Risk of New-Onset Dementia in Patients with Chronic Kidney Disease on Statin Users: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patient Selection
2.3. Study Outcome and Variables
2.4. Statistical Analysis
3. Results
3.1. Demographic Clinical Characteristics of the Study Population
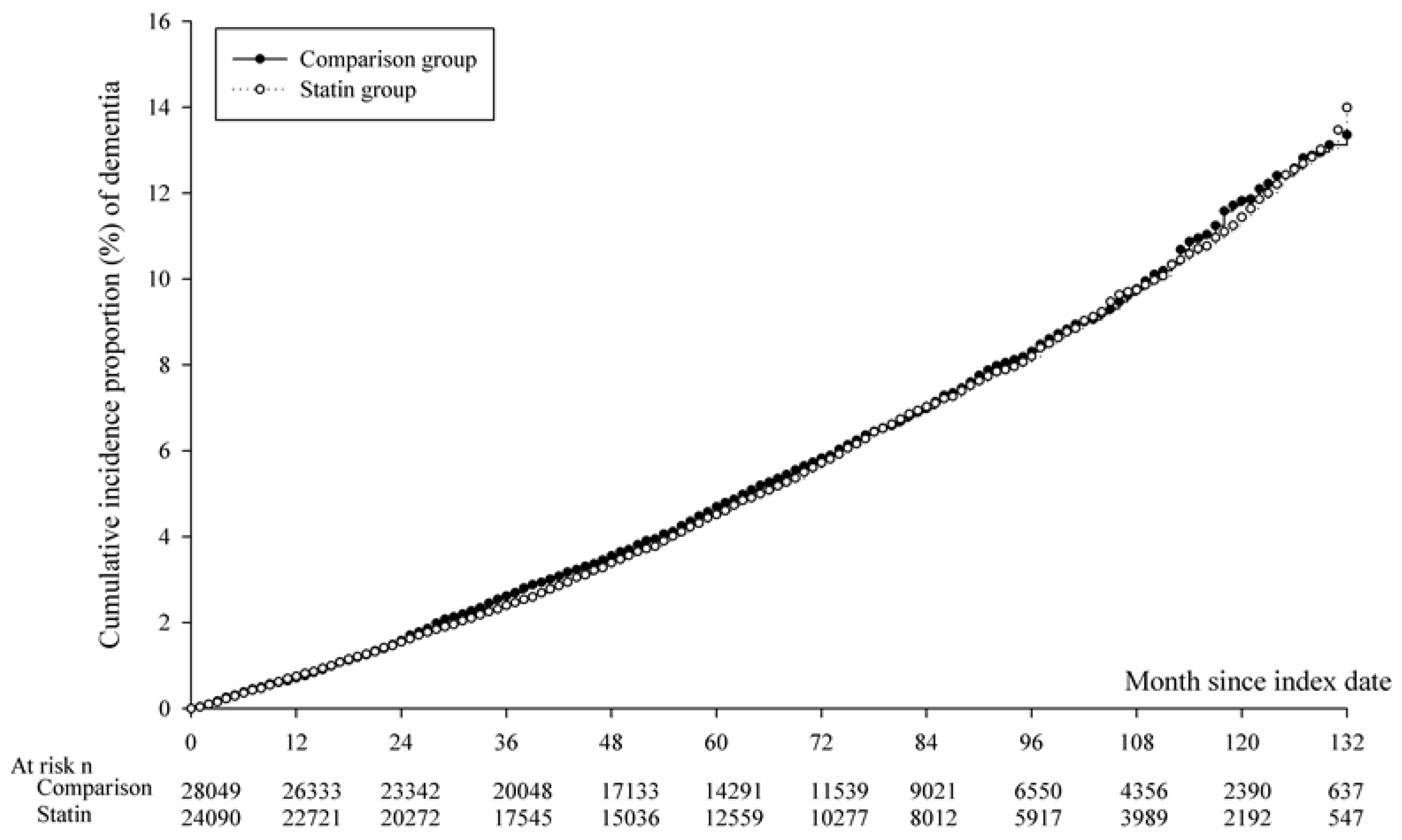
3.2. Relative Risk of NOD
3.3. Sensitivity Analysis of the Relative Risk of NOD in the Propensity Score Matching Analysis
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, A.; Dasgupta, I. Chronic Kidney Disease and Cognitive Impairment. J. Stroke Cerebrovasc. Dis. 2021, 30, 105529. [Google Scholar] [CrossRef]
- Drew, D.A.; Weiner, D.E.; Sarnak, M.J. Cognitive Impairment in CKD: Pathophysiology, Management, and Prevention. Am. J. Kidney Dis. 2019, 74, 782–790. [Google Scholar] [CrossRef]
- Xu, H.; Garcia-Ptacek, S.; Trevisan, M.; Evans, M.; Lindholm, B.; Eriksdotter, M.; Carrero Pharm, J.J. Kidney Function, Kidney Function Decline, and the Risk of Dementia in Older Adults: A Registry-Based Study. Neurology 2021, 96, e2956–e2965. [Google Scholar] [CrossRef] [PubMed]
- Krane, V.; Schmidt, K.R.; Gutjahr-Lengsfeld, L.J.; Mann, J.F.; März, W.; Swoboda, F.; Wanner, C.; 4D Study Investigators (the German Diabetes and Dialysis Study Investigators). Long-term effects following 4 years of randomized treatment with atorvastatin in patients with type 2 diabetes mellitus on hemodialysis. Kidney Int. 2016, 89, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Kimura, G.; Kasahara, M.; Ueshima, K.; Tanaka, S.; Yasuno, S.; Fujimoto, A.; Sato, T.; Imamoto, M.; Kosugi, S.; Nakao, K. Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: Assessment of clinical usefulness in CKD patients with atorvastatin (ASUCA) trial. Clin. Exp. Nephrol. 2017, 21, 417–424. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Žutelija, M.; Mavrinac, V.; Orlic, L. Dyslipidemia in patients with chronic kidney disease: Etiology and management. Int. J. Nephrol. Renov. Dis. 2017, 10, 35–45. [Google Scholar] [CrossRef]
- Kelly, D.M.; Pendlebury, S.T.; Rothwell, P.M. Associations of Chronic Kidney Disease With Dementia Before and After TIA and Stroke. Neurology 2022, 98, e711–e720. [Google Scholar] [CrossRef]
- Kaysen, G.A. Lipid-Lowering Therapy in CKD: Should We Use It and in Which Patients. Blood Purif. 2017, 43, 196–199. [Google Scholar] [CrossRef]
- Orringer, C.E.; Jacobson, T.A.; Maki, K.C. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J. Clin. Lipidol. 2019, 13, 860–872. [Google Scholar] [CrossRef]
- Chung, C.L.; Lin, M.S.; Hsu, J.T.; Hsiao, J.F.; Chang, S.T.; Pan, K.L.; Lin, C.L.; Lin, Y.S. Effects of statin therapy on cerebrovascular and renal outcomes in patients with predialysis advanced chronic kidney disease and dyslipidemia. J. Clin. Lipidol. 2017, 11, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Zammit, A.R.; Katz, M.J.; Bitzer, M.; Lipton, R.B. Cognitive impairment and dementia in older adults with chronic kidney disease: A review. Alzheimer Dis. Assoc. Disord. 2016, 30, 357–366. [Google Scholar] [CrossRef]
- Horodinschi, R.N.; Stanescu, A.M.A.; Bratu, O.G.; Pantea Stoian, A.; Radavoi, D.G.; Diaconu, C.C. Treatment with statins in elderly patients. Med. (Kaunas) 2019, 55, 721. [Google Scholar] [CrossRef] [PubMed]
- Gabin, J.M.; Romundstad, S.; Saltvedt, I.; Holmen, J. Moderately increased albuminuria, chronic kidney disease and incident dementia: The HUNT study. BMC Nephrol. 2019, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Capuano, A.W.; Leurgans, S.E.; Bennett, D.A.; Schneider, J.A. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: A cross-sectional study. Lancet Neurol. 2016, 15, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Baylis, C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 1. [Google Scholar] [CrossRef]
- Mogi, M.; Horiuchi, M. Clinical interaction between brain and kidney in small vessel disease. Cardiol. Res. Pract. 2011, 2011, 306189. [Google Scholar] [CrossRef]
- Stefani, A.; Sancesario, G.; Pierantozzi, M.; Leone, G.; Galati, S.; Hainsworth, A.H.; Diomedi, M. CSF biomarkers, impairment of cerebral hemodynamics and degree of cognitive decline in Alzheimer’s and mixed dementia. J. Neurol. Sci. 2009, 283, 109–115. [Google Scholar] [CrossRef]
- Miwa, K.; Tanaka, M.; Okazaki, S.; Furukado, S.; Yagita, Y.; Sakaguchi, M.; Mochizuki, H.; Kitagawa, K. Chronic kidney disease is associated with dementia independent of cerebral small-vessel disease. Neurology 2014, 82, 1051–1057. [Google Scholar] [CrossRef]
- Toyoda, K. Cerebral small vessel disease and chronic kidney disease. J. Stroke 2015, 17, 31–37. [Google Scholar] [CrossRef]
- van Sloten, T.T.; Sedaghat, S.; Carnethon, M.R.; Launer, L.J.; Stehouwer, C.D.A. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020, 8, 325–336. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Dimitriou, N.G.; Karalexi, M.A.; Mihas, C.; Nasothimiou, E.G.; Tousoulis, D.; Tsivgoulis, G.; Petridou, E.T. Albuminuria in association with cognitive function and dementia: A systematic review and metaanalysis. J. Am. Geriatr. Soc. 2017, 65, 1190–1198. [Google Scholar] [CrossRef]
- Dias, H.I.; Brown, C.L.; Polidori, M.C.; Lip, G.Y.; Griffiths, H.R. LDL-lipids from patients with hypercholesterolaemia and Alzheimer’s disease are inflammatory to microvascular endothelial cells: Mitigation by statin intervention. Clin. Sci. (Lond.) 2015, 129, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Olmastroni, E.; Molari, G.; De Beni, N.; Colpani, O.; Galimberti, F.; Gazzotti, M.; Zambon, A.; Catapano, A.L.; Casula, M. Statin use and risk of dementia or Alzheimer’s disease: A systematic review and meta-analysis of observational studies. Eur. J. Prev. Cardiol. 2022, 29, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Barthold, D.; Joyce, G.; Diaz Brinton, R.; Wharton, W.; Kehoe, P.G.; Zissimopoulos, J. Association of combination statin and antihypertensive therapy with reduced Alzheimer’s disease and related dementia risk. PLoS ONE 2020, 15, e0229541. [Google Scholar] [CrossRef]
- Zigman, W.B.; Schupf, N.; Jenkins, E.C.; Urv, T.K.; Tycko, B.; Silverman, W. Cholesterol level, statin use and Alzheimer’s disease in adults with Down syndrome. Neurosci. Lett. 2007, 416, 279–284. [Google Scholar] [CrossRef]
- Chadha, B.; Frishman, W.H. Review of the protective effects of statins on cognition. Cardiol. Rev. 2021, 29, 328–335. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, X.; Gasevic, D.; McLachlan, F.; Millenson, M.; Timofeeva, M.; Ioannidis, J.P.A.; Campbell, H.; Theodoratou, E. Statins and Multiple Noncardiovascular Outcomes: Umbrella Review of Meta-analyses of Observational Studies and Randomized Controlled Trials. Ann. Intern. Med. 2018, 169, 543–553. [Google Scholar] [CrossRef]
- Williamson, J.D.; Launer, L.J.; Bryan, R.N.; Coker, L.H.; Lazar, R.M.; Gerstein, H.C.; Murray, A.M.; Sullivan, M.D.; Horowitz, K.R.; Ding, J.; et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: A randomized clinical trial. JAMA Intern. Med. 2014, 174, 324–333. [Google Scholar] [CrossRef]
- Zissimopoulos, J.M.; Barthold, D.; Brinton, R.D.; Joyce, G. Sex and Race Differences in the Association Between Statin Use and the Incidence of Alzheimer Disease. JAMA Neurol. 2017, 7, 225–232. [Google Scholar] [CrossRef]
- Chitnis, A.S.; Aparasu, R.R.; Chen, H.; Kunik, M.E.; Schulz, P.E.; Johnson, M.L. Use of Statins and Risk of Dementia in Heart Failure: A Retrospective Cohort Study. Drugs Aging 2015, 32, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Uchoa, M.F.; Moser, V.A.; Pike, C.J. Interactions between inflammation, sex steroids, and Alzheimer’s disease risk factors. Front. Neuroendocrinol. 2016, 43, 60–82. [Google Scholar] [CrossRef]
- Parra, M.A.; Butler, S.; McGeown, W.J.; Brown Nicholls, L.A.; Robertson, D.J. Globalising strategies to meet global challenges: The case of ageing and dementia. J. Glob. Health 2019, 9, 020310. [Google Scholar] [CrossRef]
- Wood, W.G.; Li, L.; Müller, W.E.; Eckert, G.P. Cholesterol as a causative factor in Alzheimer’s disease: A debatable hypothesis. J. Neurochem. 2014, 129, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Samaras, K.; Makkar, S.R.; Crawford, J.D.; Kochan, N.A.; Slavin, M.J.; Wen, W.; Trollor, J.N.; Brodaty, H.; Sachdev, P.S. Effects of statins on memory, cognition, and brain volume in the elderly. J. Am. Coll. Cardiol. 2019, 74, 2554–2568. [Google Scholar] [CrossRef] [PubMed]
- Hendrie, H.C.; Hake, A.; Lane, K.; Purnell, C.; Unverzagt, F.; Smith-Gamble, V.; Murrell, J.; Ogunniyi, A.; Baiyewu, O.; Callahan, C.; et al. Statin use, incident dementia and Alzheimer disease in elderly African Americans. Ethn. Dis. 2015, 25, 345–354. [Google Scholar] [CrossRef]
- Blom, K.; Emmelot-Vonk, M.H.; Koek, H.L. The influence of vascular risk factors on cognitive decline in patients with dementia: A systematic review. Maturitas 2013, 76, 113–117. [Google Scholar] [CrossRef]

| Non-Statin | Statin User | p | |
|---|---|---|---|
| N | 28,049 | 24,090 | |
| Sex | 0.7891 | ||
| Female | 8185 (29.18%) | 7004 (29.07%) | |
| Male | 19,864 (70.82%) | 17,086 (70.93%) | |
| Age group | 0.0709 | ||
| 40–49 | 4004 (14.28%) | 3470 (14.40%) | |
| 50–59 | 9896 (35.28%) | 8557 (35.52%) | |
| 60–69 | 7886 (28.12%) | 6796 (28.21%) | |
| 70–79 | 4743 (16.91%) | 4089 (16.97%) | |
| >=80 | 1520 (5.42%) | 1178 (4.89%) | |
| Comorbidities | |||
| Coronary artery disease | 7332 (26.14%) | 7966 (33.07%) | <0.0001 |
| Hypertension | 14,600 (52.05%) | 15,850 (65.79%) | <0.0001 |
| Heart failure | 1058 (3.77%) | 2897 (12.03%) | <0.0001 |
| PAD | 2822 (10.06%) | 3573 (14.83%) | <0.0001 |
| Ischemic stroke | 3640 (12.98%) | 4223 (17.53%) | <0.0001 |
| Diabetes mellitus | 7911 (28.20%) | 9503 (39.45%) | <0.0001 |
| COPD | 5575 (19.88%) | 4754 (19.73%) | 0.6859 |
| Peptic ulcer | 13,959 (49.77%) | 11,462 (47.58%) | <0.0001 |
| Concurrent medication | |||
| Aspirin | 3264 (11.64%) | 6104 (25.34%) | <0.0001 |
| Warfarin | 154 (0.55%) | 251 (1.04%) | <0.0001 |
| Clopidogrel | 1264 (4.51%) | 4169 (17.31%) | <0.0001 |
| Diuretics | 1032 (3.68%) | 2853 (11.86%) | <0.0001 |
| Beta-blockers | 5811 (20.72%) | 7494 (31.11%) | <0.0001 |
| CCBs | 7422 (26.46%) | 9205 (38.21%) | <0.0001 |
| ACEIs | 2160 (7.70%) | 3293 (13.67%) | <0.0001 |
| ARBs | 4459 (15.90%) | 6874 (28.53%) | <0.0001 |
| Insulin | 182 (0.65%) | 361 (1.50%) | <0.0001 |
| OHAs | 811 (2.89%) | 9978 (41.42%) | <0.0001 |
| Non-Statin User | Statin User | p | |
|---|---|---|---|
| N | 28,049 | 24,090 | |
| Follow up person-months | 1,780,406 | 1,558,441 | |
| Event of dementia | 1608 | 1390 | |
| Crude hazard ratio (95% CI) | Reference | 0.98 (0.92–1.06) | 0.0894 |
| † Adjusted hazard ratio (95% CI) | Reference | 0.93 (0.87–1.00) | 0.0594 |
| Non-Statin User N = 23,812 | Statin User N = 23,812 | p | |
|---|---|---|---|
| Follow up person-months | 1,540,456 | 1,540,456 | |
| Dementia cases | 1415 | 1372 | |
| ‡ Crude HR (95% CI) | Reference | 0.92 (0.84–1.03) | 0.0673 |
| † Adjusted HR (95% CI) † | Reference | 0.91 (0.81–1.02) | 0.0598 |
| aHR | 95% CI | p | |
|---|---|---|---|
| Sex | |||
| Female | 0.87 | 0.80–0.94 | 0.0006 |
| Male | Reference | ||
| Age group | |||
| 40–49 | Reference | ||
| ≥50 | 1.42 | 1.18–1.69 | <0.0001 |
| Comorbidities | |||
| Coronary artery disease | 1.12 | 1.03–1.21 | 0.0073 |
| Hypertension | 1.02 | 0.92–1.14 | 0.6599 |
| Heart failure | 1.32 | 1.11–1.43 | <0.0001 |
| PAD | 1.12 | 1.03–1.21 | 0.0072 |
| Ischemic stroke | 1.56 | 1.43–1.69 | <0.0001 |
| Diabetes mellitus | 1.24 | 1.15–1.34 | <0.0001 |
| COPD | 1.21 | 1.11–1.31 | <0.0001 |
| Peptic ulcer | 1.11 | 1.03–1.19 | 0.0061 |
| Concurrent medication | |||
| Aspirin | 1.07 | 0.98–1.17 | 0.1110 |
| Warfarin | 1.43 | 1.08–1.90 | 0.0134 |
| Clopidogrel | 0.99 | 0.91–1.09 | 0.7677 |
| Diuretics | 1.02 | 0.93–1.12 | 0.6018 |
| Beta-blockers | 1.04 | 0.96–1.13 | 0.3657 |
| CCBs | 1.02 | 0.94–1.11 | 0.6106 |
| ACEIs | 1.01 | 0.92–1.12 | 0.8321 |
| ARBs | 0.99 | 0.90–1.08 | 0.7473 |
| Insulin | 1.01 | 0.92–1.13 | 0.8233 |
| OHAs | 1.11 | 1.02–1.20 | 0.0062 |
| Sub-Groups | aHR | 95% CI | p |
|---|---|---|---|
| With CKD | 0.931 | 0.865–1.003 | 0.0594 |
| Without CKD | 0.922 | 0.910–0.934 | <0.0001 |
| p for interaction | 0.7953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jong, G.-P.; Lin, T.-K.; Huang, J.-Y.; Liao, P.-L.; Yang, T.-Y.; Pan, L.-F. Risk of New-Onset Dementia in Patients with Chronic Kidney Disease on Statin Users: A Population-Based Cohort Study. Biomedicines 2023, 11, 1073. https://doi.org/10.3390/biomedicines11041073
Jong G-P, Lin T-K, Huang J-Y, Liao P-L, Yang T-Y, Pan L-F. Risk of New-Onset Dementia in Patients with Chronic Kidney Disease on Statin Users: A Population-Based Cohort Study. Biomedicines. 2023; 11(4):1073. https://doi.org/10.3390/biomedicines11041073
Chicago/Turabian StyleJong, Gwo-Ping, Tsung-Kun Lin, Jing-Yang Huang, Pei-Lun Liao, Tsung-Yuan Yang, and Lung-Fa Pan. 2023. "Risk of New-Onset Dementia in Patients with Chronic Kidney Disease on Statin Users: A Population-Based Cohort Study" Biomedicines 11, no. 4: 1073. https://doi.org/10.3390/biomedicines11041073
APA StyleJong, G.-P., Lin, T.-K., Huang, J.-Y., Liao, P.-L., Yang, T.-Y., & Pan, L.-F. (2023). Risk of New-Onset Dementia in Patients with Chronic Kidney Disease on Statin Users: A Population-Based Cohort Study. Biomedicines, 11(4), 1073. https://doi.org/10.3390/biomedicines11041073






