Vunakizumab-IL22, a Novel Fusion Protein, Promotes Intestinal Epithelial Repair and Protects against Gut Injury Induced by the Influenza Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Vunakizumab-IL22 (vmab-mIL22)
2.2. Influenza Virus
2.3. Animals
2.3.1. Experimental Enteritis Model Induced by H1N1 Infection
2.3.2. Experimental Enteritis Model Induced by Dextran Sodium Sulfate (DSS)
2.4. Pathological Observation and Periodic Acid-Schiff Stain (PAS)
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Immunohistochemistry (IHC) Assay
2.7. Quantitative Real-Time PCR (RT-qPCR) Analysis
2.8. Western Blotting
2.9. Data Analysis
3. Results
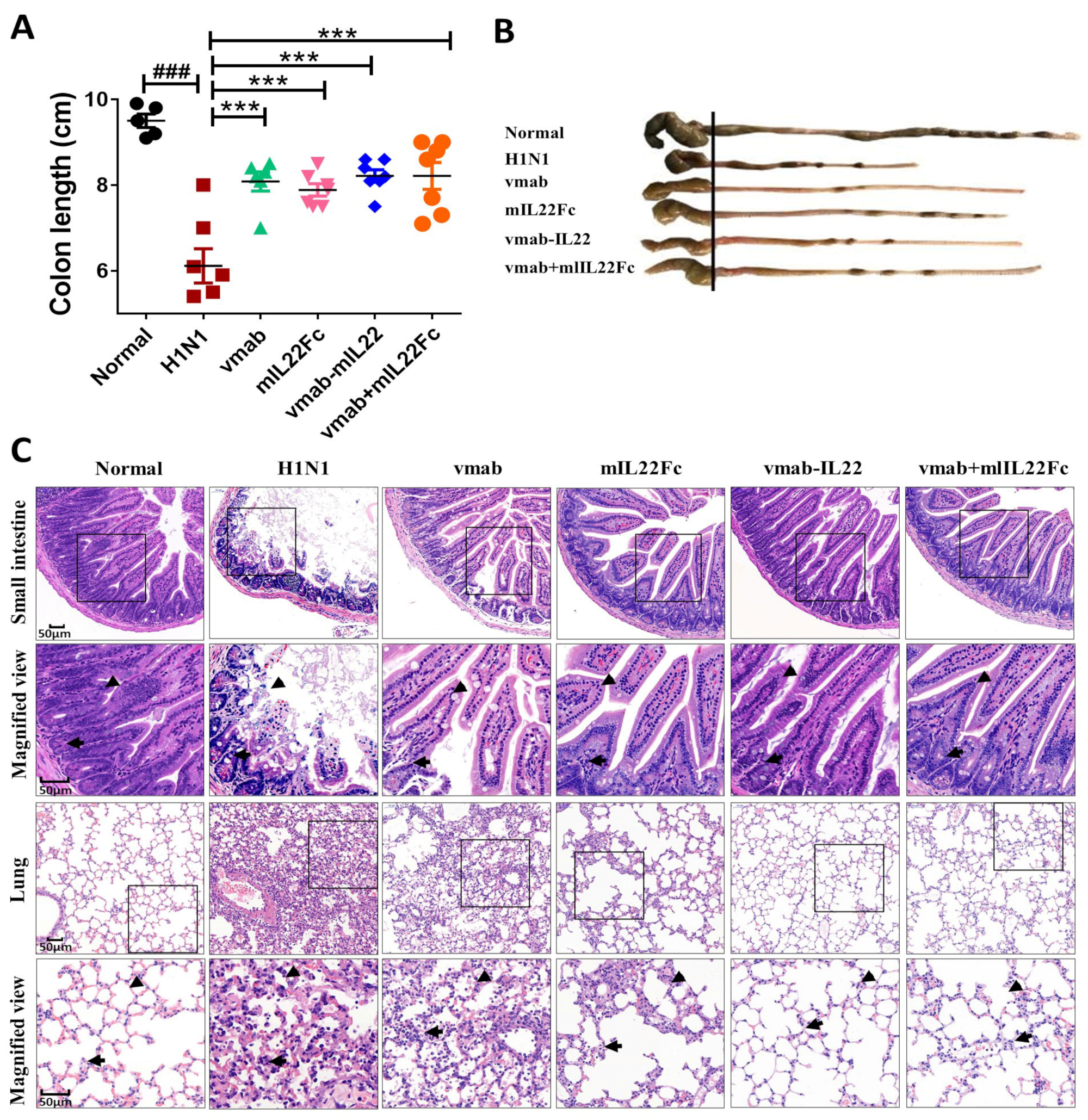
3.1. Vmab-IL22 Protects against Lung and Intestinal Injury Induced by Influenza Virus Infection
3.2. Vmab-IL22 Strengthens the Intestinal Barrier in Influenza Virus-Infected Mice
3.3. Vmab-IL22 Promotes the Expression of IL22R in the Small Intestine in Influenza Virus-Infected Mice
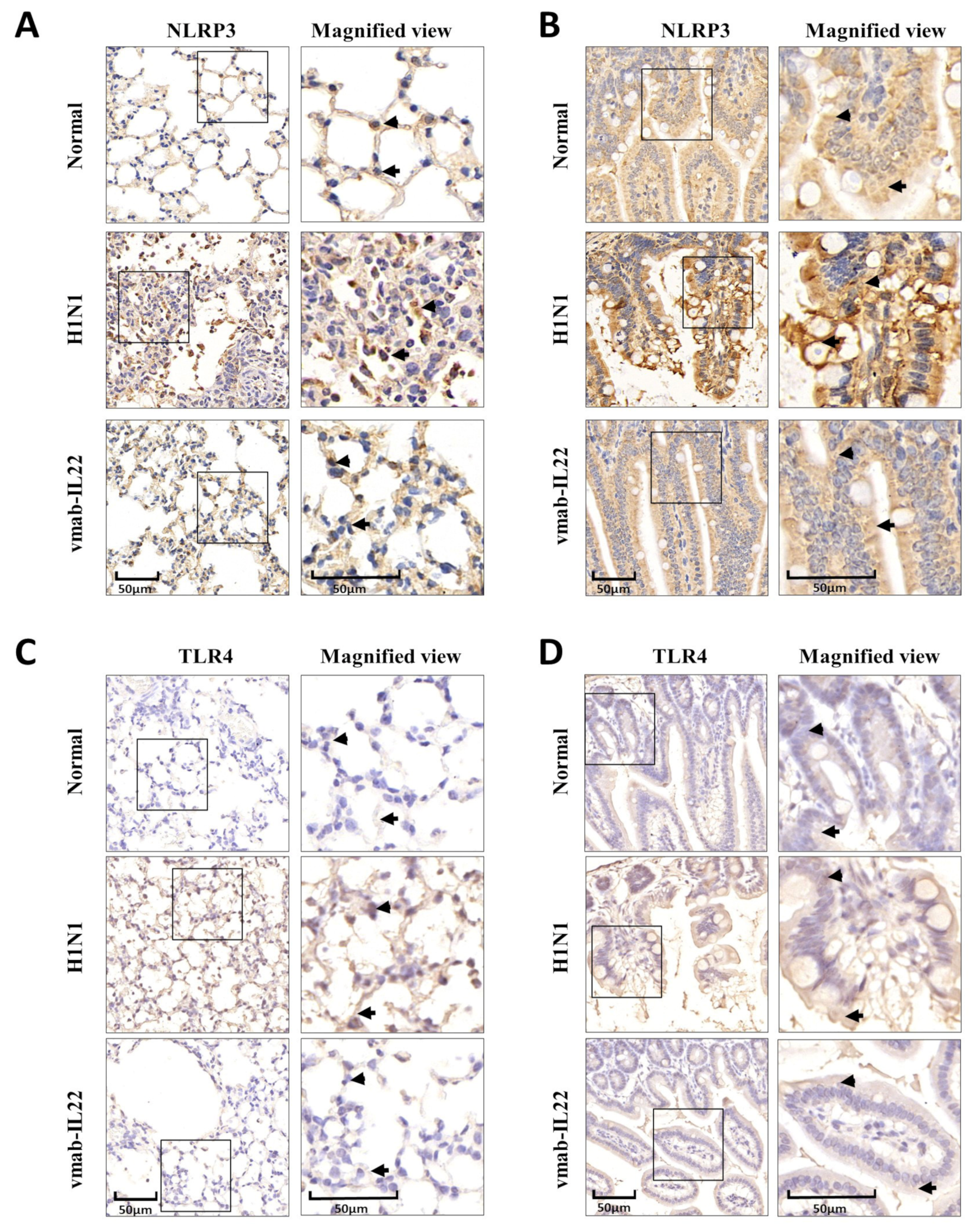
3.4. Vmab-IL22 Inhibits TLR4 and NLRP3 Inflammasome Activation in the Lung and Intestine in Influenza Virus-Infected Mice
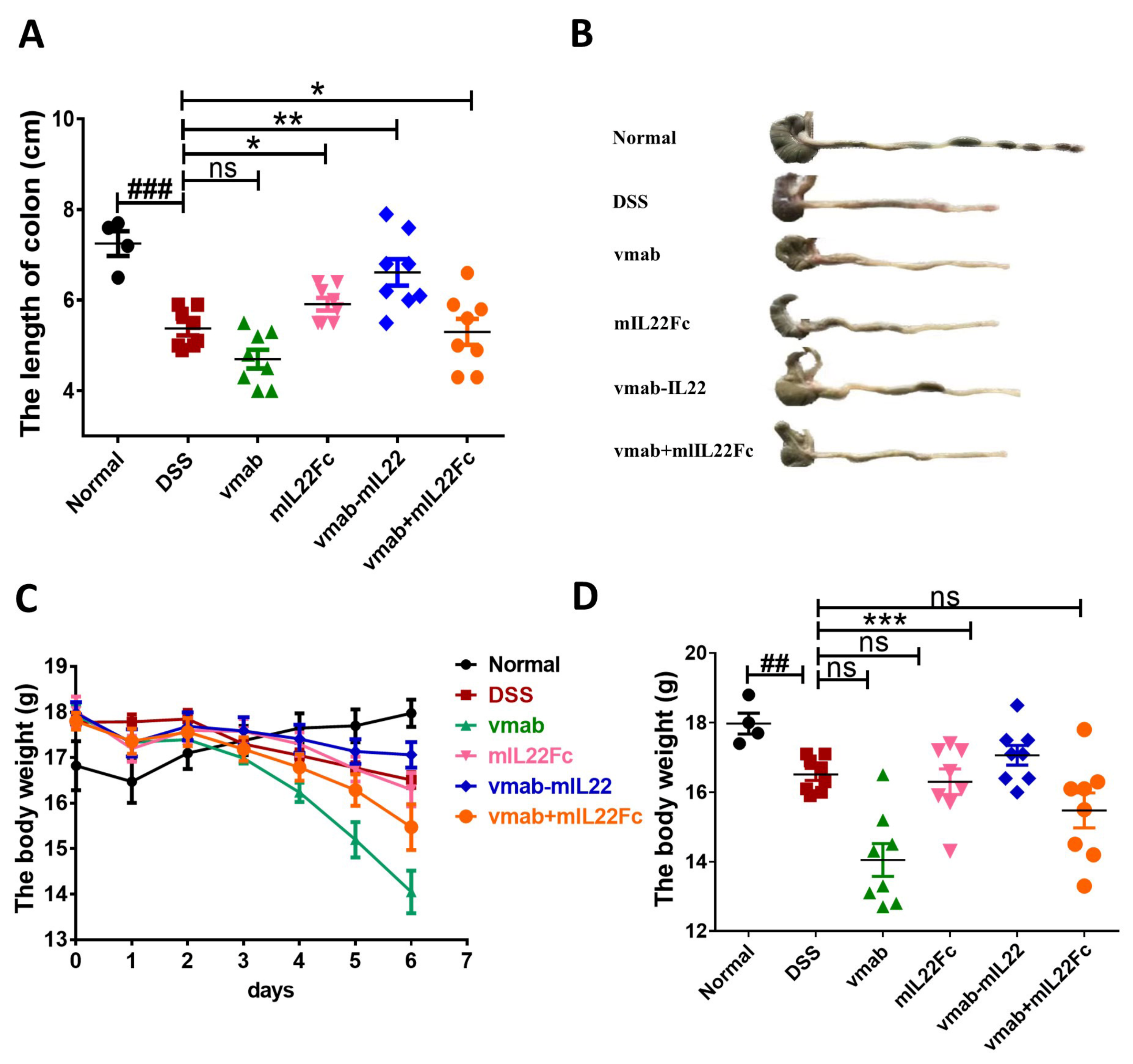
3.5. Vmab-IL22 Protects against DSS-Induced Intestinal Injury
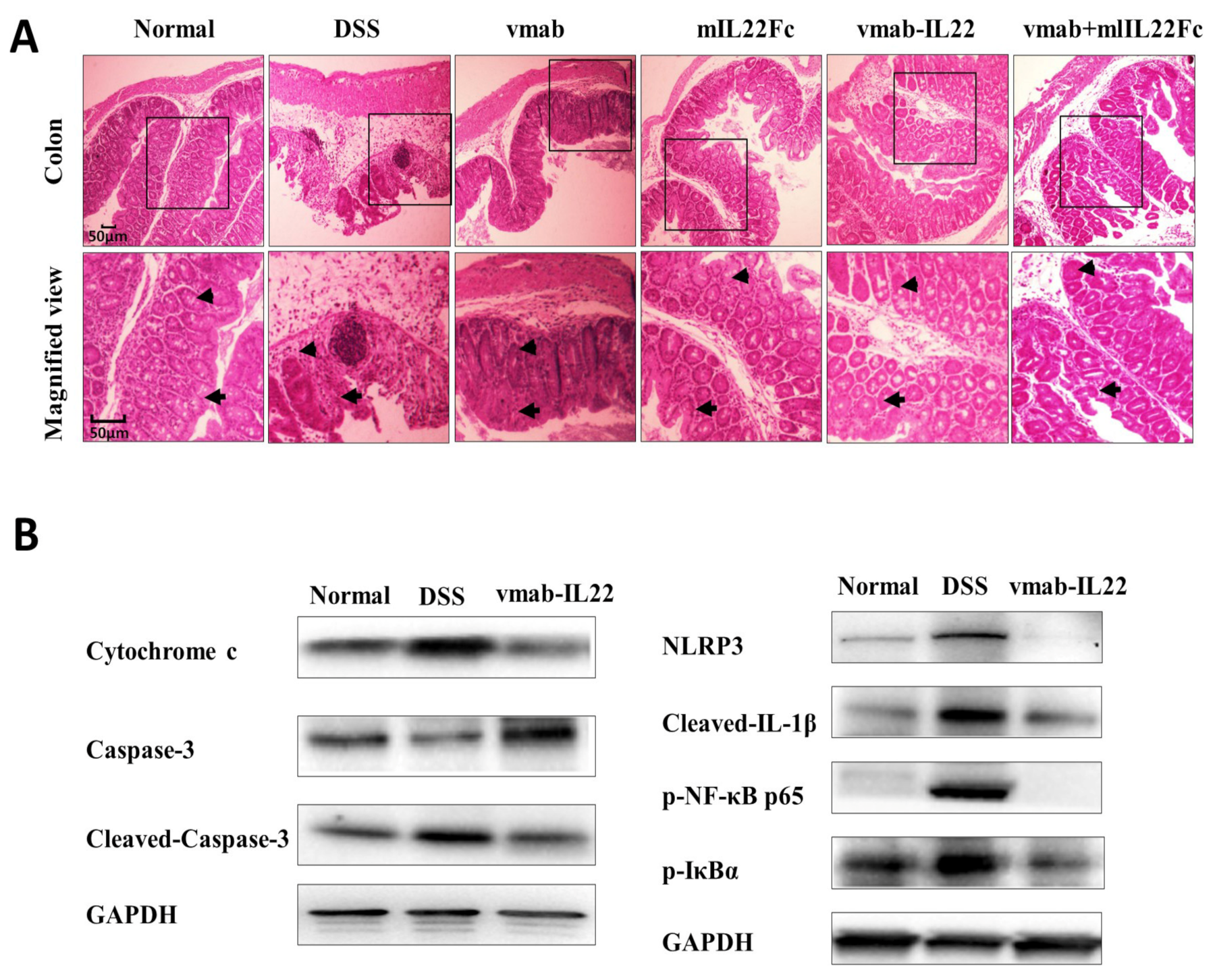
3.6. Vmab-IL22 Strengthens the Intestinal Barrier and Inhibits the Inflammatory Response and Apoptosis in DSS-Treated Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarron, M.; Kondor, R.; Zureick, K.; Griffin, C.; Fuster, C.; Hammond, A.; Lievre, M.; Vandemaele, K.; Bresee, J.; Xu, X.; et al. United States Centers for Disease Control and Prevention support for influenza surveillance, 2013–2021. Bull. World Health Organ. 2022, 100, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Zamora, E.; Trujillo, X.; Huerta, M.; Ríos-Silva, M.; Guzmán-Esquivel, J.; Benites-Godínez, V.; Mendoza-Cano, O. Survival in influenza virus-related pneumonia by viral subtype: 2016–2020. Int. J. Infect. Dis. 2021, 112, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Chen, F.; Zuo, K.; Wu, C.; Yan, Y.; Chen, W.; Lin, W.; Xie, Q. Avian Influenza Virus Subtype H9N2 Affects Intestinal Microbiota, Barrier Structure Injury, and Inflammatory Intestinal Disease in the Chicken Ileum. Viruses 2018, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, G.H.; Kuo, R.L.; Shih, S.R. Role of the intestinal microbiota in the immunomodulation of influenza virus infection. Microbes Infect. 2017, 19, 570–579. [Google Scholar] [CrossRef]
- Sencio, V.; Gallerand, A.; Gomes Machado, M.; Deruyter, L.; Heumel, S.; Soulard, D.; Barthelemy, J.; Cuinat, C.; Vieira, A.T.; Barthelemy, A.; et al. Influenza Virus Infection Impairs the Gut’s Barrier Properties and Favors Secondary Enteric Bacterial Infection through Reduced Production of Short-Chain Fatty Acids. Infect. Immun. 2021, 89, e73420. [Google Scholar] [CrossRef]
- Roach, S.N.; Fiege, J.K.; Shepherd, F.K.; Wiggen, T.D.; Hunter, R.C.; Langlois, R.A. Respiratory Influenza Virus Infection Causes Dynamic Tuft Cell and Innate Lymphoid Cell Changes in the Small Intestine. J. Virol. 2022, 96, e35222. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Wei, H.; Lian, Z.X.; Sun, R.; Tian, Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 2014, 211, 2397–2410. [Google Scholar] [CrossRef]
- Shi, C.C.; Zhu, H.Y.; Li, H.; Zeng, D.L.; Shi, X.L.; Zhang, Y.Y.; Lu, Y.; Ling, L.J.; Wang, C.Y.; Chen, D.F. Regulating the balance of Th17/Treg cells in gut-lung axis contributed to the therapeutic effect of Houttuynia cordata polysaccharides on H1N1-induced acute lung injury. Int. J. Biol. Macromol. 2020, 158, 52–66. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, L.; Li, H.; Shi, X.; Zhang, Y.; Lu, Y.; Zhu, H.; Chen, D. Intestinal microbiota metabolizing Houttuynia cordata polysaccharides in H1N1 induced pneumonia mice contributed to Th17/Treg rebalance in gut-lung axis. Int. J. Biol. Macromol. 2022, 221, 288–302. [Google Scholar] [CrossRef]
- Wen, L.; Shi, L.; Kong, X.L.; Li, K.Y.; Li, H.; Jiang, D.X.; Zhang, F.; Zhou, Z.G. Gut Microbiota Protected Against pseudomonas aeruginosa Pneumonia via Restoring Treg/Th17 Balance and Metabolism. Front. Cell. Infect. Microbiol. 2022, 12, 856633. [Google Scholar] [CrossRef]
- Matsuzaki, G.; Umemura, M. Interleukin-17 family cytokines in protective immunity against infections: Role of hematopoietic cell-derived and non-hematopoietic cell-derived interleukin-17s. Microbiol. Immunol. 2018, 62, 1–13. [Google Scholar] [CrossRef]
- Ogawa, A.; Andoh, A.; Araki, Y.; Bamba, T.; Fujiyama, Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immnnol. 2004, 110, 55–62. [Google Scholar] [CrossRef]
- Righetti, R.F.; Santos, T.M.D.; Camargo, L.D.N.; Aristóteles, L.R.C.R.B.; Fukuzaki, S.; Souza, F.C.R.D.; Santana, F.P.R.; Agrela, M.V.R.D.; Cruz, M.M.; Alonso-Vale, M.I.C.; et al. Protective Effects of Anti-IL17 on Acute Lung Injury Induced by LPS in Mice. Front. Pharmacol. 2018, 9, 1021. [Google Scholar] [CrossRef]
- Bai, J.; Bai, J.; Yang, M. Interleukin-22 Attenuates Acute Pancreatitis-Associated Intestinal Mucosa Injury in Mice via STAT3 Activation. Gut Liver 2021, 15, 771–781. [Google Scholar] [CrossRef]
- Zwarycz, B.; Gracz, A.D.; Rivera, K.R.; Williamson, I.A.; Samsa, L.A.; Starmer, J.; Daniele, M.A.; Salter-Cid, L.; Zhao, Q.; Magness, S.T. IL22 Inhibits Epithelial Stem Cell Expansion in an Ileal Organoid Model. Cell. Mol. Gastroenterol. Hepatol. 2018, 7, 1–17. [Google Scholar] [CrossRef]
- Pociask, D.A.; Scheller, E.V.; Mandalapu, S.; McHugh, K.J.; Enelow, R.I.; Fattman, C.L.; Kolls, J.K.; Alcorn, J.F. IL-22 is essential for lung epithelial repair following influenza infection. Am. J. Pathol. 2013, 182, 1286–1296. [Google Scholar] [CrossRef]
- Paget, C.; Ivanov, S.; Fontaine, J.; Renneson, J.; Blanc, F.; Pichavant, M.; Dumoutier, L.; Ryffel, B.; Renauld, J.C.; Gosset, P.; et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: Potential role in protection against lung epithelial damages. J. Biol. Chem. 2012, 287, 8816–8829. [Google Scholar] [CrossRef]
- Takahashi, Y.; Okamura, Y.; Harada, N.; Watanabe, M.; Miyanishi, H.; Kono, T.; Sakai, M.; Hikima, J.I. Interleukin-22 Deficiency Contributes to Dextran Sulfate Sodium-Induced Inflammation in Japanese Medaka, Oryzias latipes. Front. Immunol. 2021, 12, 688036. [Google Scholar] [CrossRef]
- Su, C.; Liu, S.; Ma, X.; Yang, X.; Liu, J.; Zheng, P.; Cao, Y. Decitabine attenuates dextran sodium sulfate-induced ulcerative colitis through regulation of immune regulatory cells and intestinal barrier. Int. J. Mol. Med. 2020, 46, 583–594. [Google Scholar] [CrossRef]
- Shabgah, A.G.; Navashenaq, J.G.; Shabgah, O.G.; Mohammadi, H.; Sahebkar, A. Interleukin-22 in human inflammatory diseases and viral infections. Autoimmun. Rev. 2017, 16, 1209–1218. [Google Scholar] [CrossRef]
- Geng, H.; Bu, H.F.; Liu, F.; Wu, L.; Pfeifer, K.; Chou, P.M.; Wang, X.; Sun, J.; Lu, L.; Pandey, A.; et al. In Inflamed Intestinal Tissues and Epithelial Cells, Interleukin Signaling Increases Expression of H19 Long Noncoding RNA, Which Promotes Mucosal Regeneration. Gastroenterology 2018, 155, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Patel, V.S.; Harding, J.N.; You, D.; Cormier, S.A. Exposure to combustion derived particulate matter exacerbates influenza infection in neonatal mice by inhibiting IL22 production. Part. Fibre Toxicol. 2021, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Rolla, S.; Alchera, E.; Imarisio, C.; Bardina, V.; Valente, G.; Cappello, P.; Mombello, C.; Follenzi, A.; Novelli, F.; Carini, R. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin. Sci. 2016, 130, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Nair, M.G.; Kirn, T.J.; Zaph, C.; Fouser, L.A.; Artis, D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 2010, 207, 1293–1305. [Google Scholar] [CrossRef]
- McAleer, J.P.; Kolls, J.K. Directing. traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol. Rev. 2014, 260, 129–144. [Google Scholar] [CrossRef]
- Han, L.; Shi, C.; Zeng, X.; Cen, L.; Mei, X.; Fan, J.; Ju, D.; Zhu, H. A Novel Bifunctional Fusion Protein, Vunakizumab-IL22, for Protection Against Pulmonary Immune Injury Caused by Influenza Virus. Front. Immunol. 2021, 12, 727941. [Google Scholar] [CrossRef]
- Bernard-Raichon, L.; Venzon, M.; Klein, J.; Axelrad, J.E.; Zhang, C.; Sullivan, A.P.; Hussey, G.A.; Casanovas-Massana, A.; Noval, M.G.; Valero-Jimenez, A.M.; et al. Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nat. Commun. 2022, 13, 5926. [Google Scholar] [CrossRef]
- Frieder, J.; Kivelevitch, D.; Menter, A. Secukinumab: A review of the anti-IL-17A biologic for the treatment of psoriasis. Ther. Adv. Chronic Dis. 2018, 9, 5–21. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Qiao, Y.Y.; Liu, X.Q.; Xu, C.Q.; Zhang, Z.; Xu, H.W. Interleukin-22 ameliorates acute severe pancreatitis- associated lung injury in mice. World J. Gastroenterol. 2016, 22, 5023–5032. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Z.; Cai, X.; Ren, W.; Dai, F.; Liu, H.; Chang, J.; Li, B. Interleukin 22 attenuated angiotensin II induced acute lung injury through inhibiting the apoptosis of pulmonary microvascular endothelial cells. Sci. Rep. 2017, 7, 2210. [Google Scholar] [CrossRef]


| Gene Name | Primer Sequences |
|---|---|
| GAPDH | Forward primer: 5′-AGGTCGGTGTGAACGGATTTG-3′; Reverse rimer:5′-TGTAGACCATGTAGTTGAGGTCA-3′; |
| IL22R | Forward primer: 5′-TACGTGTG CCGAGTGAAGAC-3′; Reverse primer: 5′-TAACAGAGCAAGCCGACGAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Su, C.; Cen, L.; Han, L.; Tang, J.; Wang, Z.; Shi, X.; Ju, D.; Cao, Y.; Zhu, H. Vunakizumab-IL22, a Novel Fusion Protein, Promotes Intestinal Epithelial Repair and Protects against Gut Injury Induced by the Influenza Virus. Biomedicines 2023, 11, 1160. https://doi.org/10.3390/biomedicines11041160
Shi C, Su C, Cen L, Han L, Tang J, Wang Z, Shi X, Ju D, Cao Y, Zhu H. Vunakizumab-IL22, a Novel Fusion Protein, Promotes Intestinal Epithelial Repair and Protects against Gut Injury Induced by the Influenza Virus. Biomedicines. 2023; 11(4):1160. https://doi.org/10.3390/biomedicines11041160
Chicago/Turabian StyleShi, Chenchen, Chang Su, Lifeng Cen, Lei Han, Jianguo Tang, Zetian Wang, Xunlong Shi, Dianwen Ju, Yiou Cao, and Haiyan Zhu. 2023. "Vunakizumab-IL22, a Novel Fusion Protein, Promotes Intestinal Epithelial Repair and Protects against Gut Injury Induced by the Influenza Virus" Biomedicines 11, no. 4: 1160. https://doi.org/10.3390/biomedicines11041160
APA StyleShi, C., Su, C., Cen, L., Han, L., Tang, J., Wang, Z., Shi, X., Ju, D., Cao, Y., & Zhu, H. (2023). Vunakizumab-IL22, a Novel Fusion Protein, Promotes Intestinal Epithelial Repair and Protects against Gut Injury Induced by the Influenza Virus. Biomedicines, 11(4), 1160. https://doi.org/10.3390/biomedicines11041160







