Do Epilepsy Patients with Cognitive Impairment Have Alzheimer’s Disease-like Brain Metabolism?
Abstract
1. Introduction
2. Materials and Methods
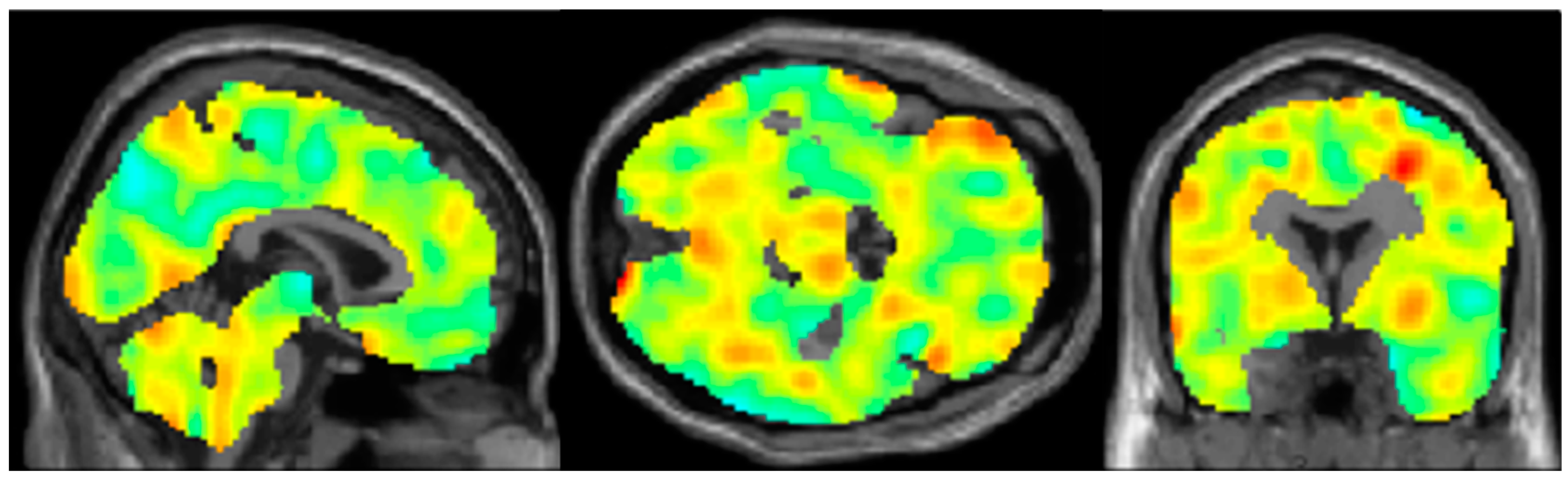
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gale, S.A.; Acar, D.; Daffner, K.R. Dementia. Am. J. Med. 2018, 131, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Miller, B.L. Alzheimer’s Disease. In Harrison’s Principles of Internal Medicine, 20e; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Blume, W.T.; Luders, H.O.; Mizrahi, E.; Tassinari, C.; van Emde Boas, W.; Engel, J., Jr. Glossary of descriptive terminology for ictal semiology: Report of the ILAE task force on classification and terminology. Epilepsia 2001, 42, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.L. Cognitive impairment in epilepsy: The role of network abnormalities. Epileptic Disord. 2015, 17, 101–116. [Google Scholar] [CrossRef]
- Ives-Deliperi, V.; Butler, J.T. Mechanisms of cognitive impairment in temporal lobe epilepsy: A systematic review of resting-state functional connectivity studies. Epilepsy Behav. 2021, 115, 107686. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Zenteno, J.F.; Patten, S.B.; Jette, N.; Williams, J.; Wiebe, S. Psychiatric comorbidity in epilepsy: A population-based analysis. Epilepsia 2007, 48, 2336–2344. [Google Scholar] [CrossRef]
- Tellez-Zenteno, J.F.; Hernandez-Ronquillo, L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res. Treat. 2012, 2012, 630853. [Google Scholar] [CrossRef]
- Allone, C.; Lo Buono, V.; Corallo, F.; Pisani, L.R.; Pollicino, P.; Bramanti, P.; Marino, S. Neuroimaging and cognitive functions in temporal lobe epilepsy: A review of the literature. J. Neurol. Sci. 2017, 381, 7–15. [Google Scholar] [CrossRef]
- Zhao, F.; Kang, H.; You, L.; Rastogi, P.; Venkatesh, D.; Chandra, M. Neuropsychological deficits in temporal lobe epilepsy: A comprehensive review. Ann. Indian Acad. Neurol. 2014, 17, 374–382. [Google Scholar] [CrossRef]
- Jokeit, H.; Ebner, A. Effects of chronic epilepsy on intellectual functions. Prog. Brain Res. 2002, 135, 455–463. [Google Scholar] [CrossRef]
- The Neuropsychiatry of Epilepsy; Cambridge University Press: Cambridge, UK, 2002. [CrossRef]
- Stefanidou, M.; Beiser, A.S.; Himali, J.J.; Peng, T.J.; Devinsky, O.; Seshadri, S.; Friedman, D. Bi-directional association between epilepsy and dementia: The Framingham Heart Study. Neurology 2020, 95, e3241–e3247. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Z.R.; Zhang, H.W.; Tseng, C.H.; Peng, H.C.; Kok, V.C.; Li, G.P.; Hsiung, C.A.; Hsu, C.Y. Late-onset epilepsy and subsequent increased risk of dementia. Aging (Albany NY) 2021, 13, 3573–3587. [Google Scholar] [CrossRef]
- Sen, A.; Capelli, V.; Husain, M. Cognition and dementia in older patients with epilepsy. Brain 2018, 141, 1592–1608. [Google Scholar] [CrossRef]
- Lam, A.D.; Deck, G.; Goldman, A.; Eskandar, E.N.; Noebels, J.; Cole, A.J. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat. Med. 2017, 23, 678–680. [Google Scholar] [CrossRef]
- Botha, H.; Mantyh, W.G.; Murray, M.E.; Knopman, D.S.; Przybelski, S.A.; Wiste, H.J.; Graff-Radford, J.; Josephs, K.A.; Schwarz, C.G.; Kremers, W.K.; et al. FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain 2018, 141, 1201–1217. [Google Scholar] [CrossRef]
- Gordon, B.A. Measures of metabolism provide insights into hippocampal sclerosis. Brain 2018, 141, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, L.G. Alzheimer Disease. Continuum 2016, 22, 419–434. [Google Scholar] [CrossRef]
- Berg, A.T.; Zelko, F.A.; Levy, S.R.; Testa, F.M. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: A prospective cohort study. Neurology 2012, 79, 1384–1391. [Google Scholar] [CrossRef]
- Xia, X.; Jiang, Q.; McDermott, J.; Han, J.-D.J. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell 2018, 17, e12802. [Google Scholar] [CrossRef]
- Vossel, K.A.; Beagle, A.J.; Rabinovici, G.D.; Shu, H.; Lee, S.E.; Naasan, G.; Hegde, M.; Cornes, S.B.; Henry, M.L.; Nelson, A.B.; et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013, 70, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Lotan, E.; Friedman, K.P.; Davidson, T.; Shepherd, T.M. Brain 18F-FDG-PET: Utility in the Diagnosis of Dementia and Epilepsy. Isr. Med. Assoc. J. 2020, 22, 178–184. [Google Scholar]
- Smailagic, N.; Vacante, M.; Hyde, C.; Martin, S.; Ukoumunne, O.; Sachpekidis, C. ¹⁸F-FDG PET for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2015, 1, Cd010632. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Tsui, W.H.; Herholz, K.; Pupi, A.; Drzezga, A.; Lucignani, G.; Reiman, E.M.; Holthoff, V.; Kalbe, E.; Sorbi, S.; et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2008, 49, 390–398. [Google Scholar] [CrossRef]
- Mahalingam, S.; Chen, M.K. Neuroimaging in Dementias. Semin. Neurol. 2019, 39, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, Y.; Hu, H.; Wang, J.; Liu, Z.; Gao, F. FDG-PET and NeuN-GFAP immunohistochemistry of hippocampus at different phases of the pilocarpine model of temporal lobe epilepsy. Int. J. Med. Sci. 2015, 12, 288–294. [Google Scholar] [CrossRef]
- Peter, J.; Houshmand, S.; Werner, T.J.; Rubello, D.; Alavi, A. Novel assessment of global metabolism by 18F-FDG-PET for localizing affected lobe in temporal lobe epilepsy. Nucl. Med. Commun. 2016, 37, 882–887. [Google Scholar] [CrossRef]
- Cendes, F.; Theodore, W.H.; Brinkmann, B.H.; Sulc, V.; Cascino, G.D. Neuroimaging of epilepsy. Handb. Clin. Neurol. 2016, 136, 985–1014. [Google Scholar] [CrossRef]
- Katako, A.; Shelton, P.; Goertzen, A.L.; Levin, D.; Bybel, B.; Aljuaid, M.; Yoon, H.J.; Kang, D.Y.; Kim, S.M.; Lee, C.S.; et al. Machine learning identified an Alzheimer’s disease-related FDG-PET pattern which is also expressed in Lewy body dementia and Parkinson’s disease dementia. Sci. Rep. 2018, 8, 13236. [Google Scholar] [CrossRef] [PubMed]
- Orrù, G.; Pettersson-Yeo, W.; Marquand, A.F.; Sartori, G.; Mechelli, A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neurosci. Biobehav. Rev. 2012, 36, 1140–1152. [Google Scholar] [CrossRef]
- Yassin, W.; Nakatani, H.; Zhu, Y.; Kojima, M.; Owada, K.; Kuwabara, H.; Gonoi, W.; Aoki, Y.; Takao, H.; Natsubori, T.; et al. Machine-learning classification using neuroimaging data in schizophrenia, autism, ultra-high risk and first-episode psychosis. Transl. Psychiatry 2020, 10, 278. [Google Scholar] [CrossRef]
- Arbabshirani, M.R.; Plis, S.; Sui, J.; Calhoun, V.D. Single subject prediction of brain disorders in neuroimaging: Promises and pitfalls. Neuroimage 2017, 145, 137–165. [Google Scholar] [CrossRef]
- Lau, A.; Beheshti, I.; Modirrousta, M.; Kolesar, T.A.; Goertzen, A.L.; Ko, J.H. Alzheimer’s Disease-Related Metabolic Pattern in Diverse Forms of Neurodegenerative Diseases. Diagnostics 2021, 11, 2023. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Taipale, H.; Gomm, W.; Broich, K.; Maier, W.; Tolppanen, A.M.; Tanskanen, A.; Tiihonen, J.; Hartikainen, S.; Haenisch, B. Use of Antiepileptic Drugs and Dementia Risk-an Analysis of Finnish Health Register and German Health Insurance Data. J. Am. Geriatr. Soc. 2018, 66, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.D.; Weaver, D.F.; Joudrey, H.R.; Carter, A.O.; Rockwood, K. Epilepsy and antiepileptic drug use in elderly people as risk factors for dementia. J. Neurol. Sci. 2007, 252, 169–172. [Google Scholar] [CrossRef]
- Jacob, L.; Bohlken, J.; Kostev, K. Is There an Association between Antiepileptic Drug Use and Dementia Risk? A Case-Control Study. J. Alzheimers Dis. 2019, 68, 97–103. [Google Scholar] [CrossRef]
- Helmstaedter, C.; Beghi, E.; Elger, C.E.; Kalviainen, R.; Malmgren, K.; May, T.W.; Perucca, E.; Trinka, E.; Witt, J.A. No proof of a causal relationship between antiepileptic drug treatment and incidence of dementia. Comment on: Use of antiepileptic drugs and dementia risk-An analysis of Finnish health register and German health insurance data. Epilepsia 2018, 59, 1303–1306. [Google Scholar] [CrossRef]
- Mattis, P.J.; Niethammer, M.; Sako, W.; Tang, C.C.; Nazem, A.; Gordon, M.L.; Brandt, V.; Dhawan, V.; Eidelberg, D. Distinct brain networks underlie cognitive dysfunction in Parkinson and Alzheimer diseases. Neurology 2016, 87, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Del Sole, A.; Clerici, F.; Chiti, A.; Lecchi, M.; Mariani, C.; Maggiore, L.; Mosconi, L.; Lucignani, G. Individual cerebral metabolic deficits in Alzheimer’s disease and amnestic mild cognitive impairment: An FDG PET study. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1357–1366. [Google Scholar] [CrossRef]
- Mosconi, L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 486–510. [Google Scholar] [CrossRef]
- Herholz, K.; Salmon, E.; Perani, D.; Baron, J.C.; Holthoff, V.; Frolich, L.; Schonknecht, P.; Ito, K.; Mielke, R.; Kalbe, E.; et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 2002, 17, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Vossel, K.A.; Tartaglia, M.C.; Nygaard, H.B.; Zeman, A.Z.; Miller, B.L. Epileptic activity in Alzheimer’s disease: Causes and clinical relevance. Lancet Neurol. 2017, 16, 311–322. [Google Scholar] [CrossRef]
- Chang, A.J.; Roth, R.; Bougioukli, E.; Ruber, T.; Keller, S.S.; Drane, D.L.; Gross, R.E.; Welsh, J.; Abrol, A.; Calhoun, V.; et al. MRI-based deep learning can discriminate between temporal lobe epilepsy, Alzheimer’s disease, and healthy controls. Commun. Med. 2023, 3, 33. [Google Scholar] [CrossRef]
- Mucke, L.; Selkoe, D.J. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012, 2, a006338. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Curr. Alzheimer Res. 2018, 15, 283–300. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front. Cell Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.S.; Wolff, M.D.; Teskey, G.C. Neurodegeneration and Pathology in Epilepsy: Clinical and Basic Perspectives. Adv. Neurobiol. 2017, 15, 317–334. [Google Scholar] [CrossRef]
- Puttachary, S.; Sharma, S.; Stark, S.; Thippeswamy, T. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed. Res. Int. 2015, 2015, 745613. [Google Scholar] [CrossRef] [PubMed]
- Portocarrero, L.K.L.; Quental, K.N.; Samorano, L.P.; Oliveira, Z.N.P.; Rivitti-Machado, M. Tuberous sclerosis complex: Review based on new diagnostic criteria. An. Bras. Dermatol. 2018, 93, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Banerjee, A.; Sur, M. Developmental Dynamics of Rett Syndrome. Neural Plast. 2016, 2016, 6154080. [Google Scholar] [CrossRef]
- McDonald, T.; Puchowicz, M.; Borges, K. Impairments in Oxidative Glucose Metabolism in Epilepsy and Metabolic Treatments Thereof. Front. Cell Neurosci. 2018, 12, 274. [Google Scholar] [CrossRef]
- Yalcin, G.; Yalcin, A. Excitotoxicity as a molecular mechanism in Epilepsy. Geriatr. Med. Care 2018, 2, 1–3. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Jones, N.C.; O’Brien, T.J.; Kwan, P.; Piperi, C.; Othman, I.; Shaikh, M.F. Tau Related Pathways as a Connecting Link between Epilepsy and Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 4199–4212. [Google Scholar] [CrossRef]
- Gourmaud, S.; Shou, H.; Irwin, D.J.; Sansalone, K.; Jacobs, L.M.; Lucas, T.H.; Marsh, E.D.; Davis, K.A.; Jensen, F.E.; Talos, D.M. Alzheimer-like amyloid and tau alterations associated with cognitive deficit in temporal lobe epilepsy. Brain 2020, 143, 191–209. [Google Scholar] [CrossRef]
- Dejakaisaya, H.; Kwan, P.; Jones, N.C. Astrocyte and glutamate involvement in the pathogenesis of epilepsy in Alzheimer’s disease. Epilepsia 2021, 62, 1485–1493. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Raichle, M.E. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol. Psychiatry 2013, 74, 340–347. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, S.; Zhao, C.; Xu, X. Comprehensive investigation of molecular signatures and pathways linking Alzheimer’s disease and Epilepsy via bioinformatic approaches. Curr. Alzheimer Res. 2022, 19, 146–160. [Google Scholar] [CrossRef]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
| Demographic | ||
|---|---|---|
| N | 20 | |
| Mean age (±SD) | 50.9 (±9.4) | |
| Sex (female:male) | 10:10 | |
| Epilepsy localization (n) | ||
| Temporal lobe | 6 | |
| Frontal lobe | 2 | |
| Frontotemporal lobe | 2 | |
| Parietal/occipital lobe | 1 | |
| Other 1 | 9 | |
| Cognition (n) | ||
| Not assessed | 9 | |
| Cognitively impaired | 6 | |
| Cognitively normal | 5 |
| MAD+ (n) | |
|---|---|
| All participants | 5/20 |
| Not assessed | 1/9 |
| Cognitively impaired | 4/6 |
| Cognitively normal | 0/5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Kolesar, T.A.; Goertzen, A.L.; Ng, M.C.; Ko, J.H. Do Epilepsy Patients with Cognitive Impairment Have Alzheimer’s Disease-like Brain Metabolism? Biomedicines 2023, 11, 1108. https://doi.org/10.3390/biomedicines11041108
He M, Kolesar TA, Goertzen AL, Ng MC, Ko JH. Do Epilepsy Patients with Cognitive Impairment Have Alzheimer’s Disease-like Brain Metabolism? Biomedicines. 2023; 11(4):1108. https://doi.org/10.3390/biomedicines11041108
Chicago/Turabian StyleHe, Michael, Tiffany A. Kolesar, Andrew L. Goertzen, Marcus C. Ng, and Ji Hyun Ko. 2023. "Do Epilepsy Patients with Cognitive Impairment Have Alzheimer’s Disease-like Brain Metabolism?" Biomedicines 11, no. 4: 1108. https://doi.org/10.3390/biomedicines11041108
APA StyleHe, M., Kolesar, T. A., Goertzen, A. L., Ng, M. C., & Ko, J. H. (2023). Do Epilepsy Patients with Cognitive Impairment Have Alzheimer’s Disease-like Brain Metabolism? Biomedicines, 11(4), 1108. https://doi.org/10.3390/biomedicines11041108





