Free Fatty Acids from Cow Urine DMSO Fraction Induce Cell Death in Breast Cancer Cells without Affecting Normal GMSCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CUDF
2.3. Cell Line Maintenance and Preparation for Drug Treatment
2.4. Trypan Blue Dye Exclusion Assay
2.5. MTT Cell Cytotoxicity Assay
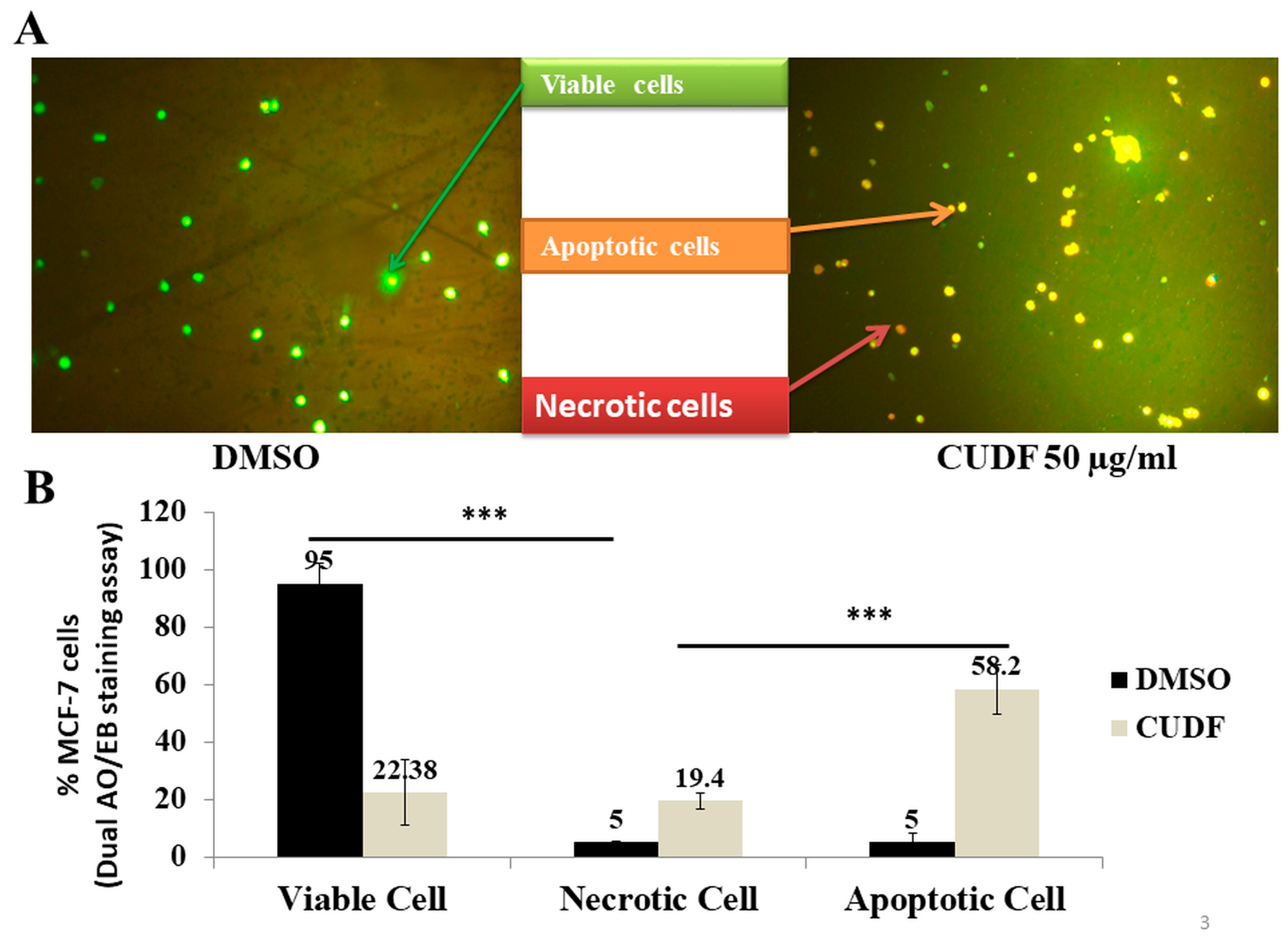
2.6. Dual AO/EB Fluorescent Staining
2.7. Flow-Cytometry-Based Apoptosis Assay
2.8. Effects on Human Gingival Mesenchymal Stem Cells (hGMSCs)
2.9. Preparation and Purification of Intracellular Metabolites by VTGE
2.10. Molecular Docking
2.11. Molecular Dynamics (MD) Simulations
3. Results
3.1. Effects of CUDF on Cell Viability
3.2. Effects of CUDF on Normal Gingival Mesenchymal Stem Cells (GMSCs)
3.3. Intracellular Metabolite Profiling
3.4. Molecular Docking
3.5. Molecular Dynamics (MD) Simulations
3.6. vNN Web Server ADMET Predictions
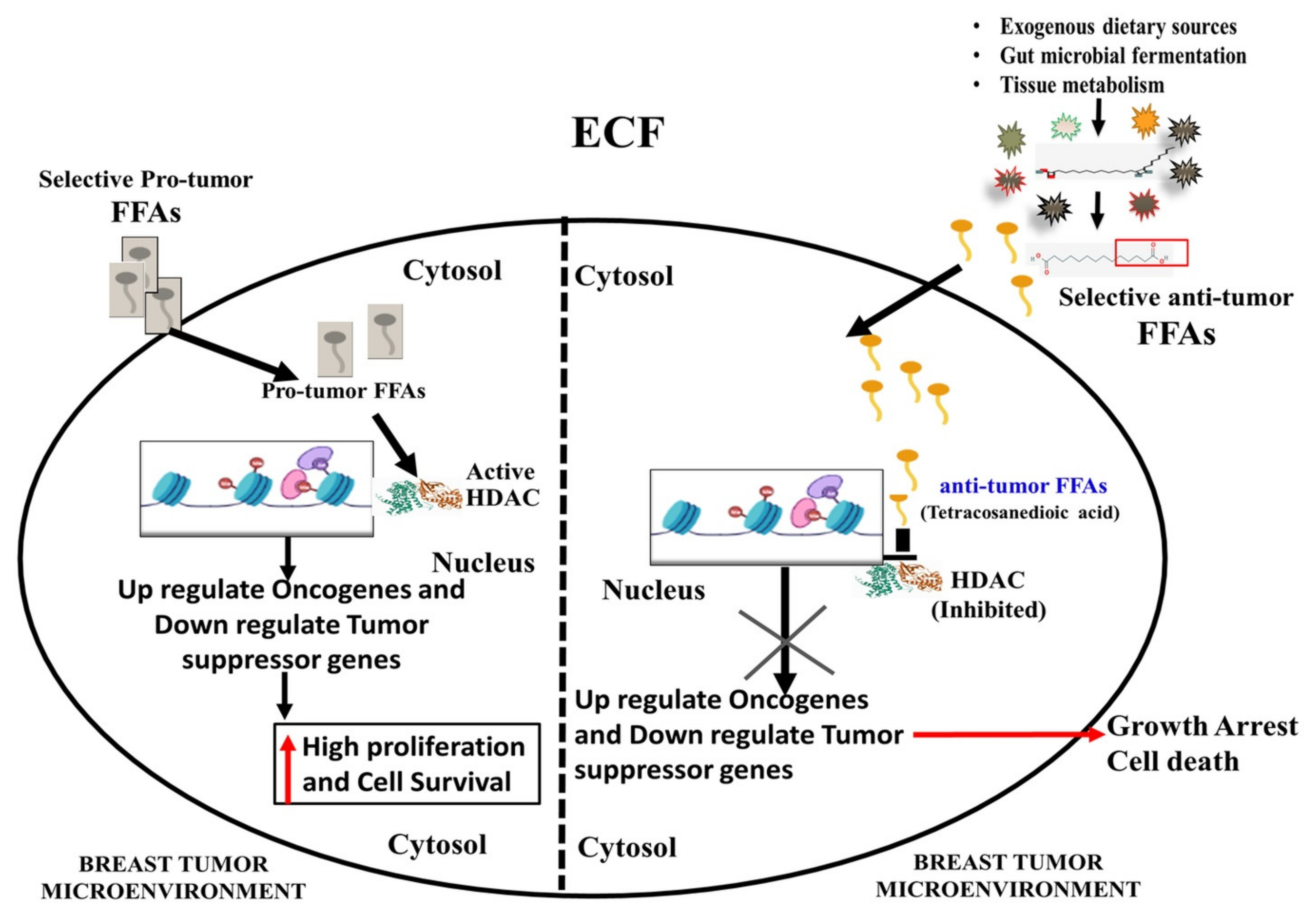
4. Discussion
5. Future Perspectives
- This paper provides a platform to discuss xeno-tumor heterogeneity, which is an interesting aspect in the cancer field to understand the contributing factors that create distinctive susceptibility and resistance across the species, such as humans versus ruminants such as cows.
- Ruminants such as cows and goats are known for rare occurrence of mammary cancers relative to humans, who show high susceptibility.
- There are various explanations for this difference, including evolution, genetic adaptations, and mutations. Nevertheless, the abundance of a set of FFAs derived from dietary and gut microbial fermentations, as well as their potential interference with cancer cell growth and proliferation, requires detailed investigations in the future to support the idea of xeno-tumor heterogeneity.
- Evaluation of FFA-enriched CUDF as antiproliferative and proapoptotic agents should be carefully interpreted based on the caspase 3 and p53 status in breast cancer cells.
- The inhibitory role of these COFFAs against HDAC is warranted at in vivo, preclinical, and clinical levels as anticancer pharmaceutical compositions.
- Our propositions with respect to the role of FFAs as inhibitors of HDAC and their potential role as a source of antiproliferative and proapoptotic agents should be viewed as a source of combinatorial therapies with specific consideration regarding the status of caspase 3 and p53 status in breast cancer cells.
- COFFAs proposed as anticancer compositions can usually cross the cell membrane in a passive diffusion process. However, the use of nanocarriers for better drug delivery of COFFAs is also proposed.
6. Conclusions
7. Novelty and Impact Statements
- This paper reports on intracellular free fatty acids (FFAs) derived from cow urine DMSO fraction (CUDF) during the treatment of MCF-7 breast cancer cells;
- This observation is the first report on the evaluation of intracellular FFAs in CUDF-treated MCF-7 cells by employing a novel in-house developed vertical tube gel electrophoresis (VTGE) system that helped in intracellular metabolite profiling;
- Furthermore, molecular docking and molecular dynamics (MD) simulations predicted the role of FFAs as inhibitors of HDACs that may potentially link the apoptotic effects in MCF-7 cells by FFA-enriched CUDF;
- This FFA fraction does not cause cell death in human gingival mesenchymal stem cells (hGMSCs), indicating its nontoxic effect on normal cells;
- This is the first report discriminating normal cells from cancer cells by employing FFAs derived from CUDF.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Suvà, M.L.; Riggi, N.; Bernstein, B.E. Epigenetic reprogramming in cancer. Science 2013, 339, 1567–1570. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Kumar, A.; Swami, S.; Sharma, N.K. Distinct DNA Metabolism and Antiproliferative Effects of Goat Urine Metabolites: An Explanation for Xeno-tumor Heterogeneity. Curr. Chem. Biol. 2020, 14, 1. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.; Thormar, H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS 2001, 109, 670–678. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef]
- Kovács, T.; Mikó, E.; Ujlaki, G.; Sári, Z.; Bai, P. The Microbiome as a Component of the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1225, 137–153. [Google Scholar]
- Vaughan, E.E.; Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar]
- Tinsley, I.J.; Schmitz, J.A.; Pierce, D.A. Influence of dietary fatty acids on the incidence of mammary tumors in the C3H mouse. Cancer Res. 1981, 41, 1460–1465. [Google Scholar]
- Waluga, M.; Żorniak, M.; Fichna, J.; Kukla, M.; Hartleb, M. Pharmacological and dietary factors in the prevention of colorectal cancer. J. Physiol. Pharmacol. 2018, 69, 325–336. [Google Scholar]
- Acevedo, C.; Amaya, C.; López-Guerra, J.L. Rare breast tumors: Review of the literature. Rep. Pract. Oncol. Radiother. 2013, 19, 267–274. [Google Scholar] [CrossRef]
- Sweet, W.W.; Matthews, C.A.; Graves, R.R. Extreme rarity of cancer in cow’s udder. J. Dairy Sci. 1940, 23, 437–446. [Google Scholar]
- Pascual, G.; Domínguez, D.; Elosúa-Bayes, M.; Beckedorff, F.; Laudanna, C.; Bigas, C.; Douillet, D.; Greco, C.; Symeonidi, A.; Hernández, I.; et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 2021, 599, 485–490. [Google Scholar] [CrossRef]
- Giulitti, F.; Petrungaro, S.; Mandatori, S.; Tomaipitinca, L.; de Franchis, V.; D’Amore, A.; Filippini, A.; Gaudio, E.; Ziparo, E.; Giampietri, C. Anti-tumor Effect of Oleic Acid in Hepatocellular Carcinoma Cell Lines via Autophagy Reduction. Front. Cell Dev. Biol. 2021, 9, 629182. [Google Scholar] [CrossRef]
- Bégin, M.E.; Ells, G.; Horrobin, D.F. Polyunsaturated fatty acid-induced cytotoxicity against tumor cells and its relationship to lipid peroxidation. J. Natl. Cancer Inst. 1988, 80, 188–194. [Google Scholar] [CrossRef]
- Abdi-Dezfuli, F.; Berge, R.K.; Rasmussen, M.; Thorsen, T.; Aakvaag, A. Effects of saturated and polyunsaturated fatty acids and their 3-thia fatty acid analogues on MCF-7 breast cancer cell growth. Ann. N. Y. Acad. Sci. 1994, 744, 306–309. [Google Scholar] [CrossRef]
- Colquhoun, A.; Curi, R. Effects of saturated and polyunsaturated fatty acids on human tumor-cell proliferation. Gen. Pharmacol. 1998, 30, 191–194. [Google Scholar] [CrossRef]
- Albino, A.P.; Juan, G.; Traganos, F.; Reinhart, L.; Connolly, J.; Rose, D.P.; Darzynkiewicz, Z. Cell cycle arrest and apoptosis of melanoma cells by docosahexaenoic acid: Association with decreased pRb phosphorylation. Cancer Res. 2000, 60, 4139–4145. [Google Scholar] [PubMed]
- Hardy, S.; El-Assaad, W.; Przybytkowski, E.; Joly, E.; Prentki, M.; Langelier, Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J. Biol. Chem. 2003, 278, 31861–31870. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharmacol. 2020, 871, 172937. [Google Scholar] [CrossRef] [PubMed]
- Nakkarach, A.; Foo, H.L.; Song, A.A.; Mutalib, N.E.A.; Nitisinprasert, S.; Withayagiat, U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb. Cell Fact. 2021, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Shen, Z.Y.; Zhan, Y.Z.; Feng, X.C.; Chen, K.L.; Li, Y.S.; Deng, H.J.; Pan, S.M.; Wu, D.H.; Ding, Y. CD36 inhibits β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat. Commun. 2019, 10, 3981. [Google Scholar] [CrossRef]
- Drury, J.; Rychahou, P.G.; He, D.; Jafari, N.; Wang, C.; Lee, E.Y.; Weiss, H.L.; Evers, B.M.; Zaytseva, Y.Y. Inhibition of Fatty Acid Synthase Upregulates Expression of CD36 to Sustain Proliferation of Colorectal Cancer Cells. Front. Oncol. 2020, 10, 1185. [Google Scholar] [CrossRef]
- Weeks, E.; Shapiro, M.; Burns, R.O.; Wakil, S.J. Control of fatty acid metabolism: Induction of the enzymes of fatty acid oxidation in Escherichia coli. J. Bacteriol. 1969, 97, 827–836. [Google Scholar] [CrossRef]
- Uchio, R.; Shiio, J. Microbial production of long-chain dicarboxylic acids from n-alkanes. Agric. Biol. Chem. 1972, 36, 426–433. [Google Scholar] [CrossRef]
- Broadway, N.M.; Dickinson, F.M.; Ratledge, C. Acyl-CoA intermediates of β-oxidation of mono- and dicarboxylic acids by extracts of Corynebacterium sp. strain 7E1C. Biochem. J. 1992, 285, 117–222. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, H.M.; Hu, G.R.; Li, F.L. Biosynthesis of nervonic acid and perspectives for its production by microalgae and other microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3027–3035. [Google Scholar] [CrossRef]
- Tsuboi, K. 2-Hydroxylated fatty acids as candidates of novel drugs to promote chemosensitivity of gastric cancer. EBioMedicine 2019, 41, 19–20. [Google Scholar] [CrossRef]
- Passi, S.; Picardo, M.; De Luca, C.; Nazzaro-Porro, M.; Rossi, L.; Rotilio, G. Saturated dicarboxylic acids as products of unsaturated fatty acid oxidation. Biochim. Biophys. Acta 1993, 1168, 190–198. [Google Scholar] [CrossRef]
- Finnin, M.S.; Donigian, J.R.; Cohen, A.; Richon, V.M.; Rifkind, R.A.; Marks, P.A.; Breslow, R.; Pavletich, N.P. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 1999, 401, 188–193. [Google Scholar] [CrossRef]
- Shao, Y.; Gao, Z.; Marks, P.A.; Jiang, X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 2004, 101, 18030–18035. [Google Scholar] [CrossRef]
- Xu, W.S.; Parmigiani, R.B.; Marks, P.A. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene 2007, 26, 5541–5552. [Google Scholar] [CrossRef]
- Dawood, M.; Fleischer, E.; Klinger, A.; Bringmann, G.; Shan, L.; Efferth, T. Inhibition of cell migration and induction of apoptosis by a novel class II histone deacetylase inhibitor, MCC2344. Pharm. Res. 2020, 160, 105076. [Google Scholar] [CrossRef]
- Sanford, J.A.; Zhang, L.J.; Williams, M.R.; Gangoiti, J.A.; Huang, C.M.; Gallo, R.L. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol. 2016, 1, eaah4609. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; To, N.B.; Lim, Y.; Cho, S.K. Odd-chain fatty acids as novel histone deacetylase 6 (HDAC6) inhibitors. Biochimie 2021, 186, 147–156. [Google Scholar] [CrossRef]
- Kumar, A.; Bhatkar, D.; Purohit, S.; Jahagirdar, D.; Sharma, N.K. Non-homologous end joining inhibitor SCR-7 to exacerbate low dose doxorubicin cytotoxicity in HeLa. J. Cancer Prev. 2017, 22, 47–54. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, S.; Bhatkar, D.; Sarode, S.C.; Sharma, N.K. A novel method to detect intracellular metabolite alterations in MCF-7 cells by doxorubicin induced cell death. Metabolomics 2021, 17, 3. [Google Scholar] [CrossRef]
- Hanwell, M.; Curtis, D.; Lonie, D.; Vandermeersch, T.; Zurek, E.; Hutchison, G. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and and Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 9, 1639–1662. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2. 0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Lokhande, K.B.; Nagar, S.; Swamy, K.V. Molecular interaction studies of Deguelin and its derivatives with Cyclin D1 and Cyclin E in cancer cell signaling pathway: The computational approach. Sci. Rep. 2019, 9, 1778. [Google Scholar] [CrossRef]
- Lopergolo, A.; Pennati, M.; Gandellini, P.; Orlotti, N.I.; Poma, P.; Daidone, M.G.; Folini, M.; Zaffaroni, N. Apollon gene silencing induces apoptosis in breast cancer cells through p53 stabilisation and caspase-3 activation. Br. J. Cancer 2009, 100, 739–746. [Google Scholar] [CrossRef]
- Ray, D.; Murphy, K.R.; Gal, S. The DNA binding and accumulation of p53 from breast cancer cell lines and the link with serine 15 phosphorylation. Cancer Biol. Ther. 2012, 13, 848–857. [Google Scholar] [CrossRef]
- Nemoto, T.; Yoshino, G.; Ojika, M.; Sakagami, Y. Amphimic acids and related long-chain fatty acids as DNA topoisomerase I inhibitors from an Australian sponge, Amphimedon sp.: Isolation, structure, synthesis, and biological evaluation. Tetrahedron 1997, 53, 16699–16710. [Google Scholar] [CrossRef]
- Carballeira, N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid. Res. 2008, 47, 50–61. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Makarova EKh Khusnutdinova, E.K.; Dzhemilev, U.M. The facile synthesis of the 5Z,9Z-dienoic acids and their topoisomerase I inhibitory activity. Chem. Commun. 2013, 49, 8401–8403. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; Makarov, A.A.; Andreev, E.N.; Makarova, E.K.; Yunusbaeva, M.M.; D’yakonov, V.A.; Dzhemilev, U.M. New 1,3-Diynoic Derivatives of Natural Lembehyne B: Stereoselective Synthesis, Anticancer, and Neuritogenic Activity. ACS Omega 2020, 5, 1974–1981. [Google Scholar] [CrossRef]
- Makarov, A.A.; Dzhemileva, L.U.; Salimova, A.R.; Makarova, E.K.; Ramazanov, I.R.; D’yakonov, V.A.; Dzhemilev, U.M. New synthetic analogues of natural 5Z,9Z-dienoic acids: Stereoselective synthesis and study of the anticancer activity. Bioorg. Chem. 2020, 104, 104303. [Google Scholar] [CrossRef]









| Sr. No | Name of Free Fatty Acid | DMSO-Treated MCF-7 Cell Lysate | CUDF-Treated MCF-7 Cell Lysate | RT | m/z | M.W. |
|---|---|---|---|---|---|---|
| 1 | 13(Z)-Docosenoic acid (monounsaturated fatty acid) | Not detectable | Detectable | 10.898 | 343.2923 | 338.6 |
| 2 | Nervonic acid (monounsaturated fatty acids) | Not detectable | 45529 | 12.835 | 371.3225 | 317.289 |
| 3 | Tetracosanedioic acid (Dicarboxylic saturated fatty acid) | Not detectable | Detectable | 14.871 | 399.3537 | 398.6 |
| 4 | 3-Hydroxytridecanoic acid (Hydroxy saturated fatty acid) | Not detectable | Detectable | 14.322 | 235.1664 | 230.34 |
| 5 | 8-Hydroxy Caprylic acid (Hydroxy saturated fatty acid) | Not detectable | Detectable | 4.433 | 824.381 | 160.21 |
| Name of Ligand (PubChem) | Protein PDB ID and Name of the Chain | Binding Affinity (−kcal/mol) | Binding Amino Acid Residues | No. of Bonds | Distance of Bonds |
|---|---|---|---|---|---|
| 13-Docosenoic acid(monounsaturated fatty acid) (PubChem CID 8216) | 1C3R-Histone deacetylase (HDAC) | −7.5 | Pro18 His21 Tyr91 Phe141 Ala197 Phe198 Phe200 Leu265 | 2 H Bonds and 8 Pi Bonds | 3.4 3.7 3.2 3.6 4.5 4.7 5.0 6.7 |
| Nervonic acid Monounsaturated (PubChem CID 5281120) | 1C3R-Histone deacetylase (HDAC) | −6.5 | Tyr91 Tyr196 Phe200 Leu265 Phe335 Phe338 | 1 H Bond and 5 Pi Bonds | 5.0 6.0 4.3 6.5 5.5 5.3 |
| Tetracosanedioic acid (saturated fatty acid) (PubChem CID 119039) | 1C3R-Histone deacetylase (HDAC) | −7.0 | Tyr297 Leu265 Phe198 Phe141 His132 Pro22 | 2 H Bonds and 8 Pi Bonds | 2.5 2.3 3.7 4.4 3.1 |
| 3-Hydroxycapric acid (saturated fatty acid) (PubChem CID 69820) | 1C3R-Histone deacetylase (HDAC) | −6.1 | Try17 Ala98 Ser103 Ser23 Tyr12 Arg27 | 5 H Bonds and 8 Pi Bonds | 2.1 3.2 4.6 5.2 2.5 4.6 |
| 3-Hydroxytridecanoic acid (saturated fatty acid) (PubChem CID 5312749) | 1C3R-Histone deacetylase (HDAC) | −6.6 | Ile25 Ser29 Arg16 Leu23 Ala98 Ala106 | 3 H Bonds and 8 Pi Bonds (1 unfavorable) | 2.7 3.5 6.4 3.5 4.6 3.8 |
| Trichostatin A(+) (PubChem CID 444732) | 1C3R-Histone deacetylase (HDAC) | −9.3 | Tyr297 Phe200 Phe198 Gly140 His132 Pro22 | 2 H Bonds and 8 Pi Bonds | 4.0 3.4 3.3 3.2 5.1 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, A.K.; Upadhyay, V.; Lokhande, K.B.; Swamy, K.V.; Bhonde, R.R.; Sarode, S.C.; Sharma, N.K. Free Fatty Acids from Cow Urine DMSO Fraction Induce Cell Death in Breast Cancer Cells without Affecting Normal GMSCs. Biomedicines 2023, 11, 889. https://doi.org/10.3390/biomedicines11030889
Raj AK, Upadhyay V, Lokhande KB, Swamy KV, Bhonde RR, Sarode SC, Sharma NK. Free Fatty Acids from Cow Urine DMSO Fraction Induce Cell Death in Breast Cancer Cells without Affecting Normal GMSCs. Biomedicines. 2023; 11(3):889. https://doi.org/10.3390/biomedicines11030889
Chicago/Turabian StyleRaj, Ajay Kumar, Vidhi Upadhyay, Kiran Bharat Lokhande, K. Venkateswara Swamy, Ramesh Ramchandra Bhonde, Sachin C. Sarode, and Nilesh Kumar Sharma. 2023. "Free Fatty Acids from Cow Urine DMSO Fraction Induce Cell Death in Breast Cancer Cells without Affecting Normal GMSCs" Biomedicines 11, no. 3: 889. https://doi.org/10.3390/biomedicines11030889
APA StyleRaj, A. K., Upadhyay, V., Lokhande, K. B., Swamy, K. V., Bhonde, R. R., Sarode, S. C., & Sharma, N. K. (2023). Free Fatty Acids from Cow Urine DMSO Fraction Induce Cell Death in Breast Cancer Cells without Affecting Normal GMSCs. Biomedicines, 11(3), 889. https://doi.org/10.3390/biomedicines11030889






