Recent Development of Nanomaterials for Transdermal Drug Delivery
Abstract
1. Introduction
2. Important NP Physical Compositions
2.1. Micellular
2.2. Magnetic NPs
2.3. Hollow NPs
2.4. Hydrogel NPs
2.5. Poloxamer Hydrogels
2.6. Acrylic Acid Copolymer Hydrogels
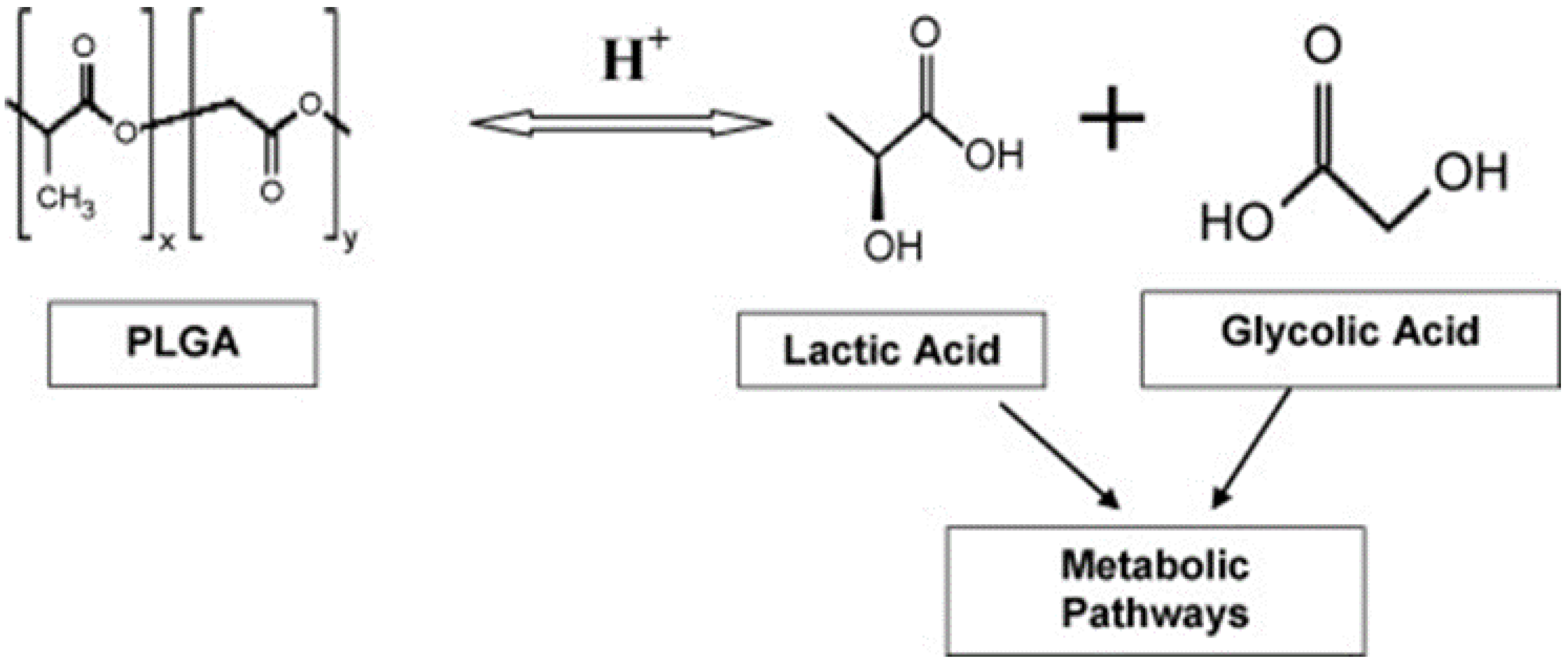
3. Poly(lactide-co-glycolide) (PLGA) NPs
3.1. Ethylene-Vinyl Acetate Flims
3.2. Poly(lactic-co-glycolic acid) Coating
| Drug Delivery System | Method | Application | Result | References |
|---|---|---|---|---|
| PGLA/collagen scaffold | Electrospinning | Human dermal fibroblast and human keratinocyte | High mechanical strength, good surface adhesion on both cell lines | [143] |
| Poly(dl-lactide-co-glycolide)-poly(ethylene glycol)-poly(dl-lactide-co-glycolide) copolymers (PLGA-PEG-PLGA) NPs | Antisolvent diffusion method | Rat skin | High thermodynamic activity, skin permeability and low irritation in PLGA-PEG-PLGA NPs | [144] |
| Gentamicin loaded PLGA (GM-PLGA) NPs | Solvent evaporation method | Rabbit | No sign of inflammation and non-toxic to all groups of rabbit | [145] |
| Hyaluronate-PGLA (HA-PGLA) NPs | Solvent evaporation method | Rat skin | No cytotoxicity, biocompatibility in cell viability, and high efficiency of transdermal delivery | [146] |
| Dictamnine-PGLA-nanocarrier (Dic-PGLA-NC) | Ultrasonication | Mouse dermatitis model | Dic-PGLA-NC can penetrate the dermal layer effectively and achieve sustained drug release | [147] |
4. Chitosan NPs
4.1. Chitosan-Sodium Alginate
4.2. Chitosan-Chondroitin
4.3. Chitosan-Nanoelmusion Films
4.4. Chitosan-Coated Lipid Carriers
5. Carbon Nanotubes CNTs
5.1. Functionalized Multi-Walled Carbon Nanotubes
5.2. Controllable CNT Membranes
5.3. “Bucky Paper”
5.4. CNT Gold NPs
5.5. CNT Hydrogel Hybrid
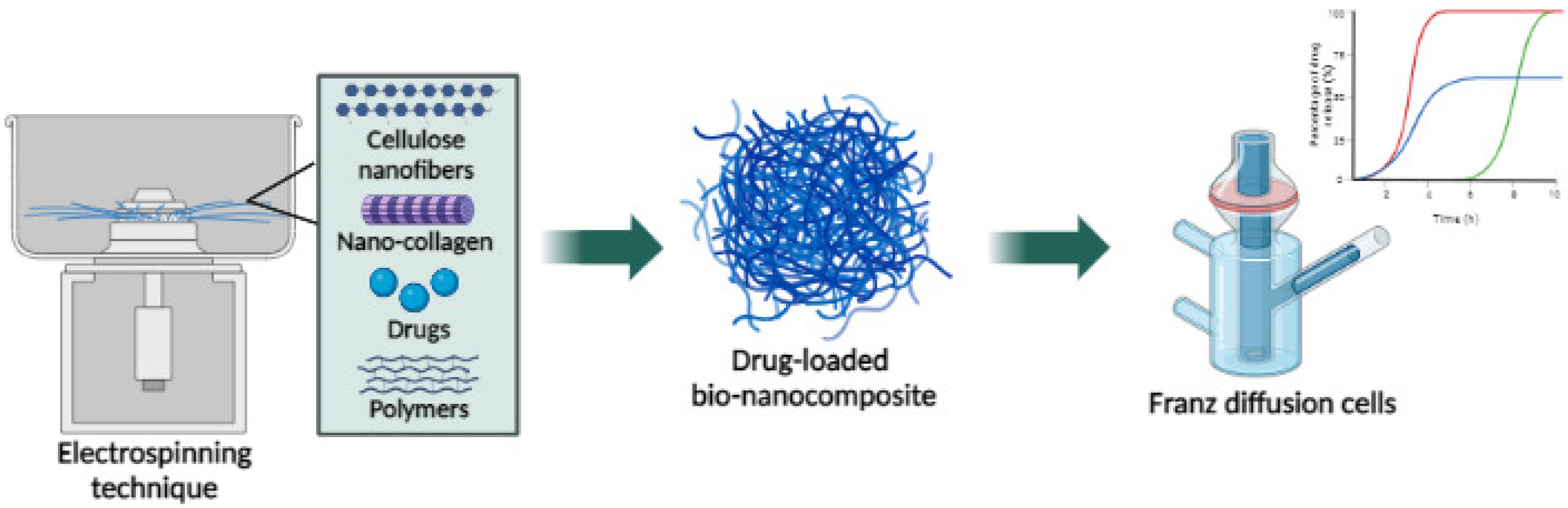
6. Nanocellulose NPs
6.1. Bacteria Nanocellulose
6.2. Cellulose Nanofibers
6.2.1. With Poly(N-isopropyl acrylamide)-Graft-Guar Gum (GG-g-PNIPAAm)
6.2.2. With CNF Transdermal Films
6.3. Cellulose Nanocrystal
6.3.1. With Methylcellulose
6.3.2. CNC-Hydrogels
6.3.3. Lanoconazole (LCZ)-Loaded CNC
7. Ionic Liquids (ILs)
7.1. Choline Geranic Acid (CAGE)
7.2. Surface Active Ionic Liquid (SAIL)
8. Natural Rubbers
Natural Rubber Layers
9. Conclusions
10. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin. Cancer Biol. 2019, 69, 166–177. [Google Scholar] [CrossRef]
- Mishra, D.; Hubenak, J.R.; Mathur, A.B. Nanoparticle systems as tools to improve drug delivery and therapeutic efficacy. J. Biomed. Mater. Res. Part A 2013, 101, 3646–3660. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Abdullah, M.A. Novel drug delivery systems based on silver nanoparticles, hyaluronic acid, lipid nanoparticles and liposomes for cancer treatment. Appl. Nanosci. 2021, 12, 3071–3096. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440. [Google Scholar] [CrossRef] [PubMed]
- Manasa, R.; Shivananjappa, M. Role of Nanotechnology-Based Materials in Drug Delivery. In Advances in Novel Formulations for Drug Delivery; Wiley: Hoboken, NJ, USA, 2023; pp. 279–307. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Biswas, A.K.; Islam, M.R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology based approaches in cancer therapeutics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043001. [Google Scholar] [CrossRef]
- Sabdoningrum, E.K.; Hidanah, S.; Chusniati, S. Characterization and Phytochemical Screening of Meniran (Phyllanthus niruri Linn) Extracts Nanoparticles Used Ball Mill Method. Pharmacogn. J. 2021, 13, 1568–1572. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Erdoğar, N.; Akkin, S.; Bilensoy, E. Nanocapsules for Drug Delivery: An Updated Review of the Last Decade. Recent Pat. Drug Deliv. Formul. 2018, 12, 252–266. [Google Scholar] [CrossRef]
- Ravindran, S.; Suthar, J.; Rokade, R.; Deshpande, P.; Singh, P.; Pratinidhi, A.; Khambadkhar, R.; Utekar, S. Pharmacokinetics, Metabolism, Distribution and Permeability of Nanomedicine. Curr. Drug Metab. 2018, 19, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhang, Z.; He, Q.; Chen, F.; Sheng, Z.; Zhang, D.; Jin, H.; Jiang, F.; Guo, L. Half-life determination of inorganic-organic hybrid nanomaterials in mice using laser-induced breakdown spectroscopy. J. Adv. Res. 2020, 24, 353–361. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.Y.; Zare, E.N.; Borzacchiello, A.; Niu, L.N.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Domanico, M.; Fukuto, A.; Tran, L.M.; Bustamante, J.-M.; Edwards, P.C.; Pinkerton, K.E.; Thomasy, S.M.; Van Winkle, L.S. Cytotoxicity of 2D engineered nanomaterials in pulmonary and corneal epithelium. Nanoimpact 2022, 26, 100404. [Google Scholar] [CrossRef] [PubMed]
- Carissimi, G.; Montalbán, M.G.; Fuster, M.G.; Víllora, G. Nanoparticles as Drug Delivery Systems. In 21st Century Nanostructured Materials—Physics, Chemistry, Classification, and Emerging Applications in Industry, Biomedicine, and Agriculture; IntechOpen: London, UK, 2021. [Google Scholar]
- Dai, H.; Chi, Y.; Wu, X.; Wang, Y.; Wei, M.; Chen, G. Biocompatible electrochemiluminescent biosensor for choline based on enzyme/titanate nanotubes/chitosan composite modified electrode. Biosens. Bioelectron. 2010, 25, 1414–1419. [Google Scholar] [CrossRef]
- John, S.A.; Chattree, A.; Ramteke, P.W.; Shanthy, P.; Nguyen, T.A.; Rajendran, S. Nanosensors for plant health monitoring. Nanosens. Smart Agric. 2022, 18, 449–461. [Google Scholar] [CrossRef]
- Lammers, T.; Ferrari, M. The success of nanomedicine. Nano Today 2020, 31, 100853. [Google Scholar] [CrossRef]
- Pham, D.T.; Tiyaboonchai, W. Fibroin nanoparticles: A promising drug delivery system. Taylors Fr. 2020, 27, 431–448. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in Medicine: Therapeutic Applications and Developments. Educ. Policy Anal. Arch. 2007, 8, 861–869. [Google Scholar] [CrossRef]
- Tomoda, K.; Makino, K. Nanoparticles for transdermal drug delivery system (TDDS). In Colloid and Interface Science in Pharmaceutical Research and Development; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–147. [Google Scholar] [CrossRef]
- Nqakala, Z.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Healing Agents. Int. J. Mol. Sci. Rev. 2021, 22, 11272. [Google Scholar] [CrossRef]
- Jamaledin, R.; Yiu, C.K.; Zare, E.N.; Niu, L.; Vecchione, R.; Chen, G.; Gu, Z.; Tay, F.R.; Makvandi, P. Advances in Antimicrobial Microneedle Patches for Combating Infections. Adv. Mater. 2020, 32, e2002129. [Google Scholar] [CrossRef]
- Kandekar, S.G.; Singhal, M.; Sonaje, K.B.; Kalia, Y.N. Polymeric micelle nanocarriers for targeted epidermal delivery of the hedgehog pathway inhibitor vismodegib: Formulation development and cutaneous biodistribution in human skin. Expert Opin. Drug Deliv. 2019, 16, 667–674. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Butler, A.E.; Badiee, A.; Sahebkar, A. Liposomal nanocarriers for statins: A pharmacokinetic and pharmacodynamics appraisal. J. Cell. Physiol. 2018, 234, 1219–1229. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef]
- Baroli, B.; Ennas, M.G.; Loffredo, F.; Isola, M.; Pinna, R.; López-Quintela, M.A. Penetration of Metallic Nanoparticles in Human Full-Thickness Skin. J. Investig. Dermatol. 2007, 127, 1701–1712. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Knorr, F.; Schäfer, U.; Lehr, C.M.; Dähne, L.; Sterry, W.; Lademann, J. Selective follicular targeting by modification of the particle sizes. J. Control. Release Off. J. Control. Release Soc. 2010, 150, 45–48. [Google Scholar] [CrossRef]
- Lademann, J.M.; Patzelt, A.; Richter, H.; Antoniou, C.; Sterry, W.; Knorr, F. Determination of the cuticula thickness of human and porcine hairs and their potential influence on the penetration of nanoparticles into the hair follicles. J. Biomed. Opt. 2009, 14, 021014. [Google Scholar] [CrossRef]
- Radtke, M.; Patzelt, A.; Knorr, F.; Lademann, J.; Netz, R.R. Ratchet effect for nanoparticle transport in hair follicles. Eur. J. Pharm. Biopharm. 2016, 116, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.J.; Oberdörster, G.; Pentland, A.P.; DeLouise, L.A. In vivo skin penetration of quantum dot nanoparticles in the murine model: The effect of UVR. Nano Lett. 2008, 8, 2779–2787. [Google Scholar] [CrossRef]
- Zhu, Y.; Choe, C.S.; Ahlberg, S.; Meinke, M.C.; Alexiev, U.; Lademann, J.; Darvin, M.E. Penetration of silver nanoparticles into porcine skinex vivousing fluorescence lifetime imaging microscopy, Raman microscopy, and surface-enhanced Raman scattering microscopy. J. Biomed. Opt. 2014, 20, 51006. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Knorr, F.; Richter, H.; Blume-Peytavi, U.; Vogt, A.; Antoniou, C.; Sterry, W.; Patzelt, A. Hair follicles--an efficient storage and penetration pathway for topically applied substances. Summary of recent results obtained at the Center of Experimental and Applied Cutaneous Physiology, Charité -Universitätsmedizin Berlin, Germany. Ski. Pharm. Physiol. 2008, 21, 150–155. [Google Scholar] [CrossRef]
- Aguilar, Z.P. Types of Nanomaterials and Corresponding Methods of Synthesis. Nanomater. Med. Appl. 2013, 33–82. [Google Scholar] [CrossRef]
- Alexander-Bryant, A.A.; Berg-Foels, W.S.V.; Wen, X. Bioengineering Strategies for Designing Targeted Cancer Therapies. Adv. Cancer Res. 2013, 118, 1–59. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Joseph, M.; Trinh, H.M.; Mitra, A.K. Peptide and Protein-Based Therapeutic Agents. Emerg. Nanotechnol. Diagn. Drug Deliv. Med. Devices 2017, 145–167. [Google Scholar] [CrossRef]
- Lee, R.W.; Shenoy, D.W.; Sheel, R. Micellar Nanoparticles: Applications for Topical and Passive Transdermal Drug Delivery. Non-Invasive Drug Deliv. Syst. 2010, 37–57. [Google Scholar]
- Jijie, R.; Barras, A.; Boukherroub, R.; Szunerits, S. Nanomaterials for transdermal drug delivery: Beyond the state of the art of liposomal structures. J. Mater. Chem. B 2017, 5, 8653–8675. [Google Scholar] [CrossRef]
- Chaudhari, Y. Nanoparticles—A paradigm for topical drug delivery. Chron. Young- Sci. 2012, 3, 82. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Bashir, M.; Ahmad, J.; Asif, M.; Khan, S.-U.; Irfan, M.; Ibrahim, A.Y.; Asghar, S.; Khan, I.U.; Iqbal, M.S.; Haseeb, A.; et al. Nanoemulgel, an Innovative Carrier for Diflunisal Topical Delivery with Profound Anti-Inflammatory Effect: In vitro and in vivo Evaluation. Int. J. Nanomed. 2021, 16, 1457–1472. [Google Scholar] [CrossRef]
- Šmejkalová, D.; Muthný, T.; Nešporová, K.; Hermannová, M.; Achbergerová, E.; Huerta-Angeles, G.; Svoboda, M.; Čepa, M.; Machalová, V.; Luptáková, D.; et al. Hyaluronan polymeric micelles for topical drug delivery. Carbohydr. Polym. 2017, 156, 86–96. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; El-Say, K.; Aljaeid, B.M.; Badr-Eldin, S.M.; Ahmed, T.A. Optimized vinpocetine-loaded vitamin E D-α-tocopherol polyethylene glycol 1000 succinate-alpha lipoic acid micelles as a potential transdermal drug delivery system: In vitro and ex vivo studies. Int. J. Nanomed. 2018, 14, 33–43. [Google Scholar] [CrossRef]
- Bachhav, Y.; Mondon, K.; Kalia, Y.; Gurny, R.; Möller, M. Novel micelle formulations to increase cutaneous bioavailability of azole antifungals. J. Control. Release 2011, 153, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Que, F.; Wei, H.; Xu, G.; Dong, X.; Zhang, H. Solubilization of Tea Seed Oil in a Food-Grade Water-Dilutable Microemulsion. PLoS ONE 2015, 10, e0127291. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Rehman, S.; Almessiere, M.A.; Khan, F.A.; Slimani, Y.; Baykal, A. Synthesis of Mn0.5Zn0.5SmxEuxFe1.8−2x O4 Nanoparticles via the Hydrothermal Approach Induced Anti-Cancer and Anti-Bacterial Activities. Nanomaterials 2019, 9, 1635. [Google Scholar] [CrossRef]
- Koblischka, M.R.; Koblischka-Veneva, A.; Zeng, X.; Hannachi, E.; Slimani, Y. Microstructure and Fluctuation-Induced Conductivity Analysis of Bi2Sr2CaCu2O8+δ (Bi-2212) Nanowire Fabrics. Crystal 2020, 10, 986. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.-J.; Zhang, G.-B.; Ling, D.; Wang, M.-Q.; Zhou, Y.; Wu, Y.-D.; Wu, T.; Hackett, M.J.; Kim, B.H.; et al. Iron oxide nanoclusters for T 1 magnetic resonance imaging of non-human primates. Nat. Biomed. Eng. 2017, 1, 637–643. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; Li, W.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Zhu, X.; Du, Y.P.; Jin, Y.; et al. Appropriate Size of Magnetic Nanoparticles for Various Bioapplications in Cancer Diagnostics and Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3092–3106. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Korkmaz, A.D.; Taskhandi, N.; Sertkol, M.; Baykal, A.; Shirsath, S.E.; Ercan, İ.; Ozçelik, B. Sonochemical synthesis of Eu3+ substituted CoFe2O4 nanoparticles and their structural, optical and magnetic properties. Ultrason. Sonochemistry 2019, 58, 104621. [Google Scholar] [CrossRef] [PubMed]
- Slimani, Y.; Almessiere, M.A.; Güner, S.; Tashkandi, N.A.; Baykal, A.; Sarac, M.F.; Nawaz, M.; Ercan, I. Calcination effect on the magneto-optical properties of vanadium substituted NiFe2O4 nanoferrites. J. Mater. Sci. Mater. Electron. 2019, 30, 9143–9154. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; Veintemillas-Verdaguer, S.; Gonzalez-Carreno, T.; Serna, C.J. ChemInform Abstract: Synthesis, Properties and Biomedical Applications of Magnetic Nanoparticles. Cheminform 2008, 39, 403–482. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.-F.; Chen, W.; Liang, X.-G.; Huang, Y.-Z.; Miao, J.; Liu, L.; Lou, Y.; Zhang, X.-G.; Wang, B.; Tang, R.-K.; et al. Epirubicin-Loaded Superparamagnetic Iron-Oxide Nanoparticles for Transdermal Delivery: Cancer Therapy by Circumventing the Skin Barrier. Small 2014, 11, 239–247. [Google Scholar] [CrossRef]
- Dhal, S.; Mohanty, A.; Yadav, I.; Uvanesh, K.; Kulanthaivel, S.; Banerjee, I.; Pal, K.; Giri, S. Magnetic nanoparticle incorporated oleogel as iontophoretic drug delivery system. Colloids Surf. B Biointerfaces 2017, 157, 118–129. [Google Scholar] [CrossRef]
- Berry, C.C.; Charles, S.; Wells, S.; Dalby, M.J.; Curtis, A.S. The influence of transferrin stabilised magnetic nanoparticles on human dermal fibroblasts in culture. Int. J. Pharm. 2003, 269, 211–225. [Google Scholar] [CrossRef]
- Sundaresan, V.; Menon, J.U.; Rahimi, M.; Nguyen, K.T.; Wadajkar, A.S. Dual-responsive polymer-coated iron oxide nanoparticles for drug delivery and imaging applications. Int. J. Pharm. 2014, 466, 1–7. [Google Scholar] [CrossRef]
- Ding, Y.; Shen, S.Z.; Sun, H.; Sun, K.; Liu, F.; Qi, Y.; Yan, J. Design and construction of polymerized-chitosan coated Fe3O4 magnetic nanoparticles and its application for hydrophobic drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 487–498. [Google Scholar] [CrossRef]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, Functionalization, and Biomedical Applications of Multifunctional Magnetic Nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef]
- Fatima, H.; Kim, K.-S. Magnetic nanoparticles for bioseparation. Korean J. Chem. Eng. 2017, 34, 589–599. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, Y.; Qiu, Y.; Yang, X.; Cao, H.; Wu, Y. Design of Functional Magnetic Nanocomposites for Bioseparation. Colloids Surf. B Biointerfaces 2020, 191, 111014. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Garu, P.; Li, C.H.; Chang, W.C.; Chen, B.W.; Sung, S.Y.; Lee, C.M.; Chen, J.Y.; Hsieh, T.F.; Sheu, W.J.; et al. Magnetoresistive Biosensors for Direct Detection of Magnetic Nanoparticle Conjugated Biomarkers on a Chip. Spin 2019, 9, 1940002. [Google Scholar] [CrossRef]
- Piñeiro, Y.; Gómez, M.G.; Alves, L.D.C.; Prieto, A.A.; Acevedo, P.G.; Gudiña, R.S.; Puig, J.; Teijeiro, C.; Vilar, S.Y.; Rivas, J. Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine. Magnetochemistry 2020, 6, 4. [Google Scholar] [CrossRef]
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Jeun, M.; Bae, S.; Tomitaka, A.; Takemura, Y.; Park, K.H.; Paek, S.H.; Chung, K.-W. Effects of particle dipole interaction on the ac magnetically induced heating characteristics of ferrite nanoparticles for hyperthermia. Appl. Phys. Lett. 2009, 95, 082501. [Google Scholar] [CrossRef]
- Zhang, L.-K.; Du, S.; Wang, X.; Jiao, Y.; Yin, L.; Zhang, Y.; Guan, Y.-Q. Bacterial cellulose based composites enhanced transdermal drug targeting for breast cancer treatment. Chem. Eng. J. 2019, 370, 749–759. [Google Scholar] [CrossRef]
- Modabberasl, A.; Pirhoushyaran, T.; Esmaeili-Faraj, S.H. Synthesis of CoFe2O4 magnetic nanoparticles for application in photocatalytic removal of azithromycin from wastewater. Sci. Rep. 2022, 12, 19171. [Google Scholar] [CrossRef]
- Kharat, P.B.; Somvanshi, S.B.; Khirade, P.P.; Jadhav, K.M. Induction Heating Analysis of Surface-Functionalized Nanoscale CoFe2O4 for Magnetic Fluid Hyperthermia toward Noninvasive Cancer Treatment. ACS Omega 2020, 5, 23378–23384. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Fu, Z.; Qu, J.; Zhong, M.; Yang, X.; Yi, Y.; Wang, C. Nanoporous Ni with High Surface Area for Potential Hydrogen Storage Application. Nanomaterials 2018, 8, 394. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, L.; Wu, M.; Wang, Y.; Lou, X.W.D. Construction of Complex Co3O4@Co3V2O8 Hollow Structures from Metal–Organic Frameworks with Enhanced Lithium Storage Properties. Adv. Mater. 2018, 30, 1702875. [Google Scholar] [CrossRef] [PubMed]
- Hany, A. A review on nanoparticles in transdermal drug delivery: Polymers at variance with semiconductors and lipids. Int. J. Eng. Appl. Sci. Technol. 2020, 5, 27–36. [Google Scholar] [CrossRef]
- Yu, L.; Yu, X.Y.; Lou, X.W. The Design and Synthesis of Hollow Micro-/Nanostructures: Present and Future Trends. Adv. Mater. 2018, 30, 1800939. [Google Scholar] [CrossRef]
- Fang, Y.; Guan, B.Y.; Luan, D.; Lou, X.W. Synthesis of CuS@CoS2 Double-Shelled Nanoboxes with Enhanced Sodium Storage Properties. Angew. Chem. Int. Ed. 2019, 58, 7739–7743. [Google Scholar] [CrossRef]
- Guo, L.; Panderi, I.; Yan, D.D.; Szulak, K.; Li, Y.; Chen, Y.-T.; Ma, H.; Niesen, D.B.; Seeram, N.; Ahmed, A.; et al. A Comparative Study of Hollow Copper Sulfide Nanoparticles and Hollow Gold Nanospheres on Degradability and Toxicity. ACS Nano 2013, 7, 8780–8793. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.; Zhou, M.; Song, S.; Huang, Q.; Hazle, J.; Li, C. Copper Sulfide Nanoparticles As a New Class of Photoacoustic Contrast Agent for Deep Tissue Imaging at 1064 nm. ACS Nano 2012, 6, 7489–7496. [Google Scholar] [CrossRef] [PubMed]
- Ain, N.U.; Nasir, J.A.; Khan, Z.; Butler, I.S.; Rehman, Z. Copper sulfide nanostructures: Synthesis and biological applications. RSC Adv. 2022, 12, 7550–7567. [Google Scholar] [CrossRef]
- Li, Q.; Sun, L.; Hou, M.; Chen, Q.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Phase-Change Material Packaged within Hollow Copper Sulfide Nanoparticles Carrying Doxorubicin and Chlorin e6 for Fluorescence-Guided Trimodal Therapy of Cancer. ACS Appl. Mater. Interfaces 2018, 11, 417–429. [Google Scholar] [CrossRef]
- Zan, P.; Than, A.; Zhang, W.; Cai, H.X.; Zhao, W.; Chen, P. Transdermal Photothermal-Pharmacotherapy to Remodel Adipose Tissue for Obesity and Metabolic Disorders. ACS Nano 2022, 16, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Liu, Q. ROS-responsive hollow mesoporous silica nanoparticles loaded with Glabridin for anti-pigmentation properties. Microporous Mesoporous Mater. 2021, 327, 111429. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, S.; Hu, W.; Lin, X.; Cao, C.; Zou, S.; Tong, Z.; Jiang, G.; Kong, X. Polymer-grafted hollow mesoporous silica nanoparticles integrated with microneedle patches for glucose-responsive drug delivery. Front. Mater. Sci. 2021, 15, 98–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, G.; Hong, W.; Gao, M.; Xu, B.; Zhu, J.; Song, G.; Liu, T. Polymeric Microneedles Integrated with Metformin-Loaded and PDA/LA-Coated Hollow Mesoporous SiO2 for NIR-Triggered Transdermal Delivery on Diabetic Rats. ACS Appl. Bio Mater. 2018, 1, 1906–1917. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, D.Y.; Seo, H.W.; Jeong, S.H.; Kim, J.H.; Kim, M.S. Preparation of erythromycin-loaded poly(vinylalcohol) film and investigation of its feasibility as a transdermal delivery carrier. Tissue Eng. Regen. Med. 2014, 11, 211–216. [Google Scholar] [CrossRef]
- An, Y.-H.; Lee, J.; Son, D.U.; Kang, D.H.; Park, M.J.; Cho, K.W.; Kim, S.; Kim, S.-H.; Ko, J.; Jang, M.-H.; et al. Facilitated Transdermal Drug Delivery Using Nanocarriers-Embedded Electroconductive Hydrogel Coupled with Reverse Electrodialysis-Driven Iontophoresis. ACS Nano 2020, 14, 4523–4535. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Tran, L.; Parks, J.; Zhao, Y.; Hai, N.; Zhong, Y.; Ji, H. Highly stretchable gelatin-polyacrylamide hydrogel for potential transdermal drug release. Nano Sel. 2020, 2, 107–115. [Google Scholar] [CrossRef]
- Jung, H.; Kim, M.K.; Lee, J.Y.; Choi, S.W.; Kim, J. Adhesive Hydrogel Patch with Enhanced Strength and Adhesiveness to Skin for Transdermal Drug Delivery. Adv. Funct. Mater. 2020, 30, 2004407. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Ahsan, A.; Tian, W.X.; Farooq, M.A.; Khan, D.H. An overview of hydrogels and their role in transdermal drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 574–584. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Materials Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Polymeric hydrogels: Characterization and biomedical applications. Designed Monomers and Polymers 2009, 12, 197–220. [Google Scholar] [CrossRef]
- Huang, D.; Sun, M.; Bu, Y.; Luo, F.; Lin, C.; Lin, Z.; Weng, Z.; Yang, F.; Wu, D. Microcapsule-embedded hydrogel patches for ultrasound responsive and enhanced transdermal delivery of diclofenac sodium. J. Mater. Chem. B 2019, 7, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xin, P.; Ou, Q.; Hollett, G.; Gu, Z.; Wu, J. Poly(ester amide)-based hybrid hydrogels for efficient transdermal insulin delivery. J. Mater. Chem. B 2018, 6, 6723–6730. [Google Scholar] [CrossRef]
- Birajdar, R.P.; Patil, S.B.; Alange, V.V.; Kulkarni, R.V. Synthesis and characterization of electrically responsive poly(acrylamide)-grafted-chondroitin sulfate hydrogel for transdermal drug delivery application. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 148–157. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Sierakowska, A.; Matysek, D. ZnO nanorods functionalized with chitosan hydrogels crosslinked with azelaic acid for transdermal drug delivery. Colloids Surf. B Biointerfaces 2020, 194, 111170. [Google Scholar] [CrossRef]
- Wei, H.; Liu, S.; Tong, Z.; Chen, T.; Yang, M.; Guo, Y.; Sun, H.; Wu, Y.; Chu, Y.; Fan, L. Hydrogel-based microneedles of chitosan derivatives for drug delivery. React. Funct. Polym. 2022, 172, 105200. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, S.J.; Song, J.Y.; Park, S.N. Synthesis and physicochemical properties of pH-sensitive hydrogel based on carboxymethyl chitosan/2-hydroxyethyl acrylate for transdermal delivery of nobiletin. J. Drug Deliv. Sci. Technol. 2019, 51, 194–203. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Pharmaceutics Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Laurano, R.; Abrami, M.; Grassi, M.; Ciardelli, G.; Boffito, M.; Chiono, V. Using Poloxamer® 407 as Building Block of Amphiphilic Poly(ether urethane)s: Effect of its Molecular Weight Distribution on Thermo-Sensitive Hydrogel Performances in the Perspective of Their Biomedical Application. Front. Mater. 2020, 7, 594515. [Google Scholar] [CrossRef]
- Shu, H.; Zhang, Y.; Zhang, M.; Wu, J.; Cui, M.; Liu, K.; Wang, J. Addition of free poloxamer 407 to a new gene vector P407-PEI-K12 solution forms a sustained-release in situ hypergel that enhances cell transfection and extends gene expression. Oncol. Lett. 2019, 17, 3085–3096. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. Expert Review A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Mayol, L.; Biondi, M.; Quaglia, F.; Fusco, S.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. Injectable Thermally Responsive Mucoadhesive Gel for Sustained Protein Delivery. Biomacromolecules 2010, 12, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wat, E.; Hui, P.C.L.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Hui, P.C.L.; Wat, E.; Ng, F.S.F.; Kan, C.-W.; Lau, C.B.S.; Leung, P.-C. Enhanced Transdermal Permeability via Constructing the Porous Structure of Poloxamer-Based Hydrogel. Polymers 2016, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hao, Y.; Zhang, W.; Wei, Y.; Shu, Y.; Wang, J. Microwave-triggered ionic liquid-based hydrogel dressing with excellent hyperthermia and transdermal drug delivery performance. Chem. Eng. J. 2021, 429, 131590. [Google Scholar] [CrossRef]
- Hamad, K.M.; Mahmoud, N.N.; Al-Dabash, S.; Al-Samad, L.A.; Abdallah, M.; Al-Bakri, A.G. Fluconazole conjugated-gold nanorods as an antifungal nanomedicine with low cytotoxicity against human dermal fibroblasts. RSC Adv. 2020, 10, 25889–25897. [Google Scholar] [CrossRef]
- Patil, S.B.; Inamdar, S.Z.; Reddy, K.R.; Raghu, A.V.; Akamanchi, K.G.; Inamadar, A.C.; Das, K.K.; Kulkarni, R.V. Functionally Tailored Electro-Sensitive Poly(Acrylamide)-g-Pectin Copolymer Hydrogel for Transdermal Drug Delivery Application: Synthesis, Characterization, In-vitro and Ex-vivo Evaluation. Drug Deliv. Lett. 2020, 10, 185–196. [Google Scholar] [CrossRef]
- Patil, S.B.; Inamdar, S.Z.; Das, K.K.; Akamanchi, K.G.; Patil, A.V.; Inamadar, A.C.; Reddy, K.R.; Raghu, A.V.; Kulkarni, R.V. Tailor-made electrically-responsive poly(acrylamide)-graft-pullulan copolymer based transdermal drug delivery systems: Synthesis, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101525. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Lather, V. Hybrid poly(lactic-co-glycolic acid) nanoparticles: Design and delivery prospectives. Drug Discov. Today 2015, 20, 95–104. [Google Scholar] [CrossRef]
- Hafez Mousa, A.; Agha Mohammad, S. Potential role of chitosan, PLGA and iron oxide nanoparticles in Parkinsons disease therapy. J. Neurol. Psychiatry Neurosurg. 2022, 58, 68. [Google Scholar]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, C. Tuning the size of poly(lactic-co-glycolic acid) (PLGA) nanoparticles fabricated by nanoprecipitation; Tuning the size of poly(lactic-co-glycolic acid) (PLGA) nanoparticles fabricated by nanoprecipitation. Biotechnol. J. 2018, 13, 1700203. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.; Esendagli, G.; Yerlikaya, F.; Caban-Toktas, S.; Yoyen-Ermis, D.; Horzum, U.; Aktas, Y.; Khan, M.; Couvreur, P.; Capan, Y. A small variation in average particle size of PLGA nanoparticles prepared by nanoprecipitation leads to considerable change in nanoparticles characteristics and efficacy of intracellular delivery. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1657–1664. [Google Scholar] [CrossRef]
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste - Its applications and degradation. International Journal of Biological Macromolecules 2023, 234, 123703. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, S. Nanoscale Metal Particles as Nanocarriers in Targeted Drug Delivery System. J. Nanomed. Res. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- Cataldi, M.; Vigliotti, C.; Mosca, T.; Cammarota, M.; Capone, D. Emerging Role of the Spleen in the Pharmacokinetics of Monoclonal Antibodies, Nanoparticles and Exosomes. Int. J. Mol. Sci. 2017, 18, 1249. [Google Scholar] [CrossRef] [PubMed]
- Karasu, İ.; Akbaş, S.; Çapan, Y. Current progress in PLGA-based nanoparticles for treatment of cancer diseases. In Poly(lactic-co-glycolic acid) Nanoparticles Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 153–177. [Google Scholar] [CrossRef]
- Dobiasch, S.; Szanyi, S.; Kjaev, A.; Werner, J.; Strauss, A.; Weis, C.; Grenacher, L.; Kapilov-Buchman, K.; Israel, L.-L.; Lellouche, J.-P.; et al. Synthesis and functionalization of protease-activated nanoparticles with tissue plasminogen activator peptides as targeting moiety and diagnostic tool for pancreatic cancer. J. Nanobiotechnology 2016, 14, 1–18. [Google Scholar] [CrossRef]
- Ambrogio, M.W.; Toro-González, M.; Keever, T.J.; McKnight, T.E.; Davern, S.M. Poly(lactic-co-glycolic acid) Nanoparticles as Delivery Systems for the Improved Administration of Radiotherapeutic Anticancer Agents. ACS Appl. Nano Mater. 2020, 3, 10565–10570. [Google Scholar] [CrossRef]
- Salaheldin, T.A.; Bharali, D.J.; Mousa, S.A. Functionalized nano-targeted moieties in management of prostate cancer. Futur. Oncol. 2020, 16, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Galindo, R.; Sánchez-López, E.; Gómara, M.J.; Espina, M.; Ettcheto, M.; Cano, A.; Haro, I.; Camins, A.; García, M.L. Development of Peptide Targeted PLGA-PEGylated Nanoparticles Loading Licochalcone-A for Ocular Inflammation. Pharmaceutics 2022, 14, 285. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular penetration of fluorometholone-loaded PEG-PLGA nanoparticles functionalized with cell-penetrating peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [CrossRef]
- Samani, S.E.; Naderimanesh, H.; Asghari, S.M.; Hoseinkhani, S. Optimization of Preparation of PEG-PLGA Nanoparticles by Solvent Evaporation Method. J. Biotechnol. 2018, 9, 201–205. [Google Scholar]
- El-Hammadi, M.M.; Arias, J.L. Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines. Nanomaterials 2022, 12, 354. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Patel, K.D.; Silva, L.B.; Park, Y.; Shakouri, T.; Keskin-Erdogan, Z.; Sawadkar, P.; Cho, K.J.; Knowles, J.C.; Chau, D.Y.; Kim, H.-W. Recent advances in drug delivery systems for glaucoma treatment. Mater. Today Nano 2022, 18, 100178. [Google Scholar] [CrossRef]
- Yadav, K.S.; Rajpurohit, R.; Sharma, S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci. 2019, 221, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Charmi, G.; Matyjaszewski, K.; Banquy, X.; Pietrasik, J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021, 123, 31–50. [Google Scholar] [CrossRef]
- Jin, Z.; Zhan, Y.; Zheng, L.; Wei, Q.; Xu, S.; Qin, Z. Cannabidiol-loaded poly lactic-co-glycolic acid nanoparticles with improved bioavailability as a potential for osteoarthritis therapeutic. Chembiochem 2022, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Pontes, A.P.; Welting, T.J.M.; Rip, J.; Creemers, L.B. Polymeric Nanoparticles for Drug Delivery in Osteoarthritis. Pharmaceutics 2022, 14, 2639. [Google Scholar] [CrossRef]
- Takeuchi, I.; Suzuki, T.; Makino, K. Skin permeability and transdermal delivery route of 50-nm indomethacin-loaded PLGA nanoparticles. Colloids Surf. B Biointerfaces 2017, 159, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Kobayashi, S.; Hida, Y.; Makino, K. Estradiol-loaded PLGA nanoparticles for improving low bone mineral density of cancellous bone caused by osteoporosis: Application of enhanced charged nanoparticles with iontophoresis. Colloids Surf. B Biointerfaces 2017, 155, 35–40. [Google Scholar] [CrossRef]
- Takeuchi, I.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for transcutaneous immunization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 608, 125607. [Google Scholar] [CrossRef]
- Bali, N.R.; Salve, P.S. Selegiline nanoparticle embedded transdermal film: An alternative approach for brain targeting in Parkinsons disease. J. Drug Deliv. Sci. Technol. 2019, 54, 101299. [Google Scholar] [CrossRef]
- Bali, N.R.; Salve, P.S. Impact of rasagiline nanoparticles on brain targeting efficiency via gellan gum based transdermal patch: A nanotheranostic perspective for Parkinsonism. Int. J. Biol. Macromol. 2020, 164, 1006–1024. [Google Scholar] [CrossRef]
- Sadeghi-Avalshahr, A.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Mahdavi-Shahri, M. Synthesis and characterization of collagen/PLGA biodegradable skin scaffold fibers. Regen. Biomater. 2017, 4, 309–314. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kagawa, A.; Makino, K. Skin permeability and transdermal delivery route of 30-nm cyclosporin A-loaded nanoparticles using PLGA-PEG-PLGA triblock copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124866. [Google Scholar] [CrossRef]
- Akhtar, N. Microneedles: An innovative approach to transdermal delivery—A review. Innovare Acad. Sci. 2014, 6, 18–25. [Google Scholar]
- Jeong, W.Y.; Kim, S.; Lee, S.Y.; Lee, H.; Han, D.-W.; Yang, S.Y.; Kim, K.S. Transdermal delivery of Minoxidil using HA-PLGA nanoparticles for the treatment in alopecia. Biomater. Res. 2019, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Hsieh, Y.-T.; Chan, L.Y.; Yang, T.-Y.; Maeda, T.; Chang, T.-M.; Huang, H.-C. Dictamnine delivered by PLGA nanocarriers ameliorated inflammation in an oxazolone-induced dermatitis mouse model. J. Control. Release 2020, 329, 731–742. [Google Scholar] [CrossRef]
- Marapureddy, S.G.; Thareja, P. Synergistic effect of chemical crosslinking and addition of graphene-oxide in Chitosan—Hydrogels, films, and drug delivery. Mater. Today Commun. 2021, 31, 103430. [Google Scholar] [CrossRef]
- Pourseif, T.; Ghafelehbashi, R.; Abdihaji, M.; Radan, N.; Kaffash, E.; Heydari, M.; Naseroleslami, M.; Mousavi-Niri, N.; Akbarzadeh, I.; Ren, Q. Chitosan-based nanoniosome for potential wound healing applications: Synergy of controlled drug release and antibacterial activity. Int. J. Biol. Macromol. 2023, 230, 123185. [Google Scholar] [CrossRef] [PubMed]
- Alhodieb, F.S.; Barkat, M.A.; Barkat, H.A.; Hadi, H.A.; Khan, M.I.; Ashfaq, F.; Rahman, M.A.; Hassan, M.Z.; Alanezi, A.A. International Journal of Biological Macromolecules Chitosan-modified nanocarriers as carriers for anticancer drug delivery: Promises and hurdles. Int. J. Biol. Macromol. 2022, 217, 457–469. [Google Scholar] [CrossRef]
- Mukhtar, M.; Fényes, E.; Bartos, C.; Zeeshan, M.; Ambrus, R. Chitosan biopolymer, its derivatives and potential applications in nano-therapeutics: A comprehensive review. Eur. Polym. J. 2021, 160, 110767. [Google Scholar] [CrossRef]
- Gorantla, S.; Dabholkar, N.; Sharma, S.; Rapalli, V.K.; Alexander, A.; Singhvi, G. Chitosan-based microneedles as a potential platform for drug delivery through the skin: Trends and regulatory aspects. Int. J. Biol. Macromol. 2021, 184, 438–453. [Google Scholar] [CrossRef]
- Abnoos, M.; Mohseni, M.; Mousavi, S.A.J.; Ashtari, K.; Ilka, R.; Mehravi, B. Chitosan-alginate nano-carrier for transdermal delivery of pirfenidone in idiopathic pulmonary fibrosis. Int. J. Biol. Macromol. 2018, 118, 1319–1325. [Google Scholar] [CrossRef]
- Talib, S.; Ahmed, N.; Khan, D.; Khan, G.M.; Rehman, A.U. Chitosan-chondroitin based artemether loaded nanoparticles for transdermal drug delivery system. J. Drug Deliv. Sci. Technol. 2021, 61, 102281. [Google Scholar] [CrossRef]
- Da Silva, T.N.; Reynaud, F.; Picciani, P.H.D.S.; Silva, K.G.D.H.E.; Barradas, T.N. Chitosan-based films containing nanoemulsions of methyl salicylate: Formulation development, physical-chemical and in vitro drug release characterization. Int. J. Biol. Macromol. 2020, 164, 2558–2568. [Google Scholar] [CrossRef]
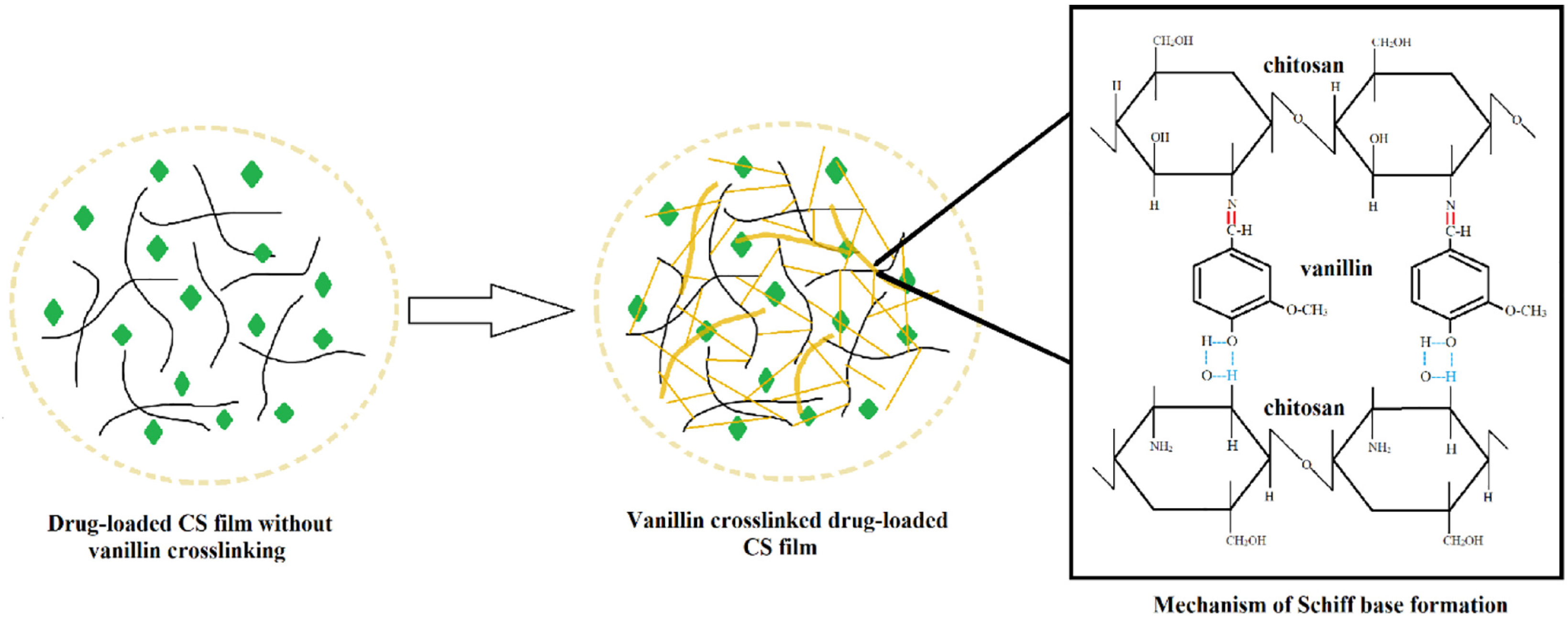
- Karakurt, I.; Ozaltin, K.; Vargun, E.; Kucerova, L.; Suly, P.; Harea, E.; Minařík, A.; Štěpánková, K.; Lehocky, M.; Humpolícek, P.; et al. Controlled release of enrofloxacin by vanillin-crosslinked chitosan-polyvinyl alcohol blends. Mater. Sci. Eng. C 2021, 126, 112125. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.H.; Alcantara, K.P.; Bulatao, B.P.I.; Sorasitthiyanukarn, F.N.; Muangnoi, C.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-coated nanostructured lipid carriers for transdermal delivery of tetrahydrocurcumin for breast cancer therapy. Carbohydr. Polym. 2022, 288, 119401. [Google Scholar] [CrossRef]
- Engkagul, V.; Klaharn, I.-Y.; Sereemaspun, A.; Chirachanchai, S. Chitosan whisker grafted with oligo(lactic acid) nanoparticles via a green synthesis pathway: Potential as a transdermal drug delivery system. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Andrés Ortiz, J.; Antonella Sepúlveda, F.; Panadero-Medianero, C.; Murgas, P.; Ahumada, M.; Palza, H.; Zapata, P.A. Cytocompatible drug delivery hydrogels based on carboxymethylagarose/chitosan pH-responsive polyelectrolyte complexes. Int. J. Biol. Macromol. 2021, 199, 96–107. [Google Scholar] [CrossRef]
- Castilla-Casadiego, D.A.; Carlton, H.; Gonzalez-Nino, D.; Miranda-Muñoz, K.A.; Daneshpour, R.; Huitink, D.; Prinz, G.; Powell, J.; Greenlee, L.; Almodovar, J. Design, characterization, and modeling of a chitosan microneedle patch for transdermal delivery of meloxicam as a pain management strategy for use in cattle. Mater. Sci. Eng. C 2020, 118, 111544. [Google Scholar] [CrossRef]
- Bigucci, F.; Abruzzo, A.; Saladini, B.; Gallucci, M.C.; Cerchiara, T.; Luppi, B. Development and characterization of chitosan/hyaluronan film for transdermal delivery of thiocolchicoside. Carbohydr. Polym. 2015, 130, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Abioye, A.O.; Issah, S.; Kola-Mustapha, A.T. Ex vivo skin permeation and retention studies on chitosan–ibuprofen–gellan ternary nanogel prepared by in situ ionic gelation technique—A tool for controlled transdermal delivery of ibuprofen. Int. J. Pharm. 2015, 490, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid electrospun chitosan-phospholipids nanofibers for transdermal drug delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Xu, F.; Gang, X.; Demirci, U.; Wei, D.; Li, Y.; Feng, Y.; Jia, D.; Zhou, Y. Hydrosoluble, UV-crosslinkable and injectable chitosan for patterned cell-laden microgel and rapid transdermal curing hydrogel in vivo. Acta Biomater. 2015, 22, 59–69. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Siu, W.S.; Kan, C.; Leung, P.C.; Wanxue, C.; Chiou, J.C. Influence of pH-responsive compounds synthesized from chitosan and hyaluronic acid on dual-responsive (pH/temperature) hydrogel drug delivery systems of Cortex Moutan. Int. J. Biol. Macromol. 2021, 168, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Takeshita, T.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for positively charged drugs. Colloids Surf. B Biointerfaces 2017, 160, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Morad, H.; Jahanshahi, M.; Akbari, J.; Saeedi, M.; Gill, P.; Enayatifard, R. Novel topical and transdermal delivery of colchicine with chitosan based biocomposite nanofiberous system; formulation, optimization, characterization, ex vivo skin deposition/permeation, and anti-melanoma evaluation. Mater. Chem. Phys. 2021, 263, 124381. [Google Scholar] [CrossRef]
- Luesakul, U.; Puthong, S.; Sansanaphongpricha, K.; Muangsin, N. Quaternized chitosan-coated nanoemulsions: A novel platform for improving the stability, anti-inflammatory, anti-cancer and transdermal properties of Plai extract. Carbohydr. Polym. 2019, 230, 115625. [Google Scholar] [CrossRef]
- Rubina, M.S.; Pestrikova, A.A.; Kazaryan, P.S.; Nikolaev, A.Y.; Chaschin, I.S.; Arkharova, N.A.; Shulenina, A.V.; Pigaleva, M.A. Supercritical impregnation of chitosan sponges with 17β-estradiol. J. CO2 Util. 2022, 62, 102106. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Kashaw, V.; Shabaaz, J.; Dahiya, R. Synthesis and ex vivo evaluation of PLGA chitosan surface modulated double walled transdermal Pluronic nanogel for the controlled delivery of Temozolomide. Int. J. Biol. Macromol. 2021, 187, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.; Wen, J.; Cheng, A.E.-M.; Kim, A.M.-J.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chen, W.; Chen, Y.; Qiu, Y.; Yi, J.; Li, X.; Lin, Q.; Guo, B. Functionalized multiwalled carbon nanotube-ethosomes for transdermal delivery of ketoprofen: Ex vivo and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2022, 69, 103098. [Google Scholar] [CrossRef]
- Gulati, G.K.; Berger, L.R.; Hinds, B.J. A preclinical evaluation of a programmable CNT membrane device for transdermal nicotine delivery in hairless Guinea pigs. J. Control. Release 2018, 293, 135–143. [Google Scholar] [CrossRef]
- Gulati, G.K.; Chen, T.; Hinds, B.J. Programmable carbon nanotube membrane-based transdermal nicotine delivery with microdialysis validation assay. Nanomed. Nanotechnol. Biol. Med. 2016, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cranford, S.W.; Buehler, M.J. In silico assembly and nanomechanical characterization of carbon nanotube buckypaper. Nanotechnology 2010, 21, 265706. [Google Scholar] [CrossRef]
- Schwengber, A.; Prado, H.J.; Bonelli, P.R.; Cukierman, A.L. Development and in vitro evaluation of potential electromodulated transdermal drug delivery systems based on carbon nanotube buckypapers. Mater. Sci. Eng. C 2017, 76, 431–438. [Google Scholar] [CrossRef]
- Anirudhan, T.; Nair, S.S. Development of voltage gated transdermal drug delivery platform to impose synergistic enhancement in skin permeation using electroporation and gold nanoparticle. Mater. Sci. Eng. C 2019, 102, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Guillet, J.-F.; Valdez-Nava, Z.; Golzio, M.; Flahaut, E. Electrical properties of double-wall carbon nanotubes nanocomposite hydrogels. Carbon 2019, 146, 542–548. [Google Scholar] [CrossRef]
- Mohd, N.H.; Ismail, N.F.H.; Zahari, J.I.; Fathilah, W.; Kargarzadeh, H.; Ramli, S.; Othaman, R. Effect of Aminosilane Modification on Nanocrystalline Cellulose Properties. J. Nanomater. 2016, 2016, e4804271. [Google Scholar] [CrossRef]
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.H.; Ismadji, S. Nanocelluloses: Sources, Pretreatment, Isolations, Modification, and Its Application as the Drug Carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Mautner, A.; Hakalahti, M.; Rissanen, V.; Tammelin, T. Crucial Interfacial Features of Nanocellulose Materials. Nanocellulose Sustain. Prod. Prop. Appl. Case Stud. 2018, 42, 87–128. [Google Scholar] [CrossRef]
- Salimi, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of Nanocellulose and Its Applications in Drug Delivery: A Critical Review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Tan, T.H.; Lee, H.V.; Yehya Dabdawb, W.A. A review of nanocellulose in the drug-delivery system. In Materials for Biomedical Engineering Nanomaterials-based Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–164. [Google Scholar]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lisdayana, N.; Fahma, F.; Sunarti, T.C.; Iriani, E.S. Thermoplastic Starch–PVA Nanocomposite Films Reinforced with Nanocellulose from Oil Palm Empty Fruit Bunches (OPEFBs): Effect of Starch Type. J. Nat. Fibers 2018, 17, 1069–1080. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Liu, S.; Qamar, S.A.; Qamar, M.; Basharat, K.; Bilal, M. Engineered nanocellulose-based hydrogels for smart drug delivery applications. Int. J. Biol. Macromol. 2021, 181, 275–290. [Google Scholar] [CrossRef]
- Quinetti, C.; Pittella, P.; Vitoria, E.M.; Francisco, G.; Junior, X. The use of bacterial nanocellulose as wound healing dressing: A scoping review. 2022. [Google Scholar]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, N.; Ahmad, I.; Zulfakar, M.H.; Kargarzadeh, H.; Ramli, S. Cetyltrimethylammonium bromide-nanocrystalline cellulose (CTAB-NCC) based microemulsions for enhancement of topical delivery of curcumin. Carbohydr. Polym. 2020, 254, 117401. [Google Scholar] [CrossRef]
- O-Chongpian, P.; Na Takuathung, M.; Chittasupho, C.; Ruksiriwanich, W.; Chaiwarit, T.; Baipaywad, P.; Jantrawut, P. Composite Nanocellulose Fibers-Based Hydrogels Loading Clindamycin HCl with Ca2+ and Citric Acid as Crosslinking Agents for Pharmaceutical Applications. Polymers 2021, 13, 4423. [Google Scholar] [CrossRef]
- Thomas, D.; Latha, M.; Thomas, K.K. Synthesis and in vitro evaluation of alginate-cellulose nanocrystal hybrid nanoparticles for the controlled oral delivery of rifampicin. J. Drug Deliv. Sci. Technol. 2018, 46, 392–399. [Google Scholar] [CrossRef]
- Pachuau, L. Application of Nanocellulose for Controlled Drug Delivery. In Nanocellulose and Nanohydrogel Matrices; Wiley: Hoboken, NJ, USA, 2017; pp. 1–19. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Mina, S.K.; Maryam, Z.; Zahra, N. Application of Bacterial Nanocellulose in Cancer Drug Delivery: A Review. Curr. Pharm. Des. 2021, 27, 3656–3665. [Google Scholar]
- Piasecka-Zelga, J.; Zelga, P.; Gronkowska, K.; Madalski, J.; Szulc, J.; Wietecha, J.; Ciechańska, D.; Dziuba, R. Toxicological and Sensitization Studies of Novel Vascular Prostheses Made of Bacterial Nanocellulose Modified with Chitosan (MBC) for Application as the Tissue-Engineered Blood Vessels. Regen. Eng. Transl. Med. 2021, 7, 218–233. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Freire, C.; Neto, C.P. Do bacterial cellulose membranes have potential in drug-delivery systems? Expert Opin. Drug Deliv. 2014, 11, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Stanisławska, A. Bacterial Nanocellulose as a Microbiological Derived Nanomaterial. Adv. Mater. Sci. 2016, 16, 45–57. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Rauchfuß, F. Nanocellulose as a natural source for groundbreaking applications in materials science: Todays state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Mota, J.P.; de Almeida, T.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef]
- Trovatti, E.; Silva, N.H.C.S.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Biocellulose Membranes as Supports for Dermal Release of Lidocaine. Biomacromolecules 2011, 12, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Trovatti, E.; Freire, C.S.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.; Neto, C.P.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Pavaloiu, R.-D.; Stoica, A.; Stroescu, M.; Dobre, T. Controlled release of amoxicillin from bacterial cellulose membranes. Cent. Eur. J. Chem. 2014, 12, 962–967. [Google Scholar] [CrossRef]
- Morais, E.S.; Silva, N.H.C.S.; Sintra, T.E.; Santos, S.A.O.; Neves, B.M.; Almeida, I.F.; Freire, C.S.R. Anti-inflammatory and antioxidant nanostructured cellulose membranes loaded with phenolic-based ionic liquids for cutaneous application. Carbohydr. Polym. 2019, 206, 187–197. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Neves, B.M.; Sèbe, G.; Coma, V.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Design of Nonsteroidal Anti-Inflammatory Drug-Based Ionic Liquids with Improved Water Solubility and Drug Delivery. ACS Sustain. Chem. Eng. 2019, 7, 14126–14134. [Google Scholar] [CrossRef]
- Fonseca, D.F.; Vilela, C.; Pinto, R.J.; Bastos, V.; Oliveira, H.; Catarino, J.; Faísca, P.; Rosado, C.; Silvestre, A.J.; Freire, C.S. Bacterial nanocellulose-hyaluronic acid microneedle patches for skin applications: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2021, 118, 111350. [Google Scholar] [CrossRef] [PubMed]
- Abba, M.; Ibrahim, Z.; Chong, C.S.; Zawawi, N.A.; Kadir, M.R.A.; Yusof, A.H.M.; Razak, S.I.A. Transdermal Delivery of Crocin Using Bacterial Nanocellulose Membrane. Fibers Polym. 2019, 20, 2025–2031. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Ghosh, A.K.; Sarkar, P.; Kundu, P. A Mussel Mimetic, Bioadhesive, Antimicrobial Patch Based on Dopamine-Modified Bacterial Cellulose/rGO/Ag NPs: A Green Approach toward Wound-Healing Applications. ACS Sustain. Chem. Eng. 2019, 7, 12083–12097. [Google Scholar] [CrossRef]
- Guccini, V.; Phiri, J.; Trifol, J.; Rissanen, V.; Mousavi, S.M.; Vapaavuori, J.; Tammelin, T.; Maloney, T.; Kontturi, E. Tuning the Porosity, Water Interaction, and Redispersion of Nanocellulose Hydrogels by Osmotic Dehydration. ACS Appl. Polym. Mater. 2021, 4, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and High Gas Barrier Films of Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation. Biomacromolecules 2008, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Kolakovic, R.; Peltonen, L.; Laaksonen, T.; Putkisto, K.; Laukkanen, A.; Hirvonen, J.T. Spray-Dried Cellulose Nanofibers as Novel Tablet Excipient. AAPS PharmSciTech 2011, 12, 1366–1373. [Google Scholar] [CrossRef]
- Hussin, M.H.; Trache, D.; Chuin, C.T.H.; Fazita, M.R.N.; Haafiz, M.K.M.; Hossain, S. Extraction of Cellulose Nanofibers and Their Eco-friendly Polymer Composites. Sustain. Polym. Compos. Nanocomposites 2019, 653–691. [Google Scholar] [CrossRef]
- Gupta, G.K.; Shukla, P. Lignocellulosic Biomass for the Synthesis of Nanocellulose and Its Eco-Friendly Advanced Applications. Front. Chem. 2020, 8, 601256. [Google Scholar] [CrossRef]
- Dutta, K.; Das, B.; Orasugh, J.T.; Mondal, D.; Adhikari, A.; Rana, D.; Banerjee, R.; Mishra, R.; Kar, S.; Chattopadhyay, D. Bio-derived cellulose nanofibril reinforced poly(N-isopropylacrylamide)-g-guar gum nanocomposite: An avant-garde biomaterial as a transdermal membrane. Polymer 2018, 135, 85–102. [Google Scholar] [CrossRef]
- Gencturk, A.; Kahraman, E.; Güngör, S.; Özhan, G.; Özsoy, Y.; Sarac, A.S. Polyurethane/hydroxypropyl cellulose electrospun nanofiber mats as potential transdermal drug delivery system: Characterization studies and in vitro assays. Artif. Cells Nanomed. Biotechnol. 2017, 45, 655–664. [Google Scholar] [CrossRef]
- Samanta, A.P.; Ali, M.S.; Orasugh, J.T.; Ghosh, S.K.; Chattopadhyay, D. Crosslinked nanocollagen-cellulose nanofibrils reinforced electrospun polyvinyl alcohol/methylcellulose/polyethylene glycol bionanocomposites: Study of material properties and sustained release of ketorolac tromethamine. Carbohydr. Polym. Technol. Appl. 2022, 3, 100195. [Google Scholar] [CrossRef]
- Sarkar, G.; Orasugh, J.T.; Saha, N.R.; Roy, I.; Bhattacharyya, A.; Chattopadhyay, A.K.; Rana, D.; Chattopadhyay, D. Cellulose nanofibrils/chitosan based transdermal drug delivery vehicle for controlled release of ketorolac tromethamine. New J. Chem. 2017, 41, 15312–15319. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose nanocrystal based composites: A review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2021, 275, 118668. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, H.; Patel, D.H.; Onkarappa, H.; Pratiksha, C.; Prasanna, G. Role of nanocellulose in industrial and pharmaceutical sectors—A review. Int. J. Biol. Macromol. 2022, 207, 1038–1047. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Fan, H.; Ullah, R.; Haq, F.; Khan, F.U.; Iqbal, M.; Wei, J. Cellulose Nanocrystals Applications in Health, Medicine and Catalysis. J. Polym. Environ. 2021, 29, 2062–2071. [Google Scholar] [CrossRef]
- Ali, M.S.; Bhunia, P.; Samanta, A.P.; Orasugh, J.T.; Chattopadhyay, D. Transdermal therapeutic system: Study of cellulose nanocrystals influenced methylcellulose-chitosan bionanocomposites. Int. J. Biol. Macromol. 2022, 218, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.I.; Kim, S.; Kim, M.-H.; Yoon, I.-S.; Lee, S.-H.; Kim, D.-D.; Cho, H.-J. Donepezil hydrochloride-reinforced cellulose nanocrystal-aggregated gel structure for long-acting drug delivery. Carbohydr. Polym. 2022, 296, 119887. [Google Scholar] [CrossRef] [PubMed]
- Hiranphinyophat, S.; Otaka, A.; Fujii, S.; Iwasaki, Y. Lanoconazole-loaded emulsion stabilized with cellulose nanocrystals decorated with polyphosphoesters reduced inflammatory edema in a mouse model. Polym. J. 2021, 53, 1493–1498. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Iwao, Y.; Zakrewsky, M.; Mitragotri, S. Transdermal Protein Delivery Using Choline and Geranate (CAGE) Deep Eutectic Solvent. Adv. Health Mater. 2017, 6, 1601411. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; Del Sesto, R.E.; Koppisch, A.T.; et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [PubMed]
- Zakrewsky, M.; Banerjee, A.; Apte, S.; Kern, T.L.; Jones, M.R.; Del Sesto, R.E.; Koppisch, A.T.; Fox, D.T.; Mitragotri, S. Choline and Geranate Deep Eutectic Solvent as a Broad-Spectrum Antiseptic Agent for Preventive and Therapeutic Applications. Adv. Health Mater. 2016, 5, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Boscariol, R.; Junior, J.M.O.; Baldo, D.A.; Balcão, V.M.; Vila, M.M. Transdermal permeation of curcumin promoted by choline geranate ionic liquid: Potential for the treatment of skin diseases. Saudi Pharm. J. 2022, 30, 382–397. [Google Scholar] [CrossRef]
- Tanner, E.E.; Ibsen, K.N.; Mitragotri, S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J. Control. Release 2018, 286, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jorge, L.R.; Harada, L.K.; Silva, E.C.; Campos, W.F.; Moreli, F.C.; Shimamoto, G.; Pereira, J.F.B.; Oliveira, J.M.J.; Tubino, M.; Vila, M.M.D.C.; et al. Non-invasive Transdermal Delivery of Human Insulin Using Ionic Liquids: In vitro Studies. Front. Pharmacol. 2020, 11, 243. [Google Scholar] [CrossRef]
- Islam, R.; Uddin, S.; Chowdhury, R.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Insulin Transdermal Delivery System for Diabetes Treatment Using a Biocompatible Ionic Liquid-Based Microemulsion. ACS Appl. Mater. Interfaces 2021, 13, 42461–42472. [Google Scholar] [CrossRef]
- Ali, K.; Moshikur, R.M.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Biocompatible Ionic Liquid-Mediated Micelles for Enhanced Transdermal Delivery of Paclitaxel. ACS Appl. Mater. Interfaces 2021, 13, 19745–19755. [Google Scholar] [CrossRef]
- Uddin, S.; Islam, R.; Moshikur, R.M.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Transdermal Delivery of Antigenic Protein Using Ionic Liquid-Based Nanocarriers for Tumor Immunotherapy. ACS Appl. Bio Mater. 2022, 5, 2586–2597. [Google Scholar] [CrossRef] [PubMed]
- Barros, N.R.; Borges, M.C.A.; Gemeinder, F.A.; Mendonça, J.L.P.; Cilli, R.J.; Herculano, R.D. Natural rubber latex: Development and in vitro characterization of a future transdermal patch for enuresis treatment. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 871–876. [Google Scholar] [CrossRef]
- Ren, X.; Barrera, C.S.; Tardiff, J.L.; Gil, A.; Cornish, K. Liquid guayule natural rubber, a renewable and crosslinkable processing aid in natural and synthetic rubber compounds. J. Clean. Prod. 2020, 276, 122933. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Das, S.S.; Sabir, F.; Sundaramahalingam, M.A.; Colmenares, J.C.; Prasannakumar, S.; Rajan, M.; Rahdar, A.; Kyzas, G.Z. Progress in natural polymer engineered biomaterials for transdermal drug delivery systems. Mater. Today Chem. 2021, 19, 100382. [Google Scholar] [CrossRef]
- Guerra, N.B.; Pegorin, G.S.; Boratto, M.H.; de Barros, N.R.; Graeff, C.F.D.O.; Herculano, R.D. Biomedical applications of natural rubber latex from the rubber tree Hevea brasiliensis. Mater. Sci. Eng. C 2021, 126, 112126. [Google Scholar] [CrossRef]
- Suksaeree, J.; Waiprib, R.; Kalkornsurapranee, E.; Pichayakorn, W. Lidocaine-pressure sensitive adhesive patches from STR-5L block rubber: Preparations, in vitro characterizations, and stability studies. J. Drug Deliv. Sci. Technol. 2021, 67, 102966. [Google Scholar] [CrossRef]
- Suksaeree, J.; Pichayakorn, W.; Monton, C.; Sakunpak, A.; Chusut, T.; Saingam, W. Rubber Polymers for Transdermal Drug Delivery Systems. Ind. Eng. Chem. Res. 2014, 53, 507–513. [Google Scholar] [CrossRef]
- Azammi, A.M.N.; Sapuan, S.M.; Ishak, M.R.; Sultan, M.T.H. Mechanical and Thermal Properties of Kenaf Reinforced Thermoplastic Polyurethane (TPU)-Natural Rubber (NR) Composites. Fibers Polym. 2018, 19, 446–451. [Google Scholar] [CrossRef]
- Hao, W.; Zeng, H.H.; Chen, X.; He, W.; Zhou, R.; Li, J.; Zhang, H. Insights into the reinforcement and mechanism of silicone mold rubber co-modified with WCB and MMQ resin. Mater. Res. Express 2021, 8, 035201. [Google Scholar] [CrossRef]
- Marcelino, M.Y.; Borges, F.A.; Costa, A.F.M.; Singulani, J.D.L.; Ribeiro, N.V.; Costa-Orlandi, C.B.; Garms, B.C.; Mendes-Giannini, M.J.S.; Herculano, R.D.; Fusco-Almeida, A.M. Antifungal activity of fluconazole-loaded natural rubber latex against Candida albicans. Future Microbiol. 2018, 13, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.V.; de Barros, N.R.; Costa-Orlandi, C.B.; Tanaka, J.L.; Moro, L.G.; Pegorin, G.S.; Oliveira, K.S.; Mendes-Gianinni, M.J.; Fusco-Almeida, A.M.; Herculano, R.D. Voriconazole-natural latex dressings for treating infected Candida spp. skin ulcers. Futur. Mircobiol. 2020, 15, 1439–1452. [Google Scholar] [CrossRef]
- Floriano, J.F.; de Barros, N.R.; Cinman, J.L.F.; da Silva, R.G.; Loffredo, A.V.; Borges, F.A.; Norberto, A.M.Q.; Chagas, A.L.D.; Garms, B.C.; Graeff, C.F.D.O.; et al. Ketoprofen Loaded in Natural Rubber Latex Transdermal Patch for Tendinitis Treatment. J. Polym. Environ. 2017, 26, 2281–2289. [Google Scholar] [CrossRef]
- Niamlang, S.; Paradee, N.; Sirivat, A. Hybrid transdermal drug delivery patch made from poly(p-phenylene vinylene)/natural rubber latex and controlled by an electric field. Polym. Int. 2018, 67, 747–754. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Ye, E.; Li, Z.; Wang, D. Control methods and applications of interface contact electrification of triboelectric nanogenerators: A review. Mater. Res. Lett. 2022, 10, 97–123. [Google Scholar] [CrossRef]
- Bejarano, J.; Navarro-Marquez, M.; Morales-Zavala, F.; Morales, J.O.; Garcia-Carvajal, I.; Araya-Fuentes, E.; Flores, Y.; Verdejo, H.E.; Castro, P.F.; Lavandero, S.; et al. Nanoparticles for diagnosis and therapy of atherosclerosis and myocardial infarction: Evolution toward prospective theranostic approaches. Theranostics 2018, 8, 4710–4732. [Google Scholar] [CrossRef]
- Karam, M.; Fahs, D.; Maatouk, B.; Safi, B.; Jaffa, A.A.; Mhanna, R. Polymeric nanoparticles in the diagnosis and treatment of myocardial infarction: Challenges and future prospects. Mater. Today Bio 2022, 14, 100249. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2018, 555, 49–62. [Google Scholar] [CrossRef]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2018, 27, 379–393. [Google Scholar] [CrossRef]
- Banerjee, R.; Jaiswal, A. Recent advances in nanoparticle-based lateral flow immunoassay as a point-of-care diagnostic tool for infectious agents and diseases. Anal. 2018, 143, 1970–1996. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities. Asian J. Pharm. Health Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef]
- Shou, P.; Yu, Z.; Wu, Y.; Feng, Q.; Zhou, B.; Xing, J.; Liu, C.; Tu, J.; Akakuru, O.U.; Ye, Z.; et al. Zn2+ Doped Ultrasmall Prussian Blue Nanotheranostic Agent for Breast Cancer Photothermal Therapy under MR Imaging Guidance. Adv. Health Mater. 2019, 9, e1900948. [Google Scholar] [CrossRef]
- Li, X.; Schumann, C.; Albarqi, H.A.; Lee, C.J.; Alani, A.W.G.; Bracha, S.; Milovancev, M.; Taratula, O.; Taratula, O. A Tumor-Activatable Theranostic Nanomedicine Platform for NIR Fluorescence-Guided Surgery and Combinatorial Phototherapy. Theranostics 2018, 8, 767–784. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
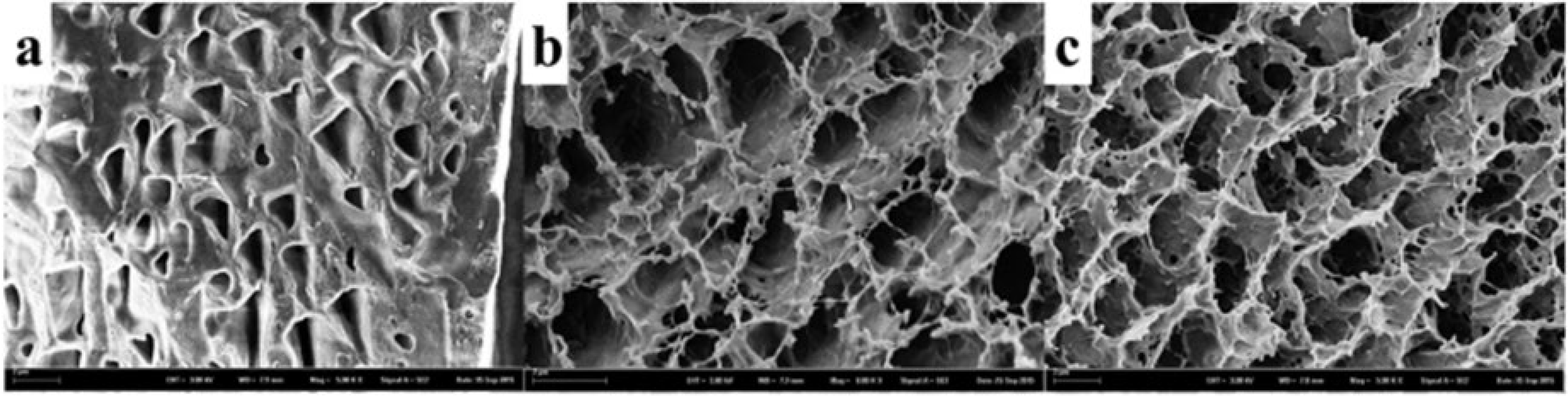
| Hydrogel | Application | Results | References |
|---|---|---|---|
| Polyethylene glycol diacrylamide (PEG-DA) hybrid hydrogel | Mouse embryonic fibroblast cell lines (NIH 3T3) | Good mechanical property, excellent swelling capacity biocompatibility, and non-toxic to skin | [96] |
| Gelatin-polyacrylamide (Gel-PAAm) hydrogel | Human skin | Non-toxic to human cells, highly stretchable, and good swelling properties | [89] |
| Polyacrylamide-grafted-chondroitin sulfate (PAAm-g-CS) hydrogel | Rat abdominal skin | No inflammatory cell infiltration, small degradation of skin, and decreased pore size | [97] |
| Chitosan-azelaic acid (CS-AZ) hydrogel | L929 mouse fibroblast | Excellent swelling, water vapor permeability, high porosity, and low cytotoxicity | [98] |
| Carboxymethyl chitosan-silk fibroin peptide/oxidized pullulan (CMCS-SFP/OPL) hydrogel | Newborn porcine skin | Good swelling, water retention properties, skin permeability, water absorption ability, excellent mechanical properties, and biocompatibility | [99] |
| Carboxymethyl chitosan-grafted-2 hydroxyethyl acrylate (CmCHT-g-pHEA) hydrogel | Micropig dorsal skin | Good pH sensitivity, pores size decreased when ratio of grafting agent increased, improved skin penetration, and non-toxic to skin | [100] |
| Nanocomposite | Transdermal Drug | Findings/Results | References |
|---|---|---|---|
| Polyelectrolyte complexes (PEC) with carboxymethylagarose (CMA) and chitosan (CS) as pH-responsive carriers | Diclofenac sodium (DS) | Immortalized human keratinocyte (HaCat) cells showed approximately 100% survival with 67% cumulative drug release after 72 h at 37 °C and pH 6.0 through the Fickian diffusion mechanism. | [159] |
| Chitosan microneedle patches (85% deacetylated, molecular weight: 1526.464 g/mol) | Meloxicam | A higher concentration of acetic acid displayed greater resistance to compressive force as temperature increased and the penetration study indicated sustained insertion of microneedles in cow’s ear cadaver skin. | [160] |
| Chitosan/hyaluronan transdermal film | Thiocolchicoside | Easy and reliable administration with high efficiency in drug release; flexible dosage, minimal drug dosage/frequency to reduce side effects. | [161] |
| Chitosan and phytagel (gellan gum) transdermal hydrogel | Ibuprofen | Chitosan improved the drug permeability to skin and increased the transdermal release rate of ibuprofen by a factor of 4. | [162] |
| Chitosan/phospholipids nanofibers | Curcumin, diclofenac, and vitamin B12 | Cytotoxicity studies confirmed the good biocompatibility of the nanofibers, the drug release rate relied eminently on the drug solubility. | [163] |
| Carboxymethyl chitosan-grafted-2 hydroxyethyl acrylate (CmCHT-g-pHEA) hydrogel | Micropig dorsal skin | Good pH sensitivity, pores size decreased when ratio of grafting agent increased, improved skin penetration, and non-toxic to skin | [100] |
| Carboxymethyl chitosan/oxidized pullulan hydrogel-based microneedles | Salvia miltiorrhiza extract | Good mechanical strength, high water absorbing capacity, good skin permeability, and rapid drug release into the targeted porcine skin. | [99] |
| N-methacryloyl chitosan (N-MAC) microgels | Bovine serum albumin (BSA) | High cell viability in N- MAC hydrogel. Rapid transdermal curing hydrogels (in vivo) for localized and sustained protein delivery. | [164] |
| N,N,N-trimethyl chitosan (TMC), polyethylene glycolate hyaluronic acid (PEG-HA), and polysaccharide-based nano-conjugate of hyaluronic acid, chitosan oligosaccharide and alanine [HA-Ala-Chito(oligo)] | Chinese medicine CortexMoutan (CM) | The ex vivo transdermal release results showed significant drug permeability into the skin. The MTT assay results showed high cell viability of human HaCaT keratinocytes, suggesting no cytotoxicity on skin cells. | [165] |
| Chitosan-coated poly(dl-lactide-co-glycolide) (PLGA) nanoparticles | Donepezil hydrochloride (DP) | Chitosan-coated PLGA nanoparticles delivered drugs to the deep hair follicles more efficiently through iontophoretic transdermal delivery, as compared to the bare PLGA nanoparticles. | [166] |
| Polyvinyl alcohol-Chitosan (PVA/CS) bioconjugate | Colchicine | Significant colchicine deposition in the skin with remarkable cytotoxicity against a melanoma cell line. | [167] |
| Chitosan-coated nanoemulsion (NE2-CS), uncoated nanoemulsion (NE1), and quaternized chitosan (QCS) | Zingiber cassumunar Roxb (Plai extract) | QCS improved the stability and transdermal properties of the Plai extract, as compared to NE1 and NE2-CS. NE2-QCS showed higher cytotoxicity to the breast (BT474) and oral cavity (KB) cancer cell lines than the Plai extract alone and had 1.5-fold higher permeability and cumulative release of the Plai extract than NE1. | [168] |
| Chitosan sponges | Hormonal drug 17β-estradiol (E2) with a purity of 99% | High drug loading was reported. Uniform distribution of E2 crystallites in the chitosan sponge volume was observed, improving the bioavailability of the drug. | [169] |
| PLGA chitosan transdermal Pluronic nanogel | Temozolomide | The in vitro drug release showed 85% transdermal release at a mildly acidic pH mimicking the skin microenvironment. Ex vivo studies displayed a penetration rate with 80% Temozolomide uptake in porcine epidermal tissue. | [170] |
| Carboxymethyl chitosan/2-hydroxyethyl acrylate hydrogel | Nobiletin | Mechanism of the nobiletin from the hydrogel was confirmed to be Fickian diffusion. In vitro skin permeation experiments showed that the hydrogel improved the transdermal delivery of nobiletin. | [100] |
| ZnO nanorods with chitosan hydrogels crosslinked with azelaic acid | Acetylsalicylic acid | The controlled drug release behaviors of nanocomposites according to the first-order kinetic model and was confirmed to be non-toxic to L929 mouse fibroblasts by XTT assay. | [98] |
| Chitosan nanoparticles mucoadhesive gel | Propranolol hydrochloride | High encapsulation efficiency and drug loading improved systemic bioavailability and therapeutic efficacy of propranolol-HCl in a transdermal delivery system. Thixotropic behavior with prolonged drug release properties was observed. | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leong, M.Y.; Kong, Y.L.; Burgess, K.; Wong, W.F.; Sethi, G.; Looi, C.Y. Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines 2023, 11, 1124. https://doi.org/10.3390/biomedicines11041124
Leong MY, Kong YL, Burgess K, Wong WF, Sethi G, Looi CY. Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines. 2023; 11(4):1124. https://doi.org/10.3390/biomedicines11041124
Chicago/Turabian StyleLeong, Moong Yan, Yeo Lee Kong, Kevin Burgess, Won Fen Wong, Gautam Sethi, and Chung Yeng Looi. 2023. "Recent Development of Nanomaterials for Transdermal Drug Delivery" Biomedicines 11, no. 4: 1124. https://doi.org/10.3390/biomedicines11041124
APA StyleLeong, M. Y., Kong, Y. L., Burgess, K., Wong, W. F., Sethi, G., & Looi, C. Y. (2023). Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines, 11(4), 1124. https://doi.org/10.3390/biomedicines11041124








