Abstract
The role of metals in the pathogenesis of Alzheimer’s disease (AD) is still debated. Although previous research has linked changes in essential metal homeostasis and exposure to environmental heavy metals to the pathogenesis of AD, more research is needed to determine the relationship between metals and AD. In this review, we included human studies that (1) compared the metal concentrations between AD patients and healthy controls, (2) correlated concentrations of AD cerebrospinal fluid (CSF) biomarkers with metal concentrations, and (3) used Mendelian randomization (MR) to assess the potential metal contributions to AD risk. Although many studies have examined various metals in dementia patients, understanding the dynamics of metals in these patients remains difficult due to considerable inconsistencies among the results of individual studies. The most consistent findings were for Zn and Cu, with most studies observing a decrease in Zn levels and an increase in Cu levels in AD patients. However, several studies found no such relation. Because few studies have compared metal levels with biomarker levels in the CSF of AD patients, more research of this type is required. Given that MR is revolutionizing epidemiologic research, additional MR studies that include participants from diverse ethnic backgrounds to assess the causal relationship between metals and AD risk are critical.
1. Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common cause of dementia worldwide (60–70% of cases), affecting over 55 million people. It is predicted that 74.7 million people will have dementia by 2030 and approximately 131.5 million by 2050. By 2030, the cost of treating and caring for dementia patients will rise to USD 2 trillion (http://www.worldalzreport2015.org/ accessed on 22 February 2023).
The accumulation of two proteins, amyloid beta (Aβ) and tau, in amyloid plaques and neurofibrillary tangles (NFTs), respectively, is thought to contribute to the development and progression of AD. Although the precise relationship between these two pathologies is unknown, there is evidence that they may interact in a bidirectional manner to promote each other’s aggregation and toxicity [1]. Overall, the relationship between Aβ and tau in AD is complex, with multiple feedback loops and interactions with other pathological processes [2].
Aβ is a small peptide derived from the amyloid precursor protein (APP). APP is a transmembrane protein with an unknown function in the brain, but it is thought to be involved in cell adhesion, signaling, and synapse formation [3]. Different enzymes can cleave APP, resulting in the production of a number of peptides of variable lengths. β-Secretase cleaves APP at the N-terminus of the Aβ domain in the amyloidogenic pathway, followed by γ-secretase cleavage within the transmembrane domain, resulting in the production of Aβ peptides with varying lengths [4]. Excessive Aβ formation, aggregation, and deposition in the brain are thought to result in the formation of amyloid plaques [5]. Aβ forms insoluble oligomers and even larger insoluble fibers when it aggregates. The longer form of the peptide (Aβ1–42) promotes abnormal Aβ peptide aggregation more strongly than the shorter form (Aβ1–40) [6]. The amyloid build-up is thought to activate microglia and cause an inflammatory response [7]. Aβ can also accumulate in the walls of meningeal and cerebral arteries, arterioles, capillaries, and veins, causing a condition known as cerebral amyloid angiopathy (CAA) [8,9,10,11].
Tau, on the other hand, is a microtubule-associated protein found primarily in the axons of healthy neurons. In this context, tau is a critical regulator of microtubule dynamics, modulating their assembly, elongation, and maturation. Tau helps stabilize microtubules and keep them aligned, which is necessary for neuronal function and transport of essential molecules and organelles [12]. Tau regulates the length, stability, and thickness of axonal microtubules by cross-linking of α and β tubulin monomers [13].
The underlying mechanisms that precede the formation of amyloid plaques and NFTs in the brains of AD patients as well as the relationships between these pathological lesions are not well understood [14,15]. The toxicity of Aβ deposits and mechanical damage to axons that impair axoplasmic transport, resulting in axonal sprouting to bridge the damaged portion of the axon, are two of the proposed mechanisms [16,17]. Axon sprouts and their microtubules should become less stable for the sprouts to form synapses, which becomes possible once tau proteins detach from the microtubules. Tau undergoes numerous post-translational modifications in AD including phosphorylation, acetylation, and O-glycosylation [18]. The phosphorylation of tau causes it to undergo a change in conformation and separate from microtubules [19]. When hyperphosphorylated, tau detaches from microtubules, the axons also disintegrate, resulting in neuronal death [20]. It is possible, however, that tau cleavage or folding occurs first, followed by phosphorylation and detachment from the microtubules [21]. In addition to amyloid and tau-related mechanisms, oxidative stress, neuroinflammation, mitochondria, lysosomes, neurovascular, and cell cycle dysfunction all play important roles in the pathological process of AD [22,23,24,25].
Previous studies have revealed that the homeostasis of essential metals is altered in AD [26,27,28], where iron (Fe), zinc (Zn), and copper (Cu) are the essential metals most commonly associated with AD pathological changes. Heavy metal concentrations have also been found to rise in AD brains [29].
This review summarizes research on the role of essential and heavy metals in AD. We included human studies that (1) compared the metal concentrations in AD patients and healthy controls, (2) compared the metal concentrations with concentrations of cerebrospinal fluid (CSF) biomarkers in AD subjects, and (3) used a Mendelian randomization methodology (MR) to assess the involvement of essential metals in AD. Two independent researchers searched Medline using the following keywords: “Alzheimer’s disease”, “aluminum”, “arsenic”, “barium”, “cobalt”, “copper”, “cadmium”, “calcium”, “iron”, “lithium”, “lead”, “mercury“, “magnesium”, “molybdenum”, “manganese”, “nickel”, “potassium”, “selenium”, “sodium”, “strontium”, “thallium”, and “zinc”. The literature search was completed on 23 February 2023.
2. Molecular Mechanisms through Which Metals Contribute to Alzheimer’s Disease Pathology
Increased metal concentration in the brain may contribute to various AD-associated pathological processes including Aβ-aggregation [30,31], hyperphosphorylation of tau protein [32,33], neuroinflammation [34], oxidative stress [35], blood–brain barrier (BBB) impairment [36], apoptosis and necrosis of neurons [37,38], and autophagy [39] (Figure 1). Experimental evidence indicates that both essential metals and heavy metals increase the aggregation of Aβ [30,40,41] and the hyperphosphorylation and aggregation of tau protein [33,42,43,44]. Furthermore, the exposure of young rats to a mixture of heavy metals induced neuroinflammation dependent on oxidative stress [45]. In addition, some essential metals such as Fe [46], Cu [47], Zn, and calcium (Ca) [39] can induce oxidative stress. Fe participates in Fenton reactions and can therefore contribute to the formation of reactive oxygen species [46]. Both the observed disruption of the BBB [48,49] and the apoptosis and necrosis of neurons [37,38] upon exposure to heavy metals may be preceded by oxidative stress, according to experimental evidence. Neurons are extremely sensitive to oxidative stress. Wang et al. [39] proposed that metal ion imbalance could induce oxidative stress, with the following downstream effects: (1) imbalance of protein kinases and phosphatases, increasing tau protein phosphorylation, and (2) imbalance of secretases, resulting in an increase in Aβ production (reviewed in [39]). On the other hand, essential metals also serve as cofactors in enzymes that combat oxidative stress. Cu, Zn, and manganese (Mn) are enzyme components of superoxide dismutase enzymes, while selenium is an enzyme component of glutathione peroxidase [50].
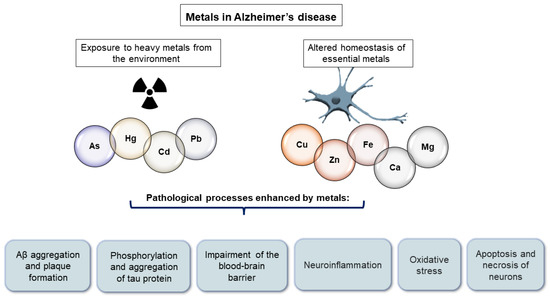
Figure 1.
Pathological processes enhanced by metals in Alzheimer’s disease.
Although there is a substantial body of evidence linking metals to AD-related pathological processes, it is unclear whether disrupted metal homeostasis is involved in the pathogenesis of AD, results from AD pathological processes, or both. Given that AD is a complex disease driven by both genetic and environmental factors, it is unlikely that AD pathogenesis will be explained by a single factor, but rather by the interaction of many.
3. Heavy Metals in Alzheimer’s Disease
Heavy metals including arsenic (As) [51], cadmium (Cd) [49], lead (Pb) [52], and mercury (Hg) [52] can cross the BBB and accumulate in the brain, or they can bypass the BBB and enter the brain directly through the olfactory pathway [53]. Some researchers have hypothesized that early exposure to heavy metals is associated with the later development of AD. Based on their observations of experimental animals, they concluded that early-life exposure to As [54], Pb [55], and Cd [56] may contribute to the development of neurodegeneration later in life, which is consistent with the developmental hypothesis of AD [57,58,59].
3.1. Arsenic
As is a metalloid that can be ingested through contaminated water, soil, and air, but primarily through drinking contaminated water. More than 220 million people are estimated to consume water that exceeds the permissible level of 10 µg/L [60]. Epidemiological studies suggest that As contributes to cognitive impairment [61] and an increased risk of AD [62], and that elevated As levels in soil are associated with an increase in AD-related mortality [63]. As exposure has also been associated with memory impairments in animal studies [64,65,66]. As exposure also increases Aβ levels [67], promotes tau hyperphosphorylation [32,68,69], tau aggregation [32], oxidative stress caused mainly by mitochondrial dysfunction [70], vascular damage [71], neuroinflammation [34], and apoptosis and the necrosis of neurons [37,38] (Figure 1). In the majority of human studies, there were no significant differences in As levels between AD patients and the controls, although some studies observed a significant increase in As levels in AD patients [72,73] and a positive association with CSF AD biomarkers [74].
3.2. Cadmium
Humans are exposed to Cd through food, air, and water [75]. Smokers have Cd levels that are two to four times higher than nonsmokers [76]. Cd may also play a role in the development of AD pathological changes. Cd has been linked in human studies to increased mortality due to AD [77,78] and cognitive decline [79,80,81]. Ruczaj and Brzoska proposed that Cd primarily exerts its effects by inducing oxidative stress [82]. Nevertheless, it also interacts with Aβ [83] and increases Aβ aggregation [30,40], promotes tau hyperphosphorylation [33] and aggregation [42], impairs the BBB [48,49], impairs cholinergic transmission and causes the death of cholinergic neurons in the basal forebrain [84], and disrupts intracellular cation homeostasis by being an anti-metabolite of Zn and replacing it in Zn enzymes [85] (Figure 1). In human studies, there is either an increase [86] or no difference [87] in Cd levels between AD patients and healthy controls (Table 1).

Table 1.
Comparison of metal levels between dementia patients and healthy controls.
3.3. Mercury
Exposure to Hg occurs through food, air, and water, with seafood consumption being the primary source of mercury poisoning [152]. Three- to 5-fold increases in Hg levels in the air and water have been documented as a result of industrialization [153]. A systematic review [154] and meta-analysis [155] demonstrated an association between Hg exposure and cognitive decline and progression of AD, but a subsequent report [156] did not confirm these findings. In addition, a neuropathological study of 286 brains by Morris et al. revealed no correlation between higher brain Hg levels and neuropathological alterations [152]. However, there are multiple molecular mechanisms through which Hg may contribute to the pathogenesis of AD. It promotes Aβ production [157] and aggregation [30], tau hyperphosphorylation [158,159] and aggregation [160], induces oxidative stress [35], and alters calcium homeostasis [161] (Figure 1). Human body fluid Hg measurements yielded contradictory results. Both an increase and a decrease were observed in Hg levels between the AD and control subjects, or there was no change (Table 1). In addition, the CSF Hg level was positively correlated with several CSF AD biomarkers [74], whereas the blood Hg level was positively correlated with the CSF Aβ1–42 level [145].
3.4. Lead
In addition to food, air, and water, humans are also exposed to lead [29] through ingestion. Epidemiological studies have demonstrated that lead exposure contributes to cognitive impairment [162,163]. Moreover, experimental studies have reported an association between Pb and AD pathological changes. Pb interacts with Aβ [31] and increases Aβ production [45,164] and aggregation [31], increases tau hyperphosphorylation [165], compromises the BBB [36], induces epigenetic modifications by altering the expression of AD-related genes [166,167], disrupts intracellular cation homeostasis by interfering with Ca homeostasis and replacing Zn ions in Zn enzymes [168], and induces oxidative stress [169]. In human studies, there was a decrease or no difference in the Pb levels between the AD patients and control subjects (Table 1), whereas a recent MR study found that higher blood Pb levels were a risk factor for AD [170].
3.5. Aluminum
Aluminum (Al), the most abundant metal in the Earth’s crust [171], is not an essential element for life; however, in its free, solvated, and trivalent forms, Al3+ is biologically reactive [172], accumulating in the central nervous system [173,174]. In AD-affected brain regions including the entorhinal cortex, hippocampal region, and amygdala, the concentration of Al is higher [175,176]. Al was co-deposited with fibrillar Aβ in amyloid plaques in a study of brain tissue samples from donors with familial AD (fAD) and the PSEN1-E280A (Glu280Ala) mutation [172,177]. Cortical Aβ levels are elevated in donors with this mutation, and this mutation is associated with an aggressive etiology of AD [178]. Aluminum’s unique association with Aβ and the high levels of Al found in these brain tissues suggest that Al plays a role in the neuropathology of fAD [177].
When Al binds to various proteins, oligomerization can occur, resulting in conformational changes that prevent proteases from degrading the proteins. In addition, Al3+ binds strongly to phosphorylated amino acids, causing highly phosphorylated cytoskeleton proteins to aggregate and accumulate [179]. As a result, Al induces the apoptotic death of neurons and glial cells. Al-Aβ co-deposition in fAD has been hypothesized, but its association with intraneuronal NFTs has not been confirmed [177,180], as demonstrated by Mold et al. [181]. While Al binding to Aβ in amyloid plaques is anticipated in the early stages of disease progression [177,178,182], an association with tau may occur in later disease stages [177,178,182]. Numerous studies have investigated the association between oral exposure to Al in drinking water and AD [183]. According to Martyn et al. [184], AD is more prevalent in regions with high levels of Al in their drinking water. In conclusion, even though Al has been proposed as a potential risk factor for AD, there is insufficient evidence to support a causal relationship (Table 2). Many studies have investigated the association between oral exposure to Al in drinking water and AD; however, more research is required to better understand how genetic, environmental, and lifestyle factors influence the onset and progression of AD.

Table 2.
Correlation between metals and CSF protein AD biomarkers.
4. Essential Metals in Alzheimer’s Disease
The homeostasis of essential metals is altered in AD patients [26,27,28]. This term refers to metals that are naturally present in the body and play a role in the function of numerous proteins and enzymes or act as second messengers. Sodium (Na), Ca, and magnesium (Mg) are the most abundant essential metals in the human body, while Fe, Cu, Zn, molybdenum (Mo), cobalt (Co), Mn, and chromium (Cr) are present in trace amounts. Many previous studies have also demonstrated the association between essential metals (primarily Fe, Cu, and Zn) and AD pathological changes.
4.1. Iron
Many biological processes in the body including the brain are regulated by Fe ions. Fe is essential for protein synthesis [197], cell growth and differentiation [198,199], the regulation of Fe-dependent enzymes [200], oxygen transport [201], and the electron transfer chain in oxidation–reduction reactions [201]. Fe is also crucial for the processes of myelination [202], development [203], and the function of numerous neurotransmitter systems [204]. Both amyloid plaques and NFTs have been found to have elevated Fe concentrations [205]. Fe is also involved in oxidative stress and the formation of reactive oxygen species in the brains of AD patients via the Fenton reaction [46]. Fe also promotes in vitro Aβ aggregation [206], tau protein phosphorylation [207,208,209], and tau aggregation [210] (Figure 1). It is interesting to note that APP is necessary for the persistence of ferroprotein (iron exporter) on the cell surface, and thus promotes Fe release [211].
In meta-analyses, a significant decrease in Fe levels was observed in the plasma [87] and serum [129] of AD patients, but no significant change was observed in the CSF [129] (Table 1). In contrast, a number of studies observed a correlation between the Fe levels in CSF and various CSF AD biomarkers [74,188,193] (Table 2). Nonetheless, in many observational studies, there was no difference in the Fe levels between the AD patients and controls (Table 1)..
4.2. Zinc
The brain has a higher Zn concentration than other organs [212]. Zn is essential for neurotransmission because, as an antagonist of glutamate NMDA (N-methyl-D-aspartate) receptors, it protects neurons from glutamate-induced excitotoxic damage [213]. Zn accumulates in amyloid plaques [214], binds to Aβ, and promotes Aβ aggregation and plaque formation [214]. Zn also promotes tau protein aggregation [215], phosphorylation [216,217], and translation [217] (Figure 1). In meta-analyses, however, a significant decrease in Zn levels was observed in the serum and plasma [123] as well as in the hair of AD patients [87], whereas there was no significant change in the CSF [123] and brain [115] levels (Table 1). To date, MR studies have not identified Zn as a risk factor for AD [218,219,220]. An in vivo study demonstrated positive effects of Zn supplementation in mouse models of AD [221], and a small double-blind clinical trial observed the stabilization of cognitive abilities in AD patients after six months [222]. Thus, adding Zn to the diet has been suggested to improve the cognitive abilities of AD patients [223], whereas Loef et al. found no significant benefit of Zn supplementation in AD [224]. In addition, in vivo studies have shown that Zn supplementation promotes the formation of NFTs [225] and Aβ deposition [226].
4.3. Copper
Normal brain function requires optimal Cu levels, as indicated by the disruption of its metabolism. Patients with Menkes syndrome, for example, suffer from intellectual deficits and neurodegeneration. This disorder is caused by a sex-linked mutation of the ATP7A gene on the X chromosome (which encodes a protein involved in the transmembrane transfer of Cu ions) and is characterized by the decreased absorption of Cu in the intestine, and consequently, a decreased concentration of Cu in the cytosol of all body cells except in the intestines and kidneys [227]. In Wilson’s disease, excessive Cu accumulation in the body is associated with psychosis, parkinsonism, and dementia [228,229]. Cu homeostasis is also impaired in AD [28]. Cu promotes the formation and accumulation of Aβ-oligomers by binding to Aβ [41]. Cu chelation can prevent the cytotoxic effect of the Cu-Aβ complex [230]. Cu accumulates in plaques [231,232], and the interaction between Cu and APP has been demonstrated [232]. Cu can induce both the phosphorylation and aggregation of tau [42,43] (Figure 1) and its interaction with apolipoprotein E (ApoE) contributes to the pathogenesis of AD. ApoE2 has the highest binding affinity for divalent Cu, Zn, and Fe ions, while ApoE4 has the lowest [233,234]. In meta-analyses, Cu levels in the serum of AD patients increased significantly [87,92,122,133], whereas Cu levels in the brains of AD patients decreased [92] (Table 1). Recent MR studies [218,220] have surprisingly found that higher Cu levels are protective against AD risk.
4.4. Calcium
Ca is an indispensable second messenger that regulates hundreds of signaling pathways crucial for the normal functioning of memory and cognition-related cells and networks [235]. Many neurodegenerative diseases including AD [236] are characterized by a disruption of cellular Ca signaling. The excessive entry of Ca ions through ionotropic glutamate receptors is a known mechanism of excitotoxic neuronal death [237,238]. Ca homeostasis disruption promotes Aβ and tau pathology [239]. However, human studies have produced contradictory results, with both decreased [240,241] and increased Ca [186,242] being risk factors. In recent MR studies, higher Ca levels were shown to reduce the risk of AD [241,243], or no association between Ca levels and AD risk has been observed [218,220] (Table 3).

Table 3.
Mendelian randomization studies that investigated the role of metals in AD.
4.5. Manganese
Mn is a crucial element for protein synthesis, lipid and glucose metabolism, and oxidative stress protection [244]. However, Mn is also an environmental toxin, and elevated Mn levels have been linked to diminished cognitive performance [187,245,246]. A rise in Mn levels has also been observed in patients with AD [109]. Nonetheless, a meta-analysis by Du et al. [89] revealed a significant decrease in Mn levels between AD and the controls.
4.6. Magnesium
Human studies have demonstrated that Mg deficiency impairs memory [247] and that Mg supplementation can improve memory in dementia patients [248,249,250]. In addition, a decrease in Mg concentration has been observed in the tissues of AD patients [251,252]. However, no change in Mg concentration was observed in the brains of AD patients in some studies (reviewed in [253]). Mg influences the processing and transport of APP, with low Mg levels favoring the β-secretase pathway and high Mg levels favoring the α-secretase pathway [254], whereas the treatment of experimental animals with Mg sulfate reduces tau phosphorylation and influences the maintenance of cognitive functions and synaptic plasticity [255]. According to the meta-analysis by Du et al. [89], the serum and plasma Mg concentrations were lower in the AD patients than in the controls, whereas the CSF Mg concentrations did not differ between groups. Thomassen et al. [95] did not find an association between the plasma Mg levels and the risk of AD in a study involving more than 100,000 participants. Kieboom et al. demonstrated that both low and high Mg concentrations were associated with an increased risk of dementia. They concluded that the relationship between Mg and the risk of dementia was U-shaped rather than linear [108].
4.7. Other Essential Metals
AD also perturbs the homeostasis of Na, K, and Co. Previous studies have associated elevated Na levels with AD [27,96,256,257]. Both increased [102] and decreased [195] K levels have been associated with AD, whereas in some studies, no change in the K levels was observed in AD. Co is an essential component of vitamin B12 and is an environmental toxin. Zheng et al. showed that mice exposed to Co develop age-related neurodegeneration [258].
5. Treatment of Alzheimer’s Disease Based on the Metal Hypothesis
So far, therapeutic interventions based on the metal hypothesis of AD have progressed in two directions. Taking metal supplements is one approach. As previously stated, there have been several clinical studies on the effect of Zn and Cu supplementation on cognitive performance [259]. Although it was previously thought that metal supplementation could delay the onset of dementia, the majority of studies have found no significant effect on cognitive function improvement [259]. Cu supplementation had no positive effects in a pilot phase 2 clinical trial in AD patients [260]. In contrast to this viewpoint, there is a theory about the therapeutic effect of chelating excess Zn, Cu, or Fe metals based on their ability to stimulate Aβ aggregation [261]. Chelators are substances that bind metals so that they cannot interact with the Aβ further. Based on promising results from in vivo studies on animal models [262], one of the compounds with chelating activity, clioquinol (CQ), has entered the second phase of clinical trials [263]. CQ can effectively bind Zn2+ and Cu2+ ions, and in vitro studies have shown that its effect can be achieved by stimulating neuroprotective signaling pathways by increasing the cellular uptake of Zn and Cu ions [264]. Furthermore, CQ can reduce the number of Aβ aggregates within the cells [264]. A clinical study found that the CQ had a promising effect on cognitive improvement, but only in patients with the worst starting point [263]. PBT-2, another similar substance, had a similar effect on AD patients. In a 2008 clinical trial, PBT-2 demonstrated an impact on Aβ metabolism by lowering the Aβ CSF concentrations, and a group of patients who received a higher dose of PBT-2 had better results in two of the executive functions tests, while other cognitive tests revealed no significant differences [265]. A recent study investigated the different capacities for chelating Cu from Cu(II)Aβ(1–42) complexes, and the results showed that CQ and B2Q were more efficient than PBT-2 [266], but it must be noted that when using such substances, they must be not too effective as chelators to disrupt the normal metal functions in the brain. Another recent in vivo study examining the effect of Cu ions on tau protein pathological changes found that lowering the Cu concentrations in the brain could help alleviate spatial memory deficits, but, neither lowering nor increasing the amounts of Cu affected the tau protein pathology [267]. A recent review study called the chelating theory into question on multiple levels [268]. As a result, there is no single metal-target approach for treating AD, and more research is needed to better understand this complex aspect of the disease.
6. Conclusions
In this review, we discussed the studies examining the role of essential metals and heavy metals in AD. Relevant studies involving human subjects were included. It is still challenging to paint a complete picture of how metals interact in AD pathogenesis because of the significant degree of variability in the results between studies. Cu and Zn showed the most consistent results, with most studies revealing that the AD patients’ Cu levels rose while their Zn levels fell (Table 1). However, several studies also failed to find such a link (Table 1). Comparing the metal levels with biomarkers from the AD subjects’ CSF has rarely been undertaken, and the results were sometimes contradictory (Table 2). The use of various methodologies to determine the metal levels and examine various body fluids may be the cause of the studies’ contradictory findings. Atomic absorption spectrophotometry (AAS) was the second most often employed method, while inductively coupled plasma mass spectrometry (ICP-MS) was the method of choice, being the method utilized in the majority of investigations into the metal measurements. Several MR studies have investigated the relationship between metals and the risk of AD (Table 3). Since MR is revolutionizing epidemiologic research [269], and given the importance of elucidating the role of metals in AD pathogenesis, additional MR studies examining the causal association between metals and AD risk and including people from various ethnic backgrounds are crucial.
Author Contributions
Conceptualization, M.B.L. and G.Š.; Validation, L.L.H., E.Š.P., K.Z. and P.R.H.; Investigation, M.B.L.; Resources, G.Š.; Writing—original draft preparation, M.B.L.; Writing—review and editing, L.L.H., E.Š.P., K.Z., M.B.L., P.R.H. and G.Š.; Visualization, M.B.L. and G.Š.; Supervision, G.Š.; Project administration, G.Š.; Funding acquisition, G.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by The Croatian Science Foundation grants IP-2019-04-3584 (“Role of the blood–brain barrier, innate immunity, and tau protein oligomerization in the pathogenesis of Alzheimer’s disease”) and IP-2014-09-9730 (“Tau protein hyperphosphorylation, aggregation, and trans-synaptic transfer in Alzheimer’s disease: cerebrospinal fluid analysis and assessment of potential neuroprotective compounds”) to GŠ, and by the Scientific Center of Excellence for Basic, Clinical, and Translational Neuroscience CoRE-NEURO (“Experimental and clinical research of hypoxic-ischemic damage in perinatal and adult brain”; GA KK01.1.1.01.0007 funded by the European Union through the European Regional Development Fund), and in part by the NIH grant P30 AG066514 to PRH.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AAS, atomic absorption spectrophotometry; Aβ, amyloid β; Aβ1–40, amyloid β1–40; Aβ1–42, amyloid β1–42; AD, Alzheimer’s disease; Al, aluminum; ALS, amyotrophic lateral sclerosis; AMD, age-related macular degeneration; ApoE, apolipoprotein E; APP, amyloid precursor protein; As, arsenic; BBB, blood-brain barrier; Ca, calcium; CAA, cerebral amyloid angiopathy; Cd, cadmium; CI, confidence interval; Co, cobalt; CQ, clioquinol; Cr, chromium; Cs, cesium; CSF, cerebrospinal fluid; Cu, copper; DLB, dementia with Lewy bodies; fAD, familial AD; FAAS, flame atomic absorption spectrometry; Fe, iron; FTD, frontotemporal dementia; GF-AAS, graphite furnace atomic absorption spectrophotometry; HC, healthy controls; Hg, mercury; HR, hazard ratio; HR-ICP-MS, high resolution inductively coupled plasma mass spectrometry; ICP-AES, inductively coupled plasma atomic emission spectrometer; ICP-DRC-MS, inductively coupled plasma dynamic reaction cell mass spectrometry; ICP-MS, inductively coupled plasma mass spectroscopy; K, potassium; Li, lithium; MCI, mild cognitive impairment; Mg, magnesium; Mn, manganese; Mo, molybdenum; MR, Mendelian randomization; MS, multiple sclerosis; Na, sodium; NFL, neurofilament light chain; NFT, neurofibrillary tangles; Ni, nickel; NMDA, N-methyl-D-aspartate; P, phosphorus; PAPP-A, pregnancy-associated plasma protein A, pappalysin-1; Pb, lead; PD, Parkinson’s disease; p-tau181, tau phosphorylated at Thr 181; p-tau231, tau phosphorylated at Thr 231; p-tau199, tau phosphorylated at Ser 199; S, sulfur; Se, selenium; SEC-ICP-MS, size exclusion chromatography inductively coupled plasma mass spectrometry; SMC, subjective memory complaint; SMD, standardized mean differences; SP, senile plaques; S100B, S100 calcium-binding protein B; t-tau, total tau; VaD, vascular dementia; VILIP-1, visinin-like protein 1; WD, Wilson disease; WMD, weighted mean difference; YKL-40, chitinase-3-like protein 1; Zn, zinc.
References
- Roda, A.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, J.; Ho, A.; Watanabe, H.; Bolshakov, V.Y.; Shen, J. APP Family Regulates Neuronal Excitability and Synaptic Plasticity but Not Neuronal Survival. Neuron 2020, 108, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Thompson, R.; Zhang, H.; Xu, H. APP processing in Alzheimer’s disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaupt, G.; Mcdonald, B.L.; Beyreuthert, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Med. Sci. 1985, 82, 4245–4249. [Google Scholar] [CrossRef]
- Jarrett, J.T.; Berger, E.P.; Lansbury, P.T. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry 1993, 32, 4693–4697. [Google Scholar] [CrossRef]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Attems, J.; Lintner, F.; Jellinger, K.A. Amyloid beta peptide 1–42 highly correlates with capillary cerebral amyloid angiopathy and Alzheimer disease pathology. Acta Neuropathol. 2004, 107, 479–480. [Google Scholar] [CrossRef]
- Jellinger, K.A. Alzheimer disease and cerebrovascular pathology: An update. J. Neural Transm. 2002, 109, 813–836. [Google Scholar] [CrossRef]
- Preston, S.D.; Steart, P.V.; Wilkinson, A.; Nicoll, J.A.R.; Weller, R.O. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: Defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol. Appl. Neurobiol. 2003, 29, 106–117. [Google Scholar] [CrossRef]
- Zekry, D.; Duyckaerts, C.; Belmin, J.; Geoffre, C.; Moulias, R.; Hauw, J.-J. Cerebral amyloid angiopathy in the elderly: Vessel walls changes and relationship with dementia. Acta Neuropathol. 2003, 106, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of tau as a microtubule-associated protein: Structural and functional aspects. Front. Aging Neurosci. 2019, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.V.; Jenkins, S.M. Tau protein in normal and Alzheimer’s disease brain. J. Alzheimers Dis. 1999, 1, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Blazquez-Llorca, L.; Valero-Freitag, S.; Rodrigues, E.F.; Merchán-Pérez, Á.; Rodríguez, J.R.; Dorostkar, M.M.; DeFelipe, J.; Herms, J. High plasticity of axonal pathology in Alzheimer’s disease mouse models. Acta Neuropathol. Commun. 2017, 5, 14. [Google Scholar] [CrossRef]
- Masliah, E.; Mallory, M.; Hansen, L.; Alford, M.; Albright, T.; DeTeresa, R.; Terry, R.; Baudier, J.; Saitoh, T. Patterns of aberrant sprouting in Alzheimer’s disease. Neuron 1991, 6, 729–739. [Google Scholar] [CrossRef]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Di Giovanni, G.; et al. Tau Protein Hyperphosphorylation and Aggregation in Alzheimer’s Disease and Other Tauopathies, and Possible Neuroprotective Strategies. Biomolecules 2016, 6, 6. [Google Scholar] [CrossRef]
- Teter, B.; Ashford, J.W. Neuroplasticity in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 402–437. [Google Scholar] [CrossRef]
- Mandelkow, E.M.; Mandelkow, E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998, 8, 425–427. [Google Scholar] [CrossRef]
- Skrabana, R.; Kontsek, P.; Mederlyova, A.; Iqbal, K.; Novak, M. Folding of Alzheimer’s core PHF subunit revealed by monoclonal antibody 423. FEBS Lett. 2004, 568, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Jurcău, M.C.; Andronie-Cioara, F.L.; Jurcău, A.; Marcu, F.; Ţiț, D.M.; Pașcalău, N.; Nistor-Cseppentö, D.C. The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer’s Disease: Therapeutic Implications and Future Perspectives. Antioxidants 2022, 11, 2167. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Španić, E.; Langer Horvat, L.; Hof, P.R. Blood-brain barrier and innate immunity in the pathogenesis of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2019, 168, 99–145. [Google Scholar] [CrossRef] [PubMed]
- Španić, E.; Langer Horvat, L.; Ilić, K.; Hof, P.R.; Šimić, G. NLRP1 Inflammasome Activation in the Hippocampal Formation in Alzheimer’s Disease: Correlation with Neuropathological Changes and Unbiasedly Estimated Neuronal Loss. Cells 2022, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, C.; Sun, J.; Shen, H.M.; Wang, J.; Yang, C. Impairment of the autophagy–lysosomal pathway in Alzheimer’s diseases: Pathogenic mechanisms and therapeutic potential. Acta Pharm. Sin. B 2022, 12, 1019–1040. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I. The metal theory of Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33 (Suppl. S1), S277–S281. [Google Scholar] [CrossRef]
- Babić Leko, M.; Jurasović, J.; Nikolac Perković, M.; Španić, E.; Sekovanić, A.; Orct, T.; Lukinović Škudar, V.; Bačić Baronica, K.; Kiđemet-Piskač, S.; Vogrinc, Ž.; et al. The Association of Essential Metals with APOE Genotype in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, 661–672. [Google Scholar] [CrossRef]
- Zubčić, K.; Hof, P.R.; Šimić, G.; Jazvinšćak Jembrek, M. The Role of Copper in Tau-Related Pathology in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 572308. [Google Scholar] [CrossRef]
- Elonheimo, H.M.; Andersen, H.R.; Katsonouri, A.; Tolonen, H. Environmental Substances Associated with Alzheimer’s Disease-A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 11839. [Google Scholar] [CrossRef]
- Yano, K.; Hirosawa, N.; Sakamoto, Y.; Katayama, H.; Moriguchi, T. Aggregations of amyloid beta-proteins in the presence of metal ions. Toxicol. Lett. 2003, 144, 134. [Google Scholar] [CrossRef]
- Wallin, C.; Sholts, S.B.; Österlund, N.; Luo, J.; Jarvet, J.; Roos, P.M.; Ilag, L.; Gräslund, A.; Wärmländer, S.K.T.S. Alzheimer’s disease and cigarette smoke components: Effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-β peptide aggregation. Sci. Rep. 2017, 7, 14423. [Google Scholar] [CrossRef] [PubMed]
- Wisessaowapak, C.; Visitnonthachai, D.; Watcharasit, P.; Satayavivad, J. Prolonged arsenic exposure increases tau phosphorylation in differentiated SH-SY5Y cells: The contribution of GSK3 and ERK1/2. Environ. Toxicol. Pharmacol. 2021, 84, 103626. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Alfaifi, M.Y. Trans-resveratrol Inhibits Tau Phosphorylation in the Brains of Control and Cadmium Chloride-Treated Rats by Activating PP2A and PI3K/Akt Induced-Inhibition of GSK3β. Neurochem. Res. 2019, 44, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yang, J.; Zhang, Y.; Li, T.; Wang, C.; Xu, L.; Hu, Q.; Wang, X.; Jiang, S.; Nie, X.; et al. Arsenic trioxide mediates HAPI microglia inflammatory response and subsequent neuron apoptosis through p38/JNK MAPK/STAT3 pathway. Toxicol. Appl. Pharmacol. 2016, 303, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Augusti, P.R.; Conterato, G.M.M.; Somacal, S.; Sobieski, R.; Spohr, P.R.; Torres, J.V.; Charão, M.F.; Moro, A.M.; Rocha, M.P.; Garcia, S.C.; et al. Effect of astaxanthin on kidney function impairment and oxidative stress induced by mercuric chloride in rats. Food Chem. Toxicol. 2008, 46, 212–219. [Google Scholar] [CrossRef]
- Gu, H.; Territo, P.R.; Persohn, S.A.; Bedwell, A.A.; Eldridge, K.; Speedy, R.; Chen, Z.; Zheng, W.; Du, Y. Evaluation of chronic lead effects in the blood brain barrier system by DCE-CT. J. Trace Elem. Med. Biol. 2020, 62, 126648. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Bhaumik, S.; Purkayastha, M.; Basu, S.; Nag Chaudhuri, A.; Das Gupta, S. Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol. Lett. 2002, 136, 65–76. [Google Scholar] [CrossRef]
- Bashir, S.; Sharma, Y.; Irshad, M.; Gupta, S.D.; Dogra, T.D. Arsenic-induced cell death in liver and brain of experimental rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 38–43. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.L.; Liu, X.Z.; Shen, P.; Zheng, Y.G.; Lan, X.R.; Lu, C.B.; Wang, J.Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 10. [Google Scholar] [CrossRef]
- Syme, C.D.; Viles, J.H. Solution 1H NMR investigation of Zn2+ and Cd2+ binding to amyloid-beta peptide (Abeta) of Alzheimer’s disease. Biochim. Biophys. Acta 2006, 1764, 246–256. [Google Scholar] [CrossRef]
- Jiao, Y.; Han, D.; Yang, P. Molecular modeling of the inhibitory mechanism of copper(II) on aggregation of amyloid β-peptide. Sci. China Ser. B 2005, 48, 580. [Google Scholar] [CrossRef]
- Kitazawa, M.; Cheng, D.; Laferla, F.M. Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J. Neurochem. 2009, 108, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-X.; Du, J.-T.; Zeng, Z.-Y.; Wu, W.-H.; Zhao, Y.-F.; Kanazawa, K.; Ishizuka, Y.; Nemoto, T.; Nakanishi, H.; Li, Y.-M. Copper (II) modulates in vitro aggregation of a tau peptide. Peptides 2007, 28, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.F.; Yao, T.M.; Zhu, Z.L.; Wang, C.; Ji, L.N. Impacts of Cd(II) on the conformation and self-aggregation of Alzheimer’s tau fragment corresponding to the third repeat of microtubule-binding domain. Biochim. Biophys. Acta 2007, 1774, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Rai, N.K.; Tripathi, S.; Bandyopadhyay, S. Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol. Sci. 2015, 143, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Kanti Das, T.; Wati, M.R.; Fatima-Shad, K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association with Alzheimer’s Disease. Arch. Neurosci. 2015, 2, e20078. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Maresca, M.; Morucci, G.; Mello, T.; Becatti, M.; Pazzagli, L.; Colzi, I.; Gonnelli, C.; Carrino, D.; Paternostro, F.; et al. Effects of Cadmium on ZO-1 Tight Junction Integrity of the Blood Brain Barrier. Int. J. Mol. Sci. 2019, 20, 6010. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Z.; Wen, L.; Lei, D.; Li, S.; Wang, J.; Huang, J.; Wang, N.; Durkan, C.; Liao, X.; et al. Cadmium-induced dysfunction of the blood-brain barrier depends on ROS-mediated inhibition of PTPase activity in zebrafish. J. Hazard. Mater. 2021, 412, 125198. [Google Scholar] [CrossRef]
- Wolonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postep. Hig. Med. Dosw. 2016, 70, 1483–1498. [Google Scholar] [CrossRef]
- Charlet, L.; Chapron, Y.; Faller, P.; Kirsch, R.; Stone, A.T.; Baveye, P.C. Neurodegenerative diseases and exposure to the environmental metals Mn, Pb, and Hg. Coord. Chem. Rev. 2012, 256, 2147–2163. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Pappa, A.; Panayiotidis, M.I.; Gonsebatt, M.E.; Franco, R. Arsenic-induced neurotoxicity: A mechanistic appraisal. J. Biol. Inorg. Chem. 2019, 24, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Sunderman Jr, F. Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann. Clin. Lab. Sci. 2001, 31, 3–24. [Google Scholar] [PubMed]
- Niño, S.A.; Vázquez-Hernández, N.; Arevalo-Villalobos, J.; Chi-Ahumada, E.; Martín-Amaya-Barajas, F.L.; Díaz-Cintra, S.; Martel-Gallegos, G.; González-Burgos, I.; Jiménez-Capdeville, M.E. Cortical Synaptic Reorganization Under Chronic Arsenic Exposure. Neurotox. Res. 2021, 39, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Bihaqi, S.W.; Bahmani, A.; Subaiea, G.M.; Zawia, N.H. Infantile exposure to lead and late-age cognitive decline: Relevance to AD. Alzheimers Dement. 2014, 10, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gustin, K.; Tofail, F.; Vahter, M.; Kippler, M. Cadmium exposure and cognitive abilities and behavior at 10 years of age: A prospective cohort study. Environ. Int. 2018, 113, 259–268. [Google Scholar] [CrossRef]
- Borenstein, A.R.; Copenhaver, C.I.; Mortimer, J.A. Early-life risk factors for Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2006, 20, 63–72. [Google Scholar] [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. Do early-life insults contribute to the late-life development of Parkinson and Alzheimer diseases? Metabolism 2008, 57 (Suppl. S2), 244–249. [Google Scholar] [CrossRef]
- Gauvrit, T.; Benderradji, H.; Buée, L.; Blum, D.; Vieau, D. Early-Life Environment Influence on Late-Onset Alzheimer’s Disease. Front. Cell Dev. Biol. 2022, 10, 834661. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Edwards, M.; Menon, C.V.; Gong, G.; Barber, R. Long-term low-level arsenic exposure is associated with poorer neuropsychological functioning: A Project FRONTIER study. Int. J. Environ. Res. Public Health 2011, 8, 861–874. [Google Scholar] [CrossRef]
- Yang, Y.W.; Liou, S.H.; Hsueh, Y.M.; Lyu, W.S.; Liu, C.S.; Liu, H.J.; Chung, M.C.; Hung, P.H.; Chung, C.J. Risk of Alzheimer’s disease with metal concentrations in whole blood and urine: A case-control study using propensity score matching. Toxicol. Appl. Pharmacol. 2018, 356, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Zhan, R.Q.; Zheng, W.; Jiang, H.; Zhang, D.F.; Shen, X.L. Positive association between soil arsenic concentration and mortality from Alzheimer’s disease in mainland China. J. Trace Elem. Med. Biol. 2020, 59, 126452. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, C.; Liu, W. Effects of arsenic on the offspring development in mice. Zhonghua Yu Fang Yi Xue Za Zhi 1994, 28, 20–23. [Google Scholar] [PubMed]
- Tyler, C.R.; Allan, A.M. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep. 2014, 1, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Jabeen, S.; Imran, I.; Zulfiqar, I.; Bilal, K. Protective effect of gallic acid against arsenic-induced anxiety-/depression- like behaviors and memory impairment in male rats. Metab. Brain Dis. 2019, 34, 1091–1102. [Google Scholar] [CrossRef]
- Zarazúa, S.; Bürger, S.; Delgado, J.M.; Jiménez-Capdeville, M.E.; Schliebs, R. Arsenic affects expression and processing of amyloid precursor protein (APP) in primary neuronal cells overexpressing the Swedish mutation of human APP. Int. J. Dev. Neurosci. 2011, 29, 389–396. [Google Scholar] [CrossRef]
- Giasson, B.I.; Sampathu, D.M.; Wilson, C.A.; Vogelsberg-Ragaglia, V.; Mushynski, W.E.; Lee, V.M.Y. The environmental toxin arsenite induces tau hyperphosphorylation. Biochemistry 2002, 41, 15376–15387. [Google Scholar] [CrossRef]
- Pakzad, D.; Akbari, V.; Sepand, M.R.; Aliomrani, M. Risk of neurodegenerative disease due to tau phosphorylation changes and arsenic exposure via drinking water. Toxicol. Res. 2021, 10, 325–333. [Google Scholar] [CrossRef]
- Hassani, S.; Yaghoubi, H.; Khosrokhavar, R.; Jafarian, I.; Mashayekhi, V.; Hosseini, M.J.; Shahraki, J. Mechanistic view for toxic effects of arsenic on isolated rat kidney and brain mitochondria. Biologia 2015, 70, 683–689. [Google Scholar] [CrossRef]
- Tsinovoi, C.L.; Xun, P.; McClure, L.A.; Carioni, V.M.O.; Brockman, J.D.; Cai, J.; Guallar, E.; Cushman, M.; Unverzagt, F.W.; Howard, V.J.; et al. Arsenic Exposure in Relation to Ischemic Stroke: The Reasons for Geographic and Racial Differences in Stroke Study. Stroke 2018, 49, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, E.; Kutuk, B.; Nalbantoglu, O.U.; Koseoglu, R.; Kendirci, M. Arsenic and selenium measurements in nail and hair show important relationships to Alzheimer’s disease in the elderly. J. Trace Elem. Med. Biol. 2021, 64, 126684. [Google Scholar] [CrossRef] [PubMed]
- Strumylaite, L.; Kregzdyte, R.; Kucikiene, O.; Baranauskiene, D.; Simakauskiene, V.; Naginiene, R.; Damuleviciene, G.; Lesauskaite, V.; Zemaitiene, R. Alzheimer’s Disease Association with Metals and Metalloids Concentration in Blood and Urine. Int. J. Environ. Res. Public Health 2022, 19, 7309. [Google Scholar] [CrossRef] [PubMed]
- Babić Leko, M.; Mihelčić, M.; Jurasović, J.; Nikolac Perković, M.; Španić, E.; Sekovanić, A.; Orct, T.; Zubčić, K.; Langer Horvat, L.; Pleić, N.; et al. Heavy Metals and Essential Metals Are Associated with Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 467. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Moore, M.R. Adverse Health Effects of Chronic Exposure to Low-Level Cadmium in Foodstuffs and Cigarette Smoke. Environ. Health Perspect. 2004, 112, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Cadmium; Agency for Toxic Substances & Disease Registry, Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Min, J.Y.; Min, K.B. Blood cadmium levels and Alzheimer’s disease mortality risk in older US adults. Environ. Health A Glob. Access Sci. Source 2016, 15, 69. [Google Scholar] [CrossRef]
- Peng, Q.; Bakulski, K.M.; Nan, B.; Park, S.K. Cadmium and Alzheimer’s disease mortality in U.S. adults: Updated evidence with a urinary biomarker and extended follow-up time. Environ. Res. 2017, 157, 44–51. [Google Scholar] [CrossRef]
- Souza-Talarico, J.N.; Marcourakis, T.; Barbosa, F.; Moraes Barros, S.B.; Rivelli, D.P.; Pompéia, S.; Caramelli, P.; Plusquellec, P.; Lupien, S.J.; Catucci, R.F.; et al. Association between heavy metal exposure and poor working memory and possible mediation effect of antioxidant defenses during aging. Sci. Total Environ. 2017, 575, 750–757. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Fu, Z.; Yan, M.; Wu, N.; Wu, H.; Yin, P. Associations between blood cadmium levels and cognitive function in a cross-sectional study of US adults aged 60 years or older. BMJ Open 2018, 8, e020533. [Google Scholar] [CrossRef]
- Huang, G.; Ren, G. Interaction between ω-6 fatty acids intake and blood cadmium on the risk of low cognitive performance in older adults from National Health and Nutrition Examination Survey (NHANES) 2011-2014. BMC Geriatr. 2022, 22, 292. [Google Scholar] [CrossRef]
- Ruczaj, A.; Brzóska, M.M. Environmental exposure of the general population to cadmium as a risk factor of the damage to the nervous system: A critical review of current data. J. Appl. Toxicol. 2022, 43, 66–88. [Google Scholar] [CrossRef]
- Notarachille, G.; Arnesano, F.; Calò, V.; Meleleo, D. Heavy metals toxicity: Effect of cadmium ions on amyloid beta protein 1–42. Possible implications for Alzheimer’s disease. Biometals 2014, 27, 371–388. [Google Scholar] [CrossRef] [PubMed]
- del Pino, J.; Zeballos, G.; Anadón, M.J.; Moyano, P.; Díaz, M.J.; García, J.M.; Frejo, M.T. Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3β enzyme, β-amyloid and tau protein levels. Arch. Toxicol. 2016, 90, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, Y.; Yu, S.; Zhao, H.; Yao, L. The effect of cadmium on Aβ levels in APP/PS1 transgenic mice. Exp. Ther. Med. 2012, 4, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Verma, A.K.; Ahmad, M.K.; Garg, R.K.; Shiuli; Mahdi, A.A.; Srivastava, S. Metals toxicity and its correlation with the gene expression in Alzheimer’s disease. Mol. Biol. Rep. 2021, 48, 3245–3252. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, A.; Mei, Y.; Zhao, J.; Zhou, Q.; Li, Y.; Yang, M.; Xu, Q. Trace elements and Alzheimer dementia in population-based studies: A bibliometric and meta-analysis. Environ. Pollut. 2023, 318, 120782. [Google Scholar] [CrossRef]
- Kazemi, T.; Moodi, M.; Rajabi, S.; Sharifi, F.; Samarghandian, S.; Khorashadizadeh, M.; Farkhondeh, T. Trace Element Concentration and Cognitive Dysfunction in Elderly Residents in Birjand. Curr. Alzheimer Res. 2022, 19, 674–680. [Google Scholar] [CrossRef]
- Du, K.; Zheng, X.; Ma, Z.T.; Lv, J.Y.; Jiang, W.J.; Liu, M.Y. Association of Circulating Magnesium Levels in Patients with Alzheimer’s Disease From 1991 to 2021: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 13, 799824. [Google Scholar] [CrossRef]
- Banerjee, G.; Forsgard, N.; Ambler, G.; Keshavan, A.; Paterson, R.W.; Foiani, M.S.; Toombs, J.; Heslegrave, A.; Thompson, E.J.; Lunn, M.P.; et al. Cerebrospinal fluid metallomics in cerebral amyloid angiopathy: An exploratory analysis. J. Neurol. 2022, 269, 1470–1475. [Google Scholar] [CrossRef]
- Socha, K.; Klimiuk, K.; Naliwajko, S.K.; Soroczyńska, J.; Puścion-jakubik, A.; Markiewicz-żukowska, R.; Kochanowicz, J. Dietary Habits, Selenium, Copper, Zinc and Total Antioxidant Status in Serum in Relation to Cognitive Functions of Patients with Alzheimer’s Disease. Nutrients 2021, 13, 287. [Google Scholar] [CrossRef]
- Squitti, R.; Ventriglia, M.; Simonelli, I.; Bonvicini, C.; Costa, A.; Perini, G.; Binetti, G.; Benussi, L.; Ghidoni, R.; Koch, G.; et al. Copper Imbalance in Alzheimer’s Disease: Meta-Analysis of Serum, Plasma, and Brain Specimens, and Replication Study Evaluating ATP7B Gene Variants. Biomolecules 2021, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Ficiarà, E.; Boschi, S.; Ansari, S.; D’Agata, F.; Abollino, O.; Caroppo, P.; Di Fede, G.; Indaco, A.; Rainero, I.; Guiot, C. Machine Learning Profiling of Alzheimer’s Disease Patients Based on Current Cerebrospinal Fluid Markers and Iron Content in Biofluids. Front. Aging Neurosci. 2021, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.Q.d.; Barros-Neto, J.A.; Vieira, N.F.L.; Menezes-Filho, J.A.; Neves, S.J.F.; Lima, S.O. Selenium concentrations in elderly people with Alzheimer’s disease: A cross-sectional study with control group. Rev. Bras. Enferm. 2021, 74, e20200984. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, J.Q.; Tolstrup, J.S.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Plasma Concentrations of Magnesium and Risk of Dementia: A General Population Study of 102 648 Individuals. Clin. Chem. 2021, 67, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.A.C.; Trebak, F.; Kumar, V.; Satou, R.; Kehoe, P.G.; Yang, W.; Wharton, W.; Earley, Y.F. Elevated cerebrospinal fluid sodium in hypertensive human subjects with a family history of Alzheimer’s disease. Physiol. Genom. 2020, 52, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Ben Zaken, S.; Radomysky, Z.; Koren, G. Association Between Serum Magnesium Levels and Alzheimer’s Disease or Mixed Dementia Patients: A Population-Based Retrospective Controlled Study. J. Alzheimer’s Dis. Rep. 2020, 4, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Giil, L.M.; Solvang, S.E.H.; Giil, M.M.; Hellton, K.H.; Skogseth, R.E.; Vik-Mo, A.O.; Hortobágyi, T.; Aarsland, D.; Nordrehaug, J.E. Serum Potassium Is Associated with Cognitive Decline in Patients with Lewy Body Dementia. J. Alzheimer’s Dis. 2019, 68, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Giuli, C.; Casoli, T.; Balietti, M.; Costarelli, L.; Provinciali, M.; Basso, A.; Piacenza, F.; Postacchini, D.; Galeazzi, R.; et al. Acetylcholinesterase inhibitors in Alzheimer’s disease influence Zinc and Copper homeostasis. J. Trace Elem. Med. Biol. 2019, 55, 58–63. [Google Scholar] [CrossRef]
- Ashraf, A.; Stosnach, H.; Parkes, H.G.; Hye, A.; Powell, J.; Soinine, H.; Tsolaki, M.; Vellas, B.; Lovestone, S.; Aarsland, D.; et al. Pattern of Altered Plasma Elemental Phosphorus, Calcium, Zinc, and Iron in Alzheimer’s Disease. Sci. Rep. 2019, 9, 3147. [Google Scholar] [CrossRef]
- Vaz, F.N.C.; Fermino, B.L.; Haskel, M.V.L.; Wouk, J.; de Freitas, G.B.L.; Fabbri, R.; Montagna, E.; Rocha, J.B.T.; Bonini, J.S. The Relationship Between Copper, Iron, and Selenium Levels and Alzheimer Disease. Biol. Trace Elem. Res. 2018, 181, 185–191. [Google Scholar] [CrossRef]
- Vintimilla, R.M.; Large, S.E.; Gamboa, A.; Rohlfing, G.D.; O’Jile, J.R.; Hall, J.R.; O’Bryant, S.E.; Johnson, L.A. The Link between Potassium and Mild Cognitive Impairment in Mexican-Americans. Dement. Geriatr. Cogn. Dis. Extra 2018, 8, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Fathabadi, B.; Dehghanifiroozabadi, M.; Aaseth, J.; Sharifzadeh, G.; Nakhaee, S.; Rajabpour-Sanati, A.; Amirabadizadeh, A.; Mehrpour, O. Comparison of Blood Lead Levels in Patients with Alzheimer’s Disease and Healthy People. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.M.; Michno, W.; Rockwood, A.L.; Blennow, K.; Strathmann, F.G.; Hanrieder, J. Association of PTHrP levels in CSF with Alzheimer’s disease biomarkers. Clin. Mass Spectrom. 2018, 14 Pt B, 124–129. [Google Scholar] [CrossRef]
- Squitti, R.; Ghidoni, R.; Simonelli, I.; Ivanova, I.D.; Colabufo, N.A.; Zuin, M.; Benussi, L.; Binetti, G.; Cassetta, E.; Rongioletti, M.; et al. Copper dyshomeostasis in Wilson disease and Alzheimer’s disease as shown by serum and urine copper indicators. J. Trace Elem. Med. Biol. 2018, 45, 181–188. [Google Scholar] [CrossRef]
- Xu, J.; Church, S.J.; Patassini, S.; Begley, P.; Kellett, K.A.B.; Vardy, E.R.L.C.; Unwin, R.D.; Hooper, N.M.; Cooper, G.J.S. Plasma metals as potential biomarkers in dementia: A case-control study in patients with sporadic Alzheimer’s disease. Biometals 2018, 31, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Dang, R.; Cui, Y.; Liu, L.; Chen, X.; Wang, X.; Zhu, J.; Li, D.; Li, J.; Wang, D. Characterization of plasma metal profiles in Alzheimer’s disease using multivariate statistical analysis. PLoS ONE 2017, 12, e0178271. [Google Scholar] [CrossRef]
- Kieboom, B.C.T.; Licher, S.; Wolters, F.J.; Ikram, M.K.; Hoorn, E.J.; Zietse, R.; Stricker, B.H.; Ikram, M.A. Serum magnesium is associated with the risk of dementia. Neurology 2017, 89, 1716–1722. [Google Scholar] [CrossRef]
- Balmuș, I.M.; Strungaru, S.A.; Ciobica, A.; Nicoara, M.N.; Dobrin, R.; Plavan, G.; Ștefănescu, C. Preliminary Data on the Interaction between Some Biometals and Oxidative Stress Status in Mild Cognitive Impairment and Alzheimer’s Disease Patients. Oxid. Med. Cell. Longev. 2017, 2017, 7156928. [Google Scholar] [CrossRef]
- Du, K.; Liu, M.; Pan, Y.; Zhong, X.; Wei, M. Association of Serum Manganese Levels with Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 231. [Google Scholar] [CrossRef]
- Reddy, V.S.; Bukke, S.; Dutt, N.; Rana, P.; Pandey, A.K. A systematic review and meta-analysis of the circulatory, erythrocellular and CSF selenium levels in Alzheimer’s disease: A metal meta-analysis (AMMA study-I). J. Trace Elem. Med. Biol. 2017, 42, 68–75. [Google Scholar] [CrossRef]
- Pu, Z.; Xu, W.; Lin, Y.; He, J.; Huang, M. Oxidative Stress Markers and Metal Ions are Correlated with Cognitive Function in Alzheimer’s Disease. Am. J. Alzheimers. Dis. Other Demen. 2017, 32, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Li, Q.X.; Fowler, C.J.; Masters, C.L.; Martins, R.N.; Ganio, K.; Lothian, A.; Mukherjee, S.; et al. Selenium Levels in Serum, Red Blood Cells, and Cerebrospinal Fluid of Alzheimer’s Disease Patients: A Report from the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL). J. Alzheimer’s Dis. 2017, 57, 183–193. [Google Scholar] [CrossRef]
- Chmatalova, Z.; Vyhnalek, M.; Laczo, J.; Hort, J.; Pospisilova, R.; Pechova, M.; Skoumalova, A.; Chmátalová, Z. Relation of Plasma Selenium and Lipid Peroxidation End Products in Patients with Alzheimer’s Disease. Physiol. Res. 2017, 66, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, M.C.; Vellas, B.; Girault, E.; Yavuz, A.C.; Sijben, J.W. Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimer’s Dement. 2017, 3, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.R.; Doecke, J.D.; Rembach, A.; Yévenes, L.F.; Fowler, C.J.; McLean, C.A.; Lind, M.; Volitakis, I.; Masters, C.L.; Bush, A.I.; et al. Rubidium and potassium levels are altered in Alzheimer’s disease brain and blood but not in cerebrospinal fluid. Acta Neuropathol. Commun. 2016, 4, 119. [Google Scholar] [CrossRef]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef]
- Hare, D.J.; Faux, N.G.; Roberts, B.R.; Volitakis, I.; Martins, R.N.; Bush, A.I. Lead and manganese levels in serum and erythrocytes in Alzheimer’s disease and mild cognitive impairment: Results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing. Metallomics 2016, 8, 628–632. [Google Scholar] [CrossRef]
- Hare, D.J.; Doecke, J.D.; Faux, N.G.; Rembach, A.; Volitakis, I.; Fowler, C.J.; Grimm, R.; Doble, P.A.; Cherny, R.A.; Masters, C.L.; et al. Decreased plasma iron in Alzheimer’s disease is due to transferrin desaturation. ACS Chem. Neurosci. 2015, 6, 398–402. [Google Scholar] [CrossRef]
- Koç, E.R.; Ilhan, A.; Aytürk, Z.; Acar, B.; Gürler, M.; Altuntaş, A.; Karapirli, M.; Bodur, A.S. A comparison of hair and serum trace elements in patients with Alzheimer disease and healthy participants. Turk. J. Med. Sci. 2015, 45, 1034–1039. [Google Scholar] [CrossRef]
- Negahdar, H.; Hosseini, S.R.; Parsian, H.; Kheirkhah, F.; Mosapour, A.; Khafri, S.; Haghighi, A.H. Homocysteine, trace elements and oxidant/antioxidant status in mild cognitively impaired elderly persons: A cross-sectional study. Rom. J. Intern. Med. 2015, 53, 336–342. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Tan, L.; Wang, H.-F.; Ma, J.; Liu, J.; Tan, M.-S.; Sun, J.-H.; Zhu, X.-C.; Jiang, T.; Yu, J.-T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimer’s Dis. 2015, 47, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Ventriglia, M.; Brewer, G.J.; Simonelli, I.; Mariani, S.; Siotto, M.; Bucossi, S.; Squitti, R. Zinc in Alzheimer’s Disease: A Meta-Analysis of Serum, Plasma, and Cerebrospinal Fluid Studies. J. Alzheimer’s Dis. 2015, 46, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Calabrò, R.S.; D’Aleo, G.; Marra, A.; Sessa, E.; Bua, D.G.; Potortì, A.G.; Dugo, G.; Bramanti, P.; et al. Heavy metals and neurodegenerative diseases: An observational study. Biol. Trace Elem. Res. 2014, 161, 151–160. [Google Scholar] [CrossRef]
- Faux, N.G.; Rembach, A.; Wiley, J.; Ellis, K.A.; Ames, D.; Fowler, C.J.; Martins, R.N.; Pertile, K.K.; Rumble, R.L.; Trounson, B.; et al. An anemia of Alzheimer’s disease. Mol. Psychiatry 2014, 19, 1227–1234. [Google Scholar] [CrossRef]
- Olde Rikkert, M.G.M.; Verhey, F.R.; Sijben, J.W.C.; Bouwman, F.H.; Dautzenberg, P.L.J.; Lansink, M.; Sipers, W.M.W.; Van Asselt, D.Z.B.; Van Hees, A.M.J.; Stevens, M.; et al. Differences in nutritional status between very mild Alzheimer’s disease patients and healthy controls. J. Alzheimer’s Dis. 2014, 41, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Lee, D.-W.; Park, K.S.; Joung, H. Serum Trace Metal Levels in Alzheimer’s Disease and Normal Control Groups. Am. J. Alzheimer’s Dis. Other Dement. 2014, 29, 76–83. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Characterization of metal profiles in serum during the progression of Alzheimer’s disease. Metallomics 2014, 6, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, Y.; Rogers, J.T.; Wang, F. Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 42, 679–690. [Google Scholar] [CrossRef]
- Rembach, A.; Hare, D.J.; Doecke, J.D.; Burnham, S.C.; Volitakis, I.; Fowler, C.J.; Cherny, R.A.; McLean, C.; Grimm, R.; Martins, R.; et al. Decreased serum zinc is an effect of ageing and not Alzheimer’s disease. Metallomics 2014, 6, 1216–1219. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.H.; Choi, D.W.; Lee, D.W.; Park, J.H.; Yoon, H.J.; Pyo, H.S.; Kwon, H.J.; Park, K.S. The Association of Heavy Metal of Blood and Serum in the Alzheimer’s Diseases. Toxicol. Res. 2012, 28, 93–98. [Google Scholar] [CrossRef]
- Hozumi, I.; Hasegawa, T.; Honda, A.; Ozawa, K.; Hayashi, Y.; Hashimoto, K.; Yamada, M.; Koumura, A.; Sakurai, T.; Kimura, A.; et al. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J. Neurol. Sci. 2011, 303, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Bucossi, S.; Ventriglia, M.; Panetta, V.; Salustri, C.; Pasqualetti, P.; Mariani, S.; Siotto, M.; Rossini, P.M.; Squitti, R. Copper in Alzheimer’s disease: A meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimer’s Dis. 2011, 24, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gerhardsson, L.; Lundh, T.; Londos, E.; Minthon, L. Cerebrospinal fluid/plasma quotients of essential and non-essential metals in patients with Alzheimer’s disease. J. Neural Transm. 2011, 118, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Chan, I.H.S.; Cheung, S.K.-K.; Goggins, W.B.; Mok, V.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; Woo, J.; et al. Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. Biometals 2010, 23, 173–179. [Google Scholar] [CrossRef]
- Boström, F.; Hansson, O.; Gerhardsson, L.; Lundh, T.; Minthon, L.; Stomrud, E.; Zetterberg, H.; Londos, E. CSF Mg and Ca as diagnostic markers for dementia with Lewy bodies. Neurobiol. Aging 2009, 30, 1265–1271. [Google Scholar] [CrossRef]
- Lavados, M.; Guillón, M.; Mujica, M.C.; Rojo, L.E.; Fuentes, P.; Maccioni, R.B. Mild cognitive impairment and Alzheimer patients display different levels of redox-active CSF iron. J. Alzheimer’s Dis. 2008, 13, 225–232. [Google Scholar] [CrossRef]
- Dong, J.; Robertson, J.D.; Markesbery, W.R.; Lovell, M.A. Serum Zinc in the Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2008, 15, 443–450. [Google Scholar] [CrossRef]
- Gerhardsson, L.; Lundh, T.; Minthon, L.; Londos, E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 25, 508–515. [Google Scholar] [CrossRef]
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Monti, M.S.; Forte, G.; Stanzione, P.; et al. Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology 2007, 28, 450–456. [Google Scholar] [CrossRef]
- Bocca, B.; Forte, G.; Petrucci, F.; Pino, A.; Marchione, F.; Bomboi, G.; Senofonte, O.; Giubilei, F.; Alimonti, A. Monitoring of chemical elements and oxidative damage in patients affected by Alzheimer’s disease. Ann. Ist. Super. Sanita 2005, 41, 197–203. [Google Scholar]
- Smorgon, C.; Mari, E.; Atti, A.R.; Dalla Nora, E.; Zamboni, P.F.; Calzoni, F.; Passaro, A.; Fellin, R. Trace elements and cognitive impairment: An elderly cohort study. Arch. Gerontol. Geriatr. 2004, 38, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, I.; Molina, J.A.; Jiménez-Jiménez, F.J.; Aguilar, M.V.; Mateos-Vega, C.J.; González-Muñoz, M.J.; de Bustos, F.; Ortí-Pareja, M.; Zurdo, M.; Berbel, A.; et al. Cerebrospinal fluid levels of selenium in patients with Alzheimer’s disease. J. Neural Transm. 1999, 106, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Snaedal, J.; Kristinsson, J.; Gunnarsdóttir, S.; Ólafsdóttir, Á.; Baldvinsson, M.; Jóhannesson, T. Copper, ceruloplasmin and superoxide dismutase in patients with Alzheimer’s disease. a case-control study. Dement. Geriatr. Cogn. Disord. 1998, 9, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Hock, C.; Drasch, G.; Golombowski, S.; Müller-Spahn, F.; Willershausen-Zönnchen, B.; Schwarz, P.; Hock, U.; Growdon, J.H.; Nitsch, R.M. Increased blood mercury levels in patients with Alzheimer’s disease. J. Neural Transm. 1998, 105, 59–68. [Google Scholar] [CrossRef]
- Molina, J.A.; Jiménez-Jiménez, F.J.; Aguilar, M.V.; Meseguer, I.; Mateos-Vega, C.J.; González-Muñoz, M.J.; de Bustos, F.; Porta, J.; Ortí-Pareja, M.; Zurdo, M.; et al. Cerebrospinal fluid levels of transition metals in patients with Alzheimer’s disease. J. Neural Transm. 1998, 105, 479–488. [Google Scholar] [CrossRef]
- Zapatero, M.D.; Garcia de Jalon, A.; Pascual, F.; Calvo, M.L.; Escanero, J.; Marro, A. Serum aluminum levels in Alzheimer’s disease and other senile dementias. Biol. Trace Elem. Res. 1995, 47, 235–240. [Google Scholar] [CrossRef]
- Basun, H.; Lind, B.; Nordberg, M.; Nordström, M.; Björkstén, K.S.; Winblad, B. Cadmium in blood in Alzheimer’s disease and non-demented subjects: Results from a population-based study. Biometals 1994, 7, 130–134. [Google Scholar] [CrossRef]
- Subhash, M.N.; Padmashree, T.S.; Srinivas, K.N.; Subbakrishna, D.K.; Shankar, S.K. Calcium and phosphorus levels in serum and CSF in dementia. Neurobiol. Aging 1991, 12, 267–269. [Google Scholar] [CrossRef]
- Sahu, R.N.; Pandey, R.S.; Subhash, M.N.; Arya, B.Y.; Padmashree, T.S.; Srinivas, K.N. CSF zinc in Alzheimer’s type dementia. Biol. Psychiatry 1988, 24, 480–482. [Google Scholar] [CrossRef]
- Hershey, C.O.; Hershey, L.A.; Varnes, A.; Vibhakar, S.D.; Lavin, P.; Strain, W.H. Cerebrospinal fluid trace element content in dementia: Clinical, radiologic, and pathologic correlations. Neurology 1983, 33, 1350–1353. [Google Scholar] [CrossRef]
- Morris, M.C.; Brockman, J.; Schneider, J.A.; Wang, Y.; Bennett, D.A.; Tangney, C.C.; van de Rest, O. Association of Seafood Consumption, Brain Mercury Level, and APOE ε4 Status with Brain Neuropathology in Older Adults. JAMA 2016, 315, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, D.R.; Fitzgerald, W.F.; Cooke, C.A.; Lamborg, C.H.; Drevnick, P.E.; Swain, E.B.; Balogh, S.J.; Balcom, P.H. Atmospheric Hg emissions from preindustrial gold and silver extraction in the Americas: A reevaluation from lake-sediment archives. Environ. Sci. Technol. 2014, 48, 6533–6543. [Google Scholar] [CrossRef] [PubMed]
- Mutter, J.; Curth, A.; Naumann, J.; Deth, R.; Walach, H. Does inorganic mercury play a role in Alzheimer’s disease? A systematic review and an integrated molecular mechanism. J. Alzheimer’s Dis. 2010, 22, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, W.; Liu, X.; Zhang, C.; Wang, P.; Zhao, X. Circulatory Levels of Toxic Metals (Aluminum, Cadmium, Mercury, Lead) in Patients with Alzheimer’s Disease: A Quantitative Meta-Analysis and Systematic Review. J. Alzheimer’s Dis. 2018, 62, 361–372. [Google Scholar] [CrossRef]
- Olayinka, O.; Olayinka, O.O.; Alemu, B.T.; Akpinar-Elci, M.; Grossberg, G.T. Toxic Environmental Risk Factors for Alzheimer’s Disease: A Systematic Review. Aging Med. Healthc. 2019, 10, 4–17. [Google Scholar] [CrossRef]
- Alattia, J.-R.; Kuraishi, T.; Dimitrov, M.; Chang, I.; Lemaitre, B.; Fraering, P.C. Mercury is a direct and potent γ-secretase inhibitor affecting Notch processing and development in Drosophila. FASEB J. 2011, 25, 2287–2295. [Google Scholar] [CrossRef]
- Olivieri, G.; Brack, C.; Müller-Spahn, F.; Stähelin, H.B.; Herrmann, M.; Renard, P.; Brockhaus, M.; Hock, C. Mercury induces cell cytotoxicity and oxidative stress and increases beta-amyloid secretion and tau phosphorylation in SHSY5Y neuroblastoma cells. J. Neurochem. 2000, 74, 231–236. [Google Scholar] [CrossRef]
- Fujimura, M.; Usuki, F.; Sawada, M.; Takashima, A. Methylmercury induces neuropathological changes with tau hyperphosphorylation mainly through the activation of the c-jun-N-terminal kinase pathway in the cerebral cortex, but not in the hippocampus of the mouse brain. Neurotoxicology 2009, 30, 1000–1007. [Google Scholar] [CrossRef]
- Yang, D.-J.; Shi, S.; Zheng, L.-F.; Yao, T.-M.; Ji, L.-N. Mercury(II) promotes the in vitro aggregation of tau fragment corresponding to the second repeat of microtubule-binding domain: Coordination and conformational transition. Biopolymers 2010, 93, 1100–1107. [Google Scholar] [CrossRef]
- Yin, X.; Sun, J.; Mei, Y.; Guo, X.; Chen, S.-I.; Wang, Z.-I.; Yang, L. Effect of Hg2+ on voltage-dependent calcium channels and intracellular free calcium in trigeminal ganglion neurons of rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2008, 26, 542–545. [Google Scholar]
- Weisskopf, M.G.; Wright, R.O.; Schwartz, J.; Spiro, A.; Sparrow, D.; Aro, A.; Hu, H. Cumulative lead exposure and prospective change in cognition among elderly men: The VA Normative Aging Study. Am. J. Epidemiol. 2004, 160, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.O.; Tsaih, S.W.; Schwartz, J.; Spiro, A.; McDonald, K.; Weiss, S.T.; Hu, H. Lead exposure biomarkers and mini-mental status exam scores in older men. Epidemiology 2003, 14, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wei, X.; Monnot, A.D.; Fontanilla, C.V.; Behl, M.; Farlow, M.R.; Zheng, W.; Du, Y. Lead exposure increases levels of β-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice. Neurosci. Lett. 2011, 490, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Bihaqi, S.W.; Eid, A.; Zawia, N.H. Lead exposure and tau hyperphosphorylation: An in vitro study. Neurotoxicology 2017, 62, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Bakulski, K.M.; Rozek, L.S.; Dolinoy, D.C.; Paulson, H.L.; Hu, H. Alzheimer’s disease and environmental exposure to lead: The epidemiologic evidence and potential role of epigenetics. Curr. Alzheimer Res. 2012, 9, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Xu, Y. Epigenetic Basis of Lead-Induced Neurological Disorders. Int. J. Environ. Res. Public Health 2020, 17, 4878. [Google Scholar] [CrossRef]
- Andrade, V.M.; Aschner, M.; Marreilha dos Santos, A.P. Neurotoxicity of Metal Mixtures. Adv. Neurobiol. 2017, 18, 227–265. [Google Scholar] [CrossRef]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative Stress in Lead and Cadmium Toxicity and Its Amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Liang, J.; Wu, C.; Zou, X. Assessing the Association Between Lead Pollution and Risk of Alzheimer’s Disease by Integrating Multigenomics. Front. Neurosci. 2022, 16, 880105. [Google Scholar] [CrossRef]
- Masten, S.; Carson, B.L. Aluminum Compounds Review of Toxicological Literature Abridged Final Report; Integrated Laboratory Systems: Morrisville, NC, USA, 2000. [Google Scholar]
- Exley, C.; Mold, M.J. The binding, transport and fate of aluminium in biological cells. J. Trace Elem. Med. Biol. 2015, 30, 90–95. [Google Scholar] [CrossRef]
- Exley, C.; Mold, M.J. Aluminium in human brain tissue: How much is too much? J. Biol. Inorg. Chem. 2019, 24, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Exley, C.; Mold, M.J. Imaging of aluminium and amyloid β in neurodegenerative disease. Heliyon 2020, 6, e03839. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.R. Brain lesions comprised of aluminum-rich cells that lack microtubules may be associated with the cognitive deficit of Alzheimer’s disease. Neurotoxicology 2009, 30, 1059–1069. [Google Scholar] [CrossRef]
- Walton, J.R. Aluminum in hippocampal neurons from humans with Alzheimer’s disease. Neurotoxicology 2006, 27, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Mold, M.; Linhart, C.; Gómez-Ramírez, J.; Villegas-Lanau, A.; Exley, C. Aluminum and Amyloid-β in Familial Alzheimer’s Disease. J. Alzheimers. Dis. 2020, 73, 1627–1635. [Google Scholar] [CrossRef]
- Lopera, F.; Ardilla, A.; Martínez, A.; Madrigal, L.; Arango-Viana, J.C.; Lemere, C.A.; Arango-Lasprilla, J.C.; Hincapié, L.; Arcos-Burgos, M.; Ossa, J.E.; et al. Clinical Features of Early-Onset Alzheimer Disease in a Large Kindred with an E280A Presenilin-1 Mutation. JAMA 1997, 277, 793–799. [Google Scholar] [CrossRef]
- Díaz-Nido, J.; Avila, J. Aluminum induces the in vitro aggregation of bovine brain cytoskeletal proteins. Neurosci. Lett. 1990, 110, 221–226. [Google Scholar] [CrossRef]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 2017, 40, 30–36. [Google Scholar] [CrossRef]
- Mold, M.J.; O’Farrell, A.; Morris, B.; Exley, C. Aluminum and Tau in Neurofibrillary Tangles in Familial Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2021, 5, 283. [Google Scholar] [CrossRef]
- Mold, M.J.; O’Farrell, A.; Morris, B.; Exley, C. Aluminum and Neurofibrillary Tangle Co-Localization in Familial Alzheimer’s Disease and Related Neurological Disorders. J. Alzheimer’s Dis. 2020, 78, 139. [Google Scholar] [CrossRef]
- Flaten, T.P. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res. Bull. 2001, 55, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Martyn, C.N.; Osmond, C.; Edwardson, J.A.; Barker, D.J.P.; Harris, E.C.; Lacey, R.F. Geographical relation between Alzheimer’s disease and aluminum in drinking water. Lancet 1989, 333, 59–62. [Google Scholar] [CrossRef]
- Luo, J.; Su, L.; He, X.; Du, Y.; Xu, N.; Wu, R.; Zhu, Y.; Wang, T.; Shao, R.; Unverzagt, F.W.; et al. Blood Selenium and Serum Glutathione Peroxidase Levels Were Associated with Serum β-Amyloid in Older Adults. Biol. Trace Elem. Res. 2022, 200, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Z.; Wang, Z.X.; Wang, Z.T.; Hou, X.H.; Shen, X.N.; Ou, Y.N.; Dong, Q.; Tan, L.; Yu, J.T. Serum Calcium Predicts Cognitive Decline and Clinical Progression of Alzheimer’s Disease. Neurotox. Res. 2021, 39, 609–617. [Google Scholar] [CrossRef]
- Mohammed, R.S.; Ibrahim, W.; Sabry, D.; El-Jaafary, S.I. Occupational metals exposure and cognitive performance among foundry workers using tau protein as a biomarker. Neurotoxicology 2020, 76, 10–16. [Google Scholar] [CrossRef]
- Shams, M.; Martola, J.; Charidimou, A.; Granberg, T.; Ferreira, D.; Westman, E.; Wintermark, M.; Iv, M.; Larvie, M.; Kristoffersen Wiberg, M.; et al. Cerebrospinal Fluid Metals and the Association with Cerebral Small Vessel Disease. J. Alzheimer’s Dis. 2020, 78, 1229–1236. [Google Scholar] [CrossRef]
- Vinceti, M.; Chiari, A.; Eichmüller, M.; Rothman, K.J.; Filippini, T.; Malagoli, C.; Weuve, J.; Tondelli, M.; Zamboni, G.; Nichelli, P.F.; et al. A selenium species in cerebrospinal fluid predicts conversion to Alzheimer’s dementia in persons with mild cognitive impairment. Alzheimers. Res. Ther. 2017, 9, 100. [Google Scholar] [CrossRef]
- Krishnan, S.; Rani, P. Evaluation of selenium, redox status and their association with plasma amyloid/tau in Alzheimer’s disease. Biol. Trace Elem. Res. 2014, 158, 158–165. [Google Scholar] [CrossRef]
- Tong, Y.; Yang, H.; Tian, X.; Wang, H.; Zhou, T.; Zhang, S.; Yu, J.; Zhang, T.; Fan, D.; Guo, X.; et al. High manganese, a risk for Alzheimer’s disease: High manganese induces amyloid-β related cognitive impairment. J. Alzheimer’s Dis. 2014, 42, 865–878. [Google Scholar] [CrossRef]
- Gerhardsson, L.; Blennow, K.; Lundh, T.; Londos, E.; Minthon, L. Concentrations of metals, beta-amyloid and tau-markers in cerebrospinal fluid in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2009, 28, 88–94. [Google Scholar] [CrossRef]
- Strozyk, D.; Launer, L.J.; Adlard, P.A.; Cherny, R.A.; Tsatsanis, A.; Volitakis, I.; Blennow, K.; Petrovitch, H.; White, L.R.; Bush, A.I. Zinc and copper modulate Alzheimer Abeta levels in human cerebrospinal fluid. Neurobiol. Aging 2009, 30, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Kessler, H.; Pajonk, F.G.; Bach, D.; Schneider-Axmann, T.; Falkai, P.; Herrmann, W.; Multhaup, G.; Wiltfang, J.; Schäfer, S.; Wirths, O.; et al. Effect of copper intake on CSF parameters in patients with mild Alzheimer’s disease: A pilot phase 2 clinical trial. J. Neural Transm. 2008, 115, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Zandi, P.P.; Blennow, K.; Gustafson, D.; Sjögren, M.; Rosengren, L.; Skoog, I. Low serum potassium in mid life associated with decreased cerebrospinal fluid Abeta42 in late life. Alzheimer Dis. Assoc. Disord. 2006, 20, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Barbati, G.; Rossi, L.; Ventriglia, M.; Dal Forno, G.; Cesaretti, S.; Moffa, F.; Caridi, I.; Cassetta, E.; Pasqualetti, P.; et al. Excess of nonceruloplasmin serum copper in AD correlates with MMSE, CSF beta-amyloid, and h-tau. Neurology 2006, 67, 76–82. [Google Scholar] [CrossRef]
- Jia, M.; Haldar, S.; Khan, M.A.; Sharma, S.D.; Merrick, W.C.; Theil, E.C.; Goss, D.J. Fe2+ binds iron responsive element-RNA, selectively changing protein-binding affinities and regulating mRNA repression and activation. Proc. Natl. Acad. Sci. USA 2012, 109, 8417–8422. [Google Scholar] [CrossRef]
- Pfeifhofer-Obermair, C.; Tymoszuk, P.; Nairz, M.; Schroll, A.; Klais, G.; Demetz, E.; Engl, S.; Brigo, N.; Weiss, G. Regulation of Th1 T Cell Differentiation by Iron via Upregulation of T Cell Immunoglobulin and Mucin Containing Protein-3 (TIM-3). Front. Immunol. 2021, 12, 637809. [Google Scholar] [CrossRef]
- Pourcelot, E.; Lénon, M.; Mobilia, N.; Cahn, J.Y.; Arnaud, J.; Fanchon, E.; Moulis, J.M.; Mossuz, P. Iron for proliferation of cell lines and hematopoietic progenitors: Nailing down the intracellular functional iron concentration. Biochim. Biophys. Acta -Mol. Cell Res. 2015, 1853, 1596–1605. [Google Scholar] [CrossRef]
- Andreini, C.; Putignano, V.; Rosato, A.; Banci, L. The human iron-proteome. Metallomics 2018, 10, 1223–1231. [Google Scholar] [CrossRef]
- Hirota, K. An intimate crosstalk between iron homeostasis and oxygen metabolism regulated by the hypoxia-inducible factors (HIFs). Free Radic. Biol. Med. 2019, 133, 118–129. [Google Scholar] [CrossRef]
- Schulz, K.; Kroner, A.; David, S. Iron efflux from astrocytes plays a role in remyelination. J. Neurosci. 2012, 32, 4841–4847. [Google Scholar] [CrossRef]
- Beard, J. Iron deficiency alters brain development and functioning. J. Nutr. 2003, 133, 1468S–1472S. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.N. Iron and the brain: Neurotransmitter receptors and magnetic resonance spectroscopy. Br. J. Nutr. 2001, 85 (Suppl. S2), S147–S150. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Harris, P.L.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W.; Ghilardi, J.R.; Rogers, S.; DeMaster, E.; Allen, C.J.; Stimson, E.R.; Maggio, J.E. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of beta-amyloid peptide. J. Neurochem. 1993, 61, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Egaña, J.T.; Zambrano, C.; Nuñez, M.T.; Gonzalez-Billault, C.; Maccioni, R.B. Iron-induced oxidative stress modify tau phosphorylation patterns in hippocampal cell cultures. Biometals 2003, 16, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xiong, S.; Xie, C.; Davies, P.; Markesbery, W.R. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J. Alzheimer’s Dis. 2004, 6, 659–671; discussion 673–681. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Cao, L.; Kalionis, B.; Murthi, P.; Xia, S.; Guan, Y. Iron Deposition Leads to Hyperphosphorylation of Tau and Disruption of Insulin Signaling. Front. Neurol. 2019, 10, 607. [Google Scholar] [CrossRef]
- Yamamoto, A.; Shin, R.-W.; Hasegawa, K.; Naiki, H.; Sato, H.; Yoshimasu, F.; Kitamoto, T. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: Implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J. Neurochem. 2002, 82, 1137–1147. [Google Scholar] [CrossRef]
- Wong, B.X.; Tsatsanis, A.; Lim, L.Q.; Adlard, P.A.; Bush, A.I.; Duce, J.A. β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS ONE 2014, 9, e114174. [Google Scholar] [CrossRef]
- Frederickson, C.J. Neurobiology of Zinc and Zinc-Containing Neurons. Int. Rev. Neurobiol. 1989, 31, 145–238. [Google Scholar] [CrossRef]
- Takeda, A.; Takada, S.; Ando, M.; Itagaki, K.; Tamano, H.; Suzuki, M.; Iwaki, H.; Oku, N. Impairment of recognition memory and hippocampal long-term potentiation after acute exposure to clioquinol. Neuroscience 2010, 171, 443–450. [Google Scholar] [CrossRef]
- Bush, A.I.; Pettingell, W.H.; de Paradis, M.; Tanzi, R.E.; Wasco, W. The amyloid beta-protein precursor and its mammalian homologues. Evidence for a zinc-modulated heparin-binding superfamily. J. Biol. Chem. 1994, 269, 26618–26621. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.-Y.; Zhu, Y.-Z.; Zhu, H.-L.; Fan, J.-B.; Chen, J.; Liang, Y. Low micromolar zinc accelerates the fibrillization of human tau via bridging of Cys-291 and Cys-322. J. Biol. Chem. 2009, 284, 34648–34657. [Google Scholar] [CrossRef] [PubMed]
- An, W.-L.; Bjorkdahl, C.; Liu, R.; Cowburn, R.F.; Winblad, B.; Pei, J.-J. Mechanism of zinc-induced phosphorylation of p70 S6 kinase and glycogen synthase kinase 3beta in SH-SY5Y neuroblastoma cells. J. Neurochem. 2005, 92, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.-J.; An, W.-L.; Zhou, X.-W.; Nishimura, T.; Norberg, J.; Benedikz, E.; Götz, J.; Winblad, B. P70 S6 kinase mediates tau phosphorylation and synthesis. FEBS Lett. 2006, 580, 107–114. [Google Scholar] [CrossRef]
- Meng, L.; Wang, Z.; Ming, Y.C.; Shen, L.; Ji, H.F. Are micronutrient levels and supplements causally associated with the risk of Alzheimer’s disease? A two-sample Mendelian randomization analysis. Food Funct. 2022, 13, 6665–6673. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, L.; Shen, L.; Ji, H.F. Impact of modifiable risk factors on Alzheimer’s disease: A two-sample Mendelian randomization study. Neurobiol. Aging 2020, 91, 167.e11–167.e19. [Google Scholar] [CrossRef]
- Cheng, W.W.; Zhu, Q.; Zhang, H.Y. Mineral Nutrition and the Risk of Chronic Diseases: A Mendelian Randomization Study. Nutrients 2019, 11, 378. [Google Scholar] [CrossRef]
- Corona, C.; Masciopinto, F.; Silvestri, E.; Del Viscovo, A.; Lattanzio, R.; La Sorda, R.; Ciavardelli, D.; Goglia, F.; Piantelli, M.; Canzoniero, L.M.T.; et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death Dis. 2010, 1, e91. [Google Scholar] [CrossRef]
- Brewer, G.J. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors 2012, 38, 107–113. [Google Scholar] [CrossRef]
- Brewer, G.J. Alzheimer’s disease causation by copper toxicity and treatment with zinc. Front. Aging Neurosci. 2014, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; von Stillfried, N.; Walach, H. Zinc diet and Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2012, 15, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Craven, K.M.; Kochen, W.R.; Hernandez, C.M.; Flinn, J.M. Zinc Exacerbates Tau Pathology in a Tau Mouse Model. J. Alzheimers. Dis. 2018, 64, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wang, T.; Zheng, W.; Zhao, B.L.; Danscher, G.; Chen, Y.H.; Wang, Z.Y. Zinc overload enhances APP cleavage and Aβ deposition in the Alzheimer mouse brain. PLoS ONE 2010, 5, e15349. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Murata, Y.; Kobayashi, M. Clinical manifestations and treatment of Menkes disease and its variants. Pediatr. Int. 1999, 41, 423–429. [Google Scholar] [CrossRef]
- Merle, U.; Schaefer, M.; Ferenci, P.; Stremmel, W. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: A cohort study. Gut 2007, 56, 115–120. [Google Scholar] [CrossRef]
- Žigman, T.; Petković Ramadža, D.; Šimić, G.; Barić, I. Inborn Errors of Metabolism Associated with Autism Spectrum Disorders: Approaches to Intervention. Front. Neurosci. 2021, 15, 624. [Google Scholar] [CrossRef]
- You, H.; Tsutsui, S.; Hameed, S.; Kannanayakal, T.J.; Chen, L.; Xia, P.; Engbers, J.D.T.; Lipton, S.A.; Stys, P.K.; Zamponi, G.W. Aβ neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Ayton, S.; Lei, P.; Bush, A.I. Metallostasis in Alzheimer’s disease. Free Radic. Biol. Med. 2013, 62, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Smith, J.D. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat. Genet. 1996, 14, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Moir, R.D.; Atwood, C.S.; Romano, D.M.; Laurans, M.H.; Huang, X.; Bush, A.I.; Smith, J.D.; Tanzi, R.E. Differential effects of apolipoprotein E isoforms on metal-induced aggregation of A beta using physiological concentrations. Biochemistry 1999, 38, 4595–4603. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, E.M.; Vivar, C.; Camandola, S. Physiology and Pathology of Calcium Signaling in the Brain. Front. Pharmacol. 2012, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Woods, N.K.; Padmanabhan, J. Neuronal Calcium Signaling and Alzheimer’s Disease. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1193–1217. [Google Scholar]
- Olney, J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 1969, 164, 719–721. [Google Scholar] [CrossRef]
- Sattler, R.; Tymianski, M. Molecular Mechanisms of Glutamate Receptor-Mediated Excitotoxic Neuronal Cell Death. Mol. Neurobiol. 2001, 24, 107–130. [Google Scholar] [CrossRef]
- Tong, B.C.K.; Wu, A.J.; Li, M.; Cheung, K.H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta -Mol. Cell Res. 2018, 1865, 1745–1760. [Google Scholar]
- Sato, K.; Mano, T.; Ihara, R.; Suzuki, K.; Tomita, N.; Arai, H.; Ishii, K.; Senda, M.; Ito, K.; Ikeuchi, T.; et al. Lower Serum Calcium as a Potentially Associated Factor for Conversion of Mild Cognitive Impairment to Early Alzheimer’s Disease in the Japanese Alzheimer’s Disease Neuroimaging Initiative. J. Alzheimer’s Dis. 2019, 68, 777–788. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, R.; Guo, Y.; Li, Q.; Zhou, H.; Yu, S.; Liang, H.; Li, Z. An Updated Mendelian Randomization Analysis of the Association Between Serum Calcium Levels and the Risk of Alzheimer’s Disease. Front. Genet. 2021, 12, 1602. [Google Scholar] [CrossRef]
- Kern, J.; Kern, S.; Blennow, K.; Zetterberg, H.; Waern, M.; Guo, X.; Börjesson-Hanson, A.; Skoog, I.; Östling, S. Calcium supplementation and risk of dementia in women with cerebrovascular disease. Neurology 2016, 87, 1674–1680. [Google Scholar] [CrossRef]
- He, Y.; Zhang, H.; Wang, T.; Han, Z.; Ni, Q.B.; Wang, K.; Wang, L.; Zhang, Y.; Hu, Y.; Jin, S.; et al. Impact of Serum Calcium Levels on Alzheimer’s Disease: A Mendelian Randomization Study. J. Alzheimer’s Dis. 2020, 76, 713–724. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [PubMed]
- Rolle-McFarland, D.; Liu, Y.; Mostafaei, F.; Zauber, S.E.; Zhou, Y.; Li, Y.; Fan, Q.; Zheng, W.; Nie, L.H.; Wells, E.M. The association of bone, fingernail and blood manganese with cognitive and olfactory function in Chinese workers. Sci. Total Environ. 2019, 666, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Barahona, A.J.; Bursac, Z.; Veledar, E.; Lucchini, R.; Tieu, K.; Richardson, J.R. Relationship of Blood and Urinary Manganese Levels with Cognitive Function in Elderly Individuals in the United States by Race/Ethnicity, NHANES 2011-2014. Toxics 2022, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, M.E.; Schultheis, P.J.; McGill, D.L.; Richmond, R.E.; Wagge, J.R. Magnesium deficiency impairs fear conditioning in mice. Brain Res. 2005, 1038, 100–106. [Google Scholar] [CrossRef]
- Cherbuin, N.; Kumar, R.; Sachdev, P.S.; Anstey, K.J. Dietary Mineral Intake and Risk of Mild Cognitive Impairment: The PATH through Life Project. Front. Aging Neurosci. 2014, 6, 4. [Google Scholar] [CrossRef]
- Glick, J. Use of magnesium in the management of dementias. Med. Sci. Res. 1990, 18, 831–833. [Google Scholar]
- Ozturk, S.; Cillier, A.E. Magnesium supplementation in the treatment of dementia patients. Med. Hypotheses 2006, 67, 1223–1225. [Google Scholar] [CrossRef]
- Andrási, E.; Igaz, S.; Molnár, Z.; Makó, S. Disturbances of magnesium concentrations in various brain areas in Alzheimer’s disease. Magnes. Res. 2000, 13, 189–196. [Google Scholar]
- Vural, H.; Demirin, H.; Kara, Y.; Eren, I.; Delibas, N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. J. Trace Elem. Med. Biol. 2010, 24, 169–173. [Google Scholar] [CrossRef]
- Cilliers, K. Trace element alterations in Alzheimer’s disease: A review. Clin. Anat. 2021, 34, 766–773. [Google Scholar] [CrossRef]
- Yu, J.; Sun, M.; Chen, Z.; Lu, J.; Liu, Y.; Zhou, L.; Xu, X.; Fan, D.; Chui, D. Magnesium modulates amyloid-beta protein precursor trafficking and processing. J. Alzheimer’s Dis. 2010, 20, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-P.; Li, L.; Bao, J.; Wang, Z.-H.; Zeng, J.; Liu, E.-J.; Li, X.-G.; Huang, R.-X.; Gao, D.; Li, M.-Z.; et al. Magnesium Protects Cognitive Functions and Synaptic Plasticity in Streptozotocin-Induced Sporadic Alzheimer’s Model. PLoS ONE 2014, 9, e108645. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Hochrainer, K.; Segarra, S.G.; Schaeffer, S.; Santisteban, M.M.; Menon, A.; Jiang, H.; Holtzman, D.M.; Anrather, J.; Iadecola, C. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 2019, 574, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Herrmann, K.; Adlung, A.; Paschke, N.; Hausner, L.; Frölich, L.; Schad, L.; Groden, C.; Ulrich, H.K. Evaluation of Sodium (23 Na) MR-imaging as a Biomarker and Predictor for Neurodegenerative Changes in Patients with Alzheimer’s Disease. In Vivo 2021, 35, 429–435. [Google Scholar] [CrossRef]
- Zheng, F.; Li, Y.; Zhang, F.; Sun, Y.; Zheng, C.; Luo, Z.; Wang, Y.L.; Aschner, M.; Zheng, H.; Lin, L.; et al. Cobalt induces neurodegenerative damages through Pin1 inactivation in mice and human neuroglioma cells. J. Hazard. Mater. 2021, 419, 126378. [Google Scholar] [CrossRef]
- Adlard, P.A.; Bush, A.I. Metals and Alzheimer’s Disease: How Far Have We Come in the Clinic? J. Alzheimer’s Dis. 2018, 62, 1369. [Google Scholar] [CrossRef]
- Kessler, H.; Bayer, T.A.; Bach, D.; Schneider-Axmann, T.; Supprian, T.; Herrmann, W.; Haber, M.; Multhaup, G.; Falkai, P.; Pajonk, F.G. Intake of copper has no effect on cognition in patients with mild Alzheimer’s disease: A pilot phase 2 clinical trial. J. Neural Transm. 2008, 115, 1181. [Google Scholar] [CrossRef]
- Chen, L.L.; Fan, Y.G.; Zhao, L.X.; Zhang, Q.; Wang, Z.Y. The metal ion hypothesis of Alzheimer’s disease and the anti-neuroinflammatory effect of metal chelators. Bioorg. Chem. 2023, 131, 106301. [Google Scholar] [CrossRef]
- Cherny, R.A.; Atwood, C.S.; Xilinas, M.E.; Gray, D.N.; Jones, W.D.; McLean, C.A.; Barnham, K.J.; Volitakis, I.; Fraser, F.W.; Kim, Y.S.; et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 2001, 30, 665–676. [Google Scholar] [CrossRef]
- Ritchie, C.W.; Bush, A.I.; Mackinnon, A.; Macfarlane, S.; Mastwyk, M.; MacGregor, L.; Kiers, L.; Cherny, R.; Li, Q.-X.; Tammer, A.; et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Arch. Neurol. 2003, 60, 1685–1691. [Google Scholar] [CrossRef]
- White, A.R.; Du, T.; Laughton, K.M.; Volitakis, I.; Sharples, R.A.; Xilinas, M.E.; Hoke, D.E.; Holsinger, R.M.D.; Evin, G.; Cherny, R.A.; et al. Degradation of the Alzheimer disease amyloid beta-peptide by metal-dependent up-regulation of metalloprotease activity. J. Biol. Chem. 2006, 281, 17670–17680. [Google Scholar] [CrossRef] [PubMed]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Harrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet. Neurol. 2008, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.L.; Roseman, G.; Schilling, K.M.; Dolgova, N.V.; Pushie, M.J.; Sokaras, D.; Kroll, T.; Harris, H.H.; Millhauser, G.L.; Pickering, I.J.; et al. Alzheimer’s Drug PBT2 Interacts with the Amyloid β 1–42 Peptide Differently than Other 8-Hydroxyquinoline Chelating Drugs. Inorg. Chem. 2022, 61, 14626–14640. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.J.; Gray, N.E.; Caruso, M.; Hunter, M.; Ralle, M.; Quinn, J.F. Copper Modulation and Memory Impairment due to Hippocampal Tau Pathology. J. Alzheimers. Dis. 2020, 78, 49–60. [Google Scholar] [CrossRef]
- Drew, S.C. The Case for Abandoning Therapeutic Chelation of Copper Ions in Alzheimer’s Disease. Front. Neurosci. 2017, 11, 317. [Google Scholar] [CrossRef]
- Pingault, J.B.; O’Reilly, P.F.; Schoeler, T.; Ploubidis, G.B.; Rijsdijk, F.; Dudbridge, F. Using genetic data to strengthen causal inference in observational research. Nat. Rev. Genet. 2018, 19, 566–580. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).