Abstract
Genomic instability and genetic mutations can lead to exhibition of several cancer hallmarks in affected cells such as sustained proliferative signaling, evasion of growth suppression, activated invasion, deregulation of cellular energetics, and avoidance of immune destruction. Similar biological changes have been observed to be a result of pathogenic viruses and, in some cases, have been linked to virus-induced cancers. Human endogenous retroviruses (HERVs), once external pathogens, now occupy more than 8% of the human genome, representing the merge of genomic and external factors. In this review, we outline all reported effects of HERVs on cancer development and discuss the HERV targets most suitable for cancer treatments as well as ongoing clinical trials for HERV-targeting drugs. We reviewed all currently available reports of the effects of HERVs on human cancers including solid tumors, lymphomas, and leukemias. Our review highlights the central roles of HERV genes, such as gag, env, pol, np9, and rec in immune regulation, checkpoint blockade, cell differentiation, cell fusion, proliferation, metastasis, and cell transformation. In addition, we summarize the involvement of HERV long terminal repeat (LTR) regions in transcriptional regulation, creation of fusion proteins, expression of long non-coding RNAs (lncRNAs), and promotion of genome instability through recombination.
1. Introduction
As the first treatment targeting a human endogenous retrovirus (HERV) protein enters into phase III clinical trials to combat multiple sclerosis (MS) progression [], HERVs are becoming increasingly promising targets in cancer diagnostics and therapies. Previously, HERVs have been identified as involved in the progression of complex diseases such as MS [,], schizophrenia [,], and type 1 diabetes [,]. Lessons learned from these phenotypes have illuminated the potential for HERVs to impact human cancer development and treatment options. For example, there is currently a phase I trial testing the safety of a HERV-E-derived peptide autologous T-cell (HERV-E TCR T-cell) therapy to treat clear cell renal cell carcinoma []. HERVs are single-stranded enveloped RNA viruses that integrated into the human germline by means of their long terminal repeats (LTRs) []. Astonishingly, over 8% of the human genome comprise HERV sequences [,]. The LTRs in simple retroviruses, such as HERVs of the gammaretroviral subfamily, flank the capsid (gag), polymerase and protease (pol), and envelope (env) genes. HERVs of the betaretroviral (e.g., HERV-K) or spumaviral (e.g., HERV-L) subfamily carry additional non-structural genes such as HERV-K rec, HERV-K np9 and HERV-L tas/bel1, and HERV-L bet, respectively []. In contrast to infectious retroviruses, about 87% of HERV sequences in the human genome are remnants of proviruses embodied by solo LTRs, while about 1.5% and 11.5% of HERV loci carry complete and truncated genomes, respectively []. For this reason, HERVs were historically considered “junk” DNA []. However, discoveries from the last two decades revealed substantial functions for various HERV elements in immune regulation [,,], cell differentiation [,], cell fusion [,], transcriptional regulation [,,,,], and cell transformation [,]. With this increasing evidence on the role of HERVs in cancer development, summaries of the current scientific knowledge are of high importance.
Even though similar endogenous retroviruses can be found in a variety of mammal species, articles on animal endogenous retroviruses (ERVs) were not considered for this review as it has been shown that data from retroviral animal systems have limited applicability to humans []. While insertions and oncogene activation through ERVs has been observed in mice [] and chickens [,], leading to the hypothesis of HERV retrotransposition as a cancer driver [,,], no de novo germline or somatic insertions of HERVs could be identified despite the sequencing of more than 4000 human cancer genomes over the last 25 years []. In the search of actively retrotransposing elements in humans, non-LTR LINE elements have been identified as better substrates for non-allelic recombination [,,,] Their central role in pathogenic recombination is further supported by their 5 times higher abundance in the human genomes than HERV sequences and the observation that reverse transcriptase (RT) inhibitors display effects as treatment in some human cancers []. Nonetheless, HERV insertion points have been reported in several cancer-related pathway genes, which utilize their viral proteins and altering transcriptional control mechanisms to initiate or promote oncogenesis.
Previous reviews of HERVs and cancer development have focused on either a specific gene product or functional region (e.g., Env [,,], LTR [], HERV-LTR7 lincROR (HGCN: LINC01419) [], a particular family (e.g., HERV-W [,], HERV-E [], HERV-K []), or a certain role of HERVs (e.g., transcriptional regulation [], promoter exaptation [], chromosomal rearrangement []). While uncovering specific aspects of HERV biology, these articles often fail to capture the interplay of different molecular mechanisms from multiple HERV families on specific cancer types. Other review articles categorize HERV effects on cancer development by molecular mechanism [,,,] and accordingly use only examples from different cancers providing no overview of the network of HERVs acting in a specific cancer type. Additionally, general review articles on HERVs and cancer tend to focus on the most common and clinically prevalent cancers, while articles on specific cancers are only available for a small number of main cancer types (e.g., breast cancer [], melanoma [], Hodgkin’s lymphoma [], and prostate cancer []).
Therefore, we conducted a review on the carcinogenic impacts of HERVs by cancer type. While the literature on HERVs is growing exponentially due to improved sequencing and bioinformatic detection methods, it becomes increasingly challenging to provide a thorough overview of the cancers associated with HERVs. Fortunately, recent advances in the digital availability of scientific articles and improvements in search algorithms have provided a way to filter and evaluate large numbers of published articles. Consequently, systematic reviews are increasing in number, making complex topics available to clinical, translational, and basic science researchers in a digestible way. With their recent publication, Grabski et al. (2019) [] were the first to conduct a systematic review employing a search algorithm for the evaluation of clinically relevant links between HERVs and cancer. While Grabski et al. provide an overview of the HERV-facilitated treatment and diagnostic options of surgical diseases, we set out to conduct a review of the molecular pathways and mechanisms affected by HERVs across the spectrum of cancer types. Our goal was to feature the integration of HERVs in known cancer pathways and to outline HERVs that are potentially suitable for the development of targeted cancer therapies.
Certain HERVs have perfectly assimilated into the cellular environment preventing oncogenesis, while others maintain their pathogenic potential and remain undercover until a time of cellular dysregulation. Accordingly, the goal of this review is to provide a comprehensive overview of the HERV influences for the human cancers listed below. Greek letters in superscript were used to denote viral functional regions or genes associated with specific observations (see Table 1).

Table 1.
Annotations used in this review. Greek letters serve as abbreviation and denote complete or partial viral origin of a gene, RNA, or protein.
2. Methodology Used to Obtain Primary Cancer-Related HERV Literature for a Qualitative Review
To allow for thorough coverage of the primary literature for this umbrella review, we identified articles on HERVs published through PubMed using the following search terms in titles and abstracts: ((HERV [Title/Abstract]) OR (ERV [Title/Abstract]) OR (endogenous retrovirus [Title/Abstract]) OR (endogenous retroviral [Title/Abstract])). Using the corresponding filter in PubMed, we identified review articles and evaluated them for articles missed by the PubMed search. We downloaded open access papers using the PubMed-Batch-Download software developed by Bill Greenwald [], supplemented with a manual download through PubMed with Texas Medical Center (TMC) library access in portable document format (PDF). We obtained articles with restricted access through the Texas Medical Center Library using the OpenAthens plugin in EndNote. Only articles accessible and available in English were assessed. We used our recently developed R package called PDF data extractor (PDE) available on CRAN (https://CRAN.R-project.org/package=PDE, version 1.4.3 accessed on 6 February 2023, Houston, TX, USA) as a pre-screening tool to separate cancer- from non-cancer-related articles []. We manually divided the articles by cancer type and later grouped them for this review. Lastly, we evaluated the identified full-text articles ad hoc for cancer-specific factors inducing HERV expression; HERV insertions, deletions, or recombinations distinctively observed in a cancer type; interactions of HERVs with known oncogenes, tumor suppressors, or other signaling pathways; HERV products with functional effects in a cancer; and HERV-based treatment approaches. The chapters in this review were similarly structured to provide a coherent hierarchy for the reader. For the qualitative synthesis, we weighted the scientific findings by number of articles reporting similar results, and excluded findings that were based on inaccurately described or performed methods (e.g., lack of detail, no technical replicates, inappropriate method for a certain conclusion). Articles on animal endogenous retroviruses were not considered for this study as it has been shown that data from animal systems are minimally applicable to human systems []. In the same way, papers on non-human cancers were included only at a minimal level in this review. Graphical summary figures were created with BioRender (accessed on 3 February 2023, Houston, TX, USA).
3. HERVs in Breast Cancer—The Rise of New Biomarkers
Breast cancer is the most common cancer and the leading cause of cancer-related deaths in women worldwide []. Due to the many breast cancer subtypes and their varying treatment responses [], targeted treatments that evolved in recent years have become a success story. However, the field is still in need of preventive and early detection methods.
HERVs might be able to close this gap providing new targets for prognostics, diagnostics, and treatments. Several groups have independently reported the overexpression of messenger RNAs (mRNAs) and proteins from multiple HERV families in breast cancer cell lines and patient tissues compared to healthy tissues []. Interestingly, the menstruation-associated hormones estradiol and progesterone were observed to increase HERV-K (HML-4) env [] and HERV-K (HML-4) RT transcripts as well as HERV-K (HML-4) RT protein levels [] in breast cancer cell lines (Figure 1). In breast cancer patients, increased HERV-K (HML-4) RT as well as HERV-K (HML-4) Env protein levels were shown to be associated with shorter metastasis-free and overall survival [,]. Conversely, Montesion et al. (2018) [] identified two HERV-K (HML-2) LTRs (HGCN: ERVK-5 at position 3q12.3 and ERVK3-4 at 11p15.4) that had specifically increased promoter activity in breast cancer while decreased activity in immortalized human mammary epithelial cells. Additionally, several stage-specific transcription factor (TF)-binding sites within the two LTRs were predicted to potentially contribute to promoter activity during neoplasia []. While the ERVK-5 (HERV-KII) was fixed in humans, the ERVK3-4 (HERV-K7) was found to be polymorphic in the human population with an allele frequency of 51%, presenting the prospect of a newly identified risk facto r []. In addition, breast cancer cell lines were shown to harbor HERV-K111 gene conversion/deletion events in the pericentromeric region of chromosome 22, suggesting a contribution to genomic instability []. Furthermore, certain HERV-K (HML-2) Env splice variants have been suggested as breast cancer-specific antigens but are still under investigation [].
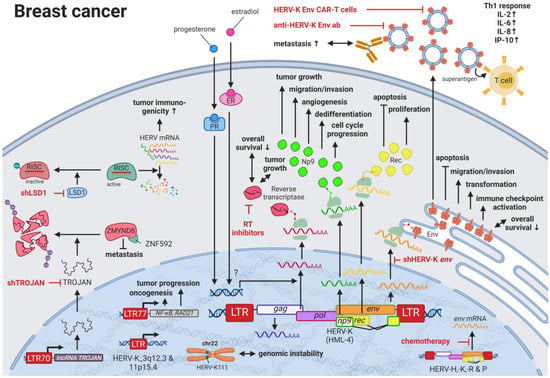
Figure 1.
The role of HERVs in breast cancer. Evaluated treatments are marked in red. Abbreviations: Th1 = T helper cell 1, ER = estradiol receptor, PR = progesterone receptor, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope. If not otherwise stated HERV-K = HML-2.
Higher expression of particular HERV-K gene products in patients with breast cancer is also signified by the pronounced immune response against such proteins. Responses include increased T-cell proliferation, Th1-specific cytokine secretion (i.e., INFγ, IL2, IL6, CXCL8, CXCL10), immune checkpoint activation, and serum antibody production against HERV-K (HML-2) proteins [,,]. Additional to higher serum HERV-K mRNA levels and serum anti-HERV-K antibody titers in women with ductal carcinoma in situ and stage I disease compared to women without cancer, Wang-Johanning et al. (2014) reported that elevated HERV-K (HML-2) antibodies and mRNA levels in the blood can be an early indicator of future metastatic disease development []. In patients undergoing chemotherapy, HERV-H, -K, -R, and -P env mRNA expression is reported to be decreased compared to patients not receiving chemotherapy []. In mouse model systems, treatment with HERV-K (HML-2) Env-directed antibodies [], short interfering RNA-mediated knockdown (RNAi) of HERV-K (HML-2) env [], and chimeric antigen receptor (CAR) T cells specific for HERV-K (HML-2) Env protein [] were able to recapitulate the effects against the engrafted human tumors. For all three treatments, specific cytotoxic effects against tumor cells were observed in the mice: reduced tumor growth, induced apoptosis, and reduced metastasis [,].
A detailed study of the HERV-K (HML-2) env knockdown through RNAi revealed the involvement of the viral gene in cellular pathways playing key roles in cancer (e.g., EGFR, TGF-β, NF-κB, MYC, p53, HRAS, KRAS, and MAPK1/3) (Figure 2) []. Overexpression of HERV-K (HML-2) env, on the other hand, increased breast cancer cell transformation, migration, and invasion, as well as restored the cancer-related signaling pathways mentioned above alongside the downregulation of p53 (HGNC: TP53) []. Additionally, microarrays identified HERV-K (HML-2) Env protein as a strong inducer of the MAPK pathway via upstream TFs [], and examinations of the DNA methylome and TF-binding data revealed several HERV LTR77-driven TFs such as NF-κB (HGNC: NFKB1) and RAD21 [,]. Besides HERV-K (HML-2) Env, HERV-K (HML-2) non-structural nuclear protein Np9 has been described to interact with cellular proteins []. Np9 destabilizes LNX1, an E3 ubiquitin ligase, that targets members of the NUMB/NOTCH1 pathway for degradation [,]. NOTCH1 regulates cell differentiation, cellular metabolism, cell cycle progression, angiogenesis, self-renewal, and immune function [] and has been shown to be deregulated in breast cancer []. Another ubiquitin ligase directly bound by Np9, which has been found upregulated in breast and other cancers, is MDM2 []. Contrary to the inhibition of p53 observed for HERV-K (HML-2) Env, Np9 has been reported to interfere with the MDM2 ubiquitin ligase activity toward p53 in the cell nucleus and thus increases p53 levels while being ubiquitinated itself []. Furthermore, Np9 was discovered to result in an upregulation of CD147 (HGNC: BSG) []. CD147 is a coreceptor for VEGFR2 (HGNC: KDR) and has been demonstrated to induce VEGFA [,] in addition to ADAMTS1 and ADAMTS9 []. Both VEGFA/VEGFR2 and ADAMTS1/9 signaling are reported inducers of metastasis and angiogenesis in several cancers [].
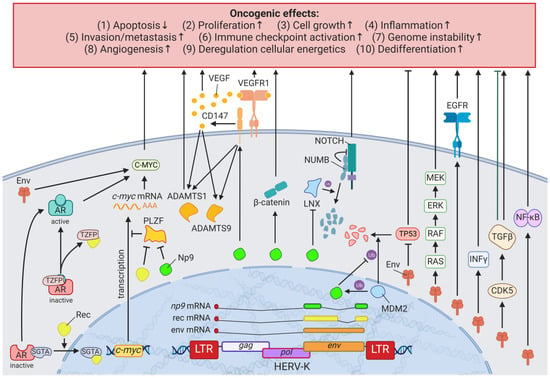
Figure 2.
The effects of HERV-K (HML-2) Np9, Rec, and Env proteins on oncogenesis. LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, env = envelope, HERV-K = HML-2.
Jin et al. (2019) [] uncovered an HERV-derived long noncoding RNA (lncRNA), named TROJANλ, which is highly expressed in human triple-negative breast cancer (TNBC). This LTR70-driven lncRNA is a promising therapeutic target, as it binds ZMYND8, a metastasis-repressing factor, and leads to its degradation by the ubiquitin-proteasome pathway []. Whereas TROJANλ overexpression stimulated TNBC proliferation and metastasis in vitro, in vivo studies in mice confirmed that RNAi targeting TROJAN can inhibit TNBC progression and reduce tumor size []. Similarly, several groups have proposed treatment strategies targeting HERV proteins as tumor-specific antigens [,]. The potential success of such approaches is supported by the findings of Sheng et al. (2018) who showed that the genetic or pharmacological ablation of the histone demethylase LSD1 (HGNC: KDM1A) enhances tumor immunogenicity by stimulating HERV expression []. In addition to enabling HERV transcription, KDM1A elimination was observed to prevent the removal of an inhibitory methyl mark on the RNA-induced silencing complex (RISC), which in its usual function promotes the degradation of HERV mRNAs [].
4. HERVs in Lymphoma—The Silent Inducers
Lymphomas are characterized by an increased proliferation of lymphocytes and are classified according to their maturity (peripheral or mature versus precursor) and cell lineage (B, T, or natural killer cell) []. In contrast to other cancers, a hallmark of lymphoma is its origin in the immune system, with known risk factors that perturb immune functions, such as immunosuppressive drugs [], autoimmune disorders [], and viral infections including Epstein–Barr virus (EBV), human immunodeficiency virus (HIV), or human T-cell lymphotropic virus (HTLV) []. As there is complex interaction between HERVs and immune system function, it is consistent with previous literature that HERV deregulation influences lymphomagenesis.
HERV-K (HML-2) was shown to have markedly different titers in the blood of patients with lymphoma (e.g., HIV infection with diffuse large B-cell lymphoma (DLBCL), non-HIV diffuse large B-cell lymphoma, and HIV infection with Hodgkin lymphoma (HL)) compared to healthy individuals []. Remission of the cancer after successful treatment was associated with a significant decrease in viral titers []. While there was a large range of titer differences between patients with lymphoma (on average 1010 copies/mL) and healthy individuals (on average 102 copies/mL), HERV-K viral particles were found in the plasma of all patients with lymphoma []. Additionally, the differences in titer between lymphoma groups (e.g., high titers of HERV-K (HML-2) type 1 with low HERV-K (HML-2) type 2 titers in HL) suggests the use of specific HERV-K titers as sensitive and specific biomarkers for lymphoma []. Furthermore, immune histochemical staining showed that cutaneous T-cell lymphoma (CTCL)-derived extracellular vesicles were positive for syncytin-1 (HGCN: ERVW-1ε) [].
While HERV-K (HML-2) was not found to be induced by HTLV [], Leung et al. (2018) reported that EBV required the activation of HERV LTRs for transcription of oncogenic genes such as HUWE1 (Figure 3A) []. In 2015, Zahn et al. identified HERV-K111 as an additional putative biomarker for lymphoma while studying the pericentromeric regions of chromosomes []. They observed the absence of the pericentromeric HERV-K111 5′ regions in cutaneous T-cell lymphoma (CTCL) lines HUT78 and H9 and mutated HERV-K111 in Jurkat cells []; however, Kaplan et al. (2019) confirmed a significant increase in the homozygous absence of the HERV-K111 5′ regions in non-Hispanic White patients with severe CTCL compared to healthy individuals []. As HERV-K111 exists in over 1000 copies in the pericentromeric region, a deletion of about 3400 Kb is suggested []. Thus, pericentromeric instability is a likely consequence of the missing sections but will have to be confirmed by additional experiments.
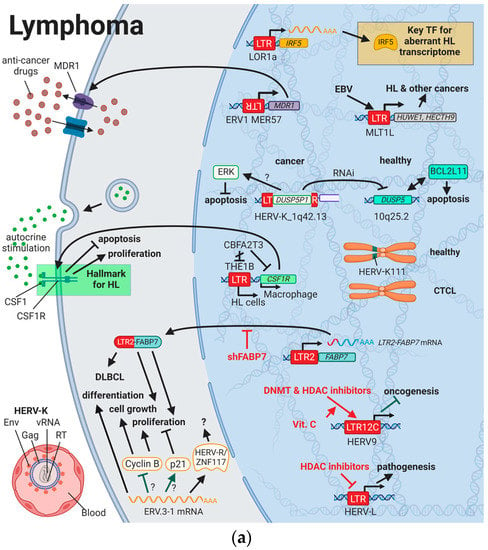
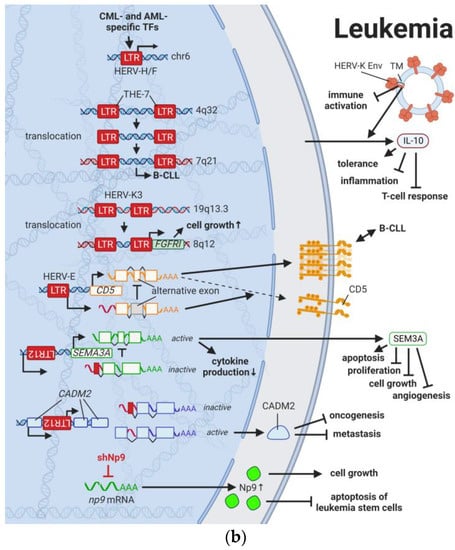
Figure 3.
The role of HERVs in (a) lymphoma and (b) leukemia. Evaluated treatments are marked in red. Abbreviations: TF = transcription factor, HL = Hodgkin’s lymphoma, EBV = Epstein–Barr virus, CTCL = cutaneous T-cell lymphoma, DLBCL = diffuse large B-cell lymphoma, DNMT = DNA methyltransferases, HDAC = histone deacetylases, LTR = long terminal repeat, CML = chronic myelogenous leukemia, AML = acute myelogenous leukemia, B-CLL = B-cell chronic lymphocytic leukemia, vRNA = viral RNA, RT = reverse transcriptase, Env = envelope protein, TM = transmembrane domain, shNp9 = siRNA targeting np9, shFABP7 = siRNA targeting FABP7. If not otherwise stated HERV-K = HML-2.
There are only two studies reporting antibodies against HERVs in 2–6% of patients with lymphoma (N = 288) [,]. This could be due to reduced immune function in these patients or the very nature of HERV integration in lymphoma development. Transcriptional activation of normally dormant oncogenes by HERV LTRs, so called promoter exaptation, appears to play a much larger role during lymphocyte transformation than the interference in cancer pathways by HERV proteins or gene products, thus providing no targets for the immune system []. The first ever described case of retroviral promoter exaptation was the expression of the CSF1 receptor (CSF1R) in HL cells discovered by Lamprecht et al. (2010) []. Autocrine stimulation by the macrophage growth factor CSF1 has been shown to be essential for the proliferation and survival of HL cells, and lineage inappropriate expression of the CSF1R has become a hallmark in HL []. In contrast to regular macrophages, CSF1R in HL cells is driven by the THE1B LTR located 6.2 kb upstream of the locus and has been linked to loss of transcriptional repressor CBFA2T3 expression []. In a similar way, normally brain-expressed fatty acid-binding protein 7 (FABP7) was observed to be expressed as a chimeric isoform with LTR2 in tissues from patients with DLBCL []. RNAi-mediated knockdown of FABP7 resulted in decreased proliferation and growth of DLBCL, suggesting a dependence on FABP7 expression []. A third example of promoter exaptation is the upregulation of IRF5 driven by the demethylated LOR1a LTR element that was specifically detected in HL cell lines []. IRF5 is a key regulator of the aberrant transcriptome in HL and crucial for HL cell survival [].
Contrary to the pathogenic effects of HERVs described above, the double-copy HERV-R on chromosomes 7q11.21 and 7q33 (HERV-R.3-1 and HERV-R.3-2, respectively) has been classified to have tumor suppressive functions []. HERV-R.3-1 Env (HGNC: ERV3-1ε) was observed to be downregulated in HL cells compared to normal blood cells [], which parallels the absence of expression seen in choriocarcinoma []. In choriocarcinoma cells, ERV3-1 overexpression inhibits cell proliferation [], while ERV3-1 is upregulated during terminal differentiation of leukemia cells and is highest in cell cycle arrested cells [,,]. Downregulation of cyclin B and upregulation of the cyclin-dependent kinase inhibitor P21 (HGNC: CDKN1A) are likely to be key mechanisms for growth inhibition []. Interestingly, 1% of healthy non-Hispanic Whites carry a stop codon in the ERV3-1 region in a homozygous state without a pathogenic phenotype, questioning the impact of HERV-R.3-1 on at least essential physiological functions []. Another interesting finding in HL cells is the interaction of a dual specificity phosphatase 5 (DUSP5) pseudogene and a specific HERV sequence. While most HERV families can be found in different species of mammals, HERVs of the family K are quite unique to the human genome []. Therefore, it was striking to find the pseudogene of DUSP5 (DUSP5P1), inserted in the HERV-K_1q42.13 sequence, indicating the prior presence of the HERV-K family in that location []. Most striking was the observation that several cancer cell lines (Burkitt’s lymphoma, leukemia, neuroblastoma, and Ewing sarcoma) as well as peripheral blood mononuclear cells (PBMC) from patients with HL displayed a significantly higher ratio of DUSP5P1/DUSP5 expression than normal cells []. While the DUSP5/DUSP5P1 ratio correlated with levels of the pro-apoptotic factor B-cell leukemia/lymphoma 2-like 11 (BCL2L11), the authors hypothesized that DUSP5P1 reduces the activity of DUSP5 via RNAi or competitive inhibition results in increased MAPK1/3 activity and subsequent inhibition of BCL2L11 []. However, a distinct function for DUSP5P1 is yet to be determined [].
Besides cancer pathways in which HERVs have incorporated themselves, advances in treatments have revealed HERVs being part of therapy-related side effects and drug resistance mechanisms. ABCB1 (MDR-1) is one of the most expansively studied drug resistance mechanisms []. The ABCB1 gene encodes a 170-kDa ATP-dependent efflux pump for the plasma membrane, which prevents intracellular drug accumulation []. Interestingly, aberrant MDR-1 transcription found in lymphoma cells is driven by the ERV1 LTR MER57 initiating transcription in the opposite direction of the viral promoter []. To reduce the transcription of detrimental genes, several DNA methyltransferases (DNMT) and histone deacetylase (HDAC) inhibitors have been incorporated in the treatment plan for hematopoietic and lymphatic malignancies in recent years []. Currently, it is not fully understood if DNMT and HDAC inhibitors provide any beneficial effects through the activation of beneficial HERVs or if the inhibitors lead to an interference with drug-induced HERV elements. Studies indicated that especially LTR12C elements from the HERV9 family are activated by DNMT and HDAC inhibitors [,] and further stimulated when vitamin C is also taken []. Even though the fear of aberrant expression of oncogenes and HERVs with unknown function is large, several LTR12 elements have been detected to induce tumor suppressor genes (see HERVs in Testicular Cancer—The Governors of Tumor Suppressor Genes) [,,]. Additionally, HDAC inhibitors have been shown to prevent the activation of HERV-L, which might participate in pathogenesis []. New research is required to evaluate the HERV-related side effects of such drugs.
5. HERVs in Leukemia—The Lifesavers for Cancer Cells
Leukemias are the most common childhood cancers worldwide [,] and among the cancers with the lowest somatic mutational burden []. Both characteristics suggest genomic risk factors that can be inherited, and when accumulated, lead to carcinogenesis. Analogous to observations made for HERV LTRs in prostate cancer (see HERVs in Prostate Cancer—The Dancing Partner of the Androgen Receptor), THE-7 LTRs were discovered as drivers of a translocation of chromosome 14q32 to chromosome 7q21 in a female patient with B-cell chronic lymphocytic leukemia (B-CLL) (Figure 3B) []. Furthermore, fibroblast growth factor receptor 1 (FGFR1) was found to be constitutively activated through the fusion between a HERV-K3 (HML-6) sequence (HGCN: ERVK3-1) and the FGFR1 gene in a male patient with an atypical stem cell myeloproliferative disorder [,]. The fusion and resulting aberrant growth signal were the result of a translocation involving chromosomes 19q13.3 and chromosome 8q12 [,]. Furthermore, deletions of pericentromeric HERV-K111 regions in adult T-cell leukemia cell lines were enriched leading to chromosomal instabilities []. Additional to these chromosomal abnormalities as prognostic markers, Schmidt et al., (2015) identified single nucleotide polymorphism (SNP) markers near two endogenous retroviral loci, HERV-K (HML-2) on chromosome 1 (HGCN: ERVK-7) and HERV-Fc1 on chromosome X (HGCN: ERVFC1) associated with multiple myeloma []. Both HERV regions encode nearly complete viral proteins, suggesting a functional involvement of the gene products in disease development [].
Similar to the observations in lymphomas, the immune response against HERV-K (HML-2) and other HERVs appears to be rather weak or unexplored (see also HERVs in Lymphoma—The Silent Inducers) []. This might also be due to the fact that HERV-K108 (HGCN: ERVK-6) Env TM has immunosuppressive properties and has been reported to induce IL10 in PBMCs []. IL10 is an anti-inflammatory cytokine, which terminates T-cell responses and leads to immune tolerance []. In a comparable way, surface CD5 expression on B cells regulates their functional fate and immunological activity. CD5 expression is tightly controlled through a HERV-E sequence located upstream of the CD5 locus (HERV-E::CD5). The HERV-E::CD5 sequence was shown to induce the integration of an alternate exon, resulting in low levels of membrane CD5 in normal B cells []. Conversely, high levels of CD5 expression in B cells caused by the absence of the alternate exon was found to be associated with CLL [].
Another oncogenic function of HERVs involves np9, which is among the HERV-K genes overexpressed in leukemias (Figure 2) [,,]. Np9 was shown to not only activate MAPK, AKT, and NOTCH1 signaling pathways (see Results HERVs in Breast Cancer—The Rise of New Biomarkers), but also to upregulate β-catenin, which is (HGNC: CTNNB1) essential for survival of leukemia stem cells [,]. Silencing of np9 in turn inhibited the growth of myeloid and lymphoblastic leukemia cells []. In several systematic studies, Sokol et al. uncovered various physiological transcription regulation mechanisms of HERV sequences and showed that HERV9 LTRs (LTR12) control splicing of the tumor suppressor genes CADM2 and SEMA3A in erythroleukemia and human embryonic stem cells [,]. Additional beneficial HERV9 functions are discussed in HERVs in Testicular Cancer—The Governors of Tumor Suppressor Genes. In addition, the HERV-H/F locus on chromosome 6 was described to carry several TF-binding sites involved in normal hematopoiesis and with reduced expression in B-cell and myeloid lineage leukemia, indicating its transcription under normal conditions [].
Further evidence for physiological functions of HERV gene products is the observation that 5-azacitidine, a DNA demethylating agent used to treat preleukemia and leukemias, activates cancer/testis antigens (CTAs) [,,] and HERV gene products specifically in tumors, triggering innate immunity [,]. This phenomenon was also observed in several other cancers including colorectal [], urothelial [], and melanoma [,].
6. HERVs in Skin Cancer—The Highly Addictive Treatment Targets
Compared to other organs, the skin is exposed to some of the highest amounts of mutagens; therefore, skin cancer is the malignancy with the highest mutational burden []. Accordingly, several physical and chemical agents with mutagenic potential have been proven to influence the regulation of HERV sequences [,]. As such, UV radiation, the primary risk factor for both melanomas and non-epithelial skin cancers, was shown to induce gag expression of HERV-K (HML-2) [] in melanoma cell lines and tumor tissues; to reduce rec and np9 expression of HERV-K (HML-2) in primary human melanocytes and melanoma []; and to reduce pol expression of HERV-K, -H, -L, -FRD, -E, and ERV9 in melanoma cell lines [], primary keratinocytes [], and skin biopsies (Figure 4) []. Furthermore, Karimi et al. (2018) demonstrated that copper (a potent antibacterial agent and deregulated nutrient in cancer) in the form of CuSO4 increases HERV-K (HML-2) and -W env transcripts in the melanoma cell line SK-Mel-37 []. HERV-K (HML-2) gene expression as a hallmark of melanoma was confirmed by multiple groups and was reported to be further induced in melanoma cells by serum starvation [,].
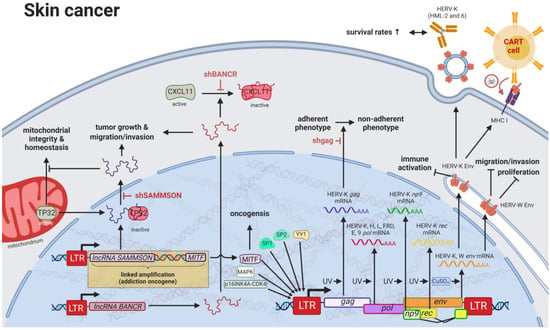
Figure 4.
The role of HERVs in skin cancer. Evaluated treatments are marked in red. Abbreviations: LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope, lncRNA = long non-coding RNA, shBANCR = siRNA targeting BANCR. If not otherwise stated HERV-K = HML-2.
Besides HERVs, many oncogenes have been found in higher levels in skin cancers, likely caused by mutated TF-binding sites in promoters []. HERV LTRs have been postulated to harbor over 64% of all human-specific TF-binding sites in human embryonic stem cells [], and chromatin immunoprecipitation (ChIP) assays as well as gene expression studies revealed that one third of p53 (HGCN: TP53) sites are located within HERV LTRs []. Of all LTR-associated p53-binding sites, approximately 70–90% are located in sequences of the ERV1 family [], while HERV-I LTRs have been shown to be repressed by TP53 and activated by TP53 mutations []. Additional to many more TF-binding sites located in HERV sequences (a web-based browser developed by Garazha et al. (2015) [] is available at https://herv.pparser.net/GenomeBrowser.php (accessed on 6 February 2023)), Sp1, Sp3, and YY1 have been described to specifically induce unmethylated HERV-K (HML-2) LTRs in melanoma cell lines []. Particularly, hypomethylation of HERV-K6 at 7q22.1 (HCGN: ERVK-6) and LINE-1 elements was observed to be associated with worse prognosis and poorer survival for melanoma patients [].
Despite the general assumption that the majority of HERV sequences in the genome are inactive due to DNA methylation and other histone marks, a study published by Jacques et al. (2013) indicated that up to 80% of HERV regions exist in an open chromatin state []. Particularly in patients with melanoma, both lncRNAs, BANCR and SAMMSON, are promoted by HERV LTRs [,,]. Both lncRNAs were confirmed to increase growth and invasiveness of melanocytes [,]. Conversely, BANCR knockdown was shown to reduce melanoma cell migration, which could be rescued by recombinant overexpression of CXCL11, indicating an inhibition of CXCL11 by BANCR []. SAMMSON was demonstrated to play an equally important role in melanoma formation, as it can be detected in more than 90% of human melanomas, functions as a lineage addiction oncogene [,] and is generally included in gene amplifications involving melanoma-specific oncogene MITF due to its proximity to the gene. MITF in turn has been reported to induce HERV-K (HML-2) gene expression by binding to E-box (CA(C/T)GTG) located within the HERV-K (HML-2) LTR [,]. SAMMSON directly binds p32, leading to its nuclear targeting and preventing its function in maintaining mitochondrial integrity and homeostasis [].
Moreover, HERV-K (HML-2) expression was shown to be induced by the activation of the MAPK and CDKN2A-CDK4 pathways in melanoma cells []. In addition to statistically significant differences noted in the seroprevalence of HERV-K (HML-2) antibodies between patients with melanoma and healthy individuals, higher serological HERV-K (HML-2) reactivity was identified as an indicator of improved survival in patients with melanoma []. Increased expression of HERV-K (HML-6) in melanoma cell lines and tumor tissues has clinically been associated with higher HERV-K (HML-6) antibody levels []. While the contribution of yellow fever virus and BCG vaccine-associated HERV-K (HML-6) to the prevention of melanoma still remains a controversial topic [,], several studies report supporting evidence for the potential use of HERV-K (HML-2)-targeting strategies as treatment for melanomas. For instance, the use of RNAi-targeting HERV-K (HML-2) was shown to prevent the transition from an adherent to a non-adherent growth phenotype of melanoma cells [], and CAR-T cells targeting HERV-K (HML-2) Env were observed to have a significant antitumor effect []. In addition to its structural functions, HERV-K (HML-2) Env also carries an immunosuppressive domain, which was shown to lead to the local immune evasion of B16 melanoma cells injected in mice []. Furthermore, HERV-K (HML-2) inhibition prevented the pathogenic formation of multinuclear atypia cells in melanoma []. Contrary to the observed reduced cell grow upon inhibition of ERVW-1ε in BeWo cancer cells [], recombinant B16F10 melanoma cells with stable expression of ERVW-1ε displayed decreased cell proliferation, migration, and invasion []. Therefore, functions of ERVW-1ε in breast cancer cells might be cell type- and concentration-specific. Most noteworthy for possible targeted treatments is the discovery of an aggressive subpopulation of melanoma cells that are highly dependent on HERV-K (HML-2) activation []. In fact, patients with melanoma and autoimmune diseases display increased survival rates, suggesting that systemic autoimmunity also targets cancer-associated HERV activities []. One potential mechanistic explanation includes HERV-K (HML-2) rec. Its depletion was demonstrated to lead to upregulation of epithelial-to-mesenchymal-associated genes and an enhanced invasion phenotype of proliferative melanoma cells []. Additionally, LSD1 depletion (see Results HERVs in Breast Cancer—The Rise of New Biomarkers for more details) in combination with anti-PD-1 antibody (HGNC: PDCD1) therapy was found to increase the immunogenicity and response to checkpoint blockade of refractory mouse melanomas, providing another way to improved treatment efficacies [].
7. HERVs in Testicular Cancer—The Governors of Tumor Suppressor Genes
Although the secretion of HERV viral particles was first detected in placenta by Kalter et al. (1973) [], three years later, testicular tumors became the first cancer tissue described to selectively release HERV viral particles []. Since then, testicular cancer cell lines serve as a model for HERV-K particle assembly and the effects of HERV-K protein expression on cell functions [,,]. Cellular transcription factor YY1 was shown to specifically activate LTRs in teratocarcinoma cell lines [] with the HERV-K (HML-2) LTR in Tera-1 cells becoming as strong of a promoter as the SV40 early promoter (Figure 5) []. Interestingly, several distinct testicular cancer cell lines and tissues were demonstrated to each specifically drive expression of reporter plasmids under different HERV-K (HML-2) LTR promoters [,]. Moreover, Mueller et al. (2018) revealed the capability of HERV-K (HML-2) to induce neighboring genes such as PRODH in germ cell tumors (GCT) that paralleled with the differentiation status of the cancer cells []. In this way, expression of genes adjacent to HERV-K (HML-2), which is highest in undifferentiated cells, may provide an additional indicator of the tumor status []. Conversely, transcription factor NFY induces HERV-9 LTR12-mediated transcription of tumor suppressors in normal testis, which is silenced in testicular cancer [,].
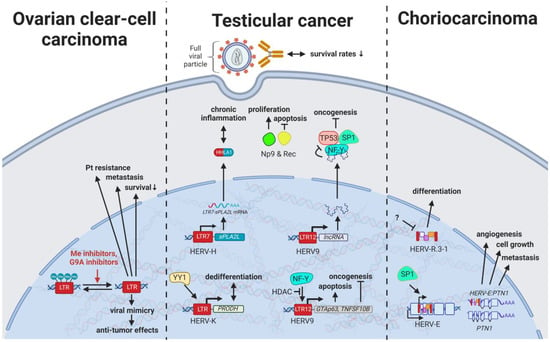
Figure 5.
The role of HERVs in genital cancers. Evaluated treatments are marked in red. Abbreviations: HDAC = histone deacetylases, Me = methylases, G9A = G9a methyltransferase, Pt = platinum treatment, HERV-K = HML-2.
With HERV viral particles being secreted by testicular tumor cells, over 60% of males with germ cell cancers have been observed to display seroreactivity to HERV-K (HML-2) antigens, while only 4% of healthy individuals showed high antibody titers []. Additionally, HERV-K(HML-2)-specific T cells could be detected in patients with a history of testicular cancer, indicating immunological memory formation that may prevent relapse in certain cases []. Most significantly, HERV-K (HML-2) antibody seropositivity in patients with GCT that persisted after treatment was associated with both lower survival rates and lower chemotherapeutic success []. Thus, HERV-K(HML-2)-specific antibodies may have great diagnostic value as surrogate measurement for HERV-K (HML-2) expression and associated oncogenic effects in GCTs.
Specifically, the two viral proteins derived from alternative transcripts of the C-terminal portion of the HERV-K (HML-2) env gene were first discovered in GCT cell lines [] and subsequently described to play a central role in the development of GCTs []. The proteins, later termed HERV-K (HML-2) Np9 [] and Rec [], associate in GCTs with the tumor suppressor PLZF (HGNC: ZBTB16), a transcriptional repressor, and chromatin remodeler and abolish its transcriptional repression of MYC, a major target of PLZF []. This leads to amplified cell proliferation and survival mediated by overexpression of MYC and corresponding MYC-regulated genes []. Furthermore, HERV-K (HML-2) rec expression in mice has been shown to result in the development of GCTs []. Np9, on the other hand, has been demonstrated to interact in addition to PLZF with multiple ubiquitin ligases modifying cancer pathways (see Results HERVs in Breast Cancer—The Rise of New Biomarkers ) [,,]. The CRISPR-Cas9-mediated knockdown of Np9 was reported to increase sensitivity to bleomycin, cisplatin, and serum starvation and reduce migration, albeit not affecting the viability of NCCIT teratocarcinoma cells, which could be restored by adding recombinant Np9 []. Possibly less significant, but still noteworthy, is the detection of a HERV-H LTR/sPLA2L fusion protein, also called HHLA1λ, in teratocarcinoma cells lines []. While this fusion protein could not be confirmed in the patient samples tested [], HHLA1λ still has several functions in digestion and is postulated to contribute to chronic inflammation [,].
Current research reveals the pivotal role of LTR12-driven transcription in treatment of testicular cancer. LTR12 activity has been suggested as biomarker for the potency of anticancer drugs [,] and might be selectively stimulated for testicular cancer treatments. As indicated above, HERV9 LTR12 induces the transcription of tumor suppressors such as germ cell-associated, transcriptionally active GTAp63 (HGNC: TP63) [,] and TNF Receptor Superfamily Member 10b (TNFRSF10B) [,]. Both tumor suppressors are frequently downregulated in testicular cancer cells and may be induced by HDACs to mediate apoptosis (see also HERVs in Lymphoma—The Silent Inducers) [,]. Moreover, lncRNAs driven by solitary HERV9 LTRs were found to function as SWI/SNF complex antagonist as well as targets of key TFs regulating proliferation, including NFY, TP53, and SP1 [].
8. HERVs in Other Genital Cancers (Ovary Cancer, Choriocarcinoma, and Endometrial Cancer)—The Ascent of New Possibilities
Despite stably falling incidences of over the last three decades [], most patients with ovarian cancer are not diagnosed until stages III (51%) and IV (29%) because they experience few or no symptoms until the disease has metastasized []. Therefore, HERVs have been investigated in different subtypes for their potential as prognostic, diagnostic, and therapy-resistance markers. Heidmann et al. (2017) proposed HEMO, a HERV MER34-derived Env protein, as a possible marker for ovarian clear-cell carcinoma (OCCC) as it was significantly increased in ovarian cancers with evidence for histiotype dependence []. In a similar way, HERV-W, HERV-E, and HERV-K (HML-2) displayed higher expression due to generalized hypomethylation in ovarian carcinomas compared to non-malignant ovarian tissues [,,]. Intriguingly, hypomethylated HERV-K (HML-2) elements in OCCC were observed to be associated with poor prognosis, platinum-based therapy resistance, and increased metastasis (Figure 5) []. Contrary to this observation, Liu et al. (2018) reported methylation inhibitors and G9A inhibitors enhancing antitumor effects through viral mimicry []. Providing a potential explanation for this apparent discrepancy, the study investigators detected the most substantial effects of the inhibitors on HERV-Fc1 LTRs with only minimal effects on HERV-K (HML-2) MER9a1, highlighting potentially counteracting forces []. This can be further reconciled by the observation that, despite insufficient reactivity against the highly expressed HERV-K gene products, potent HERV-K-specific T cells can be generated from autologous dendritic cells (DCs) pulsed with HERV-K (HML-2) Env antigens. The T cells displayed high cytotoxicity against autologous tumor cells without affecting normal cells and could be further increased upon depletion of T regulatory cells []. A possible mechanism of HERV-Ks that counteracts the immune response and drives oncogenesis could include the ovarian cancer susceptibility gene BRCA1 as it carries several HERV-K (HML-2) elements in its sequence []. As a marker for response to chemotherapy in ovarian cancer, a LTR-controlled alternate transcript of the molecular chaperone DNAJ has been suggested, as in its methylated form DNAJ is solely expressed in malignant and not surface ovarian cells [].
ERVW-1ε and ERVFRD-1ε are the most extensively studied retroviral proteins encoded in the human genome and have a vital role in placentation by facilitating trophoblast fusion and creation of an immune privileged site [,]. Nonetheless, HERVs have been implicated in furthering abnormal growth and reduced differentiation of trophoblasts resulting in the development of choriocarcinoma. HERV-E induces expression of an alternative transcript of PTN, a heparin-binding protein with central functions in growth and differentiation control of the placenta [,,]. The alternative HERV-E:PTNλγπελθ transcript is driven by the TF SP1 binding to the HERV-E LTR in untranslated exon 1 of PTN []. HERV-E:PTNλγπελθ mRNA was only detected in trophoblast cell cultures while absent in normal adult tissues (Figure 5) [,]. Interestingly, depletion of the HERV-E:PTNλγπελθ transcript was shown to prevent human choriocarcinoma growth and invasion in a mouse model []. On the contrary, HERV-R.3-1 (HGCN: ERV3-1) has been proposed as tumor suppressor since its overexpression induced differentiation of the human BeWo choriocarcinoma cell line (see also HERVs in Lymphoma—The Silent Inducers) [,].
Various HERV env transcripts and proteins have been implicated in the development of endometrial cancer (e.g., HERV-E, Fc, FRD, H, K, R, Rb, T, V1, V2, and W env, as well as ERVW-1 and ERVFRD-1 RNA) []. A demethylation-driven activation of the viral ERVW-1 LTR through a functional estrogen receptor element (ERE) was suggested in 2012 []. The higher levels of ERVW-1envW protein observed are theorized to then promote not only increased cell fusion, but also hyperproliferation, which is brought on by the interaction of ERVW-1envW with tumor growth factor beta (TGF-β; HGNC: TGFB1) [,].
9. HERVs in Colorectal and Gastrointestinal Cancers—The Hopes and Hazards of Family H
Colorectal cancers (CRCs) are among the most common cancers worldwide with strikingly low 5-year survival rates of less than 65–70% in Northern America, Australia/New Zealand, and many European countries []. While early diagnosis has markedly improved for older patients due to routine screenings in individuals >50 years of age, rising rates in the population under 45 years of age highlight the need for improved non-invasive prognostic and diagnostic tools []. In the last decade, research has started to focus on the influence of HERV elements on CRCs and has revealed interesting interactions, especially involving HERV-H. With over 1000 loci in the human genome, HERV-H is the most abundant HERV family carrying coding regions in the human genome [].
Findings by Liang et al. (2012) indicated that the number and composition of active HERV-H elements allow for the distinction between normal colon samples and colon tumor tissues, not necessarily the comparison of overall HERV H levels []. The study investigators observed 14 active elements in colon tumor tissues compared to 7 in adjacent normal tissues with some active loci located in close proximity to putative open reading frames, e.g., HERV-H_1q42.2 near viral RT and env, HERV-H_16q24.1 near viral RT, and HERV-H_19q13.31 near CREB5 (Figure 6) []. Later, Pérot et al. (2015) detected a significant increase of HERV-H gag, pol, and env RNA in patients with CRC, with HERV-H loci on chromosome Xp22.3 (HGCN: ERVH-2) and 20p11.23 having the highest expression frequencies []. Moreover, the group discovered correlations between HERV-H expression and lymph node invasion as well as microsatellite instability (MSI) of tumors []. Interestingly, CRC-associated HERV-H sequences displayed activating histone marks in their 5′ LTR regions [] and previous studies reported MYB (a proto-oncogenic transcriptional activator) binding to HERV-H LTRs, leading to a sevenfold increase in promoter activity []. In addition, the N-acetyltransferase 1 (NAT1) was shown to be crucial for self-renewal and neural differentiation of primed pluripotent stem cells, while loss of NAT1 resulted in a significant increase in the HERV-H transcript []. Furthermore, ERVH-2 performed well as a specific immunological target, since T cells stimulated with HERV-H-specific peptides resulted in proliferation of mostly CD8+ T cells, leading to increased lysis of CRC cell lines []. Additional to HERV-H expression, ERVW-1ε levels were proposed as putative prognostic markers because of a statistically significant association with decreased overall survival in rectal cancer but not in patients with colonic cancer [].
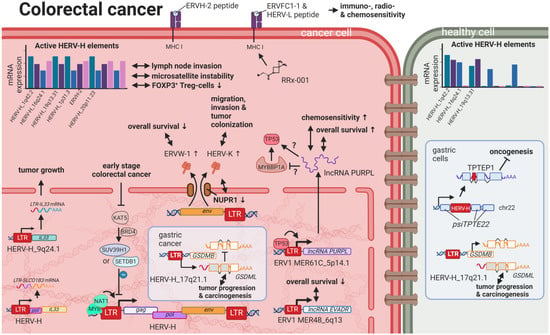
Figure 6.
The role of HERVs in colorectal cancer. Evaluated treatments are marked in red. Abbreviations: lncRNA = long non-coding RNA, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, env = envelope, HERV-K = HML-2.
A link between chronic inflammation and cancer has been established for various tumors, and, specifically for CRCs, an inflammatory microenvironment has been recognized to be a cause, hallmark, and consequence of disease []. A subset of patients with colon cancer was shown to express a HERV-H_9q24.1::IL33 fusion transcript required for tumor growth []. IL33 is a proinflammatory cytokine produced by epithelial and endothelial cells [] that has been demonstrated to correlate in its expression with CRC progression and metastasis []. The particular function of the HERV-H_9q24.1::IL33 product is unknown but, based on the function of native IL33, might include roles as a modified cytokine or nuclear factor regulating gene transcription []. Further, HERV LTR promoted chimeric transcripts detected specifically in CRC tissues and cell lines including the ion transporter SLCO1B3λπ, which is frequently mutated in CRC [].
In contrast to fusion transcripts that result in aberrant cellular genes, TIP60 (HGNC: KAT5) has been described as a regulator of the inflammatory effects of HERV expression inside the cell []. KAT5 is a tumor suppressor that is found to be repressed in early stages of CRCs and breast cancers []. A publication by Rajagopalan et al. (2018) indicated that KAT5 downregulation results in increased levels of HERV expression and associated inflammatory responses []. In normal cells, KAT5 induces H3K9 trimethylating enzymes SUV39H1 and SETDB1 in a BRD4-dependent manner, which leads to global inhibitory methylation of HERV loci []. In KAT5-repressed cancer cells, the study investigators detected induction of IRF7 mediated by the intracellular pathogen sensing STING (HGNC: TMEM173), resulting in an inflammatory response and further tumor growth [].
While viral gene products are readily detected by innate immune receptors, most cellular lncRNAs escape the surveillance mechanisms and, in this fashion, are able to interfere with regulatory pathways. For instance, the lncRNA EVADR on chromosome 6q13 was observed to be induced by the ERV1 LTR MER48 specifically in colon, rectal, lung, pancreas, and stomach adenocarcinomas []. In a similar way, the ERV1 LTR MER61C on chromosome 514.1 drives transcription of the lncRNA PURPL (LINC01021), which is increased in CRC cell lines and tumors [,]. While higher EVADR expression was associated with slightly decreased patient survival rates [], CRC tumors with higher levels of PURPL RNA resulted in improved survival rates, and induced expression in CRC cell lines lead to increased chemosensitivity according to Kaller et al. (2017) []. While Kaller et al. showed a positive correlation between PURPL RNA and TP53 target expression [], just months before, Li et al. (2017) observed suppressive effects of PURPL RNA on TP53 through the binding of MYBBP1A (a TP53 stabilizer), leading to reduced proliferation and tumor growth in a mouse model []. Despite the differing findings concerning the function and effects of PURPL RNA on TP53 levels, both publications confirmed the induction of PURPL by TP53 [,]. At this point, it should be mentioned that Kaller et al. did not divide their CRC patient population according TP53 mutation status when comparing PURPLhigh and PURPLlow cohorts. Accordingly, PURPL levels could very likely be a surrogate for TP53 status, being 50% mutated in the analyzed CMS subtype 4 group, and hence not in themselves impact survival rates. Nonetheless, EVADR and PURPL lncRNAs do present potential prognostic markers.
Additional to the potential chemo-sensitizing action of the PURPL lncRNA, RRx-001, a novel anticancer drug in phase III clinical trials [], displayed the induction of HERV-Fc2 env (HGCN: ERVFC1-1ε) and HERV-L LTRs in colon cancer cells resulting in an antiviral response, which sensitizes the cells to immuno-, radio-, and chemotherapy []. CRISPR-Cas9-mediated knockout of HERV-K (HML-2) env in DLD-1 colorectal cancer cells reduced migration, invasion, and tumor colonization and was sociated with nuclear protein-1 (NUPR1) reduction, indicating a potential connection []. Conversely, the expression of immunogenic HERVs was associated with immune checkpoint activation and microsatellite instability, a predisposition to mutation resulting from impaired DNA mismatch repair, in patients with colorectal adenocarcinoma, turning the induction of such HERVs into a potential target for therapy []. Lastly, tumors with high HERV-H level were shown to exhibit low levels of FOXP3+ T regulatory cells, rendering them more responsive to cytolytic targeting and treatment with immune checkpoint inhibitors [].
Despite the spatial and physical difference between CRC and gastric cancer, several similarities can be found between the two. Analogous to CREB5 in CRC, a HERV-H LTR on chromosome 17q21 was discovered to drive the expression of an alternative transcript of gasdermin-like (GSDML) in the stomach cancer cell line AZ521, while transcription through the cellular promoter was only detected in stomach tissues of healthy individuals []. Overexpression of GSDML has been reported to be associated with tumor progression and carcinogenesis []. On the contrary, the TPTE pseudogene on chromosome 22, containing a HERV-H element (TPTEP1), also termed psiTPTE22-HERV, was reported to be downregulated in gastric cancer compared to adjacent normal tissue samples []. The protein product of TPTEP1 has been postulated to carry tumor suppressor functions, but these have not yet been confirmed [].
10. HERVs in Liver Cancer—The Opening Chapter
Liver cancers are the third leading cause of cancer-related death in males worldwide []; however, only limited reports are available on the influence of HERVs in liver cancer oncogenesis. Ahn and Kim (2009) together with Liang et al. (2009) reported increased expression of HERV-H, HERV-R.3-1, and HERV-P in overall liver cancers without taking into consideration distinct cancer subtypes [,], while several other groups reported the distinct activation of HERV-K (HML-2) and HERV-P in hepatocellular carcinomas (HCCs) specifically [,]. However, a study by Liu et al. (2021) demonstrated that the LRP1B mutation was associated with the overexpression of HERV-H LTR-Associating 2 (HHLA2) in patients with HCC []. HCC has a high morbidity and constitutes more than three-fourths of all cases with liver cancer []. Interestingly, a large number of HERV LTRs, including LTR1, LTR12C, and THE1B, were found upregulated in human HCC tumors (Figure 7) []. HCC tumors with high LTR activation were associated with high risk of relapse [,], a more aggressive phenotype [,], poor prognosis [], and impaired cell differentiation in animal models [,]. Moreover, HERV-K (HML-2) gene products, while only moderately elevated in patients [], were described to positively correlate in their expression with tumor cell dedifferentiation, mortality rates, TNM stage, and cirrhosis in HCCs []. Furthermore, in a subgroup of patients with HCC antibodies against HERV-K (HML-2) Gag were found indicating the immunogenicity of the viral gene product [,]. Additional to its potential as prognostic marker and therapeutic target, HERV-K (HML-2) expression might also provide resolution to a dispute concerning the origin of a liver cancer cell line. It is still debated whether HepG2 cells are derived from HCC or hepatoblastoma.
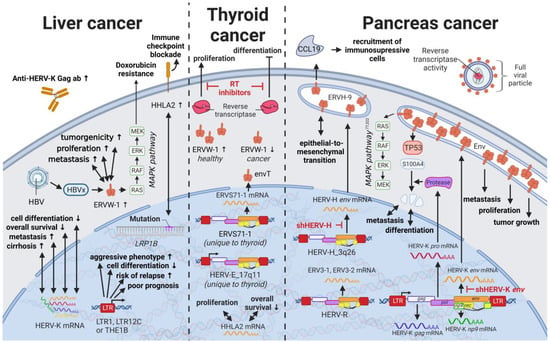
Figure 7.
The role of HERVs in liver and endocrine cancers. Evaluated treatments are marked in red. Abbreviations: HBV = hepatitis B virus, MAPK = MAP kinase, ab = antibody, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope, HERV-K = HML-2.
The absence of any HERV-K expression in HepG2 cells points to the latter [], especially since other HCC cell lines such as Hep3B display a HERV-K (HML-2) profile similar to HCCs []. Furthermore, ERVW-1ε transcription was shown to be enhanced in HCC patients and cell lines, increasing cell proliferation, metastasis, and tumorigenicity []. ERVW-1ε expression in HepG2 cells [] was demonstrated to be further induced by Hepatis B virus (HBV), the most common cause of HCC, through HBx protein-mediated NF-κB activation []. In HCC cells, ERVW-1ε actively induced the MEK/ERK pathway and suppressed doxorubicin-induced apoptosis via MEK/ERK cascade, highlighting its central role in drug resistance []. ERVW-1ε also induces cell fusion [,] and carries an immunosuppressive function [], both of which have been shown to perpetuate oncogenesis.
11. HERVs in Nervous System Cancers—The Wicked Side of HERV-W
Perhaps the best described and most widely accepted involvement of HERVs in pathogenesis has been in neurological diseases. Links between HERVs and MS, amyotrophic lateral sclerosis, and schizophrenia are supported by numerous publications and have been summarized well by Gruchot et al. (2019) [] and Dolei et al. (2019) []. Interestingly, one specific HERV-K (HML-2)-derived SINE fragment was identified by random amplified polymorphic DNA to be absent in a patient with a grade IV glioblastoma (GBM) []. Even though this finding was unique to the patient and could not be observed in 32 other gliomas, the method might be useful to identify the absence of HERV-K (HML-2) sequences normally located in tumor suppressor genes, such as BRCA2, XRCC1, and NBPFs, as a marker for oncogenesis (Figure 8) [,]. On the contrary, a much more pronounced role in inflammatory neurological disease as well as brain cancers has been confirmed for HERV-W. Substances such as caffeine and aspirin that are able to pass the blood–brain barrier have been reported to increase ERVW-1ε protein and HERV-W Gag mRNA as well as protein levels in human SH-SY5Y neuroblastoma cells []. The concentration for both substances correlated positively with HERV-W gene expression and negatively with cell survival []. Intriguingly, caffeine acted in a luciferase assay on the HERV-W promoter, while aspirin did not change the promoter activity []. Additionally, human cytomegalovirus—a betaherpesvirus implied to be present with high prevalence in many brain cancers [,,,,]—was shown to induce upregulation of HERV-W alongside HERV-T, HERV-F, ERV-9, HERV-K (HML-2 to 4 and HML-7 to 8), and HERV-L elements in GliNS1 cells []. Although less pronounced than in the active infection, UV-inactivated virus was also able to stimulate HERV expression []. While it is assumed that the majority of HERV-W gene products are made by neurons and myeloid cells, i.e., monocytes and microglia, low expression of ERVW-1ε mRNA has been detected in normal astrocytes, which is increased up to twofold in U-87 MG astrocytoma cells (26–50% and 59–74%, respectively, compared to levels in placental tissues) []. Conversely, neuroblastoma cell lines SK-N-DZ and SK-N-AS have been described as reliable producers of HERV-W RNA [,]. This expression was shown to be further induced after recovery from hypoxia [] or exposure to the antipsychotic drug valproic acid (VPA) []. VPA, a commonly prescribed medication for seizures and bipolar disorder, is a histone deacetylase inhibitor suggested to activate additional to HERV-W ERV9 promoters through chromatin remodeling in the neuroblastoma cell lines [].
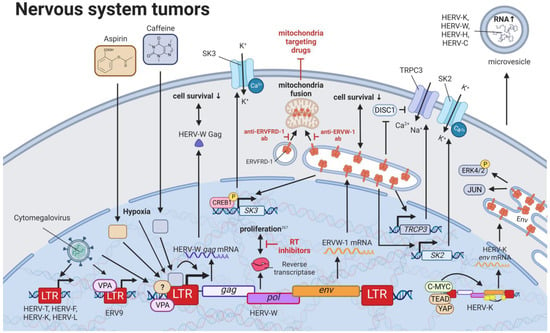
Figure 8.
The role of HERVs in nervous system tumors. Evaluated treatments are marked in red. Abbreviations: K+ = potassium, Ca2+ = calcium, ab = antibody, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope, HERV-K = HML-2.
Through studies of neuroblastoma cell lines SH-SY5Y and IMR-32, ERVW-1ε was demonstrated to stimulate the expression of SK3 [] and TRPC3 []. Additionally, ERVW-1ε-mediated SK3 channel activation was identified to be dependent on the CREB1 and documented to result in an increased potassium ion (K+) current []. On the other hand, the TRPC3 channel was postulated to be activated through either the derepression of the TRPC3 inhibitor, DISC1, or the direct induction of TRPC3 expression by ERVW-1ε []. In addition to the Ca2+ and Na+ influx caused by the TRPC3 channel, studies in recent-onset schizophrenia demonstrated the capability of ERVW-1ε to upregulate the Ca2+ induced K+ channel SK2, further escalating the potassium ion current []. While both findings point towards new functions of ERVW-1ε especially in neuronal cells, experiments showing whether the fusogenic potential of ERVW-1ε was confounding the ion influx and cell behavior observed were missing [,]. Accordingly, roles of ERVW-1ε in the aforementioned functions have to be further evaluated.
Besides expression in neuroblastoma cell lines, HERV-W RNA alongside HERV-K (HML-2), -H, and -C RNA was found to be highly enriched in microvesicles of cells derived from glioblastoma tumor specimens []. These microvesicles were reported to carry the ability of horizontal gene transfer between cancer cells, potentially leading to increased levels of HERV proteins in neighboring cells []. HERV-K (HML-2) Env expression has been reported to be upregulated in human Merlin-negative schwannoma and in all meningioma grades via the CRL4 (DCAF1) and YAP/TEAD pathway []. In addition, C-MYC was shown to bind to the HERV-K (HML-2) LTR and lead to env expression, which in turn can be inhibited by SMARCB1 binding free C-MYC []. Biallelic SMARCB1 is characteristic of atypical Teratoid Rhabdoid Tumor (AT/RT), a rare pediatric central nervous system cancer []. HERV-K (HML-2) Env in turn increases JUN and pERK4/2 when overexpressed in Schwann cells [], while retroviral protease inhibitors ritonavir, atazanavir, and lopinavir reduced proliferation of schwannoma and grade I meningioma cells []. Furthermore, ERVW-1ε and ERVFRD-1ε were demonstrated to bind to mitochondria of chemo-resistant U87RETO glioblastoma cells. allowing for their direct transfer across cell membranes []. This process was confirmed to utilize the fusion capabilities of the ERVW-1ε and ERVFRD-1ε and might have a major role in the resistance to mitochondria-targeted drugs []. Antibodies targeting ERVW-1ε and ERVFRD-1ε proteins were observed to inhibit the direct cellular uptake of mitochondria, signifying new strategies to combat drug resistance in glioblastomas [].
12. HERVs in Prostate Cancer—The Dancing Partner of the Androgen Receptor
Prostate cancer is the most frequent cancer in males in Western countries and the second most common cancer in males worldwide []. Despite increased survival rates, curative treatments, such as surgery and radiation, convey serious side effects [], so that active surveillance becomes the preferred approach for men with less-aggressive prostate cancer. Directed therapies targeting HERVs that are reportedly dysregulated in prostate cancer cell lines and tissues present a milder approach for prostate cancer treatment. A prime candidate in prostate cancer is HERV-K, which exhibits tight interactions with the testosterone receptor. While certain HERV-Ks, such as HERV-K17 on chromosome 17p13.1 (not HGCN: ERVK-17), show tissue-specific upregulation in prostate cells and downregulation in malignant cells [,], other members of the family, such as HERV-K-Mel (HML-6) on chromosome 16p11.1 [] and HERV-K (HML-2) on chromosome 22q11.23 [,], display significantly higher expression in prostate cancer tissues and cell lines compared to healthy controls (Figure 9). This increased expression was identified to be androgen-dependent with several HERV-K (HML-2) LTRs containing predicted steroid hormone receptor-binding sites []. HERV-L LTR40a was shown to exhibit ligand-dependent recruitment of the androgen receptor (AR) functioning as an enhancer for KLK3 []. KLK3 is the most well-studied biomarker for prostate cancer and is a model system to study androgen signaling []. As prostate cancer cell survival is highly dependent upon AR signaling, LTR40a induction might provide an alternative marker for disease detection. Additionally, inhibition of the LTR40a and its associated gene products could prevent the synthesis of HERV-regulated oncogenes. Reciprocal to the activation of HERV LTRs by the AR, HERV-K (HML-2) Rec has been documented to associate with the AR co-repressor, TZFP (HGNC: ZBTB32), relieving the TZFP-mediated repression of AR-induced transactivation []. Furthermore, HERV-K (HML-2) Rec binding of TZFP was reported to mitigate the direct transcriptional repression of the MYC gene promoter []. HERV-K (HML-2) Rec was also observed to inhibit the cytoplasmic negative regulator of the androgen receptor SGTA and lead to its translocation to the nucleus []. This in turn was shown to result in a higher sensitivity of the AR to its ligand in the cytoplasm and increased expression of AR-induced oncogenes as well as HERV-K (HML-2) gene products [].
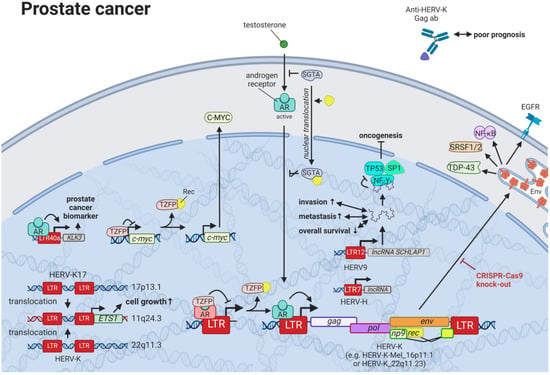
Figure 9.
The role of HERVs in prostate cancer. Evaluated treatments are marked in red. Abbreviations: AR = androgen receptor, ab = antibody, lncRNA = long non-coding RNA, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope, HERV-K = HML-2.
Targeted treatments might be especially beneficial for patients with advanced cancers, as HERV-K (HML-2) Gag antibodies are predictors of poor prognosis and correlate with prostate cancer progression [,,]. The increased antibody titers might very well indicate higher HERV-K (HML-2) protein levels, but there are no publications that have analyzed both seropositivity and HERV protein levels in the same patients. Interestingly, increased HERV-K (HML-2) expression has not only been associated with higher levels of the HERV antigen, but also with recurrent gene fusions []. While over half of prostate cancers display fusions involving the oncogenic ETS transcription factors (including ETS1), ETS1 has been described in several patients with prostate cancer involved in fusion with HERV-K17 [] and HERV-K_22q11.3 [,].
Similar to the HERV-K (HML-2) Env-mediated regulation of cellular pathways shown in breast cancer cell lines, Ibba et al. (2018) were able to demonstrate that CRISPR-Cas9-facilitated knockout of HERV-K (HML-2) env leads to significant decrease in genes implicated in prostate cell transformation such as EGFR, NF-κB, SRSF1/2, and TDP-43 (HGNC: TARDBP) []. Furthermore, several HERV LTR-driven lncRNAs are reported to hijack key TFs with tumor suppressor functions, such as TP53 and SP1 []. In particular, the HERV9 LTR-driven lncRNA SCHLAP1 was found to be upregulated in one-fourth of prostate cancers to act as an independent predictor of poor clinical outcomes and to play an essential role in tissue invasion and metastasis [,]. HERV-H provides another group of LTRs that function as promoter enhancers and lead to the transcription of lncRNAs []. Both activities are essential for human stem cells [,]—employing at times up to 40% of HERV-H sequences [], although an aberrant increase has been associated with cancer stem cell formation in many malignancies, one being prostate cancer [,]. Additionally, single-cell transcriptome analysis revealed LTR7/HERV-H network activation to be significantly increased in localized and metastatic prostate cancers compared to normal prostate, making it a putative treatment target [].
13. HERVs in Lung Cancer—The Love for Long Noncoding RNAs and Pseudogenes
Even though lung cancers are by far the deadliest cancers worldwide [] and carry an equally high mutational burden as melanomas [], only limited research on the relationship between HERVs and pulmonary diseases has been published. Being one of the leading causes of mutations in lung cancer, smoking was shown to induce HERV-K (HML-2 and 6) pol expression in multiple tissues (Figure 10) [,]. Insertional polymorphisms (absence and presence of a HERV sequences at a specific locus) can alternatively lead to differences in numerous regulatory elements at a specific locus and therefore have gained increased interest in the research community. Kayho et al. (2013) were able to demonstrate that HERV-K (HML-2)_soloLTR(1p13.2) homozygosity in never-smoker women is statistically associated with increased susceptibility to lung adenocarcinoma []. This finding is supported by the observation of a peptide-carrying sequence homology with HERV-K10 LTR specifically expressed in human lung adenocarcinoma A549 and absent in non-transformed fibroblasts []. The Krüppel-associated box domain-containing zinc-finger family protein (KZFP) is a transcriptional suppressor, which when expressed in cancer cells alters the expression of genes expression of genes related to the cell cycle and cell-matrix adhesion and suppresses cellular growth, migration, and invasion abilities [].
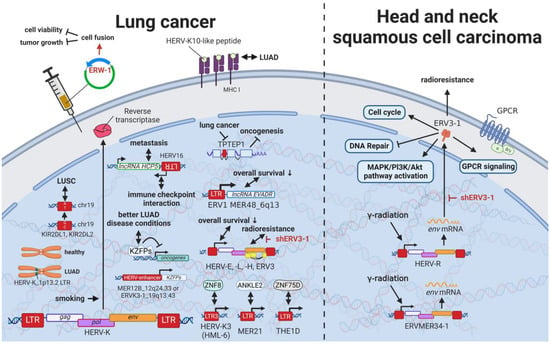
Figure 10.
The role of HERVs in lung, head, and neck cancer. Evaluated treatments are marked in red. Abbreviations: LUAD = lung adenocarcinoma, LUSC = lung squamous cell carcinoma, GPCR = G-protein-coupled receptor, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, env = envelope. If not otherwise stated HERV-K = HML-2.
Pan-cancer analysis revealed an association between HERV and KZFP expression []. In particular, CRISCR-Cas9-mediated knockout of the HERV-enhancer 1 on chromosome 12q24.33 (MER21B) and HERV-enhancer 2 on chromosome 19q13.43 (HERVK3-int, HGCN: ERVK3-1) resulted in decreased KZFP expression in lung adenocarcinoma (LUAD) cancer cell line A549 []. Overall increased KZFP expression was associated with better prognosis and lower cancer stage in patients with LUAD [], while another study demonstrated less favorable outcomes linked to increased HERV—in particular HERV-E, HERV-L, HERV-H, and ERV3 expression []. In general, HERV-E transcripts expressed in lung cancer were among the strongest predictors of outcome, whereas MER21 was associated with ANKLE2 expression, LTR3 from HERV-K3I (HML-6) with ZNF8 expression, and THE1D-int with ZNF75D expression []. Another pan-cancer study revealed several single nucleotide variants affecting KIR2DL1, KIR2DL1 (MST/MaLR) downregulation associated with lung squamous cell carcinoma (LUSC) [].
Intriguingly, in recent years, lncRNAs increasingly appeared to occupy a central position in the regulation of lung cancers. As first described, the ERV1 LTR MER48-driven lncRNA EVADR was detected in over 20% of patients with lung adenocarcinoma but undetected in normal lung tissues []. A combined statistical analysis of lung, pancreas, colon, rectal, and stomach adenocarcinomas indicated a significant association of EVADR expression with decreased survival rates []. Similarly, the regulatory lncRNA HCP5 was found overexpressed in lymph node metastasis of small cell lung cancer [,]. The HCP5 antisense HERV16 transcript in turn has been shown to interact with immune and regulatory checkpoints in various other cancers [,]. More interestingly, the TPTE pseudogene, containing a HERV-H element (TPTEP1), is downregulated in lung cancer compared to adjacent normal tissue samples []. It is known that in healthy tissues, the pseudogene is translated with the majority of the HERV sequence being spliced out, but the function of the resulting protein is still unknown []. For other roles of HERVs in lncRNA regulation, see HERVs in Other Genital Cancers (Ovary Cancer, Choriocarcinoma, and Endometrial Cancer)—The Ascent of New Possibilities. Supporting the potential use of ERVW-1ε in tumor therapies (see HERVs in Leukemia—The Lifesavers for Cancer Cells), Lin et al. (2010) were able to induce syncytia formation and, in this way, reduce cell viability and tumor growth by transfecting ERVW-1ε expression plasmids in human non-small cell lung cancer cells in vitro or by directly injecting the protein into tumors in mice [,]. Lee et al. (2016) reported that RNAi-mediated knockdown of ERV3-1 in radioresistant A549 cells increased radiosensitivity and induced apoptosis, suggesting its potential use as a drug target for new anticancer therapeutics [].
14. HERVs in Cancers of the Urinary System (Kidney and Bladder Cancer)—The Future Fire Fighters
While most HERVs operate below immune detection, research has shown that upregulation of HERVs in transformed cells can serve as a physiological tumor recognition signal, preventing the propagation of cancerous cells in early stages [,,,]. In advanced-stage cancers, such tumor suppressive functions are disrupted on multiple levels, one being through immune checkpoint activation []. Hence, newly developed immune checkpoint inhibitors have proven to be effective regimens for persistent cancers, especially for clear cell renal cell carcinoma (ccRCC), where clinically significant and robust responses have been observed []. To further enhance antitumor immune responses upon immune checkpoint blockade, Panda et al. (2018) examined HERVs as prospective inducible targets in patients with ccRCC []. The study investigators determined the subset of potentially immunogenic HERVs (piHERVs) with the greatest potential to induce immune responses, such as immune infiltration, higher cytotoxic T-cell levels, and M1 macrophage abundance (Figure 11) []. Despite lower overall survival of patients with higher expression of such HERVs, piHERVhigh patients treated with immune checkpoint inhibitors experienced significantly improved prognosis and treatment responsiveness compared to their piHERVlow counterparts []. Out of all piHERVs, HERV-R.3-2 env (ERV3-2) expression was particularly increased in responders compared to non-responders, highlighting its tumor suppressive functions mentioned for other cancers (see HERVs in Lymphoma—The Silent Inducers and HERVs in Other Genital Cancers (Ovary Cancer, Choriocarcinoma, and Endometrial Cancer)—The Ascent of New Possibilities) []. Interestingly, similar results have been observed for patients with urothelial cancer who displayed high piHERV expression []. Solovyov et al. (2018) confirmed the positive correlation of piHERV levels with overall survival, progression-free survival, and response to immune checkpoint inhibitors for urothelial cancer []. In this case, ERV3-1 was the most highly expressed HERV in responders [].
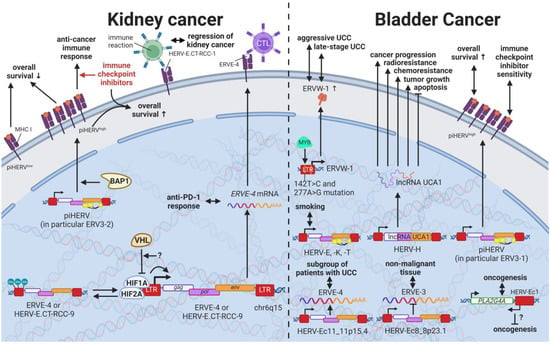
Figure 11.
The role of HERVs in cancers of the urinary system. Evaluated treatments are marked in red. Abbreviations: CTL = cytotoxic T cell, UCC = urothelial carcinoma, RCC = renal cell carcinoma, piHERV = potentially immunogenic HERVs, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, env = envelope, HERV-K = HML-2.
Several underlying mechanisms have been proposed to drive the inherently higher expression of piHERVs. On the genetic level, piHERVhigh patients demonstrated an enrichment of mutations in the BAP1 gene, which is a deubiquitinase known to functionally associate with chromatin modulators [,]. The functional disruption of BAP1 may thus lead to chromatin remodeling resulting in piHERV expression []. A subgroup of patients with ccRCC was found to express HERV-E in high amounts driven by a hypomethylated LTR []. Inactivation of the von Hippel–Lindau tumor suppressor gene (VHL) with subsequent stabilization of hypoxia-inducible transcription factors, HIF1A and -2A, was shown to induce expression of two HERV-E transcripts on chromosome 6q15 (HERV-E.CT-RCC-8 (HGCN: ERVE-4) and HERV-E.CT-RCC-9) through the HIF response element located in the viral LTR []. In other tumors and matched normal tissues, a hypermethylated LTR was reported to prevent the induction of the HERV-E.CT-RCCs []. Interestingly, recognition the HERV-E.CT-RCC-1 antigen, which carries a shorter sequence of the common region of HERV-E.CT-RCC-8 and -9, by T cells was associated with the regression of human kidney cancer following unrelated allogeneic stem cell transplantation, suggesting its potential as a target for antitumor therapies []. Strikingly, ERVE-4 expression was also able to predict the response to anti-PD-1 in metastatic ccRCC []. Additionally, the observation that ERVE-4- and hERV47000-derived epitopes were able elicit a tumor-restricted CD8+ T-cell response were the basis for an ongoing phase 1 trial evaluating the safety and efficacy of the infusion of ERVE-4 TCR-transduced CD8+/CD34+-enriched T cells (NCT03354390) []. In urothelial cell carcinoma (UCC), effects mediated by HERV-E have been found beneficial and detrimental dependent on the specific virus. For instance, HERV-Ec11 on chromosome 11p15.4 (HGCN: ERVE-2) was found to be expressed only in a subgroup of patients with UCC, while HERV-Ec8 on chromosome 8p23.1 (HGCN: ERVE-3) was detected only in non-malignant urothelial tissues []. Furthermore, HERV-Ec1 encoded on chromosome 1q31.1 has been observed to be located in antisense orientation to a cytosolic phospholipase A2 group IVA (PLA2G4A) that is dysregulated in many human tumors, and thus theorized to contribute to fine tuning of PLA2G4A and to have a potential role in oncogenesis of UCC [] (see also HERVs in Testicular Cancer—The Governors of Tumor Suppressor Genes).
Tobacco smoking has been recognized as an environmental risk factor for many cancers and is considered to be a main cause of bladder cancer [,]. HERV-E, -K, and -T have been indicated to be increased in the urothelium and a cell culture model of current smokers compared to non-smokers, making tobacco use a potential external factor related to HERV induction []. Additionally, HERV-K (HML-2) LTRs were reported to be strongly methylated in normal tissues and significantly hypomethylated in urothelial carcinomas resulting in higher HERV-K (HML-2) mRNA levels [,]. Other genetic risk factors for UCC include two mutations in the HERV-W LTR (142T > C and 277A > G) that have been identified to result in a new MYB-binding site []. Expression analysis confirmed that increased MYB binding at these sites caused significantly higher expression of ERVW-1ε, leading to amplified proliferation and viability of immortalized human uroepithelial cells []. Furthermore, pan-cancer analysis revealed several single nucleotide polymorphisms within HERVs significantly affecting ZNF99 []. Kidney cancer patients with a C2270G mutation in ZNF99 (HERV-W/HERV17/LTR17) were shown to have significantly lower survival []. In addition, ERVW-1ε overexpression was significantly associated with a late-stage and aggressive-form UCC in patients, suggesting that it may impact the degree of malignancy in UCC [].
The lncRNA urothelial carcinoma associated 1 (UCA1) is one of the best studied purely retroviral HERV gene products with major effects on cancer progression, tumor growth, apoptosis, invasion, radioresistance, chemoresistance, and metabolism []. UCA1 is a splice product of three exons located on chromosome 19p13.12 [] with three isoforms (1.7 kb: UCA1, 2.2 kb: UCA1a or CUDR, 2.7 kb: UCA1b) [,]. UCA1 was originally discovered to be upregulated in bladder transitional cell carcinoma (TCC) by Wang et al. in 2006 []. Meanwhile, it has been confirmed to be activated primarily during normal gestation with persistent expression in the heart and spleen [] and to be reactivated in every cancer type reviewed in this very report with the exception of lymphomas [,]. As it would exceed the scope of this review, we recommend the comprehensive summaries on the functions of UCA1 as regulator of miRNAs by Xuan et al. (2019) [], as chromatin remodeler in complex with other proteins by Neve et al. (2018) [], as a mediator of chemoresistance by Wang et al. (2017) [], and as a potential biomarker by Xue et al. (2016) [].
15. HERVs in Endocrine Cancers (Pancreas and Thyroid Cancer)—The Unknown Potential
Despite their rarity, endocrine tumors, particularly thyroid cancers, have dramatically increased in their incidence worldwide in the last four decades [,]. Additionally, anaplastic thyroid cancer [] and adrenocortical carcinoma [] have incredibly short survival times due to their aggressive nature, while pancreatic ductal adenocarcinoma is often associated with increased risk for metastasis and high mortality rates due to a lack of symptoms in early stages [,]. In pancreatic cancer, HERV-K (HML-2) has been suggested as a major tumor driver. Li et al. (2017) reported HERV-K (HML-2) env, gag, and np9 genes as well as HERV-K (HML-2) Env protein expression to be significantly increased in pancreatic cancer cell lines as well as patients with pancreatic cancer compared to healthy controls (Figure 7) []. The RNAi-mediated knockdown of HERV-K (HML-2) env reduced proliferation and colony formation of the cancer cell lines and resulted in decreased tumor growth and metastasis in an in vivo mouse model []. Pathway analysis by Li et al. revealed an activation of the RAS/MEK/ERK pathway and inhibition of TP53 by HERV-K (HML-2) in pancreatic cancer cells [], which was confirmed in breast cancer cells by Lemaître et al. (2017) (see Results HERVs in Breast Cancer—The Rise of New Biomarkers) []. Furthermore, the cleavage of S100A4 by the HERV-K (HML-2) protease was associated with cell cycle progression, differentiation, and metastasis in pancreatic cancer []. Moreover, Li et al. detected increased expression of ERV3-1 and ERV3-2 transcripts in prostate cancer cell lines []. These transcripts displayed an intact open reading frame in most cell lines, potentially giving rise to active Env proteins []. Interestingly, Li et al. also observed the release of viral-like particles with increased RT activities by pancreatic cancer cell lines Panc-1 and Panc-2, suggesting the potential horizontal transfer of viral proteins [].
In addition to HERV-K (HML-2) Env, HERV-H Env was described to contribute to the oncogenesis of pancreatic cancers. HERV-H on chromosome 3q26 is one of the few from the family that contain a complete open reading frame for the Env protein (HGCN: ERVH-9ε) []. This ERVH-9ε protein, also called Env60, has been upregulated in pancreatic cancer cells undergoing epithelial-to-mesenchymal transition (EMT), while also expressed in healthy pancreatic tissue []. Particularly, the immunosuppressive portion of the ERVH-9ε protein amplified EMT and induced CCL19 expression, which significantly correlated with the recruitment of immunosuppressive cells in patients []. In contrast, RNAi-mediated knockdown of HERV-H significantly decreased tumor invasion, CCL19 production, and the recruitment of immunosuppressive cells without affecting cell proliferation, suggesting the central role of ERVH-9ε in immune regulation []. In another study, HERV-H_Xp22.3 (HGCN: ERVH-2) was reported to be upregulated in 2 of 12 pancreatic cancers []. However, ERVH-2 showed higher correlations with colorectal cancers being overexpressed in almost half the tumors (see also HERVs in Other Genital Cancers (Ovary Cancer, Choriocarcinoma, and Endometrial Cancer)—The Ascent of New Possibilities) [,,]. Nonetheless, functional consequences of the overexpression of ERVH-2 must still be determined.
Unique to endocrine tissues is the high baseline expression of HERVs in non-malignant cells. For instance, ERVW-1ε was demonstrated to exhibit constitutive expression in normal pancreatic tissues, while reduced expression was detected in pancreatic adenocarcinoma []. Furthermore, two specific HERV-E transcripts derived from chromosome 17q11 were solely detected in healthy pancreatic and thyroid tissues and absent in other healthy cells (Figure 7) []. Particularly, the thyroid gland was shown to display high HERV expression levels in normal thyroid tissue, indicating possible physiological functions []. Interestingly, the thyroid is the only human tissue to express envT (HGCN: ERVS71-1ε), an env gene not produced in the placenta []. As such, ERVS71-1ε is an exceptional candidate to analyze the potential oncogenic roles of SNPs in the development of thyroid cancers as it shows a lack of conservation in primates and no implied essential physiological functions []. Along these lines, an analysis of disease-associated SNPs by Wallace et al. (2018) discovered several polymorphic HERV-K (HML-2) insertion sites uniquely associated with thyroid malignancies []. Further supporting the potential oncogenic role of polymorphic HERVs is the finding of the RT inhibitor nevirapine decreasing the proliferation and increasing the differentiation of human thyroid anaplastic carcinoma reported in a case study [,,]. In contrast to its beneficial effects in ccRCC (see HERVs in Cancers of the Urinary System (Kidney and Bladder Cancer)), HHLA2 expression was observed to be associated with poor survival in patients with papillary thyroid cancer (PTC) []. HHLA2 displayed a tumor promoter function that enhanced the proliferation of PTC cells []. Altogether, endocrine cancers display unique features that call for additional evaluation to further expand the understanding of the relationship between HERVs and cancer.
16. HERVs in Other Cancers (Osteosarcoma, Head and Neck Squamous Cell Carcinoma)—The Hodgepodge of Hope for Novel Therapies
Rare cancers, by definition, only provide limited case numbers for investigation. Accordingly, only single reports of HERVs evaluated in such cancers are available. Despite a high incidence in children, osteosarcoma is a rare malignancy in adults []. We identified only a single study on human osteosarcoma reporting the statistically significant upregulation of 35 and downregulation of 47 HERV mRNAs in osteosarcoma tissues compared to healthy controls []. The most significant HERV elements differentially expressed included LTRs of the HERV-L, HERV-K (HML-2), and ERV-1 []. The study lays the foundation for the identification of tumor-specific viral target for vaccine strategies. Proof of concept for such a strategy is the AH1 peptide (a murine endogenous retroviral (MuERV) envelope protein-derived peptide) that has been documented as a potent vaccine against murine WEHI-164 fibrosarcoma, eliciting a potent CD8+ T-cell response [,]. In addition, MuERV envelope glycoprotein gp90 has been shown to be tumor specific to murine colon carcinoma [].
Head and neck squamous cell carcinomas (HNSCC) comprise 90% of all head and neck cancers and are relatively common [,]. HNSCCs are often inoperable due to the complex anatomy, making radio- and chemotherapy the only option []. Accordingly, radioresistance poses a major problem resulting in very low survival rates [,]. Findings by Michna et al. (2016) documenting an induction of ERV3-1 and ERVMER34-1 env upon exposure of HNSCC cell lines to γ-radiation indicate a potential target to overcome radioresistance (Figure 10) []. The study investigators identified putative interactions of induced ERV3-1 with genes associated with radiation response, such as GPCR signaling, transmembrane transport of small molecules, generic transcription pathway, signaling by Rho GTPases, DNA repair, CD28-dependent Pi3K/Akt signaling, and cell cycle [] This hypothesized role of ERV3-1 is further supported by a study reporting increased levels of ERV3-1 in radioresistant A549 lung cancer cells but not in less radioresistant H460 cells []. The same study observed an increase in radiosensitivity and apoptosis upon RNAi-mediated knockdown of ERV3-1 in A549 cells [].
17. Discussion of Novel Options for Cancer Treatment Facilitated by HERVs
As described in this review, many studies have demonstrated increased HERV levels in tumor cell lines and tumor tissues compared to normal healthy tissues, suggesting two potential treatment approaches. On the one hand, strategies have been proposed that target pathways in which HERVs are involved [,,], as outlined for various cancers above. HERVs might provide another pharmacological target in this way. Moreover, HERV-derived HERV restriction factors such as suppressyn (HGCN: ERVH48-1), a HERV-F-derived inhibitor of ERVW-1ε-mediated fusion, might serve as a starting point for drug development. However, discoveries of HERV genes and LTRs involved in regulatory mechanisms are very new and still advancing with the recent development of more accurate and affordable sequencing techniques. Accordingly, we expect to see the discovery of more detailed cancer-specific signaling networks that include HERVs in the near future.
On the other hand, treatment strategies targeting HERV proteins as tumor-specific antigens have been suggested [,], assuming HERV expression is a consequence of transcriptional changes in tumors. HERVs as cancer-specific antigens in hematological cancers appear to be particularly promising. Saini et al. (2020) found HERV-specific T cells are present in 17 of the 34 patients with leukemia, recognizing 29 HERV-derived peptides representing 18 different HERV loci, among which ERVH-5, ERVW-1, and ERVE-3 had the strongest responses []. Furthermore, the ancestral retroviral HEMO envelope gene (Human Endogenous MER34 ORF) is hailed as a pan-cancer target for leukemia, lung, adrenal, thyroid, breast, ovarian, uterus, cervical, prostate, esophagus, stomach, colon, liver, pancreas, renal, bladder, brain, and skin cancer []. Vaccinations of mice with HERV epitopes were shown to be safe and able to generate tumor-specific immune cells [,,,]; however, the cancer risk-reducing properties of human vaccines contaminated with HERVs have yet to be confirmed or associated with HERVs []. Nonetheless, the advent of CAR-T cells [] and checkpoint blockade inhibitors [] might offer additional options to enhance tumor-specific immunogenicity. The generation of HERV-specific T cells recognizing tumors was demonstrated in a proof-of-concept study by Bonaventura et al. (2022), gleaning further enthusiasm for the potential use in targeted therapies [].
Furthermore, HERV-H LTR-associating proteins 1 and 2 (HHLA1 and HHLA2) on chromosomes 3q13.13 and 8q24.22 have been shown to carry immune checkpoint functions []. First described by Mager et al. in 1999, HHLA1 and HHLA2 are both members of the B7 family and obtain their polyadenylation signal through HERV-H LTR regions [,], thus revealing a control mechanism of viral origin []. While both proteins are part of oncogenic signaling pathways, HHLA2 has been detected in several human cancers []. HHLA2 was found to be overexpressed in basal breast cancer [], triple-negative breast cancer [], colorectal cancer [], lung cancer [,,], liver cancer [], bladder urothelial carcinoma [], ccRCC [,,], pancreatic cancer [,,], osteosarcoma [], oral squamous cell carcinoma [], and many other cancers [,] compared to adjacent normal tissue or healthy controls. Additionally, elevated HHLA2 protein levels were associated with tumor size, tumor stage, lymph node metastasis, and low relapse-free and overall survival in these cancers [,,,,,,,,,,,,,,]. Thus, HHLA2 might not only become a suitable prognostic marker, as shown by Zhang et al. (2021) in a Chinese cohort [], but also a potential immunotherapy target for patients who do not respond to other immune checkpoint inhibitors, such as PD-1 inhibitors. Surprisingly though, HHLA2 mRNA levels in the blood were described by Shimonosono et al. (2018) to be downregulated in patients with gastric cancer []. Furthermore, lower HHLA2 expression in the blood of patients with gastric cancer had a significant correlation with the depth of tumor invasion, poorer survival rates, distant metastasis, and tumor-node-metastasis (TNM) stage []. Correspondingly, HHLA2 expression in renal cancers was identified as higher in low pathological grades than in high pathological grades, suggesting its protective capabilities []. In addition, hypomethylation of HHLA2 was indicative of a more favorable outcome []. First attempts to use HHLA2 as treatment target demonstrated siRNA-mediated knockdown of HHLA2, resulting in the inhibition of NSCLC and reduced HCC in mice []. Additionally, Bhatt et al. (2020) were successful in developing HHLA2-targeting antibodies that specifically block its immunoinhibitory activity []. Overall, HHLA2 is a promising target with wide applicability.
In 1997, Perron et al. first described retroviral particles, which were later termed MS-associated retroviruses (MSRVs), in the leptomeningeal tissue of MS patients []. Subsequent studies of the MSRV confirmed high homology to the HERV-W family, which could be explained by the recombination of a HERV-W sequence on chromosome Xq22.3 and another defective HERV-W env on chromosome 5 []. Functional studies of the MRSV-Env protein, frequently detected in the blood and lesions of MS patients, identified it as having strong agonist functions on Toll-like receptor 4 (TLR4), leading to neuroinflammatory effects []. Accordingly, the Institut Mérieux group, INSERM, and GeNeuro developed the MSRV-Env-targeting antibody GNbAC1 (now, Temelimab), which recently completed phase IIb clinical trials for the treatment of MS []. Clinical trials showed minimal side effects and good tolerance [] as well as clinical improvements in patients with MS []. While Temelimab failed to show an effect on features of acute inflammation, it demonstrated preliminary radiological signs of possible anti-neurodegenerative effects for patients who had taken the highest dose []. Patients had less T1-hypointense lesion (associated with MS disability and progression), a reduction in brain tissue loss, and improvement in MRI markers of remyelination, suggesting that Temelimab might promote remyelination and prevent loss of nerves []. Additionally, Temelimab proved to be well-tolerated in a phase I trial for the treatment of type I diabetes []. As mentioned above, ERVW-1ε, also known as Syncytin-1, is currently the most promising HERV-related cancer therapeutic target. While clinical trials of Temelimab modeled the capabilities of HERV-W-targeting drugs in general, cross-reactivity studies of Temelimab in particular indicated binding of the antibody to ERVW-1ε at high concentrations, suggesting the possible use of Temelimab in cancers []. In conclusion, clinical studies of Temelimab show promising results laying the foundation for development and use of HERV-targeting antibodies in other diseases such as cancers.
In summary, HERVs are involved in various homeostatic and pathogenic pathways with potential effects on cancer development and progression. The usage of HERVs themselves as therapeutic agents, as well as the HERV proteins as tumor-specific targets, are promising but must be further evaluated to exclude any undesired side effects. Therefore, this review aimed to comprehensively overview HERV involvement in different cancers to provide a summary of the research possibilities.
Author Contributions
Conceptualization, E.S. and M.E.S.; methodology, E.S.; software, E.S. and M.E.S.; validation, E.S.; formal analysis, E.S.; investigation, E.S.; resources, M.E.S.; data curation, E.S.; writing—original draft preparation, E.S.; writing—review and editing, E.C.P.-G., E.S. and M.E.S.; visualization, E.S.; supervision, M.E.S.; project administration, M.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hartung, H.-P.; Derfuss, T.; Cree, B.A.; Sormani, M.P.; Selmaj, K.; Stutters, J.; Prados, F.; MacManus, D.; Schneble, H.-M.; Lambert, E.; et al. Efficacy and safety of temelimab in multiple sclerosis: Results of a randomized phase 2b and extension study. Mult. Scler. J. 2022, 28, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Nali, L.H.; Olival, G.S.; Montenegro, H.; da Silva, I.T.; Dias-Neto, E.; Naya, H.; Spangenberg, L.; Penalva-De-Oliveira, A.C.; Romano, C.M. Human endogenous retrovirus and multiple sclerosis: A review and transcriptome findings. Mult. Scler. Relat. Disord. 2021, 57, 103383. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Zhou, P.; Yan, Q.; Wu, X.; Xia, Y.; Li, W.; Li, X.; Zhu, F. ERVWE1 Reduces Hippocampal Neuron Density and Impairs Dendritic Spine Morphology through Inhibiting Wnt/JNK Non-Canonical Pathway via miR-141-3p in Schizophrenia. Viruses 2023, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Li, W.; Yan, Q.; Zhou, P.; Xia, Y.; Yao, W.; Zhu, F. HERV-W ENV Induces Innate Immune Activation and Neuronal Apoptosis via linc01930/cGAS Axis in Recent-Onset Schizophrenia. Int. J. Mol. Sci. 2023, 24, 3000. [Google Scholar] [CrossRef] [PubMed]
- Curtin, F.; Champion, B.; Davoren, P.; Duke, S.; Ekinci, E.; Gilfillan, C.; Morbey, C.; Nathow, T.; O’Moore-Sullivan, T.; O’Neal, D.; et al. A safety and pharmacodynamics study of temelimab, an antipathogenic human endogenous retrovirus type W envelope monoclonal antibody, in patients with type 1 diabetes. Diabetes Obes. Metab. 2020, 22, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Levet, S.; Charvet, B.; Bertin, A.; Deschaumes, A.; Perron, H.; Hober, D. Human Endogenous Retroviruses and Type 1 Diabetes. Curr. Diabetes Rep. 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- HERV-E TCR Transduced Autologous T Cells in People With Metastatic Clear Cell Renal Cell Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT03354390 (accessed on 26 January 2021).
- Dolei, A.; Uleri, E.; Ibba, G.; Caocci, M.; Piu, C.; Serra, C. The aliens inside human DNA: HERV-W/MSRV/syncytin-1 endogenous retroviruses and neurodegeneration. J. Infect. Dev. Ctries. 2015, 9, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Bannert, N.; Kurth, R. Retroelements and the human genome: New perspectives on an old relation. Proc. Natl. Acad. Sci. USA 2004, 101, 14572–14579. [Google Scholar] [CrossRef] [PubMed]
- Deininger, P.L.; Batzer, M.A. Mammalian retroelements. Genome Res. 2002, 12, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, D.; Steffen, I.; Pohlmann, S. Cellular entry of retroviruses. Adv. Exp. Med. Biol. 2013, 790, 128–149. [Google Scholar] [CrossRef] [PubMed]
- Tongyoo, P.; Avihingsanon, Y.; Prom-On, S.; Mutirangura, A.; Mhuantong, W.; Hirankarn, N. EnHERV: Enrichment analysis of specific human endogenous retrovirus patterns and their neighboring genes. PLoS ONE 2017, 12, e0177119. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S. Genomic impact, chromosomal distribution and transcriptional regulation of HERV elements. Mol. Cells 2012, 33, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Crosslin, D.R.; Carrell, D.S.; Burt, A.; Kim, D.S.; Underwood, J.G.; Hanna, D.S.; Comstock, B.A.; Baldwin, E.; de Andrade, M.; Kullo, I.J.; et al. Genetic variation in the HLA region is associated with susceptibility to herpes zoster. Genes Immun. 2015, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kassiotis, G. Endogenous retroviruses and the development of cancer. J. Immunol. 2014, 192, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory activities of transposable elements: From conflicts to benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, M.; Tanabe, K.; Sutou, K.; Teramoto, I.; Sawamura, Y.; Narita, M.; Nakamura, M.; Tokunaga, Y.; Nakamura, M.; Watanabe, A.; et al. Dynamic regulation of human endogenous retroviruses mediates factor-induced reprogramming and differentiation potential. Proc. Natl. Acad. Sci. USA 2014, 111, 12426–12431. [Google Scholar] [CrossRef] [PubMed]
- Durruthy-Durruthy, J.; Sebastiano, V.; Wossidlo, M.; Cepeda, D.; Cui, J.; Grow, E.J.; Davila, J.; Mall, M.; Wong, W.H.; Wysocka, J.; et al. The primate-specific noncoding RNA HPAT5 regulates pluripotency during human preimplantation development and nuclear reprogramming. Nat. Genet. 2016, 48, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Frendo, J.-L.; Olivier, D.; Cheynet, V.; Blond, J.-L.; Bouton, O.; Vidaud, M.; Rabreau, M.; Evain-Brion, D.; Mallet, F. Direct Involvement of HERV-W Env Glycoprotein in Human Trophoblast Cell Fusion and Differentiation. Mol. Cell. Biol. 2003, 23, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Soygur, B.; Sati, L. The role of syncytins in human reproduction and reproductive organ cancers. Reproduction 2016, 152, R167–R178. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.N.; Rosenberg, M.P.; Snow, C.M.; Samuelson, L.C.; Meisler, M.H. Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes Dev. 1992, 6, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Gogvadze, E.; Stukacheva, E.; Buzdin, A.; Sverdlov, E. Human-specific modulation of transcriptional activity provided by endogenous retroviral insertions. J. Virol. 2009, 83, 6098–6105. [Google Scholar] [CrossRef] [PubMed]
- Emera, D.; Casola, C.; Lynch, V.J.; Wildman, D.E.; Agnew, D.; Wagner, G.P. Convergent Evolution of Endometrial Prolactin Expression in Primates, Mice, and Elephants Through the Independent Recruitment of Transposable Elements. Mol. Biol. Evol. 2012, 29, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Suntsova, M.; Garazha, A.; Ivanova, A.; Kaminsky, D.; Zhavoronkov, A.; Buzdin, A. Molecular functions of human endogenous retroviruses in health and disease. Cell. Mol. Life Sci. 2015, 72, 3653–3675. [Google Scholar] [CrossRef] [PubMed]
- Tuan, D.; Pi, W. In Human Beta-Globin Gene Locus, ERV-9 LTR Retrotransposon Interacts with and Activates Beta- but Not Gamma-Globin Gene. Blood 2014, 124, 2686. [Google Scholar] [CrossRef]
- Chen, T.; Meng, Z.; Gan, Y.; Wang, X.; Xu, F.; Gu, Y.; Xu, X.; Tang, J.; Zhou, H.; Zhang, X.; et al. The viral oncogene Np9 acts as a critical molecular switch for co-activating β-catenin, ERK, Akt and Notch1 and promoting the growth of human leukemia stem/progenitor cells. Leukemia 2013, 27, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Heyne, K.; Kölsch, K.; Bruand, M.; Kremmer, E.; Grässer, F.A.; Mayer, J.; Roemer, K. Np9, a cellular protein of retroviral ancestry restricted to human, chimpanzee and gorilla, binds and regulates ubiquitin ligase MDM2. Cell Cycle 2015, 14, 2619–2633. [Google Scholar] [CrossRef] [PubMed]
- Jern, P.; Coffin, J.M. Effects of retroviruses on host genome function. Annu. Rev. Genet. 2008, 42, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; Eiges, R.; Gaudet, F.; Jaenisch, R.; Eden, A. Activation and transposition of endogenous retroviral elements in hypomethylation induced tumors in mice. Oncogene 2007, 27, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Jolicoeur, P. Retroviral Pathogenesis. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, H.E., Eds.; Cold Spring Harbor: New York, NY, USA, 1997. [Google Scholar]
- Fan, H.; Johnson, C. Insertional Oncogenesis by Non-Acute Retroviruses: Implications for Gene Therapy. Viruses 2011, 3, 398–422. [Google Scholar] [CrossRef] [PubMed]
- Wildschutte, J.H.; Williams, Z.H.; Montesion, M.; Subramanian, R.P.; Kidd, J.M.; Coffin, J.M. Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proc. Natl. Acad. Sci. USA 2016, 113, E2326–E2334. [Google Scholar] [CrossRef] [PubMed]
- Marchi, E.; Kanapin, A.; Magiorkinis, G.; Belshaw, R. Unfixed Endogenous Retroviral Insertions in the Human Population. J. Virol. 2014, 88, 9529–9537. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Iskow, R.; Yang, L.; Gokcumen, O.; Haseley, P.; Luquette, L.J., 3rd; Lohr, J.G.; Harris, C.C.; Ding, L.; Wilson, R.K.; et al. Landscape of Somatic Retrotransposition in Human Cancers. Science 2012, 337, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.H.; Boeke, J.D. Human Transposon Tectonics. Cell 2012, 149, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Goodier, J.L.; Kazazian, H.H., Jr. Retrotransposons Revisited: The Restraint and Rehabilitation of Parasites. Cell 2008, 135, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Hancks, D.C.; Kazazian, H.H., Jr. Active human retrotransposons: Variation and disease. Curr. Opin. Genet. Dev. 2012, 22, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Sciamanna, I.; Gualtieri, A.; Cossetti, C.; Osimo, E.F.; Ferracin, M.; Macchia, G.; Aricò, E.; Prosseda, G.; Vitullo, P.; Misteli, T.; et al. A tumor-promoting mechanism mediated by retrotransposon-encoded reverse transcriptase is active in human transformed cell lines. Oncotarget 2013, 4, 2271–2287. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, J.; Zhu, F. Human Endogenous Retroviral Envelope Protein Syncytin-1 and Inflammatory Abnormalities in Neuropsychological Diseases. Front. Psychiatry 2018, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. HERV Envelope Proteins: Physiological Role and Pathogenic Potential in Cancer and Autoimmunity. Front. Microbiol. 2018, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Hurst, T.P.; Magiorkinis, G. Epigenetic Control of Human Endogenous Retrovirus Expression: Focus on Regulation of Long-Terminal Repeats (LTRs). Viruses 2017, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, J.; Fang, H.; Li, L.; Sun, J. Relevance Function of Linc-ROR in the Pathogenesis of Cancer. Front. Cell Dev. Biol. 2020, 8, 696. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. Type W Human Endogenous Retrovirus (HERV-W) Integrations and Their Mobilization by L1 Machinery: Contribution to the Human Transcriptome and Impact on the Host Physiopathology. Viruses 2017, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Karlsson, H. Expression and regulation of human endogenous retrovirus W elements. Apmis 2016, 124, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Le Dantec, C.; Vallet, S.; Brooks, W.H.; Renaudineau, Y. Human Endogenous Retrovirus Group E and Its Involvement in Diseases. Viruses 2015, 7, 1238–1257. [Google Scholar] [CrossRef] [PubMed]
- Höhn, O.; Hanke, K.; Bannert, N. HERV-K(HML-2), the Best Preserved Family of HERVs: Endogenization, Expression, and Implications in Health and Disease. Front. Oncol. 2013, 3, 246. [Google Scholar] [CrossRef] [PubMed]
- Buzdin, A.A.; Prassolov, V.; Garazha, A.V. Friends-Enemies: Endogenous Retroviruses Are Major Transcriptional Regulators of Human DNA. Front. Chem. 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Babaian, A.; Mager, D.L. Endogenous retroviral promoter exaptation in human cancer. Mob. DNA 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Weckselblatt, B.; Rudd, M.K. Human Structural Variation: Mechanisms of Chromosome Rearrangements. Trends Genet. 2015, 31, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, J.Q.; Zheng, S. Expressional activation and functional roles of human endogenous retroviruses in cancers. Rev. Med. Virol. 2019, 29, e2025. [Google Scholar] [CrossRef] [PubMed]
- Bannert, N.; Hofmann, H.; Block, A.; Hohn, O. HERVs New Role in Cancer: From Accused Perpetrators to Cheerful Protectors. Front. Microbiol. 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, C.; Balestrieri, E.; Argaw-Denboba, A.; Sinibaldi-Vallebona, P. Human endogenous retroviruses role in cancer cell stemness. Semin. Cancer Biol. 2018, 53, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Kassiotis, G.; Stoye, J.P. Making a virtue of necessity: The pleiotropic role of human endogenous retroviruses in cancer. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160277. [Google Scholar] [CrossRef] [PubMed]
- Salmons, B.; Lawson, J.S.; Günzburg, W.H. Recent developments linking retroviruses to human breast cancer: Infectious agent, enemy within or both? J. Gen. Virol. 2014, 95, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kaye, S.; Gore, M.; McClure, M.; Bunker, C. The role of human endogenous retroviruses in melanoma. Br. J. Dermatol. 2009, 161, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Stacey, K.J.; Sagulenko, V. A clear link between endogenous retroviral LTR activity and Hodgkin’s lymphoma. Cell Res. 2010, 20, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Schulz, W.A. Does HERV-K represent a potential therapeutic target for prostate cancer? Expert Opin. Ther. Targets 2017, 21, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Grabski, D.F.; Hu, Y.; Sharma, M.; Rasmussen, S.K. Close to the Bedside: A Systematic Review of Endogenous Retroviruses and Their Impact in Oncology. J. Surg. Res. 2019, 240, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, B. Pubmed-Batch-Download. Version 3.0.0: GitHub repository. 2019. Available online: https://github.com/billgreenwald/Pubmed-Batch-Download (accessed on 17 June 2019).
- Stricker, E.; Scheurer, M.E. PDF Data Extractor (PDE)—A Free Web Application and R Package Allowing the Extraction of Tables from Portable Document Format (PDF) Files and High-Throughput Keyword Searches of Full-Text Articles. bioRxiv 2021. [Google Scholar] [CrossRef]
- Global Cancer Facts & Figures 2021 American Cancer Society. 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 15 July 2021).
- Sledge, G.W.; Mamounas, E.P.; Hortobagyi, G.N.; Burstein, H.J.; Goodwin, P.J.; Wolff, A.C. Past, Present, and Future Challenges in Breast Cancer Treatment. J. Clin. Oncol. 2014, 32, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, S.; Goudarzi, H.; Faghihloo, E. Evaluating the expression level of HERV-K env, np9, rec and gag in breast tissue. Infect. Agents Cancer 2019, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Frost, A.R.; Jian, B.; Epp, L.; Lu, D.W.; Johanning, G.L. Quantitation of HERV-K env gene expression and splicing in human breast cancer. Oncogene 2003, 22, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Golan, M.; Hizi, A.; Resau, J.H.; Yaal-Hahoshen, N.; Reichman, H.; Keydar, I.; Tsarfaty, I. Human endogenous retrovirus (HERV-K) reverse transcriptase as a breast cancer prognostic marker. Neoplasia 2008, 10, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Rycaj, K.; Geng, S.; Li, M.; Plummer, J.B.; Yin, B.; Liu, H.; Xu, X.; Zhang, Y.; Yan, Y.; et al. Expression of Human Endogenous Retrovirus Type K Envelope Protein is a Novel Candidate Prognostic Marker for Human Breast Cancer. Genes Cancer 2011, 2, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Montesion, M.; Williams, Z.H.; Subramanian, R.P.; Kuperwasser, C.; Coffin, J.M. Promoter expression of HERV-K (HML-2) provirus-derived sequences is related to LTR sequence variation and polymorphic transcription factor binding sites. Retrovirology 2018, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.K.; Mourad, M.; Kaplan, M.H.; Chefetz, I.; Malek, S.N.; Buckanovich, R.; Markovitz, D.M.; Contreras-Galindo, R. The Genomic Landscape of Centromeres in Cancers. Sci. Rep. 2019, 9, 11259. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Radvanyi, L.; Rycaj, K.; Plummer, J.B.; Yan, P.; Sastry, K.J.; Piyathilake, C.J.; Hunt, K.K.; Johanning, G.L. Human endogenous retrovirus K triggers an antigen-specific immune response in breast cancer patients. Cancer Res. 2008, 68, 5869–5877. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; De Cubas, A.A.; Stein, M.; Riedlinger, G.; Kra, J.; Mayer, T.; Smith, C.C.; Vincent, B.G.; Serody, J.S.; Beckermann, K.E.; et al. Endogenous retrovirus expression is associated with response to immune checkpoint blockade in clear cell renal cell carcinoma. JCI Insight 2018, 3, e121522. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Li, M.; Esteva, F.; Hess, K.R.; Yin, B.; Rycaj, K.; Plummer, J.B.; Garza, J.G.; Ambs, S.; Johanning, G.L. Human endogenous retrovirus type K antibodies and mRNA as serum biomarkers of early-stage breast cancer. Int. J. Cancer 2014, 134, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, D.-W.; Kang, Y.-J.; Ock, M.-S.; Eo, J.-W.; Choi, Y.-H.; Kim, W.-J.; Leem, S.-H.; Yi, J.-M.; Kim, H.-S.; Cha, H.-J. Expression of human endogenous retrovirus env genes in the blood of breast cancer patients. Int. J. Mol. Sci. 2014, 15, 9173–9183. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Rycaj, K.; Plummer, J.B.; Li, M.; Yin, B.; Frerich, K.; Garza, J.G.; Shen, J.; Lin, K.; Yan, P.; et al. Immunotherapeutic potential of anti-human endogenous retrovirus-K envelope protein antibodies in targeting breast tumors. J. Natl. Cancer Inst. 2012, 104, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, M.; Wei, Y.; Lin, K.; Lu, Y.; Shen, J.; Johanning, G.L.; Wang-Johanning, F. Activation of HERV-K Env protein is essential for tumorigenesis and metastasis of breast cancer cells. Oncotarget 2016, 7, 84093–84117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Krishnamurthy, J.; Wei, Y.; Li, M.; Hunt, K.K.; Johanning, G.L.; Cooper, L.J.; Wang-Johanning, F. Chimeric antigen receptor T cells targeting HERV-K inhibit breast cancer and its metastasis through downregulation of Ras. Oncoimmunology 2015, 4, e1047582. [Google Scholar] [CrossRef] [PubMed]
- Lemaître, C.; Tsang, J.; Bireau, C.; Heidmann, T.; Dewannieux, M. A human endogenous retrovirus-derived gene that can contribute to oncogenesis by activating the ERK pathway and inducing migration and invasion. PLoS Pathog. 2017, 13, e1006451. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hong, C.; Zhang, B.; Lowdon, R.F.; Xing, X.; Li, D.; Zhou, X.; Lee, H.J.; Maire, C.L.; Ligon, K.L.; et al. DNA hypomethylation within specific transposable element families associates with tissue-specific enhancer landscape. Nat. Genet. 2013, 45, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Armbruester, V.; Sauter, M.; Roemer, K.; Best, B.; Hahn, S.; Nty, A.; Schmid, A.; Philipp, S.; Mueller, A.; Mueller-Lantzsch, N. Np9 Protein of Human Endogenous Retrovirus K Interacts with Ligand of Numb Protein X. J. Virol. 2004, 78, 10310–10319. [Google Scholar] [CrossRef] [PubMed]
- Downey, R.F.; Sullivan, F.J.; Wang-Johanning, F.; Ambs, S.; Giles, F.J.; Glynn, S.A. Human endogenous retrovirus K and cancer: Innocent bystander or tumorigenic accomplice? Int. J. Cancer 2015, 137, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Pear, W.S.; Aster, J.C. The multifaceted role of Notch in cancer. Curr. Opin. Genet. Dev. 2007, 17, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, M.; Odorcic, S.; Chang, L.; Zhang, H.; Miller, N.; McCready, D.R.; Lockwood, G.; Egan, S.E. High-level Coexpression of JAG1 and NOTCH1 Is Observed in Human Breast Cancer and Is Associated with Poor Overall Survival. Cancer Res. 2005, 65, 8530–8537. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Del Valle, L.; Miley, W.; Whitby, D.; Ochoa, A.C.; Flemington, E.K.; Qin, Z. Transactivation of human endogenous retrovirus K (HERV-K) by KSHV promotes Kaposi’s sarcoma development. Oncogene 2018, 37, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, H.; Qu, S.; Miao, X.; Zhang, J. CD147 regulates vascular endothelial growth factor-A expression, tumorigenicity, and chemosensitivity to curcumin in hepatocellular carcinoma. IUBMB Life 2008, 60, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Nakada, M.T.; Kesavan, P.; McCabe, F.; Millar, H.; Rafferty, P.; Bugelski, P.; Yan, L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005, 65, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Trillo-Tinoco, J.; Chen, Y.; Bonstaff, K.; Del Valle, L.; Parsons, C.; Ochoa, A.C.; Zabaleta, J.; Toole, B.P.; Qin, Z. CD147 and downstream ADAMTSs promote the tumorigenicity of Kaposi’s sarcoma-associated herpesvirus infected endothelial cells. Oncotarget 2016, 7, 3806–3818. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, X.-E.; Jiang, Y.-Z.; Liu, Y.-R.; Sun, W.; Guo, Y.-J.; Ren, Y.-X.; Zuo, W.-J.; Hu, X.; Huang, S.-L.; et al. The endogenous retrovirus-derived long noncoding RNA TROJAN promotes triple-negative breast cancer progression via ZMYND8 degradation. Sci. Adv. 2019, 5, eaat9820. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Harashima, N.; Kajigaya, S.; Yokoyama, H.; Cherkasova, E.; McCoy, J.P.; Hanada, K.-I.; Mena, O.; Kurlander, R.; Abdul, T.; et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J. Clin. Investig. 2008, 118, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and Genetic Properties of Tumors Associated with Local Immune Cytolytic Activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.-H.; et al. LSD1 Ablation Stimulates Anti-tumor Immunity and Enables Checkpoint Blockade. Cell 2018, 174, 549–563.e19. [Google Scholar] [CrossRef] [PubMed]
- Elenitoba-Johnson, K.S.; Lim, M.S. New Insights into Lymphoma Pathogenesis. Annu. Rev. Pathol. 2018, 13, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Cerhan, J.R.; Wallace, R.B.; Folsom, A.R.; Potter, J.D.; Sellers, T.A.; Zheng, W.; Lutz, C.T. Medical history risk factors for non-Hodgkin’s lymphoma in older women. J. Natl. Cancer Inst. 1997, 89, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Smedby, K.E.; Vajdic, C.M.; Falster, M.; Engels, E.A.; Martínez-Maza, O.; Turner, J.; Hjalgrim, H.; Vineis, P.; Costantini, A.S.; Bracci, P.M.; et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood 2008, 111, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Cenk, H.; Sarac, G.; Karadağ, N.; Berktas, H.B.; Sahin, I.; Sener, S.; Kisaciik, D.; Kapicioglu, Y. Intravascular lymphoma presenting with paraneoplastic syndrome. Dermatol. Online J. 2020, 26. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Galindo, R.; Kaplan, M.H.; Leissner, P.; Verjat, T.; Ferlenghi, I.; Bagnoli, F.; Giusti, F.; Dosik, M.H.; Hayes, D.F.; Gitlin, S.D.; et al. Human Endogenous Retrovirus K (HML-2) Elements in the Plasma of People with Lymphoma and Breast Cancer. J. Virol. 2008, 82, 9329–9336. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, K.; Saarinen, M.; Mallet, F.; Aatonen, M.; Hau, A.; Ranki, A. Cutaneous T-Cell Lymphoma (CTCL) Cell Line-Derived Extracellular Vesicles Contain HERV-W-Encoded Fusogenic Syncytin-1. J. Investig. Dermatol. 2019, 140, 1466–1469.e4. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Leal, F.E.; Hasenkrug, A.M.; Segurado, A.C.; Nixon, D.F.; Ostrowski, M.A.; Kallas, E.G. Human endogenous retrovirus K(HML-2) Gag and Env specific T-cell responses are not detected in HTLV-I-infected subjects using standard peptide screening methods. J. Negat. Results Biomed. 2013, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Trac, C.; Kato, H.; Costello, K.R.; Chen, Z.; Natarajan, R.; Schones, D.E. LTRs activated by Epstein-Barr virus–induced transformation of B cells alter the transcriptome. Genome Res. 2018, 28, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Zahn, J.; Kaplan, M.H.; Fischer, S.; Dai, M.; Meng, F.; Saha, A.K.; Cervantes, P.; Chan, S.M.; Dube, D.; Omenn, G.S.; et al. Expansion of a novel endogenous retrovirus throughout the pericentromeres of modern humans. Genome Biol. 2015, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H.; Kaminski, M.; Estes, J.M.; Gitlin, S.D.; Zahn, J.; Elder, J.T.; Tejasvi, T.; Gensterblum, E.; Sawalha, A.H.; McGowan, J.P.; et al. Structural variation of centromeric endogenous retroviruses in human populations and their impact on cutaneous T-cell lymphoma, Sézary syndrome, and HIV infection. BMC Med. Genom. 2019, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M.; Schommer, S.; Kremmer, E.; Remberger, K.; Dölken, G.; Lemm, I.; Buck, M.; Best, B.; Neumann-Haefelin, D.; Mueller-Lantzsch, N. Human endogenous retrovirus K10: Expression of Gag protein and detection of antibodies in patients with seminomas. J. Virol. 1995, 69, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Perzova, R.; Graziano, E.; Sanghi, S.; Welch, C.; Benz, P.; Abbott, L.; Lalone, D.; Glaser, J.; Loughran, T.; Sheremata, W.; et al. Increased seroreactivity to HERV-K10 peptides in patients with HTLV myelopathy. Virol. J. 2013, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, B.; Walter, K.; Kreher, S.; Kumar, R.; Hummel, M.; Lenze, D.; Köchert, K.; Bouhlel, M.A.; Richter, J.; Soler, E.; et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat. Med. 2010, 16, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Lock, F.E.; Rebollo, R.; Miceli-Royer, K.; Gagnier, L.; Kuah, S.; Babaian, A.; Sistiaga-Poveda, M.; Lai, C.B.; Nemirovsky, O.; Serrano, I.; et al. Distinct isoform of FABP7 revealed by screening for retroelement-activated genes in diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. USA 2014, 111, E3534–E3543. [Google Scholar] [CrossRef] [PubMed]
- Babaian, A.; Romanish, M.T.; Gagnier, L.; Kuo, L.Y.; Karimi, M.M.; Steidl, C.; Mager, D. Onco-exaptation of an endogenous retroviral LTR drives IRF5 expression in Hodgkin lymphoma. Oncogene 2016, 35, 2542–2546. [Google Scholar] [CrossRef] [PubMed]
- Kreher, S.; Bouhlel, M.A.; Cauchy, P.; Lamprecht, B.; Li, S.; Grau, M.; Hummel, F.; Köchert, K.; Anagnostopoulos, I.; Jöhrens, K.; et al. Mapping of transcription factor motifs in active chromatin identifies IRF5 as key regulator in classical Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2014, 111, E4513–E4522. [Google Scholar] [CrossRef] [PubMed]
- Kewitz, S.; Staege, M.S. Expression and Regulation of the Endogenous Retrovirus 3 in Hodgkin’s Lymphoma Cells. Front. Oncol. 2013, 3, 179. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Kato, N.; Larsson, E. ERV3 human endogenous provirus mRNAs are expressed in normal and malignant tissues and cells, but not in choriocarcinoma tumor cells. J. Cell. Biochem. 1988, 36, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, B.; Rote, N.S. The cellular mechanism by which the human endogenous retrovirus ERV-3 env gene affects proliferation and differentiation in a human placental trophoblast model, BeWo. Placenta 2000, 21, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Venables, P.; Andersson, A.C.; Fan, W.; Rigby, S.; Botling, J.; Oberg, F.; Cohen, M.; Nilsson, K. Tissue and differentiation specific expression on the endogenous retrovirus ERV3 (HERV-R) in normal human tissues and during induced monocytic differentiation in the U-937 cell line. Leukemia 1997, 11 (Suppl. S3), 142–144. [Google Scholar] [PubMed]
- Åbrink, M.; Larsson, E.; Hellman, L. Demethylation of ERV3, an endogenous retrovirus regulating the Kruppel-related zinc finger gene H-plk, in several human cell lines arrested during early monocyte development. DNA Cell Biol. 1998, 17, 27–37. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, N.; Heidmann, T. Physiological knockout of the envelope gene of the single-copy ERV-3 human endogenous retrovirus in a fraction of the Caucasian population. J. Virol. 1998, 72, 3442–3445. [Google Scholar] [CrossRef] [PubMed]
- Staege, M.S.; Müller, K.; Kewitz, S.; Volkmer, I.; Mauz-Körholz, C.; Bernig, T.; Körholz, D. Expression of dual-specificity phosphatase 5 pseudogene 1 (DUSP5P1) in tumor cells. PLoS ONE 2014, 9, e89577. [Google Scholar] [CrossRef] [PubMed]
- Huff, L.M.; Wang, Z.; Iglesias, A.; Fojo, T.; Lee, J.-S. Aberrant transcription from an unrelated promoter can result in MDR-1 expression following drug selection in vitro and in relapsed lymphoma samples. Cancer Res. 2005, 65, 11694–11703. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, M.; Brocks, D.; Sheng, Y.-H.; Islam, S.; Ressnerova, A.; Assenov, Y.; Milde, T.; Oehme, I.; Witt, O.; Goyal, A.; et al. Reactivation of endogenous retroviral elements via treatment with DNMT- and HDAC-inhibitors. Cell Cycle 2018, 17, 811–822. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Beliakova-Bethell, N.; Lada, S.M.; Breen, M.S.; Hurst, T.; Spina, C.A.; Richman, D.D.; Frater, J.; Magiorkinis, G.; Woelk, C.H. Transcriptional Modulation of Human Endogenous Retroviruses in Primary CD4+ T Cells Following Vorinostat Treatment. Front. Immunol. 2018, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ohtani, H.; Zhou, W.; Ørskov, A.D.; Charlet, J.; Zhang, Y.W.; Shen, H.; Baylin, S.B.; Liang, G.; Grønbæk, K.; et al. Vitamin C increases viral mimicry induced by 5-aza-2’-deoxycytidine. Proc. Natl. Acad. Sci. USA 2016, 113, 10238–10244. [Google Scholar] [CrossRef] [PubMed]
- Beyer, U.; Dobbelstein, M. Non-hominid TP63 lacks retroviral LTRs but contains a novel conserved upstream exon. Cell Cycle 2011, 10, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Krönung, S.K.; Beyer, U.; Chiaramonte, M.L.; Dolfini, D.; Mantovani, R.; Dobbelstein, M. LTR12 promoter activation in a broad range of human tumor cells by HDAC inhibition. Oncotarget 2016, 7, 33484–33497. [Google Scholar] [CrossRef] [PubMed]
- Beyer, U.; Krönung, S.K.; Leha, A.; Walter, L.; Dobbelstein, M. Comprehensive identification of genes driven by ERV9-LTRs reveals TNFRSF10B as a re-activatable mediator of testicular cancer cell death. Cell Death Differ. 2016, 23, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.E.; Lupo, P.J.; Bondy, M.L. Epidemiology of childhood cancer. In Principles and Practices of Pediatric Oncology, 7th ed.; Pizzo, P.A., Poplack, D.G., Eds.; Lippincott: Philadelphia, PA, USA, 2016. [Google Scholar]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Wahbi, K.; Hayette, S.; Callanan, M.; Gadoux, M.; Charrin, C.; Magaud, J.-P.; Rimokh, R. Involvement of a human endogenous retroviral sequence (THE-7) in a t(7;14)(q21;q32) chromosomal translocation associated with a B cell chronic lymphocytic leukemia. Leukemia 1997, 11, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Guasch, G.; Popovici, C.; Mugneret, F.; Chaffanet, M.; Pontarotti, P.; Birnbaum, D.; Pébusque, M.-J. Endogenous retroviral sequence is fused to FGFR1 kinase in the 8p12 stem-cell myeloproliferative disorder with t(8;19)(p12;q13.3). Blood 2003, 101, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Mugneret, F.; Chaffanet, M.; Maynadie, M.; Guasch, G.; Favre, B.; Casasnovas, O.; Birnbaum, D.; Pebusque, M.-J. The 8p12 myeloproliferative disorder. t(8;19)(p12;q13.3): A novel translocation involving the FGFR1 gene. Br. J. Haematol. 2000, 111, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.L.; Vangsted, A.J.; Hansen, B.; Vogel, U.B.; Hermansen, N.E.U.; Jensen, S.B.; Laska, M.J.; Nexø, B.A. Synergy of two human endogenous retroviruses in multiple myeloma. Leuk. Res. 2015, 39, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.A.; Thi, V.L.D.; Denner, J. The transmembrane protein of the human endogenous retrovirus--K (HERV-K) modulates cytokine release and gene expression. PLoS ONE 2013, 8, e70399. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Renaudineau, Y.; Hillion, S.; Saraux, A.; Mageed, R.A.; Youinou, P. An alternative exon 1 of the CD5 gene regulates CD5 expression in human B lymphocytes. Blood 2005, 106, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Armbruester, V.; Sauter, M.; Krautkraemer, E.; Meese, E.; Kleiman, A.; Best, B.; Roemer, K.; Mueller-Lantzsch, N. A novel gene from the human endogenous retrovirus K expressed in transformed cells. Clin. Cancer Res. 2002, 8, 1800–1807. [Google Scholar] [PubMed]
- Fischer, S.; Echeverría, N.; Moratorio, G.; Landoni, A.I.; Dighiero, G.; Cristina, J.; Oppezzo, P.; Moreno, P. Human endogenous retrovirus np9 gene is over expressed in chronic lymphocytic leukemia patients. Leuk. Res. Rep. 2014, 3, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Arco, P.G.-D.; Kashiwagi, M.; Jackson, A.F.; Naito, T.; Zhang, J.; Liu, F.; Kee, B.; Vooijs, M.; Radtke, F.; Redondo, J.M.; et al. Alternative Promoter Usage at the Notch1 Locus Supports Ligand-Independent Signaling in T Cell Development and Leukemogenesis. Immunity 2010, 33, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Sokol, M.; Jessen, K.M.; Pedersen, F.S. Human endogenous retroviruses sustain complex and cooperative regulation of gene-containing loci and unannotated megabase-sized regions. Retrovirology 2015, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Sokol, M.; Jessen, K.M.; Pedersen, F.S. Utility of next-generation RNA-sequencing in identifying chimeric transcription involving human endogenous retroviruses. APMIS 2016, 124, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Patzkea, S.; Lindeskogb, M.; Munthea, E.; Aasheim, H.-C. Characterization of a Novel Human Endogenous Retrovirus, HERV-H/F, Expressed in Human Leukemia Cell Lines. Virology 2002, 303, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Gang, A.O.; Frøsig, T.M.; Brimnes, M.K.; Lyngaa, R.; Treppendahl, M.B.; Grønbæk, K.; Dufva, I.H.; Straten, P.T.; Hadrup, S.R. 5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies. Blood Cancer J. 2014, 4, e197. [Google Scholar] [CrossRef] [PubMed]
- Dubovsky, J.A.; McNeel, D.G.; Powers, J.J.; Gordon, J.; Sotomayor, E.M.; Pinilla-Ibarz, J.A. Treatment of chronic lymphocytic leukemia with a hypomethylating agent induces expression of NXF2, an immunogenic cancer testis antigen. Clin. Cancer Res. 2009, 15, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, O.; Agathanggelou, A.; Novitzky-Basso, I.; Siddique, S.; McSkeane, T.; Ryan, G.; Vyas, P.; Cavenagh, J.; Stankovic, T.; Moss, P.; et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood 2010, 116, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Roulois, D.; Loo Yau, H.; Singhania, R.; Wang, Y.; Danesh, A.; Shen, S.Y.; Han, H.; Liang, G.; Jones, P.A.; Pugh, T.J.; et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell 2015, 162, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, U.; Schulz, W.A.; Koch, A.; Niegisch, G.; Goering, W. HERV-K and LINE-1 DNA Methylation and Reexpression in Urothelial Carcinoma. Front. Oncol. 2013, 3, 255. [Google Scholar] [CrossRef] [PubMed]
- Stengel, S.; Fiebig, U.; Kurth, R.; Denner, J. Regulation of human endogenous retrovirus-K expression in melanomas by CpG methylation. Genes Chromosom. Cancer 2010, 49, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Reichrath, J.; Roesch, A.; Meese, E.; Mayer, J. Transcriptional profiling of human endogenous retrovirus group HERV-K(HML-2) loci in melanoma. Genome Biol. Evol. 2013, 5, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Reiche, J.; Pauli, G.; Ellerbrok, H. Differential expression of human endogenous retrovirus K transcripts in primary human melanocytes and melanoma cell lines after UV irradiation. Melanoma Res. 2010, 20, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Schanab, O.; Humer, J.; Gleiss, A.; Mikula, M.; Sturlan, S.; Grunt, S.; Okamoto, I.; Muster, T.; Pehamberger, H.; Waltenberger, A. Expression of human endogenous retrovirus K is stimulated by ultraviolet radiation in melanoma. Pigment. Cell Melanoma Res. 2011, 24, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Hohenadl, C.; Germaier, H.; Walchner, M.; Hagenhofer, M.; Herrmann, M.; Stürzl, M.; Kind, P.; Hehlmann, R.; Erfle, V.; Leib-Mösch, C. Transcriptional activation of endogenous retroviral sequences in human epidermal keratinocytes by UVB irradiation. J. Investig. Dermatol. 1999, 113, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Schön, U.; Seifarth, W.; Baust, C.; Hohenadl, C.; Erfle, V.; Leib-Mösch, C. Cell type-specific expression and promoter activity of human endogenous retroviral long terminal repeats. Virology 2001, 279, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Sheervalilou, R.; Kahroba, H. A New Insight on Activation of Human Endogenous Retroviruses (HERVs) in Malignant Melanoma upon Exposure to CuSO4. Biol. Trace Element Res. 2019, 191, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Serafino, A.; Balestrieri, E.; Pierimarchi, P.; Matteucci, C.; Moroni, G.; Oricchio, E.; Rasi, G.; Mastino, A.; Spadafora, C.; Garaci, E.; et al. The activation of human endogenous retrovirus K (HERV-K) is implicated in melanoma cell malignant transformation. Exp. Cell Res. 2009, 315, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Argaw-Denboba, A.; Gambacurta, A.; Cipriani, C.; Bei, R.; Serafino, A.; Sinibaldi-Vallebona, P.; Matteucci, C. Human Endogenous Retrovirus K in the Crosstalk Between Cancer Cells Microenvironment and Plasticity: A New Perspective for Combination Therapy. Front. Microbiol. 2018, 9, 1448. [Google Scholar] [CrossRef] [PubMed]
- Golob, M.; Buettner, R.; Bosserhoff, A. Characterization of a Transcription Factor Binding Site, Specifically Activating MIA Transcription in Melanoma. J. Investig. Dermatol. 2000, 115, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, G.V. Transposable Elements and DNA Methylation Create in Embryonic Stem Cells Human-Specific Regulatory Sequences Associated with Distal Enhancers and Noncoding RNAs. Genome Biol. Evol. 2015, 7, 1432–1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zeng, J.; Lowe, C.B.; Sellers, R.G.; Salama, S.R.; Yang, M.; Burgess, S.M.; Brachmann, R.K.; Haussler, D. Species-specific endogenous retroviruses shape the transcriptional network of the human tumor suppressor protein p53. Proc. Natl. Acad. Sci. USA 2007, 104, 18613–18618. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; LoVerso, P.R.; Fisk, J.N.; Zhurkin, V.B.; Cui, F. p53 binding sites in normal and cancer cells are characterized by distinct chromatin context. Cell Cycle 2017, 16, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-T.; Yang, W.K.; Huang, H.-C.; Yeh, K.-W.; Wu, C.-W. The transcriptional activity of HERV-I LTR is negatively regulated by its cis-elements and wild type p53 tumor suppressor protein. J. Biomed. Sci. 2007, 14, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Garazha, A.; Ivanova, A.; Suntsova, M.; Malakhova, G.; Roumiantsev, S.; Zhavoronkov, A.; Buzdin, A. New bioinformatic tool for quick identification of functionally relevant endogenous retroviral inserts in human genome. Cell Cycle 2015, 14, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, N.; Kraft, M.; Tondera, C.; Hanschmann, K.-M.; Löwer, J.; Löwer, R. Expression of the human endogenous retrovirus (HERV) group HML-2/HERV-K does not depend on canonical promoter elements but is regulated by transcription factors Sp1 and Sp3. J. Virol. 2011, 85, 3436–3448. [Google Scholar] [CrossRef] [PubMed]
- Cardelli, M.; van Doorn, R.; Larcher, L.; Di Donato, M.; Piacenza, F.; Pierpaoli, E.; Giacconi, R.; Malavolta, M.; Rachakonda, S.; Gruis, N.A.; et al. Association of HERV-K and LINE-1 hypomethylation with reduced disease-free survival in melanoma patients. Epigenomics 2020, 12, 1689–1706. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.; Jeyakani, J.; Bourque, G. The Majority of Primate-Specific Regulatory Sequences Are Derived from Transposable Elements. PLoS Genet. 2013, 9, e1003504. [Google Scholar] [CrossRef] [PubMed]
- Flockhart, R.J.; Webster, D.E.; Qu, K.; Mascarenhas, N.; Kovalski, J.; Kretz, M.; Khavari, P.A. BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res. 2012, 22, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Leucci, E.; Vendramin, R.; Spinazzi, M.; Laurette, P.; Fiers, M.; Wouters, J.; Radaelli, E.; Eyckerman, S.; Leonelli, C.; Vanderheyden, K.; et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature 2016, 531, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Cai, H.; Bunse, M.; Feschotte, C.; Izsvák, Z. Human Endogenous Retrovirus K Rec forms a Regulatory Loop with MITF that Opposes the Progression of Melanoma to an Invasive Stage. Viruses 2020, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Katoh, I.; Mírová, A.; Kurata, S.-I.; Murakami, Y.; Horikawa, K.; Nakakuki, N.; Sakai, T.; Hashimoto, K.; Maruyama, A.; Yonaga, T.; et al. Activation of the long terminal repeat of human endogenous retrovirus K by melanoma-specific transcription factor MITF-M. Neoplasia 2011, 13, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sheng, T.; Wan, X.; Liu, T.; Wu, H.; Dong, J. Expression of HERV-K correlates with status of MEK-ERK and p16INK4A-CDK4 pathways in melanoma cells. Cancer Investig. 2010, 28, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Ugurel, S.; Hanschmann, K.-M.; Strobel, H.; Tondera, C.; Schadendorf, D.; Löwer, J.; Löwer, R. Serological response to human endogenous retrovirus K in melanoma patients correlates with survival probability. AIDS Res. Hum. Retroviruses 2008, 24, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Humer, J.; Waltenberger, A.; Grassauer, A.; Kurz, M.; Valencak, J.; Rapberger, R.; Hahn, S.; Löwer, R.; Wolff, K.; Bergmann, M.; et al. Identification of a melanoma marker derived from melanoma-associated endogenous retroviruses. Cancer Res. 2006, 66, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Salata, C.; Weiderpass, E.; Vineis, P.; Palù, G.; Mastrangelo, G. Human endogenous retroviruses and cancer prevention: Evidence and prospects. BMC Cancer 2013, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, G.; Krone, B.; Fadda, E.; Buja, A.; Grange, J.; Rausa, G.; de Vries, E.; Koelmel, K. Does yellow fever 17D vaccine protect against melanoma? Vaccine 2009, 27, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, J.; Rabinovich, B.A.; Mi, T.; Switzer, K.C.; Olivares, S.; Maiti, S.N.; Plummer, J.B.; Singh, H.; Kumaresan, P.R.; Huls, H.M.; et al. Genetic Engineering of T Cells to Target HERV-K, an Ancient Retrovirus on Melanoma. Clin. Cancer Res. 2015, 21, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Mangeney, M.; Heidmann, T. Tumor cells expressing a retroviral envelope escape immune rejection in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 14920–14925. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, J.; Wang, F.; Oliver, M.T.; Tipton, T.; Gao, Y.; Jiang, S.-W. Syncytin-1 modulates placental trophoblast cell proliferation by promoting G1/S transition. Cell. Signal. 2013, 25, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Ouyang, D.-Y.; Xu, L.; Gao, Q.; He, X.-H. Human endogenous retroviral syncytin exerts inhibitory effect on invasive phenotype of B16F10 melanoma cells. Chin. J. Cancer Res. 2013, 25, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Argaw-Denboba, A.; Balestrieri, E.; Serafino, A.; Cipriani, C.; Bucci, I.; Sorrentino, R.; Sciamanna, I.; Gambacurta, A.; Sinibaldi-Vallebona, P.; Matteucci, C. HERV-K activation is strictly required to sustain CD133+ melanoma cells with stemness features. J. Exp. Clin. Cancer Res. 2017, 36, 20. [Google Scholar] [CrossRef] [PubMed]
- Ellerhorst, J.A.; Cooksley, C.D.; Grimm, E.A. Autoimmunity and hypothyroidism in patients with uveal melanoma. Melanoma Res. 2001, 11, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Kalter, S.S.; Helmke, R.J.; Heberling, R.L.; Panigel, M.; Fowler, A.K.; Strickland, J.E.; Hellman, A. Brief communication: C-type particles in normal human placentas. J. Natl. Cancer Inst. 1973, 50, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Bronson, D.L.; Ritzi, D.M.; Fraley, E.E.; Dalton, A.J. Morphologic evidence for retrovirus production by epithelial cells derived from a human testicular tumor metastasis. J. Natl. Cancer Inst. 1978, 60, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Boller, K.; Janssen, O.; Schuldes, H.; Tönjes, R.R.; Kurth, R. Characterization of the antibody response specific for the human endogenous retrovirus HTDV/HERV-K. J. Virol. 1997, 71, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, K.; Ferreira, H.; Flockerzi, A.; Wahl, S.; Sauter, M.; Mayer, J.; Mueller-Lantzsch, N. Human endogenous retrovirus family HERV-K(HML-2) RNA transcripts are selectively packaged into retroviral particles produced by the human germ cell tumor line Tera-1 and originate mainly from a provirus on chromosome 22q11.21. J. Virol. 2008, 82, 10008–10016. [Google Scholar] [CrossRef] [PubMed]
- Boller, K.; Schönfeld, K.; Lischer, S.; Fischer, N.; Hoffmann, A.; Kurth, R.; Tönjes, R.R. Human endogenous retrovirus HERV-K113 is capable of producing intact viral particles. J. Gen. Virol. 2008, 89, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Knössl, M.; Löwer, R.; Löwer, J. Expression of the human endogenous retrovirus HTDV/HERV-K is enhanced by cellular transcription factor YY1. J. Virol. 1999, 73, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Ruda, V.M.; Akopov, S.B.; Trubetskoy, D.O.; Manuylov, N.L.; Vetchinova, A.S.; Zavalova, L.L.; Nikolaev, L.G.; Sverdlov, E.D. Tissue specificity of enhancer and promoter activities of a HERV-K(HML-2) LTR. Virus Res. 2004, 104, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, T.V.; Leppik, L.; Nikolaev, L.G.; Akopov, S.B.; Kleiman, A.M.; Senyuta, N.B.; Sverdlov, E.D. Solitary human endogenous retroviruses-K LTRs retain transcriptional activity in vivo, the mode of which is different in different cell types. Virology 2001, 290, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, D.; Gogvadze, E.; Kholodenko, R.; Grzela, D.P.; Mityaev, M.; Vinogradova, T.; Kopantzev, E.; Malakhova, G.; Suntsova, M.; Sokov, D.; et al. Novel strong tissue specific promoter for gene expression in human germ cells. BMC Biotechnol. 2010, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Hantsch, C.; Volkmer, I.; Staege, M.S. Differentiation-Dependent Regulation of Human Endogenous Retrovirus K Sequences and Neighboring Genes in Germ Cell Tumor Cells. Front. Microbiol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Kuebler, P.J.; Heymann, J.J.; Sheehy, M.E.; Ortiz, G.M.; Ogg, G.S.; Barbour, J.D.; Lenz, J.; Steinfeld, A.D.; Nixon, D.F. Detection of T lymphocytes specific for human endogenous retrovirus K (HERV-K) in patients with seminoma. AIDS Res. Hum. Retroviruses 2006, 22, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, A.; Senyuta, N.; Tryakin, A.; Sauter, M.; Karseladze, A.; Tjulandin, S.; Gurtsevitch, V.; Mueller-Lantzsch, N. HERV-K(HML-2) GAG/ENV antibodies as indicator for therapy effect in patients with germ cell tumors. Int. J. Cancer 2004, 110, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Löwer, R.; Tönjes, R.R.; Korbmacher, C.; Kurth, R.; Löwer, J. Identification of a Rev-related protein by analysis of spliced transcripts of the human endogenous retroviruses HTDV/HERV-K. J. Virol. 1995, 69, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.; Sauter, M.; Galli, U.; Best, B.; Herbst, H.; Mayer, J.; Kremmer, E.; Roemer, K.; Mueller-Lantzsch, N. Human endogenous retrovirus protein cORF supports cell transformation and associates with the promyelocytic leukemia zinc finger protein. Oncogene 2000, 19, 4328–4336. [Google Scholar] [CrossRef] [PubMed]
- Magin-Lachmann, C.; Hahn, S.; Strobel, H.; Held, U.; Löwer, J.; Löwer, R. Rec (formerly Corf) function requires interaction with a complex, folded RNA structure within its responsive element rather than binding to a discrete specific binding site. J. Virol. 2001, 75, 10359–10371. [Google Scholar] [CrossRef] [PubMed]
- Denne, M.; Sauter, M.; Armbruester, V.; Licht, J.D.; Roemer, K.; Mueller-Lantzsch, N. Physical and functional interactions of human endogenous retrovirus proteins Np9 and rec with the promyelocytic leukemia zinc finger protein. J. Virol. 2007, 81, 5607–5616. [Google Scholar] [CrossRef] [PubMed]
- Galli, U.M.; Sauter, M.; Lecher, B.; Maurer, S.; Herbst, H.; Roemer, K.; Mueller-Lantzsch, N. Human endogenous retrovirus rec interferes with germ cell development in mice and may cause carcinoma in situ, the predecessor lesion of germ cell tumors. Oncogene 2005, 24, 3223–3228. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.; Sapir, T.; Park, S.-S.; Rual, J.-F.; Contreras-Galindo, R.; Reiner, O.; Markovitz, D.M. The HERV-K accessory protein Np9 controls viability and migration of teratocarcinoma cells. PLoS ONE 2019, 14, e0212970. [Google Scholar] [CrossRef] [PubMed]
- Feuchter-Murthy, A.E.; Freeman, J.D.; Mager, D.L. Splicing of a human endogenous retrovirus to a novel phospholipase A2 related gene. Nucleic Acids Res. 1993, 21, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.E.; Freeman, J.; Mager, D. Intergenic splicing between a HERV-H endogenous retrovirus and two adjacent human genes. Genomics 1999, 57, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Elkahloun, A.G.; Candotti, F.; Grajkowski, A.; Beaucage, S.L.; Petricoin, E.F.; Calvert, V.; Juhl, H.; Mills, F.; Mason, K.; et al. A novel function of RNAs arising from the long terminal repeat of human endogenous retrovirus 9 in cell cycle arrest. J. Virol. 2013, 87, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A. Real-world evidence in the treatment of ovarian cancer. Ann. Oncol. 2017, 28, viii61–viii65. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Heidmann, O.; Béguin, A.; Paternina, J.; Berthier, R.; Deloger, M.; Bawa, O.; Heidmann, T. HEMO, an ancestral endogenous retroviral envelope protein shed in the blood of pregnant women and expressed in pluripotent stem cells and tumors. Proc. Natl. Acad. Sci. USA 2017, 114, E6642–E6651. [Google Scholar] [CrossRef] [PubMed]
- Menendez, L.; Benigno, B.B.; McDonald, J.F. L1 and HERV-W retrotransposons are hypomethylated in human ovarian carcinomas. Mol. Cancer 2004, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Iramaneerat, K.; Rattanatunyong, P.; Khemapech, N.; Triratanachat, S.; Mutirangura, A. HERV-K hypomethylation in ovarian clear cell carcinoma is associated with a poor prognosis and platinum resistance. Int. J. Gynecol. Cancer 2011, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L.; Kitova, M.; Maldener, E.; Meese, E.; Mayer, J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2). J. Virol. 2005, 79, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Thomas, S.L.; DeWitt, A.K.; Zhou, W.; Madaj, Z.B.; Ohtani, H.; Baylin, S.B.; Liang, G.; Jones, P.A. Dual Inhibition of DNA and Histone Methyltransferases Increases Viral Mimicry in Ovarian Cancer Cells. Cancer Res. 2018, 78, 5754–5766. [Google Scholar] [CrossRef] [PubMed]
- Rycaj, K.; Plummer, J.B.; Yin, B.; Li, M.; Garza, J.; Radvanyi, L.; Ramondetta, L.M.; Lin, K.; Johanning, G.L.; Tang, D.G.; et al. Cytotoxicity of human endogenous retrovirus K-specific T cells toward autologous ovarian cancer cells. Clin. Cancer Res. 2015, 21, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Black, D.M.; Brown, M.A.; Griffiths, B.L.; Nicolai, H.M.; Chambers, J.A.; Bonjardim, M.; Xu, C.F.; Boyd, M.; McFarlane, R. The detailed characterisation of a 400 kb cosmid walk in the BRCA1 region: Identification and localisation of 10 genes including a dual-specificity phosphatase. Hum. Mol. Genet. 1994, 3, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Sin, H.-S.; Huh, J.-W.; Kim, D.-S.; Kang, D.W.; Min, D.S.; Kim, T.-H.; Ha, H.-S.; Kim, H.-H.; Lee, S.-Y.; Kim, H.-S. Transcriptional control of the HERV-H LTR element of the GSDML gene in human tissues and cancer cells. Arch. Virol. 2006, 151, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.; Carmody, M.; Wynne, F.; Dockery, P.; Aigner, A.; Cameron, I.; Higgins, J.; Smith, S.; Aplin, J.; Moore, T. Expression of pleiotrophin and its receptors in human placenta suggests roles in trophoblast life cycle and angiogenesis. Placenta 2009, 30, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.M.; Lai, S.; Kurtz, A.; Czubayko, F.; Riegel, A.T.; Wellstein, A. Human trophoblast and choriocarcinoma expression of the growth factor pleiotrophin attributable to germ-line insertion of an endogenous retrovirus. Proc. Natl. Acad. Sci. USA 1996, 93, 14759–14764. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.M.; Malerczyk, C.; Cabal-Manzano, R.; Gajarsa, J.J.; List, H.-J.; Riegel, A.T.; Wellstein, A. Influence of the human endogenous retrovirus-like element HERV-E.PTN on the expression of growth factor pleiotrophin: A critical role of a retroviral Sp1-binding site. Oncogene 2000, 19, 3988–3998. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, B.; Rote, N.S. Expression of endogenous retrovirus ERV-3 induces differentiation in BeWo, a choriocarcinoma model of human placental trophoblast. Placenta 1999, 20, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Strissel, P.L.; Ruebner, M.; Thiel, F.; Wachter, D.; Ekici, A.B.; Wolf, F.; Thieme, F.; Ruprecht, K.; Beckmann, M.W.; Strick, R. Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: Emergence of new molecular targets. Oncotarget 2012, 3, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Strick, R.; Ackermann, S.; Langbein, M.; Swiatek, J.; Schubert, S.W.; Hashemolhosseini, S.; Koscheck, T.; Fasching, P.A.; Schild, R.L.; Beckmann, M.W.; et al. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral Syncytin-1 and regulated by TGF-beta. J. Mol. Med. 2007, 85, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, B.A.; Marshall, J.L.; Salem, M.E. The Growing Challenge of Young Adults With Colorectal Cancer. Oncology 2017, 31, 381–389. [Google Scholar] [PubMed]
- Bannert, N.; Kurth, R. The Evolutionary Dynamics of Human Endogenous Retroviral Families. Annu. Rev. Genom. Hum. Genet. 2006, 7, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Xu, Z.; Xu, R.; Wu, L.; Zheng, S. Expression Patterns of Non-Coding Spliced Transcripts from Human Endogenous Retrovirus HERV-H Elements in Colon Cancer. PLoS ONE 2012, 7, e29950. [Google Scholar] [CrossRef] [PubMed]
- Pérot, P.; Mullins, C.S.; Naville, M.; Bressan, C.; Hühns, M.; Gock, M.; Kühn, F.; Volff, J.-N.; Trillet-Lenoir, V.; Linnebacher, M.; et al. Expression of young HERV-H loci in the course of colorectal carcinoma and correlation with molecular subtypes. Oncotarget 2015, 6, 40095–40111. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, N.; Forrest, G.; Venables, P.J.; Heidmann, T. ERV-3 envelope expression and congenital heart block: What does a physiological knockout teach us. Autoimmunity 1999, 30, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Jeong, D.; Wang, S.; Narita, M.; Jin, X.; Iwasaki, M.; Perli, S.D.; Conklin, B.R.; Yamanaka, S. Critical Roles of Translation Initiation and RNA Uridylation in Endogenous Retroviral Expression and Neural Differentiation in Pluripotent Stem Cells. Cell Rep. 2020, 31, 107715. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.S.; Linnebacher, M. Endogenous retrovirus sequences as a novel class of tumor-specific antigens: An example of HERV-H env encoding strong CTL epitopes. Cancer Immunol. Immunother. 2012, 61, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M.; Christensen, I.J.; Nielsen, H.J.; Hansen, U.; Bjerregaard, B.; Talts, J.F.; Larsson, L.-I. Syncytin immunoreactivity in colorectal cancer: Potential prognostic impact. Cancer Lett. 2009, 280, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Karin, M. Tumor-Elicited Inflammation and Colorectal Cancer. Adv. Cancer Res. 2015, 128, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Lock, F.E.; Babaian, A.; Zhang, Y.; Gagnier, L.; Kuah, S.; Weberling, A.; Karimi, M.M.; Mager, D.L. A novel isoform of IL-33 revealed by screening for transposable element promoted genes in human colorectal cancer. PLoS ONE 2017, 12, e0180659. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M. Role of IL-33 in inflammation and disease. J. Inflamm. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Qi, H.; Gundersen, M.D.; Yang, H.; Christiansen, I.; Sørbye, S.W.; Goll, R.; Florholmen, J. Dynamics of the IL-33/ST2 network in the progression of human colorectal adenoma to sporadic colorectal cancer. Cancer Immunol. Immunother. 2015, 64, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, D.; Magallanes, R.T.; Bhatia, S.S.; Teo, W.S.; Sian, S.; Hora, S.; Lee, K.K.; Zhang, Y.; Jadhav, S.P.; Wu, Y.; et al. TIP60 represses activation of endogenous retroviral elements. Nucleic Acids Res. 2018, 46, 9456–9470. [Google Scholar] [CrossRef] [PubMed]
- Gibb, E.A.; Warren, R.L.; Wilson, G.W.; Brown, S.D.; Robertson, G.A.; Morin, G.B.; Holt, R.A. Activation of an endogenous retrovirus-associated long non-coding RNA in human adenocarcinoma. Genome Med. 2015, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Kaller, M.; Götz, U.; Hermeking, H. Loss of p53-inducible long non-coding RNA LINC01021 increases chemosensitivity. Oncotarget 2017, 8, 102783–102800. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Subramanian, M.; Jones, M.F.; Chaudhary, R.; Singh, D.K.; Zong, X.; Gryder, B.; Sindri, S.; Mo, M.; Schetter, A.; et al. Long Noncoding RNA PURPL Suppresses Basal p53 Levels and Promotes Tumorigenicity in Colorectal Cancer. Cell Rep. 2017, 20, 2408–2423. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ao-Ieong, E.S.Y.; Yalcin, O.; Carter, C.A.; Cabrales, P. Cardioprotective Effect of Phase 3 Clinical Anticancer Agent, RRx-001, in Doxorubicin-Induced Acute Cardiotoxicity in Mice. Mol. Pharm. 2019, 16, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ning, S.; Nolley, R.; Scicinski, J.; Oronsky, B.; Knox, S.J.; Peehl, D.M. The immunomodulatory anticancer agent, RRx-001, induces an interferon response through epigenetic induction of viral mimicry. Clin. Epigenetics 2017, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.-J.; Ock, M.-S.; Choi, Y.-H.; Iovanna, J.; Mun, S.; Han, K.; Kim, H.-S.; Cha, H.-J. Human Endogenous Retrovirus (HERV)-K env Gene Knockout Affects Tumorigenic Characteristics of nupr1 Gene in DLD-1 Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 3941. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Sajed, D.; Arora, K.S.; Solovyov, A.; Rajurkar, M.; Bledsoe, J.R.; Sil, S.; Amri, R.; Tai, E.; MacKenzie, O.C.; et al. Diverse repetitive element RNA expression defines epigenetic and immunologic features of colon cancer. JCI Insight 2017, 2, e91078. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yang, J.; Xing, G.; Sun, Q.; Zhang, L.; He, F. Expression of GSDML Associates with Tumor Progression in Uterine Cervix Cancer. Transl. Oncol. 2008, 1, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ding, J.; Xu, R.; Xu, Z.; Zheng, S. The novel human endogenous retrovirus-related gene, psiTPTE22-HERV, is silenced by DNA methylation in cancers. Int. J. Cancer 2010, 127, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today Lyon: International Agency for Research on Cancer. 2022. Available online: https://gco.iarc.fr/today (accessed on 14 June 2022).
- Ahn, K.; Kim, H.-S. Structural and quantitative expression analyses of HERV gene family in human tissues. Mol. Cells 2009, 28, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ding, J.; Xu, R.; Xu, Z.; Zheng, S. Identification of a novel human endogenous retrovirus and promoter activity of its 5’ U3. Biochem. Biophys. Res. Commun. 2009, 382, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; Jungbluth, A.A.; Frosina, D.; Holz, M.; Ritter, E.; Nakayama, E.; Ishida, T.; Obata, Y.; Carver, B.; Scher, H.; et al. Prostate Cancer Progression Correlates with Increased Humoral Immune Response to a Human Endogenous Retrovirus GAG Protein. Clin. Cancer Res. 2013, 19, 6112–6125. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Hong, Z.; Liu, H.; Chen, X.; Ding, L.; Liu, Z.; Zhou, F.; Yuan, Y. Human Endogenous Retroviruses-K (HML-2) Expression Is Correlated with Prognosis and Progress of Hepatocellular Carcinoma. BioMed Res. Int. 2016, 2016, 8201642. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Suzuki, A.M.; Dos Santos, A.; Desterke, C.; Collino, A.; Ghisletti, S.; Braun, E.; Bonetti, A.; Fort, A.; Qin, X.-Y.; et al. CAGE profiling of ncRNAs in hepatocellular carcinoma reveals widespread activation of retroviral LTR promoters in virus-induced tumors. Genome Res. 2015, 25, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hou, W.; Liang, J.; Zhu, L.; Luo, C. LRP1B mutation: A novel independent prognostic factor and a predictive tumor mutation burden in hepatocellular carcinoma. J. Cancer 2021, 12, 4039–4048. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sachs, F.; Ramsay, L.; Jacques, P.; Göke, J.; Bourque, G.; Ng, H.-H. The retrovirus HERVH is a long noncoding RNA required for human embryonic stem cell identity. Nat. Struct. Mol. Biol. 2014, 21, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Obata, Y.; Ohara, N.; Matsushita, H.; Sato, S.; Uenaka, A.; Saika, T.; Miyamura, T.; Chayama, K.; Nakamura, Y.; et al. Identification of the HERV-K gag antigen in prostate cancer by SEREX using autologous patient serum and its immunogenicity. Cancer Immun. 2008, 8, 15. [Google Scholar]
- Liu, C.; Liu, L.; Wang, X.; Liu, Y.; Wang, M.; Zhu, F. HBV X Protein induces overexpression of HERV-W env through NF-kappaB in HepG2 cells. Virus Genes 2017, 53, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Liu, Y.; Zhou, P.; Yan, Q.; Yu, H.; Chen, X.; Zhu, F. Implication of human endogenous retrovirus W family envelope in hepatocellular carcinoma promotes MEK/ERK-mediated metastatic invasiveness and doxorubicin resistance. Cell Death Discov. 2021, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.-M.; Kim, H.-M. Expression of the human endogenous retrovirus HERV-W family in various human tissues and cancer cells. J. Gen. Virol. 2004, 85, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.-I.; Holck, S.; Christensen, I.J. Prognostic role of syncytin expression in breast cancer. Hum. Pathol. 2007, 38, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Lokossou, A.G.; Toudic, C.; Barbeau, B. Implication of Human Endogenous Retrovirus Envelope Proteins in Placental Functions. Viruses 2014, 6, 4609–4627. [Google Scholar] [CrossRef] [PubMed]
- Gruchot, J.; Kremer, D.; Küry, P. Neural Cell Responses Upon Exposure to Human Endogenous Retroviruses. Front. Genet. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Dolei, A.; Ibba, G.; Piu, C.; Serra, C. Expression of HERV Genes as Possible Biomarker and Target in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3706. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Chosdol, K.; Sarkar, C.; Mahapatra, A.K.; Sinha, S. Alteration of a sequence with homology to human endogenous retrovirus (HERV-K) in primary human glioma: Implications for viral repeat mediated rearrangement. Mutat. Res. 2001, 484, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Abrarova, N.; Simonova, L.; Vinogradova, T.; Sverdlov, E. Different transcription activity of HERV-K LTR-containing and LTR-lacking genes of the KIAA1245/NBPF gene subfamily. Genetica 2011, 139, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Y.; Li, S.; Yu, H.; Zeng, J.; Wang, X.; Zhu, F. Activation of elements in HERV-W family by caffeine and aspirin. Virus Genes 2013, 47, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Cobbs, C.S.; Soroceanu, L.; Denham, S.; Zhang, W.; Kraus, M.H. Modulation of oncogenic phenotype in human glioma cells by cytomegalovirus IE1-mediated mitogenicity. Cancer Res. 2008, 68, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.; Orrego, A.; Peredo, I.; Dzabic, M.; Wolmer-Solberg, N.; Strååt, K.; Stragliotto, G.; Söderberg-Nauclér, C. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J. Clin. Virol. 2013, 57, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.; Stragliotto, G.; Orrego, A.; Peredo, I.; Taher, C.; Willems, J.; Söderberg-Naucler, C. Low levels of Human Cytomegalovirus Infection in Glioblastoma multiforme associates with patient survival; -a case-control study. Herpesviridae 2012, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Wolmer-Solberg, N.; Baryawno, N.; Rahbar, A.; Fuchs, D.; Odeberg, J.; Taher, C.; Wilhelmi, V.; Milosevic, J.; Mohammad, A.-A.; Martinsson, T.; et al. Frequent detection of human cytomegalovirus in neuroblastoma: A novel therapeutic target? Int. J. Cancer 2013, 133, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Baryawno, N.; Rahbar, A.; Wolmer-Solberg, N.; Taher, C.; Odeberg, J.; Darabi, A.; Khan, Z.; Sveinbjörnsson, B.; Fuskevåg, O.-M.; Segerström, L.; et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J. Clin. Investig. 2011, 121, 4043–4055. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A.; Yaiw, K.-C.; Göttesdorfer, I.; Leib-Mösch, C.; Söderberg-Nauclér, C. Human Cytomegalovirus (HCMV) induces Human Endogenous Retrovirus (HERV) transcription. Retrovirology 2013, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Machnik, G.; Skudrzyk, E.; Bułdak, Ł.; Ruczyński, J.; Kozłowska, A.; Mucha, P.; Rekowski, P.; Szkróbka, W.; Basiak, M.; Bołdys, A.; et al. Monitoring the Transcriptional Activity of Human Endogenous Retroviral HERV-W Family Using PNA Strand Invasion into Double-Stranded DNA. Mol. Biotechnol. 2018, 60, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Uzhameckis, D.; Hedborg, F.; Blomberg, J. Dynamic and selective HERV RNA expression in neuroblastoma cells subjected to variation in oxygen tension and demethylation. APMIS 2016, 124, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Diem, O.; Schäffner, M.; Seifarth, W.; Leib-Mösch, C. Influence of antipsychotic drugs on human endogenous retrovirus (HERV) transcription in brain cells. PLoS ONE 2012, 7, e30054. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Z.; Yin, S.; Chen, Y.; Yu, H.; Zeng, J.; Zhang, Q.; Zhu, F. Human endogenous retrovirus W family envelope gene activates the small conductance Ca2+-activated K+ channel in human neuroblastoma cells through CREB. Neuroscience 2013, 247, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, Q.; Zhou, P.; Li, S.; Zhu, F. HERV-W env regulates calcium influx via activating TRPC3 channel together with depressing DISC1 in human neuroblastoma cells. J. NeuroVirology 2019, 25, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yan, Q.; Liu, L.; Xue, X.; Yao, W.; Li, X.; Li, W.; Ding, S.; Xia, Y.; Zhang, D.; et al. Domesticated HERV-W env contributes to the activation of the small conductance Ca2+-activated K+ type 2 channels via decreased 5-HT4 receptor in recent-onset schizophrenia. Virol. Sin. 2023, 38, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.-J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef] [PubMed]
- Maze, E.A.; Agit, B.; Reeves, S.; Hilton, D.A.; Parkinson, D.B.; Laraba, L.; Ercolano, E.; Kurian, K.M.; Hanemann, C.O.; Belshaw, R.D.; et al. Human Endogenous Retrovirus Type K Promotes Proliferation and Confers Sensitivity to Antiretroviral Drugs in Merlin-Negative Schwannoma and Meningioma. Cancer Res. 2022, 82, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Doucet-O’Hare, T.T.; DiSanza, B.L.; DeMarino, C.; Atkinson, A.L.; Rosenblum, J.S.; Henderson, L.J.; Johnson, K.R.; Kowalak, J.; Garcia-Montojo, M.; Allen, S.J.; et al. SMARCB1 deletion in atypical teratoid rhabdoid tumors results in human endogenous retrovirus K (HML-2) expression. Sci. Rep. 2021, 11, 12893. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Carballo, D.; Klein, J.; Acikelli, A.H.; Wilk, C.; Saka, S.; Jastrow, H.; Wennemuth, G.; Dammann, P.; Giger-Pabst, U.; Khosrawipour, V.; et al. Cytotoxic stress induces transfer of mitochondria-associated human endogenous retroviral RNA and proteins between cancer cells. Oncotarget 2017, 8, 95945. [Google Scholar] [CrossRef] [PubMed]
- Applebaum, M.A.; Desai, A.V.; Bender, J.L.G.; Cohn, S.L. Emerging and investigational therapies for neuroblastoma. Expert Opin. Orphan Drugs 2017, 5, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.A.; Laxman, B.; Dhanasekaran, S.M.; Helgeson, B.E.; Cao, X.; Morris, D.S.; Menon, A.; Jing, X.; Cao, Q.; Han, B.; et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 2007, 448, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Hermans, K.G.; van der Korput, H.A.; van Marion, R.; van de Wijngaart, D.J.; der Made, A.Z.-V.; Dits, N.F.; Boormans, J.L.; van der Kwast, T.H.; van Dekken, H.; Bangma, C.H.; et al. Truncated ETV1, Fused to Novel Tissue-Specific Genes, and Full-Length ETV1 in Prostate Cancer. Cancer Res. 2008, 68, 7541–7549. [Google Scholar] [CrossRef] [PubMed]
- Schiavetti, F.; Thonnard, J.; Colau, D.; Boon, T.; Coulie, P.G. A human endogenous retroviral sequence encoding an antigen recognized on melanoma by cytolytic T lymphocytes. Cancer Res. 2002, 62, 5510–5516. [Google Scholar] [PubMed]
- Goering, W.; Ribarska, T.; Schulz, W. Selective changes of retroelement expression in human prostate cancer. Carcinogenesis 2011, 32, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.G.; Stephens, C.R.; Need, E.F.; Lai, J.; Buchanan, G.; Clements, J.A. Long terminal repeats act as androgen-responsive enhancers for the PSA-kallikrein locus. Endocrinology 2012, 153, 3199–3210. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.G.; Lai, J.; Clements, J. Kallikreins on steroids: Structure, function, and hormonal regulation of prostate-specific antigen and the extended kallikrein locus. Endocr. Rev. 2010, 31, 407–446. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.; Sauter, M.; Schmitt, M.; Baumert, B.; Best, B.; Boese, A.; Roemer, K.; Mueller-Lantzsch, N. Human endogenous retrovirus protein Rec interacts with the testicular zinc-finger protein and androgen receptor. J. Gen. Virol. 2010, 91, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Hanke, K.; Chudak, C.; Kurth, R.; Bannert, N. The Rec protein of HERV-K(HML-2) upregulates androgen receptor activity by binding to the human small glutamine-rich tetratricopeptide repeat protein (hSGT). Int. J. Cancer 2013, 132, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Ali, A.; Tan, S.-H.; Banerjee, S.; Chen, Y.; Cullen, J.; Xavier, C.P.; Mohamed, A.A.; Ravindranath, L.; Srivastav, J.; et al. Autoantibodies against oncogenic ERG protein in prostate cancer: Potential use in diagnosis and prognosis in a panel with C-MYC, AMACR and HERV-K Gag. Genes Cancer 2016, 7, 394–413. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, B.E.; Tomlins, S.A.; Shah, N.; Laxman, B.; Cao, Q.; Prensner, J.R.; Cao, X.; Singla, N.; Montie, J.E.; Varambally, S.; et al. Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res. 2008, 68, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ibba, G.; Piu, C.; Uleri, E.; Serra, C.; Dolei, A. Disruption by SaCas9 Endonuclease of HERV-Kenv, a Retroviral Gene with Oncogenic and Neuropathogenic Potential, Inhibits Molecules Involved in Cancer and Amyotrophic Lateral Sclerosis. Viruses 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Prensner, J.R.; Iyer, M.K.; Sahu, A.; Asangani, I.A.; Cao, Q.; Patel, L.; Vergara, I.A.; Davicioni, E.; Erho, N.; Ghadessi, M.; et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat. Genet. 2013, 45, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Santoni, F.A.; Guerra, J.; Luban, J. HERV-H RNA is abundant in human embryonic stem cells and a precise marker for pluripotency. Retrovirology 2012, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Agliano, A.; Calvo, A.; Box, C. The challenge of targeting cancer stem cells to halt metastasis. Semin. Cancer Biol. 2017, 44, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi-Aoi, M.; Ohnuki, M.; Takahashi, K.; Okita, K.; Noma, H.; Sawamura, Y.; Teramoto, I.; Narita, M.; Sato, Y.; Ichisaka, T.; et al. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 20569–20574. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, G.V. Single cell genomics reveals activation signatures of endogenous SCAR’s networks in aneuploid human embryos and clinically intractable malignant tumors. Cancer Lett. 2016, 381, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, U.; Steidler, A.; Trojan, L.; Michel, M.S.; Seifarth, W.; Fabarius, A.; Hanke, K.; Hohn, O.; Bannert, N.; Ohishi, T.; et al. Smoking increases transcription of human endogenous retroviruses in a newly established in vitro cell model and in normal urothelium. AIDS Res. Hum. Retroviruses 2010, 26, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Kahyo, T.; Tao, H.; Shinmura, K.; Yamada, H.; Mori, H.; Funai, K.; Kurabe, N.; Suzuki, M.; Tanahashi, M.; Niwa, H.; et al. Identification and association study with lung cancer for novel insertion polymorphisms of human endogenous retrovirus. Carcinogenesis 2013, 34, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, S.; Hashizume, S.; Katakura, Y.; Tachibana, H.; Murakami, H. Molecular cloning of yeast cytochrome c-like polypeptide expressed in human lung carcinoma: An antigen recognizable by lung cancer-specific human monoclonal antibody. Vitr. Cell. Dev. Biol.-Anim. 1995, 31, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Kimura, I.; Soper, A.; Coudray, A.; Koyanagi, Y.; Nakaoka, H.; Inoue, I.; Turelli, P.; Trono, D.; Sato, K. Endogenous retroviruses drive KRAB zinc-finger protein family expression for tumor suppression. Sci. Adv. 2020, 6, eabc3020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, H.; Aglave, M.; Lainé, A.; Gallopin, M.; Gautheret, D. The contribution of uncharted RNA sequences to tumor identity in lung adenocarcinoma. NAR Cancer 2022, 4, zcac001. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-C.; Goud, S.; Torcivia, J.; Hu, Y.; Pan, Q.; Kahsay, R.; Blomberg, J.; Mazumder, R. Investigation of somatic single nucleotide variations in human endogenous retrovirus elements and their potential association with cancer. PLoS ONE 2019, 14, e0213770. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, B.; Sun, L.; Yan, X.; Xu, J. Identification of candidate genes or microRNAs associated with the lymph node metastasis of SCLC. Cancer Cell Int. 2018, 18, 161. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yan, Z.; Wu, C.; Zhang, Q.; Zhu, Y.; Li, K.; Xu, Y. Integrated analysis of dosage effect lncRNAs in lung adenocarcinoma based on comprehensive network. Oncotarget 2017, 8, 71430–71446. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.; Boss, J.M. CTCF controls expression and chromatin architecture of the human major histocompatibility complex class II locus. Mol. Cell. Biol. 2010, 30, 4211–4223. [Google Scholar] [CrossRef] [PubMed]
- Kulski, J.K. Long Noncoding RNA HCP5, a Hybrid HLA Class I Endogenous Retroviral Gene: Structure, Expression, and Disease Associations. Cells 2019, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.-H.; Salon, C.; Brambilla, E.; Lavillette, D.; Szecsi, J.; Cosset, F.-L.; Coll, J.-L. Fusogenic membrane glycoproteins induce syncytia formation and death in vitro and in vivo: A potential therapy agent for lung cancer. Cancer Gene Ther. 2010, 17, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Jung, Y.D. Effects of HERV-R env Knockdown in Combination with Ionizing Radiation on Apoptosis-Related Gene Expression in A549 Lung Cancer Cells. Biochem. Physiol. Open Access 2016, 1. [Google Scholar] [CrossRef]
- Kim, J.W.; Eder, J.P. Prospects for targeting PD-1 and PD-L1 in various tumor types. Oncology 2014, 28 (Suppl. S3), 15–28. [Google Scholar] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Solovyov, A.; Vabret, N.; Arora, K.S.; Snyder, A.; Funt, S.A.; Bajorin, D.F.; Rosenberg, J.E.; Bhardwaj, N.; Ting, D.T.; Greenbaum, B.D. Global Cancer Transcriptome Quantifies Repeat Element Polarization between Immunotherapy Responsive and T Cell Suppressive Classes. Cell Rep. 2018, 23, 512–521. [Google Scholar] [CrossRef] [PubMed]
- LaFave, L.M.; Béguelin, W.; Koche, R.; Teater, M.; Spitzer, B.; Chramiec, A.; Papalexi, E.; Keller, M.D.; Hricik, T.; Konstantinoff, K.; et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat. Med. 2015, 21, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, E.E.; Malinzak, E.E.; Rao, S.; Takahashi, Y.; Senchenko, V.N.; Kudryavtseva, A.V.; Nickerson, M.L.; Merino, M.; Hong, J.A.; Schrump, D.S.; et al. Inactivation of the von Hippel–Lindau tumor suppressor leads to selective expression of a human endogenous retrovirus in kidney cancer. Oncogene 2011, 30, 4697–4706. [Google Scholar] [CrossRef] [PubMed]
- Ficial, M.; Jegede, O.A.; Sant’Angelo, M.; Hou, Y.; Flaifel, A.; Pignon, J.-C.; Braun, D.A.; Wind-Rotolo, M.; Sticco-Ivins, M.A.; Catalano, P.J.; et al. Expression of T-Cell Exhaustion Molecules and Human Endogenous Retroviruses as Predictive Biomarkers for Response to Nivolumab in Metastatic Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2021, 27, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Gosenca, D.; Gabriel, U.; Steidler, A.; Mayer, J.; Diem, O.; Erben, P.; Fabarius, A.; Leib-Mösch, C.; Hofmann, W.-K.; Seifarth, W. HERV-E-mediated modulation of PLA2G4A transcription in urothelial carcinoma. PLoS ONE 2012, 7, e49341. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Dulay, P.; Rivera, M.N.; Aramouni, C.; Saxena, V. Neoplastic Pathogenesis Associated with Cigarette Carcinogens. Cureus 2019, 11, e3955. [Google Scholar] [CrossRef] [PubMed]
- Florl, A.R.; Löwer, R.; Schmitz-Dräger, B.J.; Schulz, W.A. DNA methylation and expression of LINE-1 and HERV-K provirus sequences in urothelial and renal cell carcinomas. Br. J. Cancer 1999, 80, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, T.; Zhao, Z.; Chen, Y.; Zeng, J.; Liu, S.; Zhu, F. Mutations in 3′-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene 2014, 33, 3947–3958. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Yu, H.; Zhang, X.; Song, D. Crosstalk between the lncRNA UCA1 and microRNAs in cancer. FEBS Lett. 2019, 593, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Zhang, Z.; Wang, H.-C.; Cai, J.-L.; Xu, Q.-W.; Li, M.-Q.; Chen, Y.-C.; Qian, X.-P.; Lu, T.-J.; Yu, L.-Z.; et al. Rapid Identification of UCA1 as a Very Sensitive and Specific Unique Marker for Human Bladder Carcinoma. Clin. Cancer Res. 2006, 12, 4851–4858. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Wong, T.W.; Cheung, A.H.; Co, C.N.; Kwok, T.T. Induction of drug resistance and transformation in human cancer cells by the noncoding RNA CUDR. RNA 2007, 13, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Chen, W.; Li, X. Urothelial cancer associated 1: A long noncoding RNA with a crucial role in cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, X.; Xie, X.; Zhao, L.; Chen, W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett. 2008, 582, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Neve, B.; Jonckheere, N.; Vincent, A.; Van Seuningen, I. Epigenetic Regulation by lncRNAs: An Overview Focused on UCA1 in Colorectal Cancer. Cancers 2018, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, Z.; He, K.; Qian, J.; Cao, J.; Teng, L. LncRNA UCA1 in anti-cancer drug resistance. Oncotarget 2017, 8, 64638–64650. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Dehghan, M.H.; Dastmalchi, R.; Mashayekhi, F.J.; Salami, S.; Hedayati, M. The roles and role-players in thyroid cancer angiogenesis. Endocr. J. 2019, 66, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Marlow, L.; Copland, J.A. Anaplastic thyroid cancer: Molecular pathogenesis and emerging therapies. Endocr.-Relat. Cancer 2009, 16, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S.E.; Evans, D.B.; Lee, J.E.; Perrier, N.D. Adrenocortical carcinoma. Surg. Oncol. Clin. N. Am. 2006, 15, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhang, H.; Hao, S.; Liu, C.; Xu, J.; Ning, J.; Wu, G.; Jiang, L.; Li, G.; Zheng, H.; et al. Patterns and clinical significance of cervical lymph node metastasis in papillary thyroid cancer patients with Delphian lymph node metastasis. Oncotarget 2017, 8, 57089–57098. [Google Scholar] [CrossRef] [PubMed]
- Fogel, E.L.; Shahda, S.; Sandrasegaran, K.; DeWitt, J.; Easler, J.J.; Agarwal, D.M.; Eagleson, M.; Zyromski, N.J.; House, M.G.; Ellsworth, S.; et al. A Multidisciplinary Approach to Pancreas Cancer in 2016: A Review. Am. J. Gastroenterol. 2017, 112, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Radvanyi, L.; Yin, B.; Rycaj, K.; Li, J.; Chivukula, R.; Lin, K.; Lu, Y.; Shen, J.; Chang, D.Z.; et al. Downregulation of Human Endogenous Retrovirus Type K (HERV-K) Viral env RNA in Pancreatic Cancer Cells Decreases Cell Proliferation and Tumor Growth. Clin. Cancer Res. 2017, 23, 5892–5911. [Google Scholar] [CrossRef] [PubMed]
- Rigogliuso, G.; Biniossek, M.L.; Goodier, J.L.; Mayer, B.; Pereira, G.C.; Schilling, O.; Meese, E.; Mayer, J. A human endogenous retrovirus encoded protease potentially cleaves numerous cellular proteins. Mob. DNA 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, N.; Françoiscasellaa, J.; Gressinb, L.; Heidmann, T. Characterization of the three HERV-H proviruses with an open envelope reading frame encompassing the immunosuppressive domain and evolutionary history in primates. Virology 2001, 279, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Yura, M.; Yamamoto, R.; Kawakami, Y. Induction of immunoregulatory CD271+ cells by metastatic tumor cells that express human endogenous retrovirus H. Cancer Res. 2014, 74, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Coy, J.F.; Knaebel, H.-P.; Linnebacher, M.; Wilz, B.; Gebert, J.; Doeberitz, M.V.K. Expression of an endogenous retroviral sequence from the HERV-H group in gastrointestinal cancers. Int. J. Cancer 2007, 121, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, J.; Senkowski, C.; Tang, Z.; Wang, J.; Huang, T.; Wang, X.; Terry, K.; Brower, S.; Glasgow, W.; et al. Promoter Hypermethylation and Decreased Expression of Syncytin-1 in Pancreatic Adenocarcinomas. PLoS ONE 2015, 10, e0134412. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, T.; Sugimoto, J.; Oda, T.; Jinno, Y.; Kanaya, F. Search for active endogenous retroviruses: Identification and characterization of a HERV-E gene that is expressed in the pancreas and thyroid. J. Hum. Genet. 2001, 46, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Seifarth, W.; Frank, O.; Zeilfelder, U.; Spiess, B.; Greenwood, A.D.; Hehlmann, R.; Leib-Mösch, C. Comprehensive analysis of human endogenous retrovirus transcriptional activity in human tissues with a retrovirus-specific microarray. J. Virol. 2005, 79, 341–352. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, N.; Lazar, V.; Casella, J.-F.; Benit, L.; Heidmann, T. Survey of Human Genes of Retroviral Origin: Identification and Transcriptome of the Genes with Coding Capacity for Complete Envelope Proteins. J. Virol. 2003, 77, 10414–10422. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, N.; Diop, G.; Blaise, S.; Helle, F.; Vasilescu, A.; Matsuda, F.; Heidmann, T. Comprehensive search for intra- and inter-specific sequence polymorphisms among coding envelope genes of retroviral origin found in the human genome: Genes and pseudogenes. BMC Genom. 2005, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.D.; Wendt, G.; Barcellos, L.F.; De Smith, A.J.; Walsh, K.; Metayer, C.; Costello, J.F.; Wiemels, J.L.; Francis, S.S. To ERV Is Human: A Phenotype-Wide Scan Linking Polymorphic Human Endogenous Retrovirus-K Insertions to Complex Phenotypes. Front. Genet. 2018, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, M.; Spadafora, C.; Cignarelli, M.; Barone, C. Anti-tumor activity of non-nucleosidic reverse transcriptase inhibitors. Curr. Pharm. Des. 2007, 13, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, M.; Modoni, S.; Fabiano, A.; Fersini, A.; Barone, C.; Ambrosi, A.; Cignarelli, M. Cell differentiation and iodine-131 uptake in poorly differentiated thyroid tumour in response to nevirapine. Lancet Oncol. 2006, 7, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Modoni, S.; Landriscina, M.; Fabiano, A.; Fersini, A.; Urbano, N.; Ambrosi, A.; Cignarelli, M. Reinduction of cell differentiation and 131I uptake in a poorly differentiated thyroid tumor in response to the reverse transcriptase (RT) inhibitor nevirapine. Cancer Biother. Radiopharm. 2007, 22, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Huang, Y.; Dong, A.; Sun, Y. Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 Possesses Prognostic Significance and Promotes Progression of Papillary Thyroid Cancer. Int. J. Gen. Med. 2022, 15, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Ho, X.D.; Nguyen, H.G.; Trinh, L.H.; Reimann, E.; Prans, E.; Kõks, G.; Maasalu, K.; Le, V.Q.; Nguyen, V.H.; Le, N.T.N.; et al. Analysis of the Expression of Repetitive DNA Elements in Osteosarcoma. Front. Genet. 2017, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Probst, P.; Kopp, J.; Oxenius, A.; Colombo, M.P.; Ritz, D.; Fugmann, T.; Neri, D. Sarcoma Eradication by Doxorubicin and Targeted TNF Relies upon CD8+ T-cell Recognition of a Retroviral Antigen. Cancer Res 2017, 77, 3644–3654. [Google Scholar] [CrossRef] [PubMed]
- Probst, P.; Stringhini, M.; Ritz, D.; Fugmann, T.; Neri, D. Antibody-based Delivery of TNF to the Tumor Neovasculature Potentiates the Therapeutic Activity of a Peptide Anticancer Vaccine. Clin. Cancer Res. 2019, 25, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Mimura-Kimura, Y.; Doores, K.; Golgher, D.; Davis, B.G.; Dwek, R.A.; Rudd, P.M.; Elliott, T. Folding of an MHC class II-restricted tumor antigen controls its antigenicity via MHC-guided processing. Proc. Natl. Acad. Sci. USA 2007, 104, 5983–5988. [Google Scholar] [CrossRef] [PubMed]
- Michna, A.; Schötz, U.; Selmansberger, M.; Zitzelsberger, H.; Lauber, K.; Unger, K.; Hess, J. Transcriptomic analyses of the radiation response in head and neck squamous cell carcinoma subclones with different radiation sensitivity: Time-course gene expression profiles and gene association networks. Radiat. Oncol. 2016, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.M.; Ellisen, L.W. The molecular pathogenesis of head and neck squamous cell carcinoma. J. Clin. Investig. 2012, 122, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, V.N.; Thylur, D.S.; Bauschard, M.; Schmale, I.; Sinha, U.K. Overcoming radioresistance in head and neck squamous cell carcinoma. Oral Oncol. 2016, 63, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-R.; Ahn, K.; Kim, Y.-J.; Jung, Y.-D.; Kim, H.-S. Radiation-Induced Human Endogenous Retrovirus (HERV)-R env Gene Expression by Epigenetic Control. Radiat. Res. 2012, 178, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Johanning, G.L.; Malouf, G.G.; Zheng, X.; Esteva, F.J.; Weinstein, J.N.; Wang-Johanning, F.; Su, X. Expression of human endogenous retrovirus-K is strongly associated with the basal-like breast cancer phenotype. Sci. Rep. 2017, 7, 41960. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.S.; Linnebacher, M. Human endogenous retroviruses and cancer: Causality and therapeutic possibilities. World J. Gastroenterol. 2012, 18, 6027–6035. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.K.; Ørskov, A.D.; Bjerregaard, A.-M.; Unnikrishnan, A.; Holmberg-Thydén, S.; Borch, A.; Jensen, K.V.; Anande, G.; Bentzen, A.K.; Marquard, A.M.; et al. Human endogenous retroviruses form a reservoir of T cell targets in hematological cancers. Nat. Commun. 2020, 11, 5660. [Google Scholar] [CrossRef] [PubMed]
- Kasperek, A.; Béguin, A.; Bawa, O.; De Azevedo, K.; Job, B.; Massard, C.; Scoazec, J.; Heidmann, T.; Heidmann, O. Therapeutic potential of the human endogenous retroviral envelope protein HEMO: A pan-cancer analysis. Mol. Oncol. 2022, 16, 1451–1473. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J.B.; Kim, I.-J.; Chen, L.; Ullah, J.H.; Goodwin, D.A.; Simmons, H.A.; Schenkman, D.I.; von Pelchrzim, F.; Gifford, R.J.; Nimityongskul, F.A.; et al. Vaccination with cancer- and HIV infection-associated endogenous retrotransposable elements is safe and immunogenic. J. Immunol. 2012, 189, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Kraus, B.; Fischer, K.; Sliva, K.; Schnierle, B.S. Vaccination directed against the human endogenous retrovirus-K (HERV-K) gag protein slows HERV-K gag expressing cell growth in a murine model system. Virol. J. 2014, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, G.; Pavanello, S.; Fadda, E.; Buja, A.; Fedeli, U. Yellow fever vaccine 17D administered to healthy women aged between 40 and 54 years halves breast cancer risk: An observational study. Eur. J. Cancer Prev. 2018, 27, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Alcazer, V.; Mutez, V.; Tonon, L.; Martin, J.; Chuvin, N.; Michel, E.; Boulos, R.E.; Estornes, Y.; Valladeau-Guilemond, J.; et al. Identification of shared tumor epitopes from endogenous retroviruses inducing high-avidity cytotoxic T cells for cancer immunotherapy. Sci. Adv. 2022, 8, eabj3671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chinai, J.M.; Buhl, S.; Scandiuzzi, L.; Ray, A.; Jeon, H.; Ohaegbulam, K.C.; Ghosh, K.; Zhao, A.; Scharff, M.D.; et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc. Natl. Acad. Sci. USA 2013, 110, 9879–9884. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, J.; Sugimoto, M.; Bernstein, H.; Jinno, Y.; Schust, D. A novel human endogenous retroviral protein inhibits cell-cell fusion. Sci. Rep. 2013, 3, 01462. [Google Scholar] [CrossRef] [PubMed]
- Malfavon-Borja, R.; Feschotte, C. Fighting Fire with Fire: Endogenous Retrovirus Envelopes as Restriction Factors. J. Virol. 2015, 89, 4047–4050. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.L.; Hunter, D.; Schertzer, M.; Freeman, J. Endogenous Retroviruses Provide the Primary Polyadenylation Signal for Two New Human Genes (HHLA2 and HHLA3). Genomics 1999, 59, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, M.; Chinai, J.M.; Fineberg, S.; Fiser, A.; Montagna, C.; Medavarapu, R.; Castano, E.; Jeon, H.; Ohaegbulam, K.C.; Zhao, R.; et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin. Cancer Res. 2015, 21, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Dong, W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. OncoTargets Ther. 2018, 11, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Borczuk, A.; Janakiram, M.; Ren, X.; Lin, J.; Assal, A.; Halmos, B.; Perez-Soler, R.; Zang, X. Wide Expression and Significance of Alternative Immune Checkpoint Molecules, B7x and HHLA2, in PD-L1-Negative Human Lung Cancers. Clin. Cancer Res. 2018, 24, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Janakiram, M.; Borczuk, A.; Lin, J.; Qiu, W.; Liu, H.; Chinai, J.M.; Halmos, B.; Perez-Soler, R.; Zang, X. HHLA2, a New Immune Checkpoint Member of the B7 Family, Is Widely Expressed in Human Lung Cancer and Associated with EGFR Mutational Status. Clin. Cancer Res. 2017, 23, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Farrag, M.S.; Ibrahim, E.M.; El-Hadidy, T.A.; Akl, M.F.; Elsergany, A.R.; Abdelwahab, H.W. Human Endogenous Retrovirus-H Long Terminal Repeat- Associating Protein 2 (HHLA2) is a Novel Immune Checkpoint Protein in Lung Cancer which Predicts Survival. Asian Pac. J. Cancer Prev. 2021, 22, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.-Y.; Fu, Y.-P.; Yi, Y.; Zhang, M.-X.; Zheng, S.-S.; Huang, J.-L.; Gan, W.; Xu, X.; Lin, J.-J.; Zhang, J.; et al. HHLA2 in intrahepatic cholangiocarcinoma: An immune checkpoint with prognostic significance and wider expression compared with PD-L1. J. Immunother. Cancer 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Ye, H.; Wang, J.; Chen, S.; Chen, X.; Zhang, C. Immune Checkpoint Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 is Upregulated and Independently Predicts Unfavorable Prognosis in Bladder Urothelial Carcinoma. Nephron 2019, 141, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ran, Z.; Liu, M.; Ou, Y. Prognostic Significance of Potential Immune Checkpoint Member HHLA2 in Human Tumors: A Comprehensive Analysis. Front. Immunol. 2019, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, W.; Xu, Y.; Zhu, M.; Xiao, Y.; Shen, Y.; Zhu, S.; Cao, C.; Xu, X. Upregulated immune checkpoint HHLA2 in clear cell renal cell carcinoma: A novel prognostic biomarker and potential therapeutic target. J. Med. Genet. 2019, 56, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, D.; Feng, J.; Zhou, Y.; Wang, Q.; Feng, H.; Zhang, J.; Jiang, J. Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qiu, W.; de Gonzalez, A.K.K.; Wei, J.-S.; Tu, M.; Xi, C.-H.; Yang, Y.-R.; Peng, Y.-P.; Tsai, W.-Y.; Remotti, H.E.; et al. HHLA2 is a novel immune checkpoint protein in pancreatic ductal adenocarcinoma and predicts post-surgical survival. Cancer Lett. 2019, 442, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.T.; Paniccia, A.; Kaplan, J.; Koenig, M.; Kahn, N.; Wilson, L.; Chen, L.; Schulick, R.D.; Edil, B.H.; Zhu, Y. Expression of the Novel Costimulatory Molecule B7-H5 in Pancreatic Cancer. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S1574–S1579. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yang, Y.; Zhang, N.; Soto, C.; Jiang, X.; An, Z.; Zheng, W. Human Endogenous Retroviruses in Glioblastoma Multiforme. Microorganisms 2021, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Roth, M.E.; Gill, J.; Chinai, J.M.; Ewart, M.R.; Piperdi, S.; Geller, D.S.; Hoang, B.H.; Fatakhova, Y.V.; Ghorpade, M.; et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci. Rep. 2016, 6, 31154. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, H.; Yang, L.-L.; Mao, L.; Wu, C.-C.; Zhang, W.-F.; Sun, Z.-J. The Expression Patterns and Associated Clinical Parameters of Human Endogenous Retrovirus-H Long Terminal Repeat-Associating Protein 2 and Transmembrane and Immunoglobulin Domain Containing 2 in Oral Squamous Cell Carcinoma. Dis. Markers 2019, 2019, 5421985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, J.; Ye, J.; Zhang, X. Prognostic value of HHLA2 expression in solid tumors: A meta-analysis based on the Chinese population. Medicine 2021, 100, e26789. [Google Scholar] [CrossRef] [PubMed]
- Shimonosono, M.; Arigami, T.; Yanagita, S.; Matsushita, D.; Uchikado, Y.; Kijima, Y.; Kurahara, H.; Kita, Y.; Mori, S.; Sasaki, K.; et al. The association of human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2) expression with gastric cancer prognosis. Oncotarget 2018, 9, 22069–22078. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.S.; Berjis, A.; Konge, J.C.; Mahoney, K.M.; Klee, A.N.; Freeman, S.S.; Chen, C.-H.; Jegede, O.A.; Catalano, P.J.; Pignon, J.-C.; et al. KIR3DL3 Is an Inhibitory Receptor for HHLA2 that Mediates an Alternative Immunoinhibitory Pathway to PD1. Cancer Immunol. Res. 2021, 9, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Perron, H.; Garson, J.A.; Bedin, F.; Beseme, F.; Paranhos-Baccala, G.; Komurian-Pradel, F.; Mallet, F.; Tuke, P.W.; Voisset, C.; Blond, J.L.; et al. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. The Collaborative Research Group on Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 1997, 94, 7583–7588. [Google Scholar] [CrossRef] [PubMed]
- Ryan, F.P. Human endogenous retroviruses in multiple sclerosis: Potential for novel neuro-pharmacological research. Curr. Neuropharmacol. 2011, 9, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Diebold, M.; Derfuss, T. The monoclonal antibody GNbAC1: Targeting human endogenous retroviruses in multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419833574. [Google Scholar] [CrossRef] [PubMed]
- Porchet, H.; Vidal, V.; Kornmann, G.; Malpass, S.; Curtin, F. A High-dose Pharmacokinetic Study of a New IgG4 Monoclonal Antibody Temelimab/GNbAC1 Antagonist of an Endogenous Retroviral Protein pHERV-W Env. Clin. Ther. 2019, 41, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Curtin, F.; Perron, H.; Kromminga, A.; Porchet, H.; Lang, A.B. Preclinical and early clinical development of GNbAC1, a humanized IgG4 monoclonal antibody targeting endogenous retroviral MSRV-Env protein. MAbs 2015, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).