Abstract
Renal cell carcinoma (RCC) is the seventh most common cancer in men and the ninth most common cancer in women worldwide. There is plenty of evidence about the role of the immune system in surveillance against tumors. Thanks to a better understanding of immunosurveillance mechanisms, immunotherapy has been introduced as a promising cancer treatment in recent years. Renal cell carcinoma (RCC) has long been thought chemoresistant but highly immunogenic. Considering that up to 30% of the patients present metastatic disease at diagnosis, and around 20–30% of patients undergoing surgery will suffer recurrence, we need to identify novel therapeutic targets. The introduction of immune checkpoint inhibitors (ICIs) in the clinical management of RCC has revolutionized the therapeutic approach against this tumor. Several clinical trials have shown that therapy with ICIs in combination or ICIs and the tyrosine kinase inhibitor has a very good response rate. In this review article we summarize the mechanisms of immunity modulation and immune checkpoints in RCC and discuss the potential therapeutic strategies in renal cancer treatment.
1. Introduction
Renal cell carcinoma (RCC) is the seventh most common cancer in men and the ninth most common cancer in women worldwide. In the United States, it is estimated that there will be about 81,800 new cases of kidney cancer (including RCC) and about 14,890 deaths from this disease in 2023 [1].
Recent studies have suggested that RCC can be considered a metabolic disease, as changes in metabolism contribute to the development and progression of this cancer [2,3,4,5,6,7,8,9,10]. One of the main metabolic alterations in RCC is the activation of the hypoxia-inducible factor (HIF) pathway. HIF is a transcription factor that is activated in response to low oxygen levels, or hypoxia. In RCC, HIF is constitutively activated, leading to increased expression of genes involved in glycolysis, angiogenesis, and survival pathways. The metabolic alterations observed in RCC, in association with intratumor heterogeneity, have an important role in the chemo-resistant mechanisms described in this tumor [11,12,13,14]. Furthermore, considering that up to 30% of these patients present metastatic disease at diagnosis, and around 20–30% of patients undergoing surgery will suffer recurrence, we need to identify novel therapeutic targets [15,16,17,18,19,20]. In recent years, the introduction of immune checkpoint inhibitors (ICIs) in the clinical management of RCC has revolutionized the therapeutic approach against this tumor. In this review article, we summarize the mechanisms of immunity modulation and immune checkpoints in RCC and discuss the potential therapeutic strategies in renal cancer treatment [21].
2. Cancer Immune Surveillance and Escape Mechanisms
Cancer cells develop and proliferate when internal and external checking systems fail [22]. The internal checking system mainly consists of tumor suppressor genes. Apoptosis is eventually activated when uncorrectable genomic errors are detected. The external checking system is mediated by our immune system. There is plenty of evidence about the role of the immune system in the surveillance against tumors. For example, immunodeficient individuals (either primary or secondary) carry a higher risk to develop cancer than immune-competent ones [23]. In addition, some “paraneoplastic syndromes” may develop because of immune responses to cancer (anemia, nephropathy, neuromyopathy, Stauffer syndrome, vasculopathy, coagulopathy, amyloidosis, etc.) [24]. Finally, thanks to a better understanding of immunosurveillance mechanisms, immunotherapy has been introduced as a further treatment of cancer in recent years. Two types of immune responses against cancer cells are known as well as against microbes. Innate immunity represents an early but aspecific reaction, while adaptative immunity provides a very specific but delayed response. In this scenario, the ultimate cause of cancer can be explained as the uncontrolled proliferation of cells that have evaded immune system attack. Phagocytic cells (neutrophils and macrophages), dendritic cells (DCs), NK cells, and other lymphoid cells are the main effectors of innate immunity. A series of elements have been documented to interfere with DC maturation [25,26,27]. Notably, IL-35 has been reported to lower the expression of the costimulatory molecules CD40, CD80, and CD86 as well as HLA-DR and CD83. Similarly, IL-6 has been noted to inhibit DC maturation by reducing MHC class II and CD86 expression. CD47 on tumor cells may block macrophage phagocytosis by binding SIRP-α (signal regulatory protein α—an inhibitory receptor on phagocytes) [28]. Recently, CD47 expression has been associated with more aggressive phenotypes of clear cell renal cell carcinoma (ccRCC) and poorer patient prognosis [29]. NK cells destroy several tumor cell types, particularly those with decreased MHC I expression or that express ligands for NK-activating receptors. NK cells are thought to be the first line of protection against blood-borne metastatic tumor cells. Patients with metastatic disease show aberrant NK cell activity, and low NK cell levels may predict impending metastases. There are two types of adaptive immune responses: cellular (T cells) and humoral (B-cells). Besides blocking the function of their target, antibodies produced by B cells may enhance the elimination of their target in a process named “antibody-dependent cell-mediated cytotoxicity” (ADCC). To be activated, adaptive immune cells demand antigen-presenting cells (APCs) such as dendritic cells and their antigen-presenting structure represented by the major histocompatibility complex (MHC). Antigen peptides in MHC are recognized by T cell receptors; CD8 T cells bind class I MHC, whereas CD4 T cells bind class II MHC. CD4 and CD8 are T cell coreceptors that bind non-polymorphic regions of MHC molecules. Moreover, CD28 co-stimulation is necessary for T cell activation since CD28 binds B7-1 (CD80) and B7-2 (CD86) on activated macrophages, dendritic cells, and B lymphocytes. Ongoing mutations may lead cancer cells to reduce or even to turn off tumor-specific antigens’ expression such as class I MHC, β-2 microglobulin, or components of the antigen-processing machinery (i.e., tapasin and TAP) [30,31]. A low tumor mutation burden (TMB) has already been associated with a reduced response to immunotherapy since tumor cells tend to express fewer neoantigens on MHC to immune cell effectors. In line with this, the loss of the most immunostimulatory neoantigens is thought to be a mechanism of immunoediting that may pave the way to resistance to immunotherapies [32]. Effector T cells’ homing to tumor sites is impaired as well. VEGF promotes the growth of aberrant blood vessels, and it downregulates adhesion molecules’ expression (such as ICAM-1 and VCAM-1), which limit T cells’ extravasation to tumor microenvironment (TME), as does endothelin B receptor overexpression [33,34,35]. The expression of “immune checkpoint” molecules also contributes to the active suppression of immunological responses (Figure 1) [36].
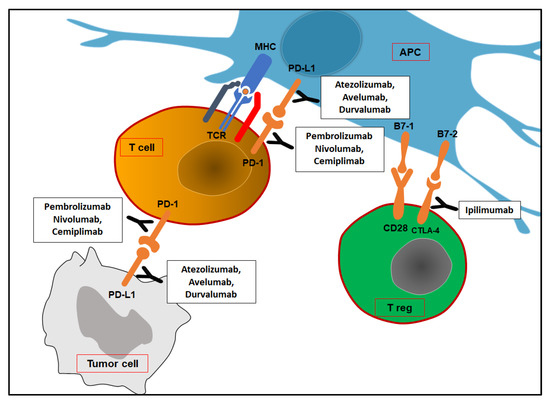
Figure 1.
Immune checkpoints and corresponding inhibitors. At tumor sites, PD-1/PD-L1 interaction leads to T cells’ death or inhibition. Treg cells may express CTLA-4 at the lymph nodes, which provides negative feedback signals. APC: antigen-presenting cell; TCR: T cell receptor; MHC: major histocompatibility complex; PD-1: programmed death-1; PD-L1: programmed death ligand-1; CTLA-4: cytotoxic T lymphocyte antigen-4.
Most human solid cancers express PD-L1, a B7 family protein that binds T cells’ inhibitory receptor PD-1 (programmed death-1). In tumor sites, PD-L1/PD-1 interaction leads activated immune cells to either die or lose their function. IFN-γ produced by activated T cells may block cancer cell proliferation by interfering with DNA duplication. At the same time, IFN- γ induces PD-L1 expression on cancer cells. However, APCs may express PD-L1 to avoid T cell over-activation. Tumor-infiltrating dendritic cells are known to express PD-L2 (also called B7-DC), which is another ligand of PD-1 (not expressed by most human cancer cells) [37,38]. At the lymph node level, activated T cells may express CTLA-4 (cytotoxic T lymphocyte antigen-4), which provides negative feedback signals for T cell activation. The presentation of tumor antigens by APCs in the absence of robust innate immunity and consequently with low levels of B7 costimulators has been proposed as a potential explanation for the participation of CTLA-4 in this process. Autoimmune responses have been described as a side effect of immune checkpoint blockade therapy. Other immune checkpoint molecules have been discovered (B7-H3, B7-H4, VISTA, PD-1H, Tim-3, LAG3, TIGIT, etc.), and clinical trials have already tested their clinical relevance [39,40]. Moreover, tumor cells and tumor-associated macrophages (TAMs- M2 phenotype) may release products (TGF-β, IL-10, VEGF, prostaglandin E2, etc.), which are able to block the proliferation and functions of lymphocytes and macrophages. M2 phenotype depends on alternative macrophage activation by type 2 CD4 T cells (TH2)’ cytokines (IL-4 and IL-13). TAMs express arginase and IDO as well as PD-L1 and PD-L2, whose expression is enhanced by macrophage’s chemokine CXCL8 [41,42]. A recent study investigated the role of MUC1 in ccRCC. Overexpression of the anaphylatoxin C3a (C3aR) and C5a (C5aR) receptors was seen in MUC1-expressing ccRCCs (MUC1H). MUC1H ccRCC characterized by high microvessel density, high M2-TAM (IDO+) infiltrates, and altered metabolism can be recognized as an immunologically silent subset of renal cancer [43,44,45,46].
Cancer cells may recruit myeloid-derived suppressor cells (MDSCs) to inhibit the immune responses at tumor or lymph node sites. MDSCs are bone marrow cells whose process of differentiation into APCs is disrupted. They may accumulate also at sites of chronic inflammation. Their recruitment depends on proinflammatory mediators such as prostaglandin E2, IL-6, VEGF, and complement fragment C5a. At tumor sites, MDSCs release IL-10 and free radicals such as peroxynitrite, and they also express indolamine 2,3 dioxygenase (IDO1) and arginase-1 (ARG1). IDO1 transforms L-tryptophan (TRP) to kynurerine (KYN), whereas arginase-1 reduces L-arginine availability. ARG1-expressing cells also include M2-TAMs and T regs [47]. Reduced levels of these amino acids impair T cell proliferation [48,49]. In addition, regulatory T cells (Treg) may dampen T cell responses at tumor sites and lymph nodes. To date, different mechanisms of Tregs’ immunosuppressive activity are known besides the expression of inhibitor checkpoint molecules. Tregs may secrete IL-2, IL-10, TGF-β, adenosine, granzyme, and/or perforin, thus limiting the activity of effector CD8 T cells [50,51,52]. This has been supported by the finding that reducing the Treg population significantly slows tumor growth and raises the proportion of CD8 T cells in tumor sites [53].
3. Immunometabolic Rewiring of Cancer
Current evidence points out that crosstalk between cancer metabolic reprogramming and anti-tumor immune response occurs [54,55]. Cancer cells can reduce immune responses by competing for and depleting vital nutrients, increasing oxygen consumption, and producing reactive nitrogen and oxygen intermediates. The proliferation, differentiation, activation, and function of immune cells may also be significantly influenced by aberrant metabolites and intermediates in the TME. Interestingly, immune cells may activate different metabolic pathways according to their functional state (T cells above all) [56]. The primary source of energy for activated neutrophils, M1 macrophages, and iNOS-expressed DCs is glycolysis. Oxidative phosphorylation (OXPHOS) from fatty acid oxidation is the main energy source of M2 macrophages and Tregs [57]. Competitive uptake of glucose by cancer cells may inhibit the function of tumor-infiltrating T cells. An inverse relationship between GLUT1 expression and infiltrating CD8 T cell number has been outlined in RCC specimens [58]. Aberrant aerobic glycolysis of tumor cells (Warburg effect) results in lactate accumulation in TME and its acidification. An acidic TME has been shown to limit the activity of both T cells and myeloid immune cells. Lactate may reduce IFN-γ production by NK cells by silencing the nuclear factor of activated T cells (NFAT) signals [59]. Glutamine plays a crucial role in many activities of immune cells, including cell proliferation, antigen presentation, phagocytosis, production of cytokines, NO, and peroxide. Subsequently, these may be dampened by glutamine deprivation by cancer cells. Increased lipogenesis for membrane phospholipids and signaling molecules is frequently observed in tumor cells. Hence, it has been demonstrated that metabolic reprogramming causes tumor-infiltrating myeloid cells (including MDSCs, DCs, and TAMs) to skew towards immunosuppressive and anti-inflammatory phenotypes. This may be caused by the aberrant accumulation of lipid metabolites (such as short-chain fatty acids, long-chain fatty acids, cholesterol, etc.). Cholesterol concentration in cancer cells has been shown to be higher than in immune cells: a high amount of sterol promotes immune checkpoint molecules’ expression [60]. A high rate of cholesterol esterification in the tumor can impair immune responses; as a result, disrupting cholesterol esterification to increase the concentration of cholesterol in immune cells’ plasma membranes may promote the proliferation of these cells and enhance their ability to function as effectors. Therefore, future deeper insights into immune cells’ metabolism might be useful to develop metabolism-targeting therapies, enhancing the possibility for immunotherapy synergy.
4. Immunotherapy in RCC
Renal cell carcinoma (RCC) has long been thought chemoresistant but highly immunogenic. In the 1960s, spontaneous remission of metastatic patients was observed after the surgical removal of primary tumors [61]. Immunotherapy agents do not directly destroy their targets, but they stimulate immune responses to destroy them. IL-2 and IFNα2b have been the first immunotherapy regimens used to treat metastatic RCC until the development of new agents in 2005 [62,63]. With effects on both effector and regulatory T cells, IL-2 was already known to promote T cell proliferation and differentiation. Therefore, high dose- IL-2 was authorized in 1992 for the treatment of metastatic RCC. Because of the pharmacokinetics of a pegylated form (Bempegaldesleukin), high doses of IL-2 may be avoided [64]. Currently, the administration of IFN is approved in combination with bevacizumab for metastatic RCC [65,66]. Advanced RCC systemic therapy has evolved over the past 20 years from a non-specific immune strategy (the cytokine era) to targeted therapy against vascular endothelial growth factor (VEGF), VEGF receptor (VEGFR), and immune checkpoint inhibitors (ICIs) [67] (Figure 2).
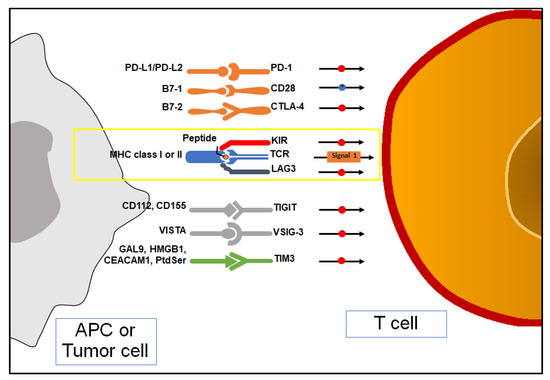
Figure 2.
Antigen presentation, co-stimulation, and immune checkpoint inhibition (ICIs) of T cell. APC: antigen-presenting cell; MHC: major histocompatibility complex; PD-1: programmed death-1; PD-L1/PD-L2: programmed death ligand-1/2; TIM-3: T cell Immunoglobulin and mucin domain-containing 3; Gal9: galectin9; HMBGB1: high-motility group box-1; CEACAM1: carcinoembryonic antigen cell adhesion molecule; Ptdser: phosphatidylserine; TIGIT: T cell Immunoreceptor with immunoglobulin and ITIM domain; TCR: T cell receptor; CTLA-4: cytotoxic T lymphocyte antigen-4; LAG-3: lymphocyte activating-gene-3; VISTA: V-domain immunoglobulin suppressor of T cell activation; VSIG-3: V-set and immunoglobulin domain containing protein 3.
5. Mechanism of Action of ICIs
5.1. Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4)
Costimulatory signals are necessary for T cell activation. This is achieved when B7-1 and B7-2 on APCs bind CD28 on naïve T cells. CTLA-4 is a member of the CD28 receptor family, so, when binding to B7, it negatively regulates T cell activation. The engagement of CTLA-4 and its ligand B7 activates the serine/threonine phosphatase PP2A, thus reducing TCR- and CD28-associated signaling pathway (reduced AKT activity) [68,69]. A strong association between CTLA-4 and T cell infiltration has been observed in several cancer tissues, where it has been reported to be significantly expressed (including in ccRCC) [70].
5.2. Programmed Cell Death Protein-1 and Its Ligand (PD-1/PD-L1)
PD-1 and its ligands PD-L1 and PD-L2 may downregulate the TCR signaling pathway. It has been noted that PD-1 expression increases upon the exposure of naïve T cells to antigens, and it decreases as the antigen disappears. In the case of persistent antigen exposure, PD-1 is continuously highly expressed as it happens in ccRCC. Hence, PD-L1 and PD-L2 are highly expressed in primary and metastatic sites of ccRCC [71]. After their binding, the PI3K-AKT and the RAS-MEK-ERK pathways will be downregulated. This will lead to significantly reduced T cell proliferation, cytotoxic molecule production, and killing capacity. In contrast, regulatory T cells’ maturation and function will be kept [72,73,74].
A recent study highlighted that PD-L1 level was reduced in ccRCC characterized by increased expression of MUC1 [44].
The increased nuclear grade was associated with PD-L1 expression. In metastatic ccRCC, it may also be positively linked to higher sarcomatoid features, advanced T stage, primary tumor size, International Metastatic RCC Database Consortium (IMDC), or Memorial Sloan Kettering Cancer Centre (MSKCC) risk scoring, as well as the occurrence of numerous metastases, although these correlations are less evident [75,76].
5.3. T Cell Immunoglobulin and Mucin Domain-Containing 3 (TIM-3)
T cell immunoglobulin and mucin domain-containing 3 (TIM-3) is a type I trans-membrane protein with coinhibitory activity that has been found on IFN-γ producing T cells, FoxP3+ Treg cells, and innate immune cells (macrophages and dendritic cells) [77]. TIM-3 locus maps on chromosome 5q33.2 in the human genome near the IL-4 gene cluster [78]. Previous studies have already demonstrated the association of TIM-3 with autoimmune and allergic disorders in both murine and human models. Effector T cells produce IFN-γ, which promotes both direct anti-tumor activity and expansion of myeloid-derived suppressor cells (MDSCs). MDSCs produce high levels of Galectin-9 (Gal9); by binding to TIM-3, effector CD8+ T cells lead to apoptosis [79]. Moreover, TIM-3+ FoxP3+ Tregs release high amounts of molecules with an inhibitory effect on effector T cells (i.e., IL-10, etc.). Dendritic cells have been shown to express more TIM-3 in a tumor microenvironment (TME) than in healthy tissue. High-mobility group box 1 (HMBG1) allows the tumor-derived nucleic acids transport into dendritic cells. Upon binding to HMBG1, TIM-3 prevents the latter endosomal trafficking, thus limiting innate immune responses to tumor-derived nucleic acids [80]. To date, other TIM-3 ligands have been identified such as carcinoembryonic antigen cell adhesion molecule (Ceacam1) and phosphatidylserine (PtdSer). Therefore, overexpression of TIM-3 is associated with T cell exhaustion (T cell suppression and dysfunction) in tumor-associated leukocytes (TILs). TIM-3 expression has been found to be closely linked to PD-1 expression. In ccRCC, VHL loss results in an increased expression of VEGF, which has recently been associated with the upregulation of PD-1 and TIM-3 on CD8-T cells [81]. Granier et al. has already demonstrated that RCC patients with tumor-infiltrating CD8 cells co-expressing PD-1 and TIM-3 experienced a more aggressive phenotype, as shown by a high Fuhrman grade, a larger tumor size, and more advanced TNM and UISS (UCLA Integrated Staging System) scores [82]. Targeting both the TIM-3 and PD-1 pathways simultaneously is believed to be more efficient than targeting either pathway alone. Nonetheless, some data suggest that TIM-3 may even exert co-stimulatory effects on CTL and other immune effectors [83,84].
5.4. T Cell Immunoreceptor with Immunoglobulin and ITIM Domain (TIGIT)
T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT) is a novel promising co-inhibitory receptor that is upregulated in NK cells, activated T cells, memory T cells, and FoxP3+ T regs [85,86]. It belongs to the poliovirus receptor (PVR) family. TIGIT competes with the activator receptor CD226 for the same ligands CD155 (PVR) and CD112 (PVRL2) that are expressed by tumor cells and APCs in the TME. Different mechanisms of action have been described. First, because of the engagement of TIGIT by CD155, inhibitory signals in T and NK cells are triggered. TIGIT binds to CD155 on APCs to increase the production of IL-10 and reduce the production of IL-12, which indirectly suppresses T cells (tolerogenic dendritic cells). At the same time, T regs’ immunosuppressive functions are enhanced. Ligation of TIGIT promotes the release of inhibitory molecules IL-10 and Fgl2 (fibrinogen-like protein 2) by T regs. In TIGIT+ T-regs, TIGIT upregulates TIM-3 expression, so it synergizes with TIM-3 and LAG-3 [87,88]. TIGIT can decrease CD8 T cell proliferation and immunosuppression by interacting with the PD-1/PD-L1 pathway as well [89,90]. In this regard, Hong et al. investigated the biological functions of TIGIT and PD-1 in the development, invasion, and metastasis of RCC, as well as their relationship with the clinicopathological features of RCC [91].
5.5. Lymphocyte Activation Gene-3 (LAG-3)
Lymphocyte activation gene-3 (LAG-3) is expressed on Treg cells, NK cells, CD4 and CD8 T cells as a response to persistent antigen stimulation [92]. It shows ammino acid homology to CD4. LAG-3 binds with higher affinity to the peptide-MHCII complex than CD4, thus limiting CD4 T cells’ activation [93,94]. CD8 T cells are inhibited because of LAG-3 recruitment, although mechanisms have not been elucidated yet; other ligands may likely exist (such as Galectin-3, lectin LSECtin, and fibrinogen-related protein FGL-1) [95,96,97]. LAG-3+ Treg cells release inhibitory cytokines (such as IL-10 and TGF-β), which further suppress antitumor T cell’s activities [98]. The most common inhibitory receptor combination in CD4 and CD8 T lymphocytes in ccRCC tissues was identified to be LAG-3 and PD-1. Co-expression of LAG-3 and PD-1 is associated with intratumoral T cell dysfunction [99]. LAG-3 was upregulated in response to PD-1 inhibition, and enhanced IFN release was observed after dual blockade of both during in vitro stimulation. Zelba et al. indicated that PD-1 and LAG-3 co-blockade might be a potential treatment option for advanced ccRCC since these inhibitor receptors (IRs) were found to be similarly expressed. These results were obtained from samples of primary RCC tumors, and IR expression may also vary according to the metastatic sites [100,101]. Metalloproteinases may remove LAG-3 and TIM-3 from cell surfaces, and their soluble forms are then released [102,103].
5.6. Indoleamine 2,3-Dioxyegenase 1 (IDO1)
Indoleamine 2,3-dioxyegenase 1 (IDO1) catalyzes the rate-limiting step in the KYN pathway. Besides MDSCs, IDO1 has been found expressed in mature DC, as well as in macrophages, cancer, endothelial and stromal cells in ccRCC [48,104]. TRP deprivation has been noted to induce apoptosis in T cells to limit their proliferation. TRP catabolism causes autophagy in T cells and inhibits the immunomodulatory kinases mTOR and protein kinases C [105]. Additionally, further studies have revealed that KYN inhibits antitumor immune responses and stimulates T cell differentiation into FoxP3+ T reg cells via aryl hydrocarbon receptor (AHR) [106]. For these reasons, IDO1 is consequently thought to be a possible cancer immunological checkpoint.
5.7. V-Domain Immunoglobulin Suppressor of T Cell Activation (VISTA)
V-domain immunoglobulin suppressor of T cell activation (VISTA or B7-H5) is overexpressed in different tumor cells and in immune cells in TME. By interacting with inhibitory receptors on T cells, VISTA limits their proliferation and activation, whereas it induces FoxP3 expression. Hence, VISTA expression is associated with a state of tumor immunosuppression [107,108]. On the other hand, VISTA may play a stimulatory checkpoint role in anti-cancer immunity in specific malignancies (i.e., esophageal, gastric, liver, and ovarian cancers). VISTA was discovered to be markedly elevated in ccRCC, even beyond the level of PD-L1. CD14+ HLA-DR+ macrophages were revealed to express higher levels of VISTA in ccRCC [109]. A more significant efficacy was noted when anti-VISTA therapy was combined with PD-1 or CTLA-4 blockade than in monotherapy [110]. At present, ten members of the B7 family have been identified: B7-1 (CD80), B7-H1 (PD-L1), B7-DC (PD-L2), B7-H2, B7-H3, B7-H4, B7-H5, B7-H6, and B7-H7.
They are considered to form receptor–ligand networks, which may regulate immune responses differentially in a context-dependent manner in different human malignancies [74].
6. Use of Immune Checkpoint Inhibitors (ICIs) in Clinical Settings
Considering the increased expression of different immune checkpoint molecules in ccRCC (Figure 3), in recent years, many clinical trials have been conducted to evaluate the use of ICIs as a novel therapeutic approach in this tumor.
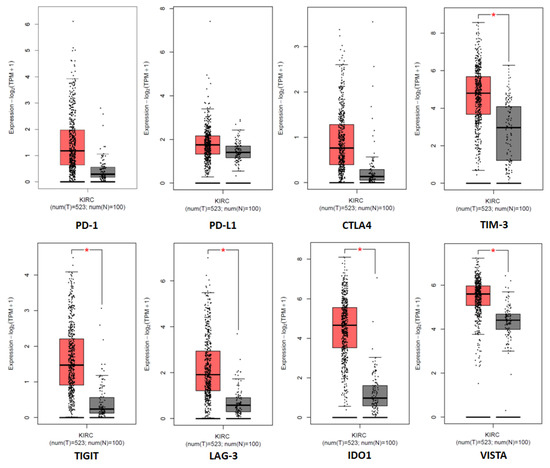
Figure 3.
Tissue expression of the immune checkpoint genes in the cancer genome atlas (TCGA) clear cell RCC patient cohort (KIRC). * denotes p < 0.05.
A phase II clinical trial (NCT00057889) was conducted to evaluate the efficacy of ipilimumab (CTLA-4 inhibitor), which became the first immune checkpoint inhibitor (ICI) used to treat patients with metastatic ccRCC [111]. For the first time, tumor remission was confirmed in patients who did not respond to IL2 treatment. A phase III clinical trial (CheckMate 025, NCT001668784) compared everolimus to nivolumab (PD-1 inhibitor) in patients with advanced ccRCC. Patients’ overall survival (OS), overall response rate (ORR), and treatment-related adverse events (AEs) were demonstrated to improve with the PD-1 inhibitor. Therefore, nivolumab significantly outperformed everolimus as a second-line treatment for advanced ccRCC in terms of survival and safety [112,113]. The first combination immune blockade treatment for ccRCC was nivolumab plus ipilimumab, which was first assessed for efficacy and safety in a phase I trial (Checkmate 016, NCT01472081) [114]. Different doses of the combination were used, but the lower dose ipilimumab combination appeared less toxic. In an international multicenter phase 3 trial (CheckMate 214), where patients were randomly assigned, a combination of nivolumab plus ipilimumab was found to have a higher ORR and longer progression-free survival (PFS) in intermediate and poor-risk patients than sunitinib [115,116]. Phase II clinical trial KEYNOTE-427 (NCT02853344) assessed pembrolizumab (another PD-1 inhibitor) as a single therapeutic agent for ccRCC, and it appeared as tolerable as for patients with other tumor types [117]. Phase II trial IMmotion 150 (NCT01984242) evaluated atezolizumab (PD-L1 blocker) as a first-line therapy for RCC [118]. Another PD-L1 blocker that has recently been developed is spartalizumab [119].
In recent years, several trials combining ICIs with anti-VEGF therapy have taken place with different results. Given the weak results in terms of OS within phase III trial IMmotion151 (NCT02420821), bevacizumab (anti-VEGF) plus atezolizumab failed to be approved by FDA for advanced RCC [120]. Avelumab (anti-PD-L1) plus axitinib (a tyrosine kinase inhibitor-TKI) were evaluated in the phase III trial JAVELIN Renal 101 (NCT02684006). Compared to the sunitinib group, patients receiving combination therapy had higher median PFS and ORR; however, long-term follow-up data are still needed to demonstrate the true benefit [121]. The results of phase III trial KEYNOTE-426 (NCT02853331) led to the approval by the FDA of pembrolizumab plus axitininb as first-line therapy for advanced ccRCC [122]. In a randomized controlled phase III trial CLEAR (NCT02811861), a longer median PFS and superior OS benefit were achieved in the lenvatinib (TKI) plus pembrolizumab group than in sunitinib one [123]. Nivolumab and cabozantinib (TKI) together also showed greater oncological efficacy than sunitinib [124]. Finally, in a COSMIC-313 phase 3 randomized controlled study, the triple regimen of cabozantinib, nivolumab, and ipilimumab was evaluated as the first-line systemic treatment for metastatic ccRCC [125]. In turn, although both groups receive considerable benefits from ICI combination regimens over sunitinib, PD-L1+ patients seem to respond to anti-PD-1/PD-L1 drugs more favorably than PD-L1- patients [75]. Several clinical trials are ongoing or have evaluated the use of new ICIs for the treatment of renal cell carcinoma (Table 1).

Table 1.
Novel immune checkpoint inhibitors: ongoing clinical trials in RCC.
CD8+ T cell activation and tumor growth inhibition were induced in ccRCC patients by IMP231, a recombinant soluble LAG-3Ig fusion protein [126,127]. On the other hand, despite having a good tolerability profile, sabatolimab (anti-TIM3) did not produce significant benefits in advanced solid cancers, either in monotherapy or in combination with spartalizumab [128,129]. Navoximod (IDO1 inhibitor) plus atezolizumab were evaluated in a phase I trial involving seven patients with advanced RCC (ORR 43%) [130]. There are current clinical trials for VISTA and PD-L1 blockade together in advanced cancers (NCT02812875) [131]. Different results were achieved in patients with non-clear-cell RCC (nccRCC) with ICIs [132,133]. Histology represented one of the main factors affecting the outcomes: better responses were obtained in papillary and sarcomatoid dedifferentiated tumors, whereas poorer responses were found in chromophobe renal cancers. Nevertheless, rarer histologic subtypes (such as translocation renal cell carcinoma) and aggressive renal cancers (such as collecting duct tumors) were documented to respond to ICI-based therapy [134,135,136]. To further understand the true level of efficacy of ICI monotherapy in the nccRCCs, more prospective trials are required, since better outcomes were associated with ICI combination regimens. Another intriguing role of immunotherapy may be its pre-operative administration in metastatic and neoadjuvant settings. Meaningful reductions in the primary tumor were demonstrated by first-line combination regimens in the metastatic setting, thus facilitating cytoreductive nephrectomy. Early evidence from the neoadjuvant setting supports the use of VEGFR-TKIs either alone or in combination with ICIs, whilst the outcomes of single neoadjuvant ICI are disappointing [137,138].
7. Conclusions
Despite the clinical success of current anti-CTLA-4, anti-PD-1/PD-L1 agents, a significant number of RCC patients remain unresponsive or even develop resistance. In such a complicated tumor immune environment, blocking a single checkpoint may result in the activation of other immune modulators. Targeting novel ICIs (LAG-3, TIM-3, and TIGIT) and B7-family ligands alone or in association with first-series ICIs may be future promising approaches for RCC treatment. In addition, further preclinical or clinical studies need to assess the validity and applicability of prognostic biomarkers, which might help to personalize checkpoint combination therapy and ultimately increase the clinical response rate.
Author Contributions
Conceptualization, F.L. and G.L.; methodology, F.L.; software, G.L.; validation, F.L. and G.L.; formal analysis, F.L.; investigation, F.L. and G.L.; resources, G.L.; data curation, F.L., N.A.d.M., M.R., M.M., M.F., S.D.P., F.C., O.S.T., R.A., M.B., P.D. and G.L.; writing—original draft preparation, F.L.; writing—review and editing, G.L.; visualization, F.L.; supervision, G.L.; project administration, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- di Meo, N.A.; Lasorsa, F.; Rutigliano, M.; Loizzo, D.; Ferro, M.; Stella, A.; Bizzoca, C.; Vincenti, L.; Pandolfo, S.D.; Autorino, R.; et al. Renal Cell Carcinoma as a Metabolic Disease: An Update on Main Pathways, Potential Biomarkers, and Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 14360. [Google Scholar] [CrossRef]
- Lucarelli, G.; Rutigliano, M.; Sallustio, F.; Ribatti, D.; Giglio, A.; Signorile, M.L.; Grossi, V.; Sanese, P.; Napoli, A.; Maiorano, E.; et al. Integrated multi-omics characterization reveals a distinctive metabolic signature and the role of NDUFA4L2 in promoting angiogenesis, chemoresistance, and mitochondrial dysfunction in clear cell renal cell carcinoma. Aging 2018, 10, 3957–3985. [Google Scholar] [CrossRef]
- Lucarelli, G.; Loizzo, D.; Franzin, R.; Battaglia, S.; Ferro, M.; Cantiello, F.; Castellano, G.; Bettocchi, C.; Ditonno, P.; Battaglia, M. Metabolomic insights into pathophysiological mechanisms and biomarker discovery in clear cell renal cell carcinoma. Expert Rev. Mol. Diagn. 2019, 19, 397–407. [Google Scholar] [CrossRef]
- Lucarelli, G.; Ferro, M.; Loizzo, D.; Bianchi, C.; Terracciano, D.; Cantiello, F.; Bell, L.N.; Battaglia, S.; Porta, C.; Gernone, A.; et al. Integration of Lipidomics and Transcriptomics Reveals Reprogramming of the Lipid Metabolism and Composition in Clear Cell Renal Cell Carcinoma. Metabolites 2020, 10, 509. [Google Scholar] [CrossRef]
- De Marco, S.; Torsello, B.; Minutiello, E.; Morabito, I.; Grasselli, C.; Bombelli, S.; Zucchini, N.; Lucarelli, G.; Strada, G.; Perego, R.A.; et al. The cross-talk between Abl2 tyrosine kinase and TGFβ1 signalling modulates the invasion of clear cell Renal Cell Carcinoma cells. FEBS Lett. 2022. [Google Scholar] [CrossRef]
- Bianchi, C.; Meregalli, C.; Bombelli, S.; Di Stefano, V.; Salerno, F.; Torsello, B.; De Marco, S.; Bovo, G.; Cifola, I.; Mangano, E.; et al. The glucose and lipid metabolism reprogramming is grade-dependent in clear cell renal cell carcinoma primary cultures and is targetable to modulate cell viability and proliferation. Oncotarget 2017, 8, 113502–113515. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Rutigliano, M.; Sanguedolce, F.; Galleggiante, V.; Giglio, A.; Cagiano, S.; Bufo, P.; Maiorano, E.; Ribatti, D.; Ranieri, E.; et al. Increased Expression of the Autocrine Motility Factor is Associated With Poor Prognosis in Patients With Clear Cell–Renal Cell Carcinoma. Medicine 2015, 94, e2117. [Google Scholar] [CrossRef]
- Lucarelli, G.; Galleggiante, V.; Rutigliano, M.; Sanguedolce, F.; Cagiano, S.; Bufo, P.; Lastilla, G.; Maiorano, E.; Ribatti, D.; Giglio, A.; et al. Metabolomic profile of glycolysis and the pentose phosphate pathway identifies the central role of glucose-6-phosphate dehydrogenase in clear cell-renal cell carcinoma. Oncotarget 2015, 6, 13371–13386. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Ferro, M.; Ditonno, P.; Battaglia, M. The urea cycle enzymes act as metabolic suppressors in clear cell renal cell carcinoma. Transl. Cancer Res. 2018, 7, S766–S769. [Google Scholar] [CrossRef]
- Bombelli, S.; Torsello, B.; De Marco, S.; Lucarelli, G.; Cifola, I.; Grasselli, C.; Strada, G.; Bovo, G.; Perego, R.A.; Bianchi, C. 36-kDa Annexin A3 Isoform Negatively Modulates Lipid Storage in Clear Cell Renal Cell Carcinoma Cells. Am. J. Pathol. 2020, 190, 2317–2326. [Google Scholar] [CrossRef]
- Lucarelli, G.; Ferro, M.; Battaglia, M. Multi-omics approach reveals the secrets of metabolism of clear cell—Renal cell carcinoma. Transl. Androl. Urol. 2016, 5, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Ragone, R.; Sallustio, F.; Piccinonna, S.; Rutigliano, M.; Vanessa, G.; Palazzo, S.; Lucarelli, G.; Ditonno, P.; Battaglia, M.; Fanizzi, F.P.; et al. Renal Cell Carcinoma: A Study through NMR-Based Metabolomics Combined with Transcriptomics. Diseases 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- di Meo, N.A.; Lasorsa, F.; Rutigliano, M.; Milella, M.; Ferro, M.; Battaglia, M.; Ditonno, P.; Lucarelli, G. The dark side of lipid metabolism in prostate and renal carcinoma: Novel insights into molecular diagnostic and biomarker discovery. Expert Rev. Mol. Diagn. 2023, 1–17. [Google Scholar] [CrossRef]
- Ferro, M.; Musi, G.; Marchioni, M.; Maggi, M.; Veccia, A.; Del Giudice, F.; Barone, B.; Crocetto, F.; Lasorsa, F.; Antonelli, A.; et al. Radiogenomics in Renal Cancer Management—Current Evidence and Future Prospects. Int. J. Mol. Sci. 2023, 24, 4615. [Google Scholar] [CrossRef]
- Tataru, O.S.; Marchioni, M.; Crocetto, F.; Barone, B.; Lucarelli, G.; Del Giudice, F.; Busetto, G.M.; Veccia, A.; Giudice, A.L.; Russo, G.I.; et al. Molecular Imaging Diagnosis of Renal Cancer Using 99mTc-Sestamibi SPECT/CT and Girentuximab PET-CT-Current Evidence and Future Development of Novel Techniques. Diagnostics 2023, 13, 593. [Google Scholar] [CrossRef]
- Papale, M.; Vocino, G.; Lucarelli, G.; Rutigliano, M.; Gigante, M.; Rocchetti, M.T.; Pesce, F.; Sanguedolce, F.; Bufo, P.; Battaglia, M.; et al. Urinary RKIP/p-RKIP is a potential diagnostic and prognostic marker of clear cell renal cell carcinoma. Oncotarget 2017, 8, 40412–40424. [Google Scholar] [CrossRef]
- Gigante, M.; Lucarelli, G.; Divella, C.; Netti, G.S.; Pontrelli, P.; Cafiero, C.; Grandaliano, G.; Castellano, G.; Rutigliano, M.; Stallone, G.; et al. Soluble Serum αKlotho Is a Potential Predictive Marker of Disease Progression in Clear Cell Renal Cell Carcinoma. Medicine 2015, 94, e1917. [Google Scholar] [CrossRef] [PubMed]
- Galleggiante, V.; Rutigliano, M.; Sallustio, F.; Ribatti, D.; Ditonno, P.; Bettocchi, C.; Selvaggi, F.P.; Lucarelli, G.; Battaglia, M. CTR2 Identifies a Population of Cancer Cells with Stem Cell-like Features in Patients with Clear Cell Renal Cell Carcinoma. J. Urol. 2014, 192, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Lunardini, S.; Magli, I.A.; Campi, R.; Primiceri, G.; Berardinelli, F.; Amparore, D.; Terracciano, D.; Lucarelli, G.; Schips, L.; et al. Micro-RNAs Predict Response to Systemic Treatments in Metastatic Renal Cell Carcinoma Patients: Results from a Systematic Review of the Literature. Biomedicines 2022, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Lucarelli, G. The Role of Renal Surgery in the Era of Targeted Therapy: The Urologist’s Perspective. Urol. J. 2015, 82, 137–138. [Google Scholar] [CrossRef]
- The Basics of Cancer Immunotherapy; Springer Science+Business Media: New York, NY, USA, 2018; ISBN 978-3-319-70621-4.
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 8th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; ISBN 978-0-323-22275-4. [Google Scholar]
- Lundon, D.J.; Kelly, B.D.; Nusrat, N.B.; Foley, R.W.; D’Arcy, F.T.; Jaffry, S.Q. Renal Cell Carcinoma Presenting as Painless Jaundice and Unintentional Weight Loss. Am. J. Clin. Exp. Urol. 2022, 10, 408–411. [Google Scholar] [PubMed]
- Chen, X.; Hao, S.; Zhao, Z.; Liu, J.; Shao, Q.; Wang, F.; Sun, D.; He, Y.; Gao, W.; Mao, H. Interleukin 35: Inhibitory regulator in monocyte-derived dendritic cell maturation and activation. Cytokine 2018, 108, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Ohno, Y.; Toyoshima, Y.; Ohtake, J.; Homma, S.; Kawamura, H.; Takahashi, N.; Taketomi, A. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017, 108, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Tyurin, V.A.; Mohammadyani, D.; Blasi, M.; Duperret, E.K.; Donthireddy, L.; Hashimoto, A.; Kapralov, A.; Amoscato, A.; Angelini, R.; et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat. Commun. 2017, 8, 2122. [Google Scholar] [CrossRef]
- Tseng, D.; Volkmer, J.-P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody–mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef]
- Park, H.; Jee, S.; Bang, S.; Son, H.; Cha, H.; Myung, J.; Sim, J.; Kim, Y.; Paik, S.; Kim, H. CD47 Expression Predicts Unfavorable Prognosis in Clear Cell Renal Cell Carcinoma after Curative Resection. Diagnostics 2022, 12, 2291. [Google Scholar] [CrossRef]
- Ling, A.; Löfgren-Burström, A.; Larsson, P.; Li, X.; Wikberg, M.L.; Öberg, Å.; Stenling, R.; Edin, S.; Palmqvist, R. TAP1 down-regulation elicits immune escape and poor prognosis in colorectal cancer. Oncoimmunology 2017, 6, e1356143. [Google Scholar] [CrossRef]
- Shionoya, Y.; Kanaseki, T.; Miyamoto, S.; Tokita, S.; Hongo, A.; Kikuchi, Y.; Kochin, V.; Watanabe, K.; Horibe, R.; Saijo, H.; et al. Loss of tapasin in human lung and colon cancer cells and escape from tumor-associated antigen-specific CTL recognition. Oncoimmunology 2017, 6, e1274476. [Google Scholar] [CrossRef]
- Yarchoan, M.; Johnson, B.A.; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Erratum: Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 569. [Google Scholar] [CrossRef]
- Dirkx, A.E.M.; Egbrink, M.G.A.O.; E Kuijpers, M.J.; Van Der Niet, S.T.; Heijnen, V.V.T.; Steege, J.C.A.B.-T.; Wagstaff, J.; Griffioen, A.W. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003, 63, 2322–2329. [Google Scholar]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Buckanovich, R.J.; Facciabene, A.; Kim, S.; Benencia, F.; Sasaroli, D.; Balint, K.; Katsaros, D.; O’Brien-Jenkins, A.; A Gimotty, P.; Coukos, G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 2008, 14, 28–36. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Tseng, S.-Y.; Otsuji, M.; Gorski, K.; Huang, X.; Slansky, J.E.; Pai, S.I.; Shalabi, A.; Shin, T.; Pardoll, D.M.; Tsuchiya, H. B7-Dc, a New Dendritic Cell Molecule with Potent Costimulatory Properties for T Cells. J. Exp. Med. 2001, 193, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhu, Y.; Chen, L. Advances in targeting cell surface signalling molecules for immune modulation. Nat. Rev. Drug Discov. 2013, 12, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, K.; Tanaka, N.; Hakozaki, K.; Takahashi, R.; Teranishi, Y.; Murakami, T.; Kufukihara, R.; Niwa, N.; Mikami, S.; Shinojima, T.; et al. Profiling the inhibitory receptors LAG-3, TIM-3, and TIGIT in renal cell carcinoma reveals malignancy. Nat. Commun. 2021, 12, 5547. [Google Scholar] [CrossRef]
- Lin, C.; He, H.; Liu, H.; Li, R.; Chen, Y.; Qi, Y.; Jiang, Q.; Chen, L.; Zhang, P.; Zhang, H.; et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 2019, 68, 1764–1773. [Google Scholar] [CrossRef]
- Tamma, R.; Rutigliano, M.; Lucarelli, G.; Annese, T.; Ruggieri, S.; Cascardi, E.; Napoli, A.; Battaglia, M.; Ribatti, D. Microvascular density, macrophages, and mast cells in human clear cell renal carcinoma with and without bevacizumab treatment. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 355.e11–355.e19. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Rutigliano, M.; Loizzo, D.; di Meo, N.A.; Lasorsa, F.; Mastropasqua, M.; Maiorano, E.; Bizzoca, C.; Vincenti, L.; Battaglia, M.; et al. MUC1 Tissue Expression and Its Soluble Form CA15-3 Identify a Clear Cell Renal Cell Carcinoma with Distinct Metabolic Profile and Poor Clinical Outcome. Int. J. Mol. Sci. 2022, 23, 13968. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Netti, G.S.; Rutigliano, M.; Lasorsa, F.; Loizzo, D.; Milella, M.; Schirinzi, A.; Fontana, A.; Di Serio, F.; Tamma, R.; et al. MUC1 Expression Affects the Immunoflogosis in Renal Cell Carcinoma Microenvironment through Complement System Activation and Immune Infiltrate Modulation. Int. J. Mol. Sci. 2023, 24, 4814. [Google Scholar] [CrossRef] [PubMed]
- Netti, G.S.; Lucarelli, G.; Spadaccino, F.; Castellano, G.; Gigante, M.; Divella, C.; Rocchetti, M.T.; Rascio, F.; Mancini, V.; Stallone, G.; et al. PTX3 modulates the immunoflogosis in tumor microenvironment and is a prognostic factor for patients with clear cell renal cell carcinoma. Aging 2020, 12, 7585–7602. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Ditonno, P.; Bettocchi, C.; Vavallo, A.; Rutigliano, M.; Galleggiante, V.; LaRocca, A.M.V.; Castellano, G.; Gesualdo, L.; Grandaliano, G.; et al. Diagnostic and Prognostic Role of Preoperative Circulating CA 15-3, CA 125, and Beta-2 Microglobulin in Renal Cell Carcinoma. Dis. Markers 2014, 2014, 689795. [Google Scholar] [CrossRef]
- Phillips, M.M.; Sheaff, M.T.; Szlosarek, P.W. Targeting Arginine-Dependent Cancers with Arginine-Degrading Enzymes: Opportunities and Challenges. Cancer Res. Treat. 2013, 45, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Rutigliano, M.; Ferro, M.; Giglio, A.; Intini, A.; Triggiano, F.; Palazzo, S.; Gigante, M.; Castellano, G.; Ranieri, E.; et al. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 461.e15–461.e27. [Google Scholar] [CrossRef]
- Lasorsa, F.; di Meo, N.A.; Rutigliano, M.; Ferro, M.; Terracciano, D.; Tataru, O.S.; Battaglia, M.; Ditonno, P.; Lucarelli, G. Emerging Hallmarks of Metabolic Reprogramming in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 910. [Google Scholar] [CrossRef]
- Di Gennaro, P.; Gerlini, G.; Caporale, R.; Sestini, S.; Brandani, P.; Urso, C.; Pimpinelli, N.; Borgognoni, L. T regulatory cells mediate immunosuppresion by adenosine in peripheral blood, sentinel lymph node and TILs from melanoma patients. Cancer Lett. 2018, 417, 124–130. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Gigante, M.; Pontrelli, P.; Herr, W.; Gigante, M.; D’Avenia, M.; Zaza, G.; Cavalcanti, E.; Accetturo, M.; Lucarelli, G.; Carrieri, G.; et al. miR-29b and miR-198 overexpression in CD8+ T cells of renal cell carcinoma patients down-modulates JAK3 and MCL-1 leading to immune dysfunction. J. Transl. Med. 2016, 14, 84. [Google Scholar] [CrossRef]
- Taylor, N.A.; Vick, S.C.; Iglesia, M.D.; Brickey, W.J.; Midkiff, B.R.; McKinnon, K.P.; Reisdorf, S.; Anders, C.K.; Carey, L.A.; Parker, J.S.; et al. Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. J. Clin. Investig. 2017, 127, 3472–3483. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Lian, X.; Yang, K.; Li, R.; Li, M.; Zuo, J.; Zheng, B.; Wang, W.; Wang, P.; Zhou, S. Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol. Cancer 2022, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, S.; Manfrini, N.; Alfieri, R.; Calamita, P.; Crosti, M.C.; Gallo, S.; Müller, R.; Pagani, M.; Abrignani, S.; Biffo, S. The Translational Machinery of Human CD4+ T Cells Is Poised for Activation and Controls the Switch from Quiescence to Metabolic Remodeling. Cell Metab. 2018, 28, 895–906.e5. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic Pathways in Immune Cell Activation and Quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.; Kastenberger, M.; Gottfried, E.; Hammerschmied, C.G.; Büttner, M.; Aigner, M.; Seliger, B.; Walter, B.; Schlösser, H.; Hartmann, A.; et al. Warburg phenotype in renal cell carcinoma: High expression of glucose-transporter 1 (GLUT-1) correlates with low CD8+ T-cell infiltration in the tumor. Int. J. Cancer 2011, 128, 2085–2095. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Perrone, F.; Minari, R.; Bersanelli, M.; Bordi, P.; Tiseo, M.; Favari, E.; Sabato, R.; Buti, S. The Prognostic Role of High Blood Cholesterol in Advanced Cancer Patients Treated With Immune Checkpoint Inhibitors. J. Immunother. 2020, 43, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Barré, C.; Vérine, J.L.; Régnier, J.; Enon, B.; Houssin, A.; Chaigné, P.; Soret, J.Y. Spontaneous regression of regressive pulmonary metastases from kidney cancer. Myth or reality? Apropos of 2 cases. Ann. Durologie 1986, 20, 275–279. [Google Scholar]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef]
- Dekernion, J.; Sarna, G.; Figlin, R.; Lindner, A.; Smith, R.B. The Treatment of Renal Cell Carcinoma with Human Leukocyte Alpha-Interferon. J. Urol. 1983, 130, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Charych, D.H.; Hoch, U.; Langowski, J.L.; Lee, S.R.; Addepalli, M.K.; Kirk, P.B.; Sheng, D.; Liu, X.; Sims, P.W.; VanderVeen, L.A.; et al. NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin. Cancer Res. 2016, 22, 680–690. [Google Scholar] [CrossRef]
- Escudier, B.; Pluzanska, A.; Koralewski, P.; Ravaud, A.; Bracarda, S.; Szczylik, C.; Chevreau, C.; Filipek, M.; Melichar, B.; Bajetta, E.; et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet 2008, 370, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Halabi, S.; Rosenberg, J.E.; Stadler, W.M.; Vaena, D.A.; Ou, S.-S.; Archer, L.; Atkins, J.N.; Picus, J.; Czaykowski, P.; et al. Bevacizumab Plus Interferon Alfa Compared With Interferon Alfa Monotherapy in Patients With Metastatic Renal Cell Carcinoma: CALGB 90206. J. Clin. Oncol. 2008, 26, 5422–5428. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Zhang, Z.-C.; Wang, S.-Y.; Fu, S.-Q.; Cheng, X.-F.; Chen, R.; Sun, T. Immune checkpoint inhibitor-based therapy for advanced clear cell renal cell carcinoma: A narrative review. Int. Immunopharmacol. 2022, 110, 108900. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Bradshaw, J.; Greene, J.; Peach, R.; Bennett, K.L.; Mittler, R.S. Intracellular Trafficking of CTLA-4 and Focal Localization Towards Sites of TCR Engagement. Immunity 1996, 4, 535–543. [Google Scholar] [CrossRef]
- Schildberg, F.A.; Klein, S.R.; Freeman, G.J.; Sharpe, A.H. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 2016, 44, 955–972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Song, Q.; Sun, X.; Xue, M.; Yang, Z.; Shang, J. Comprehensive analysis of CTLA-4 in the tumor immune microenvironment of 33 cancer types. Int. Immunopharmacol. 2020, 85, 106633. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, X.; Zhang, H.; Sun, G.; Yang, Y.; Chen, J.; Zhu, X.; Zhao, P.; Zhao, J.; Liu, J.; et al. Differential expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic sites in renal cell carcinoma. BMC Cancer 2019, 19, 360. [Google Scholar] [CrossRef]
- Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L.; Boussiotis, V.A. Selective Effects of PD-1 on Akt and Ras Pathways Regulate Molecular Components of the Cell Cycle and Inhibit T Cell Proliferation. Sci. Signal. 2012, 5, ra46. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Yano, H.; Vignali, D.A.A. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: Breakthroughs or backups. Nat. Immunol. 2019, 20, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Sweeney, P.L.; Barata, P.C.; Koshkin, V.S. PD-L1 Expression and Treatment Implications in Metastatic Clear Cell Renal Cell Carcinoma: A Systematic Review. Kidney Cancer 2021, 5, 31–46. [Google Scholar] [CrossRef]
- Kammerer-Jacquet, S.-F.; Brunot, A.; Lefort, M.; Bayat, S.; Peyronnet, B.; Verhoest, G.; Mathieu, R.; Lespagnol, A.; Mosser, J.; Laguerre, B.; et al. Metastatic Clear-cell Renal Cell Carcinoma With a Long-term Response to Sunitinib: A Distinct Phenotype Independently Associated With Low PD-L1 Expression. Clin. Genitourin. Cancer 2019, 17, 169–176.e1. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef]
- Meyers, J.H.; Sabatos, C.A.; Chakravarti, S.; Kuchroo, V.K. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol. Med. 2005, 11, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D.; et al. Tumor-infiltrating DCs suppress nucleic acid–mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.-L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef]
- Granier, C.; Dariane, C.; Combe, P.; Verkarre, V.; Urien, S.; Badoual, C.; Roussel, H.; Mandavit, M.; Ravel, P.; Sibony, M.; et al. Tim-3 Expression on Tumor-Infiltrating PD-1+CD8+ T Cells Correlates with Poor Clinical Outcome in Renal Cell Carcinoma. Cancer Res. 2017, 77, 1075–1082. [Google Scholar] [CrossRef]
- Gorman, J.V.; Starbeck-Miller, G.; Pham, N.-L.L.; Traver, G.L.; Rothman, P.B.; Harty, J.T.; Colgan, J.D. Tim-3 Directly Enhances CD8 T Cell Responses to Acute Listeria monocytogenes Infection. J. Immunol. 2014, 192, 3133–3142. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.K.; Lenvik, T.R.; McCullar, V.; Felices, M.; O’Brien, M.S.; Cooley, S.A.; Verneris, M.R.; Cichocki, F.; Holman, C.J.; Panoskaltsis-Mortari, A.; et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012, 119, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; A Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2008, 10, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.-M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef]
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V.; et al. Treg Cells Expressing the Coinhibitory Molecule TIGIT Selectively Inhibit Proinflammatory Th1 and Th17 Cell Responses. Immunity 2014, 40, 569–581. [Google Scholar] [CrossRef]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.-F.; Joller, N.; Tan, D.J.; Teng, M.; Smyth, M.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Investig. 2015, 125, 4053–4062. [Google Scholar] [CrossRef]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; Sharpe, A.H.; Kuchroo, V.K. Cutting Edge: TIGIT Has T Cell-Intrinsic Inhibitory Functions. J. Immunol. 2011, 186, 1338–1342. [Google Scholar] [CrossRef]
- Chauvin, J.-M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.-H.T.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 impair tumor antigen–specific CD8+ T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X.; Wang, T.; Zhang, X. Correlation of T Cell Immunoglobulin and ITIM Domain (TIGIT) and Programmed Death 1 (PD-1) with Clinicopathological Characteristics of Renal Cell Carcinoma May Indicate Potential Targets for Treatment. Experiment 2018, 24, 6861–6872. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Maruhashi, T.; Okazaki, I.-M.; Sugiura, D.; Takahashi, S.; Maeda, T.K.; Shimizu, K.; Okazaki, T. LAG-3 inhibits the activation of CD4+ T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat. Immunol. 2018, 19, 1415–1426. [Google Scholar] [CrossRef]
- Huard, B.; Mastrangeli, R.; Prigent, P.; Bruniquel, D.; Donini, S.; El-Tayar, N.; Maigret, B.; Dréano, M.; Triebel, F. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc. Natl. Acad. Sci. USA 1997, 94, 5744–5749. [Google Scholar] [CrossRef]
- Xu, F.; Liu, J.; Liu, D.; Liu, B.; Wang, M.; Hu, Z.; Du, X.; Tang, L.; He, F. LSECtin Expressed on Melanoma Cells Promotes Tumor Progression by Inhibiting Antitumor T-cell Responses. Cancer Res. 2014, 74, 3418–3428. [Google Scholar] [CrossRef] [PubMed]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347.e12. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, J.; Qin, Y.; Wu, Y.; Zhu, L.; Lu, L.; Tang, G.; Shen, Q. Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small-cell lung cancer patients. Am. J. Cancer Res. 2015, 5, 2190–2201. [Google Scholar] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef]
- Zelba, H.; Bedke, J.; Hennenlotter, J.; Mostböck, S.; Zettl, M.; Zichner, T.; Chandran, P.A.; Stenzl, A.; Rammensee, H.-G.; Gouttefangeas, C. PD-1 and LAG-3 Dominate Checkpoint Receptor–Mediated T-cell Inhibition in Renal Cell Carcinoma. Cancer Immunol. Res. 2019, 7, 1891–1899. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Becht, E.; Pagès, F.; Skliris, G.P.; Verkarre, V.; Vano, Y.; Mejean, A.; Saint-Aubert, N.; Lacroix, L.; Natario, I.; et al. Orchestration and Prognostic Significance of Immune Checkpoints in the Microenvironment of Primary and Metastatic Renal Cell Cancer. Clin. Cancer Res. 2015, 21, 3031–3040. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Forbes, K.; Vignali, K.M.; Heale, B.S.; Saftig, P.; Hartmann, D.; A Black, R.; Rossi, J.J.; Blobel, C.P.; et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007, 26, 494–504. [Google Scholar] [CrossRef]
- Möller-Hackbarth, K.; Dewitz, C.; Schweigert, O.; Trad, A.; Garbers, C.; Rose-John, S.; Scheller, J. A Disintegrin and Metalloprotease (ADAM) 10 and ADAM17 Are Major Sheddases of T Cell Immunoglobulin and Mucin Domain 3 (Tim-3). J. Biol. Chem. 2013, 288, 34529–34544. [Google Scholar] [CrossRef] [PubMed]
- Théate, I.; van Baren, N.; Pilotte, L.; Moulin, P.; Larrieu, P.; Renauld, J.-C.; Hervé, C.; Gutierrez-Roelens, I.; Marbaix, E.; Sempoux, C.; et al. Extensive Profiling of the Expression of the Indoleamine 2,3-Dioxygenase 1 Protein in Normal and Tumoral Human Tissues. Cancer Immunol. Res. 2015, 3, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Metz, R.; Rust, S.; DuHadaway, J.B.; Mautino, M.R.; Munn, D.H.; Vahanian, N.N.; Link, C.J.; Prendergast, G.C. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 2012, 1, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA Is an Immune Checkpoint Molecule for Human T Cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Li, E.; Zhang, G.; Wang, X.; Tang, T.; Bai, X.; Liang, T. VISTA: An immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 83. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Q.; Xia, H.; Zhu, G.; Feng, Y.; Wang, Q.; Zhang, Z.; He, W.; Lu, J.; Dong, C.; et al. Analysis of VISTA expression and function in renal cell carcinoma highlights VISTA as a potential target for immunotherapy. Protein Cell 2019, 10, 840–845. [Google Scholar] [CrossRef]
- Roux, D.T.-L.; Sautreuil, M.; Bentriou, M.; Vérine, J.; Palma, M.B.; Daouya, M.; Bouhidel, F.; Lemler, S.; LeMaoult, J.; Desgrandchamps, F.; et al. Comprehensive landscape of immune-checkpoints uncovered in clear cell renal cell carcinoma reveals new and emerging therapeutic targets. Cancer Immunol. Immunother. 2020, 69, 1237–1252. [Google Scholar] [CrossRef]
- Yang, J.C.; Hughes, M.; Kammula, U.; Royal, R.; Sherry, R.M.; Topalian, S.L.; Suri, K.B.; Levy, C.; Allen, T.; Mavroukakis, S.; et al. Ipilimumab (Anti-CTLA4 Antibody) Causes Regression of Metastatic Renal Cell Cancer Associated With Enteritis and Hypophysitis. J. Immunother. 2007, 30, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Plimack, E.R.; Procopio, G.; McDermott, D.F.; et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020, 126, 4156–4167. [Google Scholar] [CrossRef] [PubMed]
- Hammers, H.J.; Plimack, E.R.; Infante, J.R.; Rini, B.I.; McDermott, D.F.; Lewis, L.D.; Voss, M.H.; Sharma, P.; Pal, S.K.; Razak, A.R.A.; et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J. Clin. Oncol. 2017, 35, 3851–3858. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef]
- McDermott, D.F.; Lee, J.-L.; Ziobro, M.; Suarez, C.; Langiewicz, P.; Matveev, V.B.; Wiechno, P.; Gafanov, R.A.; Tomczak, P.; Pouliot, F.; et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non–Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2021, 39, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef]
- Naing, A.; Gainor, J.F.; Gelderblom, H.; Forde, P.M.; O Butler, M.; Lin, C.-C.; Sharma, S.; de Olza, M.O.; Varga, A.; Taylor, M.; et al. A first-in-human phase 1 dose escalation study of spartalizumab (PDR001), an anti–PD-1 antibody, in patients with advanced solid tumors. J. Immunother. Cancer 2019, 8, e000530. [Google Scholar] [CrossRef]
- Motzer, R.J.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Alekseev, B.Y.; Lee, J.-L.; Suarez, C.; Stroyakovskiy, D.; De Giorgi, U.; et al. Final Overall Survival and Molecular Analysis in IMmotion151, a Phase 3 Trial Comparing Atezolizumab Plus Bevacizumab vs Sunitinib in Patients With Previously Untreated Metastatic Renal Cell Carcinoma. JAMA Oncol. 2022, 8, 275. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Powles, T.; Plimack, E.R.; Soulières, D.; Waddell, T.; Stus, V.; Gafanov, R.; Nosov, D.; Pouliot, F.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 1563–1573. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Msaouel, P. Less is More? First Impressions From COSMIC-313. Cancer Investig. 2022, 41, 101–106. [Google Scholar] [CrossRef]
- Brignone, C.; Escudier, B.; Grygar, C.; Marcu, M.; Triebel, F. A Phase I Pharmacokinetic and Biological Correlative Study of IMP321, a Novel MHC Class II Agonist, in Patients with Advanced Renal Cell Carcinoma. Clin. Cancer Res. 2009, 15, 6225–6231. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, D.A.; Merkin, R.D.; Moutafi, M.; Martinez, S.; Adeniran, A.; Kumar, D.; Jilaveanu, L.; Hurwitz, M.; Rimm, D.L.; Kluger, H.M. Location matters: LAG3 levels are lower in renal cell carcinoma metastatic sites compared to primary tumors, and expression at metastatic sites only may have prognostic importance. Front. Oncol. 2022, 12, 990367. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.-C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti–TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti–PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef]
- Gutierrez, M.E.; Tang, S.-C.; Powderly, J.D.; Balmanoukian, A.S.; Janik, J.; Hoyle, P.; Wei, W.; Gong, X.; Hamid, O. 730MO First-in-human phase I study of INCAGN02390, a TIM-3 monoclonal antibody antagonist in patients with advanced malignancies. Ann. Oncol. 2022, 33, S876–S877. [Google Scholar] [CrossRef]
- Jung, K.H.; LoRusso, P.; Burris, H.; Gordon, M.; Bang, Y.-J.; Hellmann, M.D.; Cervantes, A.; de Olza, M.O.; Marabelle, A.; Hodi, F.S.; et al. Phase I Study of the Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Navoximod (GDC-0919) Administered with PD-L1 Inhibitor (Atezolizumab) in Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 3220–3228. [Google Scholar] [CrossRef]
- Tagliamento, M.; Agostinetto, E.; Borea, R.; Brandão, M.; Poggio, F.; Addeo, A.; Lambertini, M. VISTA: A Promising Target for Cancer Immunotherapy? ImmunoTargets Ther. 2021, 10, 185–200. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, A.F.P.; Simão, D.; Odeny, T.; Rodrigues, C.; Fontes-Sousa, M.; da Luz, R.; Chowdry, R.P.; Welsh, S.J.; Paller, C.; Barata, P.C. A Systematic Review of Immune Checkpoint Inhibitors in Non-Clear-Cell Renal Cancer. Kidney Cancer 2022, 6, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Koshkin, V.S.; Barata, P.C.; Zhang, T.; George, D.J.; Atkins, M.B.; Kelly, W.J.; Vogelzang, N.J.; Pal, S.K.; Hsu, J.; Appleman, L.J.; et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J. Immunother. Cancer 2018, 6, 9. [Google Scholar] [CrossRef]
- Boilève, A.; Carlo, M.I.; Barthélémy, P.; Oudard, S.; Borchiellini, D.; Voss, M.H.; George, S.; Chevreau, C.; Landman-Parker, J.; Tabone, M.-D.; et al. Immune checkpoint inhibitors in MITF family translocation renal cell carcinomas and genetic correlates of exceptional responders. J. Immunother. Cancer 2018, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- de Vries-Brilland, M.; Gross-Goupil, M.; Seegers, V.; Boughalem, E.; Beuselinck, B.; Thibault, C.; Chevreau, C.; Ladoire, S.; Barthélémy, P.; Negrier, S.; et al. Are immune checkpoint inhibitors a valid option for papillary renal cell carcinoma? A multicentre retrospective study. Eur. J. Cancer 2020, 136, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.K.; Msaouel, P.; Hess, K.R.; Yu, K.-J.; Matin, S.F.; Sircar, K.; Tamboli, P.; Jonasch, E.; Wood, C.G.; Karam, J.A.; et al. Outcomes of Patients with Renal Cell Carcinoma and Sarcomatoid Dedifferentiation Treated with Nephrectomy and Systemic Therapies: Comparison between the Cytokine and Targeted Therapy Eras. J. Urol. 2017, 198, 530–537. [Google Scholar] [CrossRef]
- Jones, J.O.; Ince, W.H.J.; Welsh, S.J.; Stewart, G.D. Activity of Immunotherapy Regimens on Primary Renal Tumours: A Systematic Review. Kidney Cancer 2022, 6, 221–236. [Google Scholar] [CrossRef]
- Gulati, S.; Lara, P.N. Immune Checkpoint Inhibitors in the Pre-operative Setting and Impact on the Primary Renal Tumor. Kidney Cancer 2022, 6, 201–203. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).