Bag-1 Protects Nucleus Pulposus Cells from Oxidative Stress by Interacting with HSP70
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of NP Cells, Hypoxic Culture Conditions, and Cell Treatments
2.2. Bag-1 Overexpression
2.3. Immunohistological Studies
2.4. Cell Viability Assay
2.5. Measurement of Intracellular ROS
2.6. Detection of Mitochondrial Membrane Potential
2.7. Real-Time RT-PCR Analysis
2.8. Protein Extraction, Western Blot Analysis, and Immunoprecipitation
2.9. Statistical Analysis
3. Results
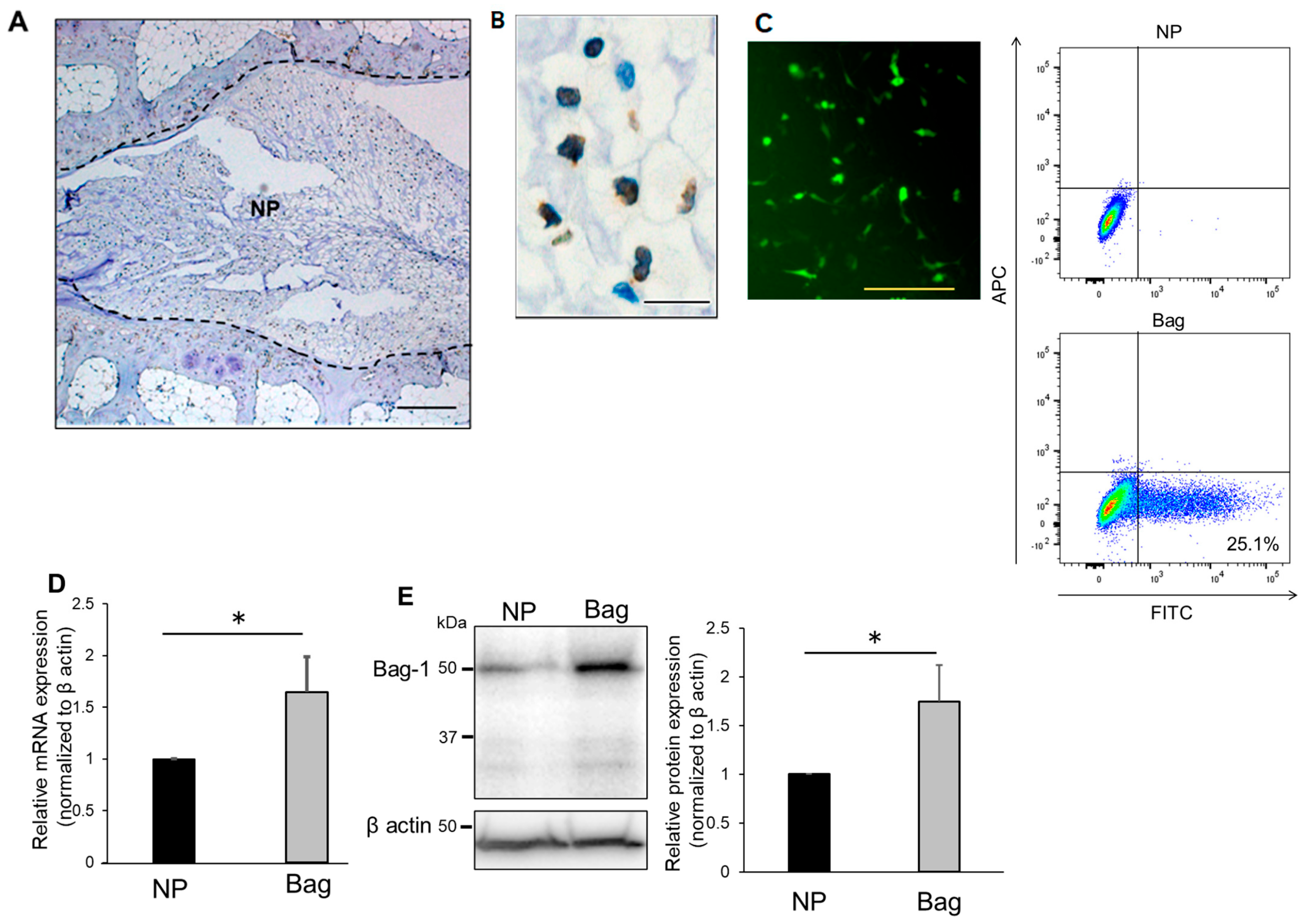
3.1. Evaluation of Bag-1 Expression in NP Cells
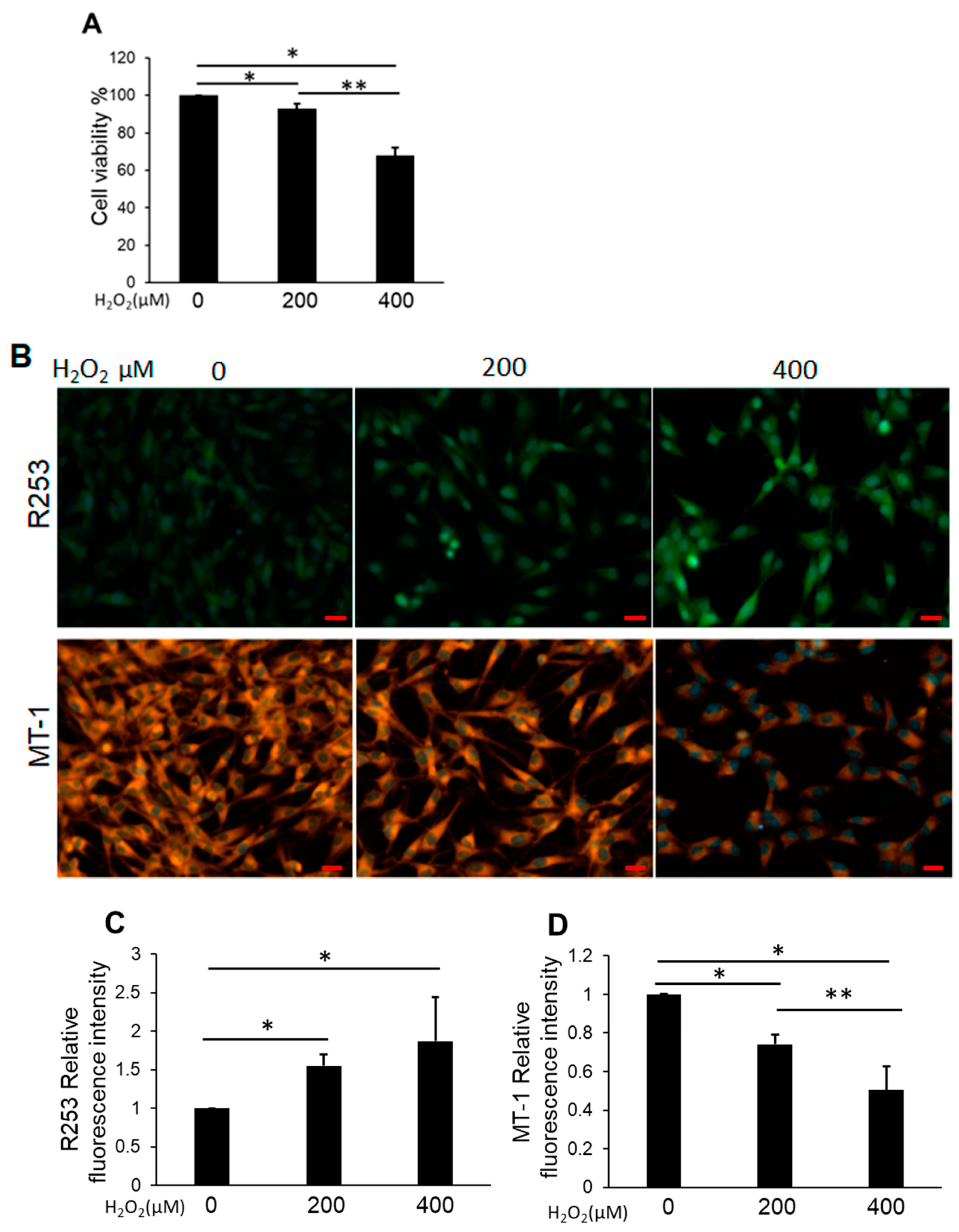
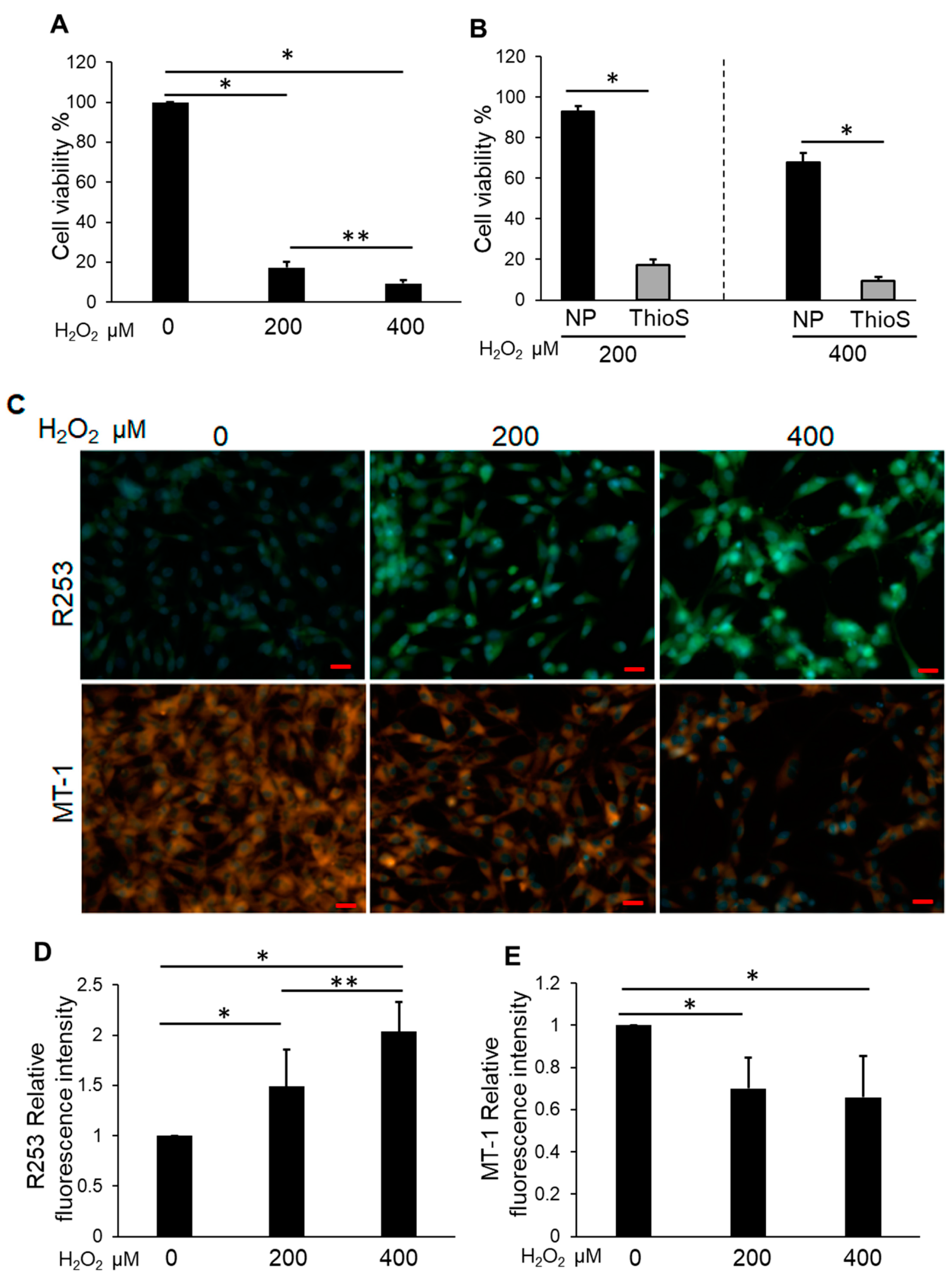
3.2. H2O2 Impaired NP Cell Viability and Mitochondrial Function and Increased Intracellular ROS
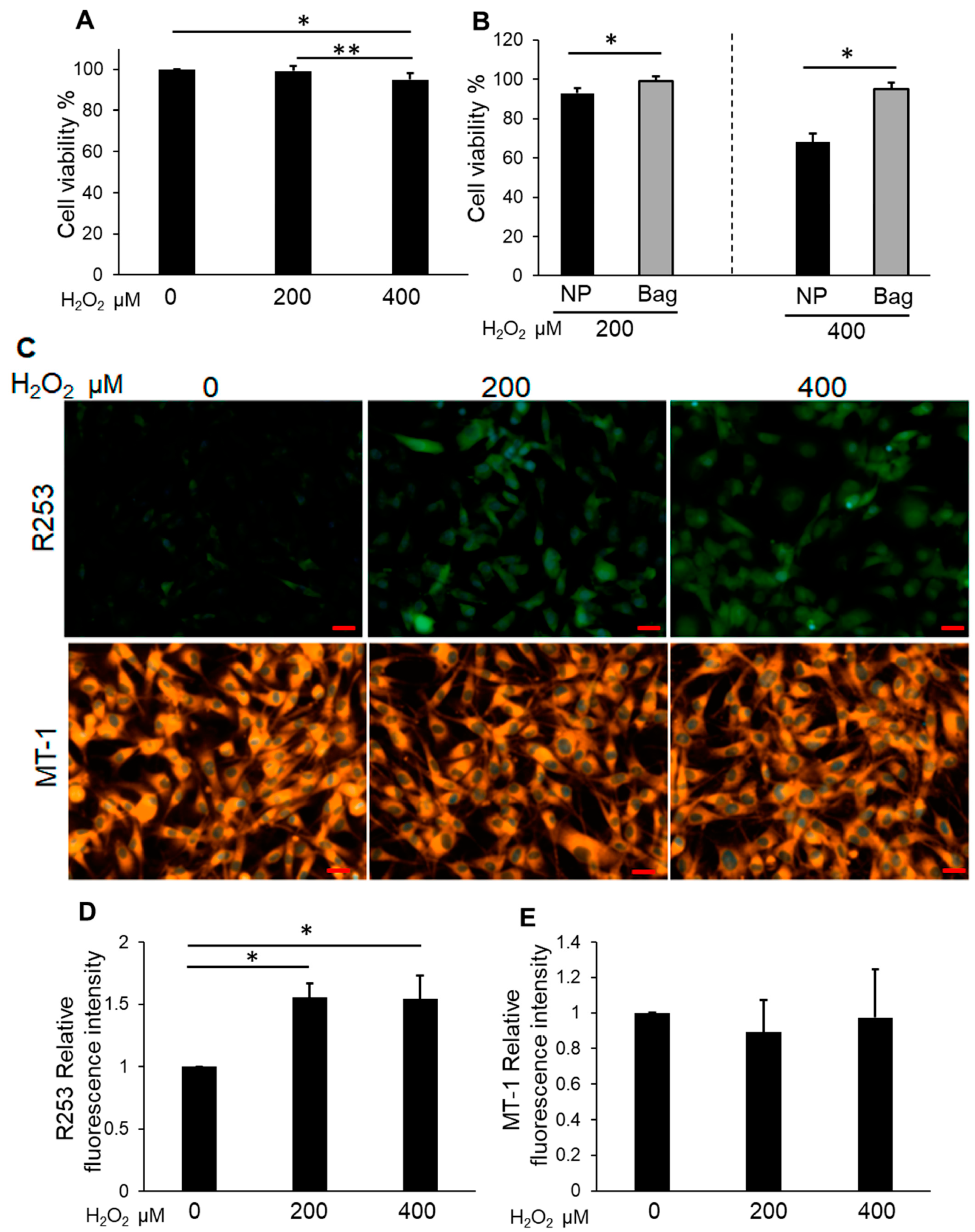
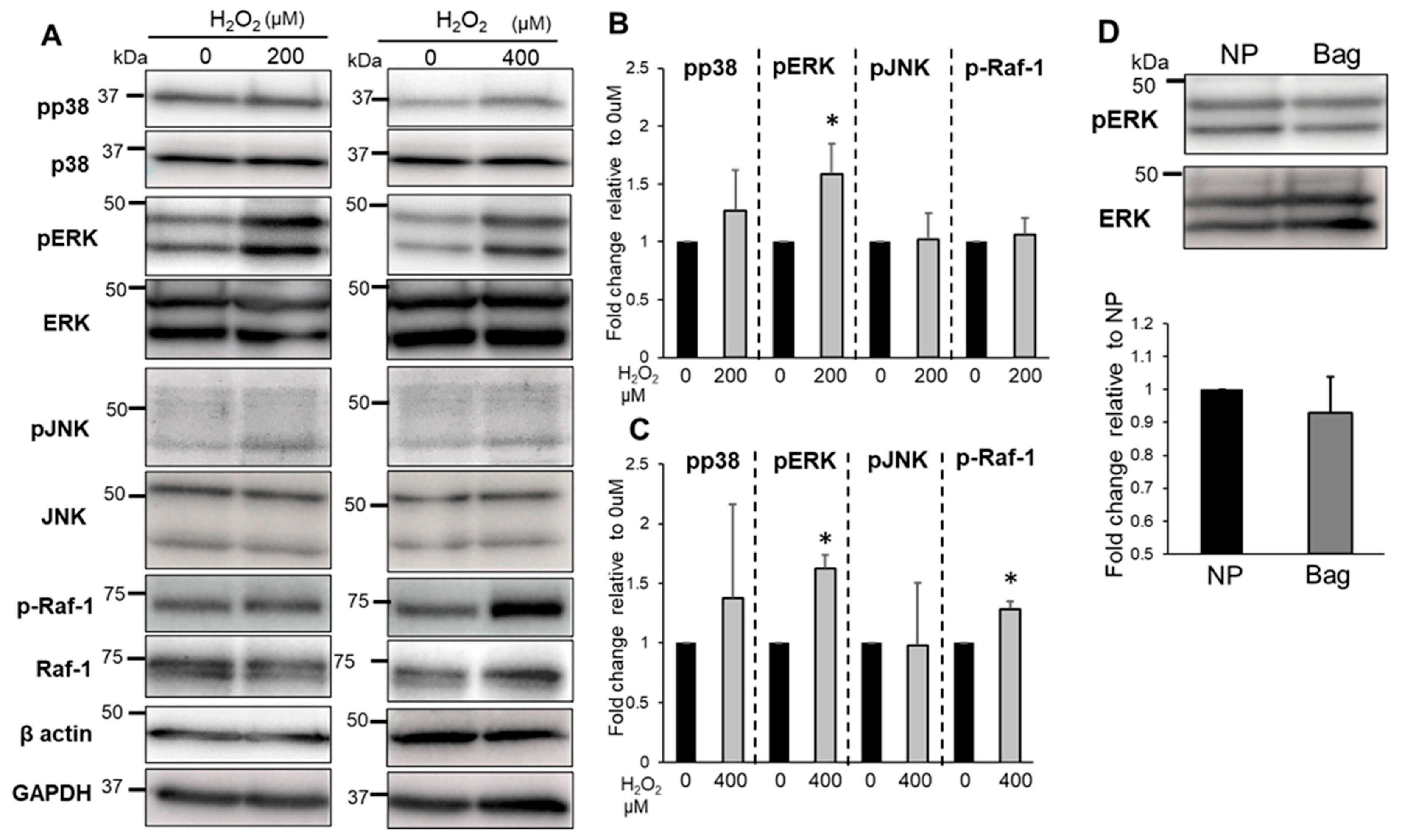
3.3. Bag-1-Overexpressing NP Cells Attenuate the Effect of H2O2 on Cell Viability, Mitochondrial Function, and Increased Intracellular ROS Levels
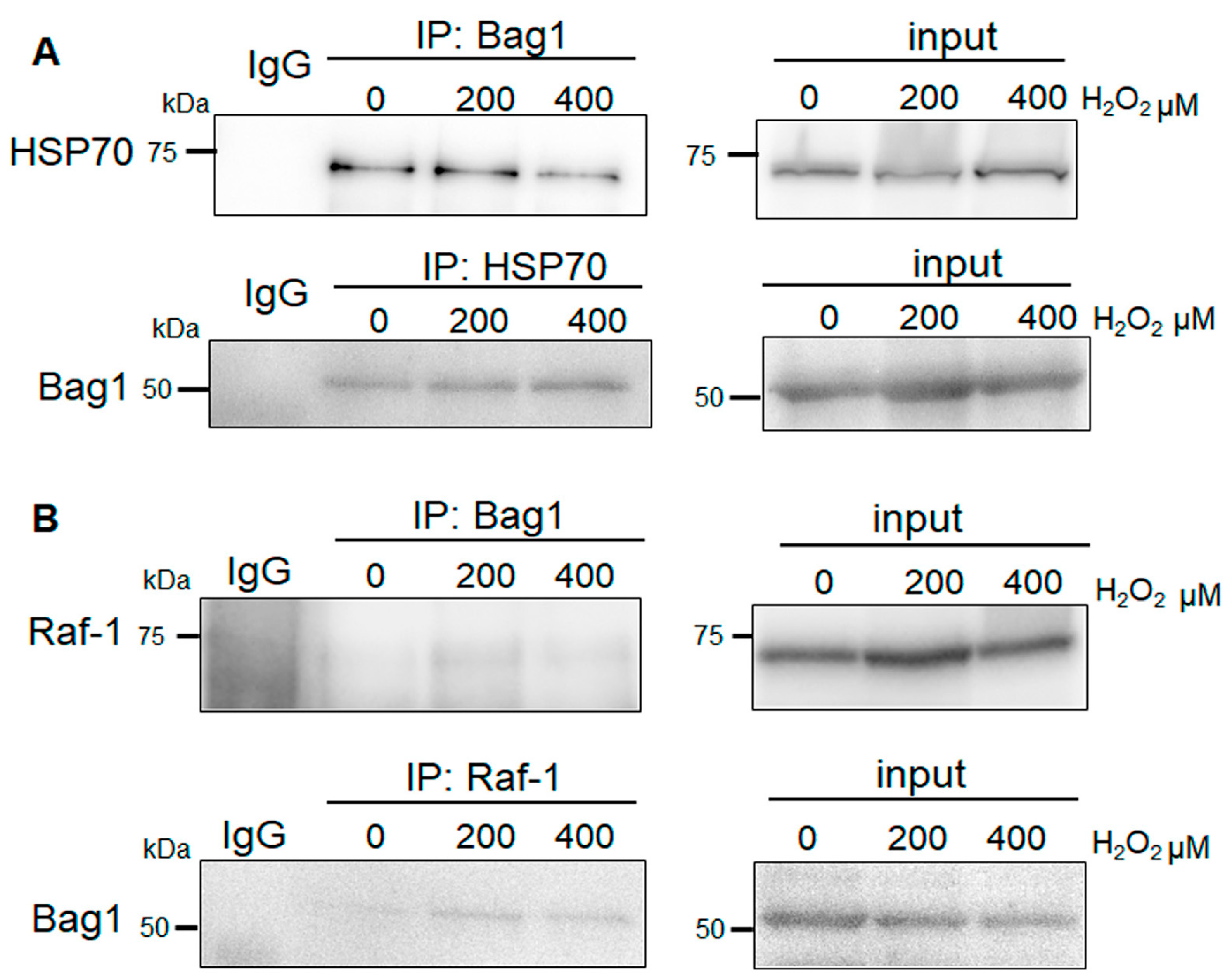
3.4. Bag-1 Binds to HSP70 in NP Cells, but Does Exhibit Obvious Raf-1 Binding
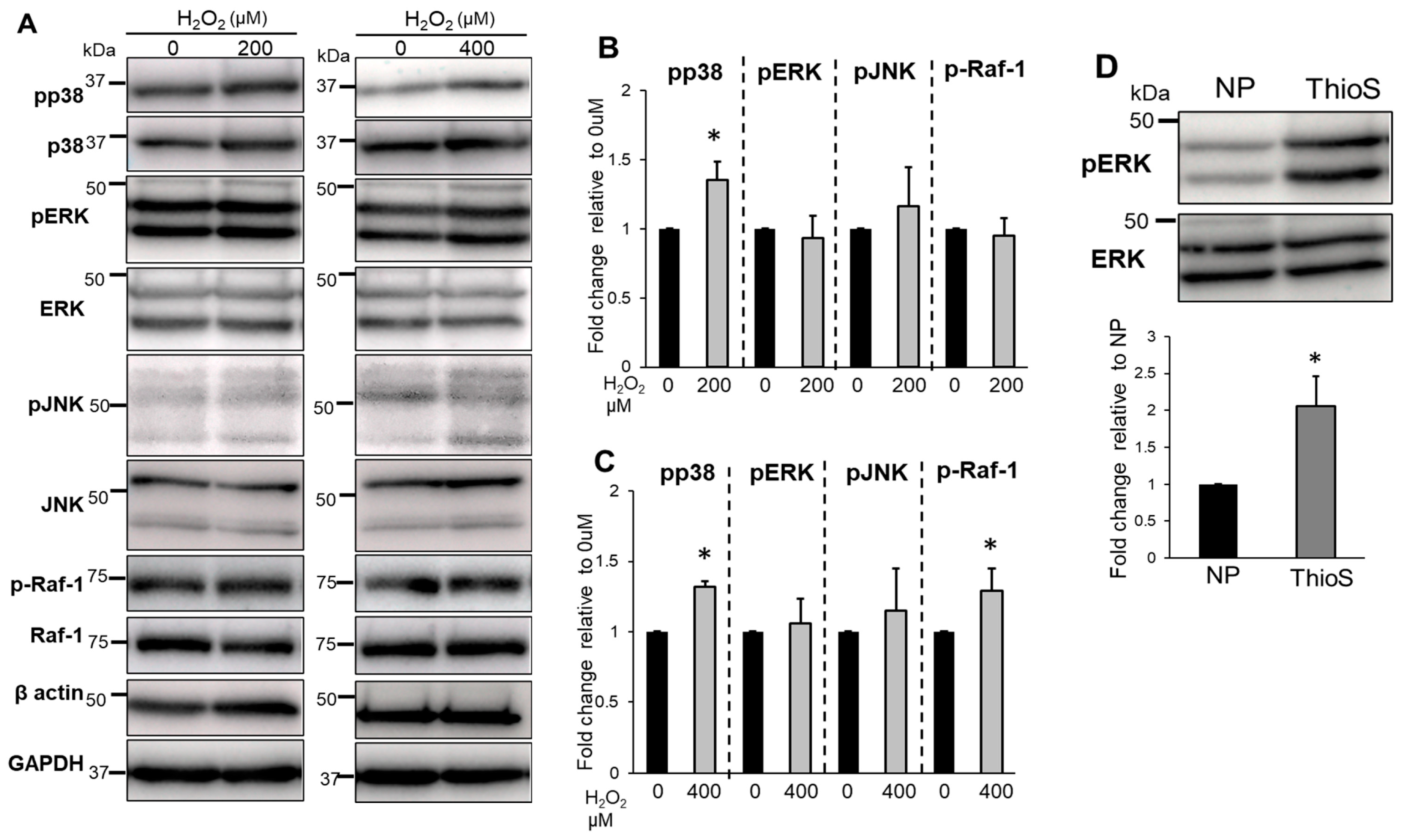
3.5. Treatment of Bag-1 with an Inhibitor of the Binding Site for HSP70 and Raf-1 Attenuates NP Cell Viability, Mitochondrial Function, and Increased Intracellular ROS Levels
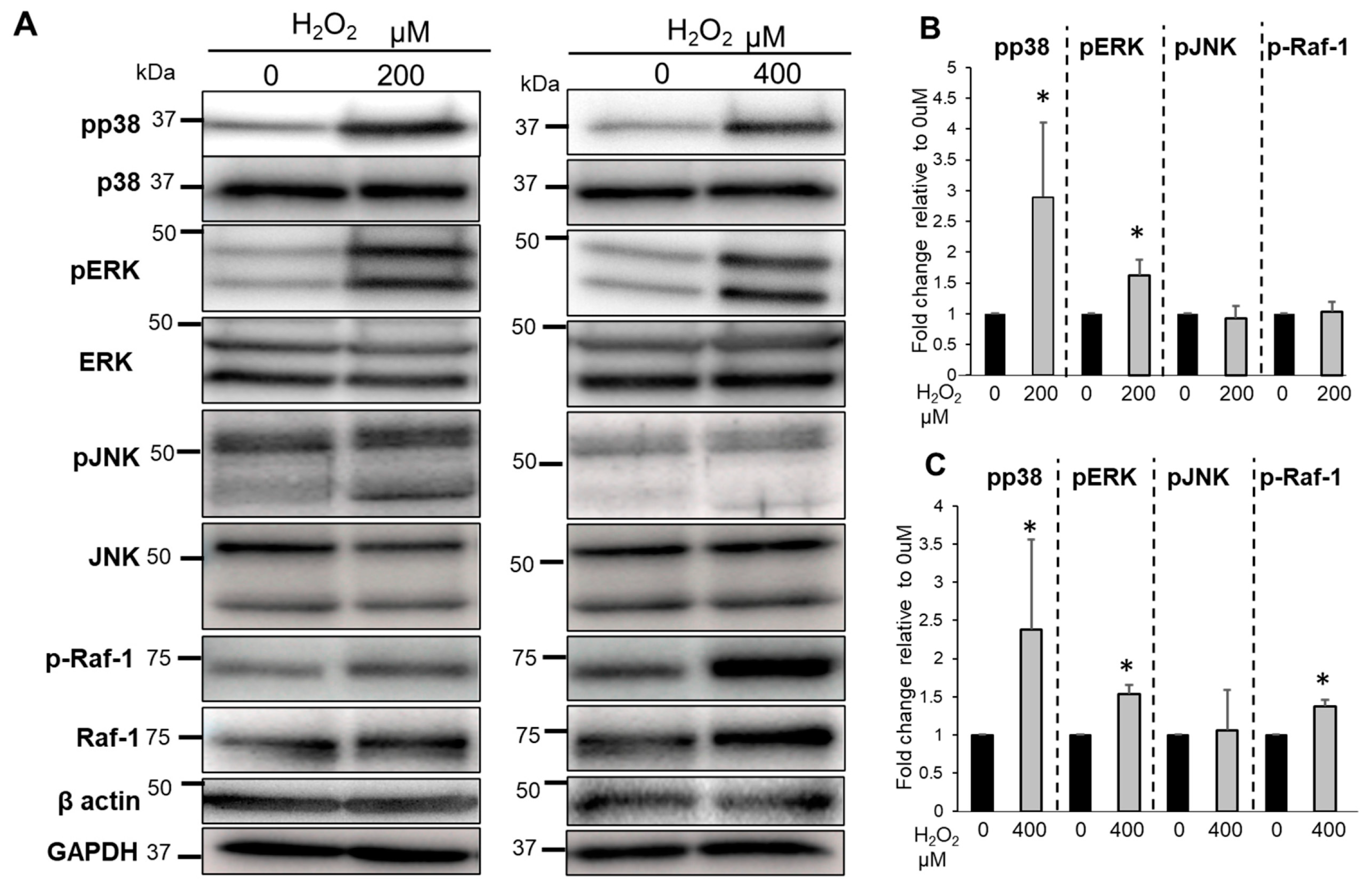
3.6. The Effects of Bag-1 on MAPKs and Raf-1 Activation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, B.I.; Deyo, R.A.; Mirza, S.K.; Turner, J.A.; Comstock, B.A.; Hollingworth, W.; Sullivan, S.D. Expenditures and Health Status among Adults with Back and Neck Problems. JAMA 2008, 299, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.V.; Hartman, R.A.; Patil, P.R.; Risbud, M.V.; Kletsas, D.; Iatridis, J.C.; Hoyland, J.A.; Le Maitre, C.L.; Sowa, G.A.; Kang, J.D. Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res. 2016, 34, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, X.; Shen, H.; Zhang, C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int. J. Mol. Med. 2016, 37, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Falabella, M.; Saeed, A.; Lee, D.; Kaufman, B.; Shiva, S.; Croix, C.S.; Van Houten, B.; Niedernhofer, L.J.; Robbins, P.D.; et al. Oxidative stress-induced senescence markedly increases disc cell bioenergetics. Mech. Ageing Dev. 2019, 180, 97–106. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Yang, Z.; Xie, C.; Ling, L.; Hu, H.; Cao, Y.; Huang, Y.; Hua, Y. Rutin maintains redox balance to relieve oxidative stress induced by TBHP in nucleus pulposus cells. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 448–456. [Google Scholar] [CrossRef]
- Feng, C.; Liu, H.; Yang, M.; Zhang, Y.; Huang, B.; Zhou, Y. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways. Cell Cycle 2016, 15, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, B.; Wang, J.; Cheng, F.; Shi, K.; Ying, L.; Wang, C.; Xia, K.; Huang, X.; Gong, Z.; et al. Cell Senescence: A Nonnegligible Cell State under Survival Stress in Pathology of Intervertebral Disc Degeneration. Oxid. Med. Cell. Longev. 2020, 2020, 9503562. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, Z.; Wang, J.; Feng, X.; Hua, W.; Luo, R.; Wang, B.; Liao, Z.; Ma, L.; Li, G.; et al. Allicin Attenuated Advanced Oxidation Protein Product-Induced Oxidative Stress and Mitochondrial Apoptosis in Human Nucleus Pulposus Cells. Oxid. Med. Cell. Longev. 2020, 2020, 6685043. [Google Scholar] [CrossRef]
- Song, Y.; Lu, S.; Geng, W.; Feng, X.; Luo, R.; Li, G.; Yang, C. Mitochondrial quality control in intervertebral disc degeneration. Exp. Mol. Med. 2021, 53, 1124–1133. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Wang, P.; Hu, B.; Lv, X.; Chen, S.; Wang, B.; Shao, Z. Activation of HSP70 impedes tert-butyl hydroperoxide (t-BHP)-induced apoptosis and senescence of human nucleus pulposus stem cells via inhibiting the JNK/c-Jun pathway. Mol. Cell. Biochem. 2021, 476, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- Dimozi, A.; Mavrogonatou, E.; Sklirou, A.; Kletsas, D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur. Cell. Mater. 2015, 30, 89–102, discussion 103. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Sato, T.; Krajewski, S.; Kochel, K.; Irie, S.; Milian, J.A.; Reed, J.C. Cloning and functional analysis of BAG-1: A novel Bcl-2-binding protein with anti-cell death activity. Cell 1995, 80, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Shi, L.; Zhai, C.; Wang, G.; Qu, Y. Bag-1L Protects against Cell Apoptosis in an In Vitro Model of Lung Ischemia-Reperfusion Injury through the C-Terminal “Bag” Domain. BioMed Res. Int. 2021, 2021, 8822807. [Google Scholar] [CrossRef]
- Mariotto, E.; Viola, G.; Zanon, C.; Aveic, S. A BAG’s life: Every connection matters in cancer. Pharmacol. Ther. 2020, 209, 107498. [Google Scholar] [CrossRef]
- Denizce Can, N.; Id, E.B.; Kizilboga, T.; Mehmet Akcay, I.; Dingiloglu, B.; Tatli, O.; Acar, S.; Kilbas, P.O.; Elbeyli, E.; Muratcioglu, S.; et al. Interactome analysis of Bag-1 isoforms reveals novel interaction partners in endoplasmic reticulum-associated degradation. PLoS ONE 2021, 16, e0256640. [Google Scholar] [CrossRef]
- Anderson, L.R.; Sutherland, R.L.; Butt, A.J. BAG-1 overexpression attenuates luminal apoptosis in MCF-10A mammary epithelial cells through enhanced RAF-1 activation. Oncogene 2010, 29, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Townsend, P.A.; Cutress, R.I.; Sharp, A.; Brimmell, M.; Packham, G. BAG-1: A multifunctional regulator of cell growth and survival. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2003, 1603, 83. [Google Scholar] [CrossRef]
- Takayama, S.; Reed, J.C. Molecular chaperone targeting and regulation by BAG family proteins. Nat. Cell Biol. 2001, 3, E237–E241. [Google Scholar] [CrossRef]
- Takayama, S.; Bimston, D.N.; Matsuzawa, S.; Freeman, B.C.; Aime-Sempe, C.; Xie, Z.; Morimoto, R.I.; Reed, J.C. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997, 16, 4887–4896. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta Rev. Cancer. 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, W.; Wu, S.; Perrett, S. Hsp70 in Redox Homeostasis. Cells 2022, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, V.R.; Orr, M.E.; Rodriguez, K.A.; Buffenstein, R. Mechanisms of oxidative stress resistance in the brain: Lessons learned from hypoxia tolerant extremophilic vertebrates. Arch. Biochem. 2015, 576, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-c.; Xu, X.; Xing, Y.; Wei, Z.-l.; Takahashi, H.; Masuda, S.; Takano, Y. Nuclear or cytoplasmic localization of Bag-1 distinctly correlates with pathologic behavior and outcome of gastric carcinomas. Hum. Pathol. 2010, 41, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Ozfiliz, P.; Arisan, E.D.; Coker-Gurkan, A.; Obakan, P.; Eralp, T.N.; Dinler-Doganay, G.; Palavan-Unsal, N. Bag-1L is a Stress-withstand Molecule Prevents the Downregulation of Mcl-1 and c-Raf Under Control of Heat Shock Proteins in Cisplatin Treated HeLa Cervix Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 15, 4475–4482. [Google Scholar] [CrossRef]
- Wang, H.G.; Takayama, S.; Rapp, U.R.; Reed, J.C. Bcl-2 Interacting Protein BAG-1, Binds to and Activates the Kinase Raf-1. Proc. Natl. Acad. Sci. USA 1996, 93, 7063–7068. [Google Scholar] [CrossRef]
- Song, J.; Takeda, M.; Morimoto, R.I. Bag-1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat. Cell Biol. 2001, 3, 276–282. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, Y.; Cai, Y.; Shui, C.; Wang, X.; Zhu, J. circ_0000045 promotes proliferation, migration, and invasion of head and neck squamous cell carcinomas via regulating HSP70 and MAPK pathway. BMC Cancer 2022, 22, 799. [Google Scholar] [CrossRef]
- Fan, W.; Gao, X.K.; Rao, X.S.; Shi, Y.P.; Liu, X.C.; Fei, Y.; Wang; Liu, Y.F.; Cong, X.X.; He, M.Y.; et al. Hsp70 Interacts with Mitogen-Activated Protein Kinase (MAPK)-Activated Protein Kinase 2 To Regulate p38MAPK Stability and Myoblast Differentiation during Skeletal Muscle Regeneration. Mol. Cell Biol. 2018, 38, e00211-18. [Google Scholar] [CrossRef]
- Risbud, M.V.; Guttapalli, A.; Stokes, D.G.; Hawkins, D.; Danielson, K.G.; Schaer, T.P.; Albert, T.J.; Shapiro, I.M. Nucleus pulposus cells express HIF-1α under normoxic culture conditions: A metabolic adaptation to the intervertebral disc microenvironment. J. Cell. Biochem. 2006, 98, 152–159. [Google Scholar] [CrossRef]
- Enthammer, M.; Papadakis, E.S.; Salomé Gachet, M.; Deutsch, M.; Schwaiger, S.; Koziel, K.; Ashraf, M.I.; Khalid, S.; Wolber, G.; Packham, G.; et al. Isolation of a Novel Thioflavin S–Derived Compound That Inhibits BAG-1–Mediated Protein Interactions and Targets BRAF Inhibitor–Resistant Cell Lines. Mol. Cancer Ther. 2013, 12, 2400–2414. [Google Scholar] [CrossRef]
- Sharp, A.; Crabb, S.J.; Johnson, P.W.M.; Hague, A.; Cutress, R.; Townsend, P.A.; Ganesan, A.; Packham, G. Thioflavin S (NSC71948) Interferes with Bcl-2-Associated Athanogene (BAG-1)-Mediated Protein-Protein Interactions. J. Pharmacol. Exp. Ther. 2009, 331, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, C.; Li, Q.; Xie, C.; Zhang, N.; Qu, Y. BAG-1L Protects SH-SY5Y Neuroblastoma Cells Against Hypoxia/Re-oxygenation Through Up-Regulating HSP70 and Activating PI3K/AKT Signaling Pathway. Neurochem. Res. 2017, 42, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, J.; Chen, Y.; Liu, H.; Zhou, H.; Bai, Z.; Hu, Z.; Guo, X. PINK1 protects against oxidative stress induced senescence of human nucleus pulposus cells via regulating mitophagy. Biochem. Biophys. Res. Commun. 2018, 504, 406–414. [Google Scholar] [CrossRef]
- Cedikova, M.; Pitule, P.; Kripnerova, M.; Markova, M.; Kuncova, J. Multiple Roles of Mitochondria in Aging Processes. Physiol. Res. 2016, 65, S519–S531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liao, H.; Bai, D.; Wang, Z.; Xie, X. MAPK/ERK signaling pathway: A potential target for the treatment of intervertebral disc degeneration. Biomed. Pharmacother. 2021, 143, 112170. [Google Scholar] [CrossRef]
- Freund, A.; Patil, C.K.; Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011, 30, 1536–1548. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Liu, G.; Zhu, M.; Wu, Y.; Jie, Z.; Xie, Z.; Wang, S.; Ma, Q.; Fan, S.; et al. Oxidative stress abrogates the degradation of KMT2D to promote degeneration in nucleus pulposus. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1866, 165888. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Yan, M.; Yang, M.; Wang, S.; Pan, J.; Li, L.; Tan, J. Oxidative damage induces apoptosis and promotes calcification in disc cartilage endplate cell through ROS/MAPK/NF-κB pathway: Implications for disc degeneration. Biochem. Biophys. Res. Commun. 2019, 516, 1026–1032. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, H.; Wang, Q.; Xu, W.; Liu, K.; Zhang, J.; Zhao, H.; Hou, G. Aquaporin-3 Attenuates Oxidative Stress-Induced Nucleus Pulposus Cell Apoptosis Through Regulating the P38 MAPK Pathway. Cell. Physiol. Biochem. 2018, 50, 1687–1697. [Google Scholar] [CrossRef]
- Zuiderweg, E.R.P.; Bertelsen, E.B.; Rousaki, A.; Mayer, M.P.; Gestwicki, J.E.; Ahmad, A. Allostery in the Hsp70 Chaperone Proteins. Top. Curr. Chem. 2013, 328, 99–153. [Google Scholar] [CrossRef]
- Kizilboga, T.; Baskale, E.A.; Yildiz, J.; Akcay, I.M.; Zemheri, E.; Can, N.D.; Ozden, C.; Demir, S.; Ezberci, F.; Dinler-Doganay, G. Bag-1 stimulates Bad phosphorylation through activation of Akt and Raf kinases to mediate cell survival in breast cancer. BMC Cancer 2019, 19, 1254. [Google Scholar] [CrossRef]
- Tekari, A.; Marazza, A.; Crump, K.; Bermudez-lekerika, P.; Gantenbein, B. Inhibition of the extracellular signal-regulated kinase pathway reduces the inflammatory component in nucleus pulposus cells. J. Orthop. Res. 2022, 40, 2362. [Google Scholar] [CrossRef]
- Risbud, M.V.; Guttapalli, A.; Albert, T.J.; Shapiro, I.M. Hypoxia activates MAPK activity in rat nucleus pulposus cells: Regulation of integrin expression and cell survival. Spine 2005, 30, 2503–2509. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suyama, K.; Sakai, D.; Hayashi, S.; Qu, N.; Terayama, H.; Kiyoshima, D.; Nagahori, K.; Watanabe, M. Bag-1 Protects Nucleus Pulposus Cells from Oxidative Stress by Interacting with HSP70. Biomedicines 2023, 11, 863. https://doi.org/10.3390/biomedicines11030863
Suyama K, Sakai D, Hayashi S, Qu N, Terayama H, Kiyoshima D, Nagahori K, Watanabe M. Bag-1 Protects Nucleus Pulposus Cells from Oxidative Stress by Interacting with HSP70. Biomedicines. 2023; 11(3):863. https://doi.org/10.3390/biomedicines11030863
Chicago/Turabian StyleSuyama, Kaori, Daisuke Sakai, Shogo Hayashi, Ning Qu, Hayato Terayama, Daisuke Kiyoshima, Kenta Nagahori, and Masahiko Watanabe. 2023. "Bag-1 Protects Nucleus Pulposus Cells from Oxidative Stress by Interacting with HSP70" Biomedicines 11, no. 3: 863. https://doi.org/10.3390/biomedicines11030863
APA StyleSuyama, K., Sakai, D., Hayashi, S., Qu, N., Terayama, H., Kiyoshima, D., Nagahori, K., & Watanabe, M. (2023). Bag-1 Protects Nucleus Pulposus Cells from Oxidative Stress by Interacting with HSP70. Biomedicines, 11(3), 863. https://doi.org/10.3390/biomedicines11030863






