossRef]
- Bennett, J.P.; O’Brien, L.C.; Brohawn, D.G. Pharmacological properties of microneurotrophin drugs developed for treatment of amyotrophic lateral sclerosis. Biochem. Pharmacol. 2016, 117, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Tsika, C.; Tzatzarakis, M.N.; Antimisiaris, S.G.; Tsoka, P.; Efstathopoulos, P.; Charalampopoulos, I.; Gravanis, A.; Tsilimbaris, M.K. Quantification of BNN27, a novel neuroprotective 17-spiroepoxy dehydroepiandrosterone derivative in the blood and retina of rodents, after single intraperitoneal administration. Pharmacol. Res. Perspect. 2021, 9, e00724. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Moon, L.D.F.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.B.; Alabed, Y.; Fournier, A. Molecular Targets to Promote Central Nervous System Regeneration. Curr. Neurovascular Res. 2004, 1, 61–75. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K. Glial Cells Shape Pathology and Repair After Spinal Cord Injury. Neurotherapeutics 2018, 15, 554–577. [Google Scholar] [CrossRef]
- Spilker, M.H.; Yannas, I.V.; Kostyk, S.K.; Norregaard, T.V.; Hsu, H.P.; Spector, M. The Effects of Tubulation on Healing and Scar Formation after Transection of the Adult Rat Spinal Cord. Restor. Neurol. Neurosci. 2021, 18, 23–38. [Google Scholar]
- Li, X.; Liu, S.; Zhao, Y.; Li, J.; Ding, W.; Han, S.; Chen, B.; Xiao, Z.; Dai, J. Training Neural Stem Cells on Functional Collagen Scaffolds for Severe Spinal Cord Injury Repair. Adv. Funct. Mater. 2016, 26, 5835–5847. [Google Scholar] [CrossRef]
- Lu, P.; Jones, L.; Snyder, E.; Tuszynski, M. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol. 2003, 181, 115–129. [Google Scholar] [CrossRef]
- Lepore, A.; Fischer, I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp. Neurol. 2005, 194, 230–242. [Google Scholar] [CrossRef]
- Hwang, D.H.; Shin, H.Y.; Kwon, M.J.; Choi, J.Y.; Ryu, B.-Y.; Kim, B.G. Survival of Neural Stem Cell Grafts in the Lesioned Spinal Cord Is Enhanced by a Combination of Treadmill Locomotor Training via Insulin-Like Growth Factor-1 Signaling. J. Neurosci. 2014, 34, 12788–12800. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.M.; Kulbatski, I.; Tator, C.H. Transplantation of Adult Rat Spinal Cord Stem/Progenitor Cells for Spinal Cord Injury. J. Neurotrauma 2007, 24, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Z. Employing Endogenous NSCs to Promote Recovery of Spinal Cord Injury. Stem Cells Int. 2019, 2019, 1958631. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.T.; Satkunendrarajah, K.; Zuccato, J.A.; Nassiri, F.; Fehlings, M.G. Neural Precursor Cell Transplantation Enhances Functional Recovery and Reduces Astrogliosis in Bilateral Compressive/Contusive Cervical Spinal Cord Injury. STEM CELLS Transl. Med. 2014, 3, 1148–1159. [Google Scholar] [CrossRef]
- Younsi, A.; Zheng, G.; Riemann, L.; Scherer, M.; Zhang, H.; Tail, M.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 13106. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef]
- Cao, Q.L.; Zhang, Y.P.; Howard, R.M.; Walters, W.M.; Tsoulfas, P.; Whittemore, S.R. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol. 2001, 167, 48–58. [Google Scholar] [CrossRef]
- Zhu, Y.; Uezono, N.; Yasui, T.; Nakashima, K. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev. Dyn. 2017, 247, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Fan, C.; Chen, B.; Zhao, Y.; Xiao, Z.; Dai, J. Direct neuronal differentiation of neural stem cells for spinal cord injury repair. STEM CELLS 2021, 39, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Lien, B.V.; Tuszynski, M.H.; Lu, P. Astrocytes migrate from human neural stem cell grafts and functionally integrate into the injured rat spinal cord. Exp. Neurol. 2019, 314, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-Distance Growth and Connectivity of Neural Stem Cells after Severe Spinal Cord Injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Lachyankar, M.B.; Condon, P.J.; Quesenberry, P.J.; Litofsky, N.; Recht, L.D.; Ross, A.H. Embryonic Precursor Cells That Express Trk Receptors: Induction of Different Cell Fates by NGF, BDNF, NT-3, and CNTF. Exp. Neurol. 1997, 144, 350–360. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, A.K.; Shukla, R.K.; Tripathi, V.K.; Jahan, S.; Pandey, A.; Srivastava, A.; Agrawal, M.; Yadav, S.; Khanna, V.K.; et al. Molecular Mechanism of Switching of TrkA/p75NTR Signaling in Monocrotophos Induced Neurotoxicity. Sci. Rep. 2015, 5, srep14038. [Google Scholar] [CrossRef]
- Oliveira, S.L.; Trujillo, C.A.; Negraes, P.D.; Ulrich, H. Effects of ATP and NGF on Proliferation and Migration of Neural Precursor Cells. Neurochem. Res. 2015, 40, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, K.T. Neural growth factor stimulates proliferation of spinal cord derived-neural precursor/stem cells. J. Korean Neurosurg. Soc. 2016, 59, 437–441. [Google Scholar] [CrossRef]
- Ceto, S.; Sekiguchi, K.J.; Takashima, Y.; Nimmerjahn, A.; Tuszynski, M.H. Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury. Cell Stem Cell 2020, 27, 430–440.e5. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Cao, X.; Yu, B. Revascularization After Traumatic Spinal Cord Injury. Front. Physiol. 2021, 12, 631500. [Google Scholar] [CrossRef] [PubMed]
- Tsivelekas, K.K.; Evangelopoulos, D.S.; Pallis, D.; Benetos, I.S.; Papadakis, S.A.; Vlamis, J.; Pneumaticos, S.G. Angiogenesis in Spinal Cord Injury: Progress and Treatment. Cureus 2022, 14, e25475. [Google Scholar] [CrossRef]
- Kumagai, G.; Okada, Y.; Yamane, J.; Nagoshi, N.; Kitamura, K.; Mukaino, M.; Tsuji, O.; Fujiyoshi, K.; Katoh, H.; Okada, S.; et al. Roles of ES Cell-Derived Gliogenic Neural Stem/Progenitor Cells in Functional Recovery after Spinal Cord Injury. PLoS ONE 2009, 4, e7706. [Google Scholar] [CrossRef] [PubMed]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E.; et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef] [PubMed]
- Rogdakis, T.; Charou, D.; Latorrata, A.; Papadimitriou, E.; Tsengenes, A.; Athanasiou, C.; Papadopoulou, M.; Chalikiopoulou, C.; Katsila, T.; Ramos, I.; et al. Development and Biological Characterization of a Novel Selective TrkA Agonist with Neuroprotective Properties against Amyloid Toxicity. Biomedicines 2022, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Rogdakis, T.; Latorrata, A.; Thanou, E.; Karadima, E.; Papadimitriou, E.; Siapi, E.; Li, K.W.; Katsila, T.; Calogeropoulou, T.; et al. ENT-A010, a Novel Steroid Derivative, Displays Neuroprotective Functions and Modulates Microglial Responses. Biomolecules 2022, 12, 424. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgelou, K.; Saridaki, E.-A.; Karali, K.; Papagiannaki, A.; Charalampopoulos, I.; Gravanis, A.; Tzeranis, D.S. Microneurotrophin BNN27 Reduces Astrogliosis and Increases Density of Neurons and Implanted Neural Stem Cell-Derived Cells after Spinal Cord Injury. Biomedicines 2023, 11, 1170. https://doi.org/10.3390/biomedicines11041170
Georgelou K, Saridaki E-A, Karali K, Papagiannaki A, Charalampopoulos I, Gravanis A, Tzeranis DS. Microneurotrophin BNN27 Reduces Astrogliosis and Increases Density of Neurons and Implanted Neural Stem Cell-Derived Cells after Spinal Cord Injury. Biomedicines. 2023; 11(4):1170. https://doi.org/10.3390/biomedicines11041170
Chicago/Turabian StyleGeorgelou, Konstantina, Erasmia-Angeliki Saridaki, Kanelina Karali, Argyri Papagiannaki, Ioannis Charalampopoulos, Achille Gravanis, and Dimitrios S. Tzeranis. 2023. "Microneurotrophin BNN27 Reduces Astrogliosis and Increases Density of Neurons and Implanted Neural Stem Cell-Derived Cells after Spinal Cord Injury" Biomedicines 11, no. 4: 1170. https://doi.org/10.3390/biomedicines11041170
APA StyleGeorgelou, K., Saridaki, E.-A., Karali, K., Papagiannaki, A., Charalampopoulos, I., Gravanis, A., & Tzeranis, D. S. (2023). Microneurotrophin BNN27 Reduces Astrogliosis and Increases Density of Neurons and Implanted Neural Stem Cell-Derived Cells after Spinal Cord Injury. Biomedicines, 11(4), 1170. https://doi.org/10.3390/biomedicines11041170





