Impact of Ovarian Endometrioma and Surgery on Reproductive Outcomes: A Single-Center Spanish Cohort Study
Abstract
1. Introduction
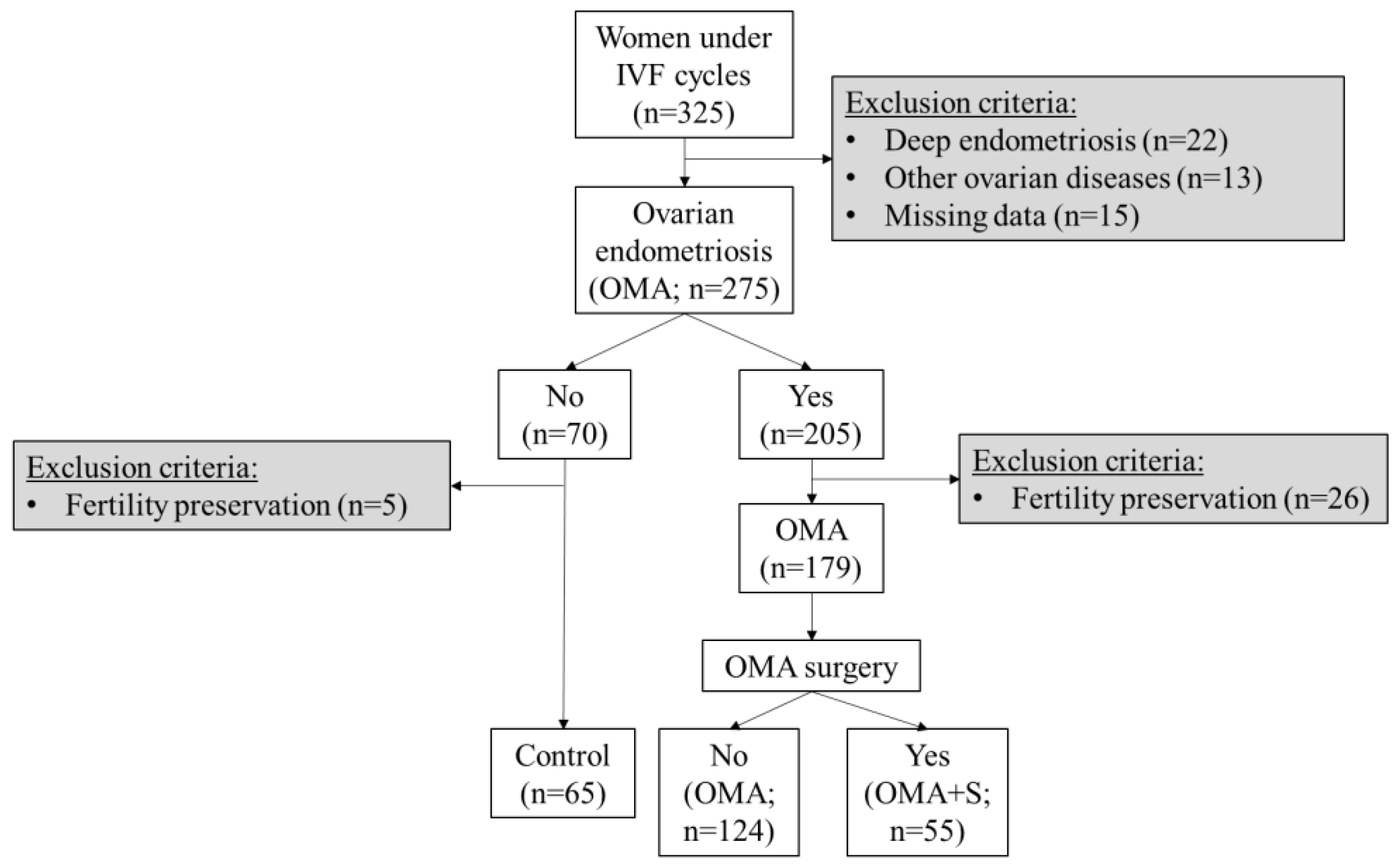
2. Materials and Methods
2.1. Study Design
2.2. Biochemical, Reproductive, and Obstetrical Variables
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Cohort
3.2. Reproductive Characteristics in Women Undergoing IVF
3.3. Pregnancy Outcomes in Women Undergoing IVF
3.4. Endometriosis Association Factors with Reproductive Variables and Pregnancy Outcomes
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Streuli, I.; de Ziegler, D.; Gayet, V.; Santulli, P.; Bijaoui, G.; de Mouzon, J.; Chapron, C. In Women with Endometriosis Anti-Mullerian Hormone Levels Are Decreased Only in Those with Previous Endometrioma Surgery. Hum. Reprod. 2012, 27, 3294–3303. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Nie, X. Effect of Endometrioma and Its Surgical Excision on Fertility (Review). Exp. Ther. Med. 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Leone Roberti Maggiore, U.; Scala, C.; Venturini, P.L.; Remorgida, V.; Ferrero, S. Endometriotic Ovarian Cysts Do Not Negatively Affect the Rate of Spontaneous Ovulation. Hum. Reprod. 2015, 30, 299–307. [Google Scholar] [CrossRef]
- Muzii, L.; di Tucci, C.; di Feliciantonio, M.; Galati, G.; di Donato, V.; Musella, A.; Palaia, I.; Panici, P.B. Antimüllerian Hormone Is Reduced in the Presence of Ovarian Endometriomas: A Systematic Review and Meta-Analysis. Fertil. Steril. 2018, 110, 932–940.e1. [Google Scholar] [CrossRef]
- Salihoğlu, K.N.; Dilbaz, B.; Cırık, D.A.; Ozelci, R.; Ozkaya, E.; Mollamahmutoğlu, L. Short-Term Impact of Laparoscopic Cystectomy on Ovarian Reserve Tests in Bilateral and Unilateral Endometriotic and Nonendometriotic Cysts. J. Minim. Invasive Gynecol. 2016, 23, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pei, H.; Chang, Y.; Chen, M.; Wang, H.; Xie, H.; Yao, S. The Impact of Endometrioma and Laparoscopic Cystectomy on Ovarian Reserve and the Exploration of Related Factors Assessed by Serum Anti-Mullerian Hormone: A Prospective Cohort Study. J. Ovarian. Res. 2014, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Uncu, G.; Kasapoglu, I.; Ozerkan, K.; Seyhan, A.; Oral Yilmaztepe, A.; Ata, B. Prospective Assessment of the Impact of Endometriomas and Their Removal on Ovarian Reserve and Determinants of the Rate of Decline in Ovarian Reserve. Hum. Reprod. 2013, 28, 2140–2145. [Google Scholar] [CrossRef]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; de Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE Guideline: Management of Women with Endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of Endometriosis. Obstet. Gynecol. 2010, 116, 223–236. [Google Scholar] [CrossRef]
- Leyland, N.; Casper, R.; Laberge, P.; Singh, S.S. SOGC Endometriosis: Diagnosis and Management. J. Obstet. Gynaecol. Can. 2010, 32, S1–S32. [Google Scholar] [CrossRef]
- Ozkan, S.; Murk, W.; Arici, A. Endometriosis and Infertility. Ann. N. Y. Acad. Sci. 2008, 1127, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and Infertility: A Committee Opinion. Fertil. Steril. 2012, 98, 591–598. [Google Scholar] [CrossRef]
- Bukman, A. Ovarian Reserve Testing and the Use of Prognostic Models in Patients with Subfertility. Hum. Reprod. Update 2001, 7, 581–590. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, I.A.J. Serum Anti-Mullerian Hormone Levels: A Novel Measure of Ovarian Reserve. Hum. Reprod. 2002, 17, 3065–3071. [Google Scholar] [CrossRef]
- Hehenkamp, W.J.K.; Looman, C.W.N.; Themmen, A.P.N.; de Jong, F.H.; te Velde, E.R.; Broekmans, F.J.M. Anti-Müllerian Hormone Levels in the Spontaneous Menstrual Cycle Do Not Show Substantial Fluctuation. J. Clin. Endocrinol. Metab. 2006, 91, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Raad, J.; Dahan, Y.; Marcellin, L.; Maignien, C.; Even, M.; Pocate-Cheriet, K.; Lamau, M.C.; Santulli, P.; Chapron, C. Endometriosis and ART: A Prior History of Surgery for OMA Is Associated with a Poor Ovarian Response to Hyperstimulation. PLoS ONE 2018, 13, e0202399. [Google Scholar] [CrossRef]
- González-Foruria, I.; Soldevila, P.B.; Rodríguez, I.; Rodríguez-Purata, J.; Pardos, C.; García, S.; Pascual, M.Á.; Barri, P.N.; Polyzos, N.P. Do Ovarian Endometriomas Affect Ovarian Response to Ovarian Stimulation for IVF/ICSI? Reprod. Biomed. Online 2020, 41, 37–43. [Google Scholar] [CrossRef]
- Ata, B.; Mumusoglu, S.; Aslan, K.; Seyhan, A.; Kasapoglu, I.; Avcı, B.; Urman, B.; Bozdag, G.; Uncu, G. Which Is Worse? Comparison of ART Outcome between Women with Primary or Recurrent Endometriomas. Hum. Reprod. 2017, 32, 1427–1431. [Google Scholar] [CrossRef]
- Deckers, P.; Ribeiro, S.C.; dos Santos Simões, R.; da Fonseca Miyahara, C.B.; Baracat, E.C. Systematic Review and Meta-Analysis of the Effect of Bipolar Electrocoagulation during Laparoscopic Ovarian Endometrioma Stripping on Ovarian Reserve. Int. J. Gynecol. Obstet. 2018, 140, 11–17. [Google Scholar] [CrossRef]
- Hamdan, M.; Dunselman, G.; Li, T.C.; Cheong, Y. The Impact of Endometrioma on IVF/ICSI Outcomes: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2015, 21, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Kwee, J.; Hendriks, D.J.; Mol, B.W.; Lambalk, C.B. A Systematic Review of Tests Predicting Ovarian Reserve and IVF Outcome. Hum. Reprod. Update 2006, 12, 685–718. [Google Scholar] [CrossRef] [PubMed]
- Younis, J.S.; Shapso, N.; Fleming, R.; Ben-Shlomo, I.; Izhaki, I. Impact of Unilateral versus Bilateral Ovarian Endometriotic Cystectomy on Ovarian Reserve: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2019, 25, 375–391. [Google Scholar] [CrossRef]
- Wu, M.-H.; Tsai, S.-J.; Pan, H.-A.; Hsiao, K.-Y.; Chang, F.-M. Three-Dimensional Power Doppler Imaging of Ovarian Stromal Blood Flow in Women with Endometriosis Undergoing in Vitro Fertilization. Ultrasound Obstet. Gynecol. 2003, 21, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Kasapoglu, I.; Ata, B.; Uyaniklar, O.; Seyhan, A.; Orhan, A.; Yildiz Oguz, S.; Uncu, G. Endometrioma-Related Reduction in Ovarian Reserve (ERROR): A Prospective Longitudinal Study. Fertil. Steril. 2018, 110, 122–127. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, R.; Lan, J.; Lin, H.; Jiao, X.; Zhang, Q. Ovarian Endometrioma Negatively Impacts Oocyte Quality and Quantity But Not Pregnancy Outcomes in Women Undergoing IVF/ICSI Treatment: A Retrospective Cohort Study. Front. Endocrinol. 2021, 12, 739228. [Google Scholar] [CrossRef] [PubMed]
- Maneschi, F.; Marasá, L.; Incandela, S.; Mazzarese, M.; Zupi, E. Ovarian Cortex Surrounding Benign Neoplasms: A Histologic Study. Am. J. Obstet. Gynecol. 1993, 169, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Schubert, B.; Canis, M.; Darcha, C.; Artonne, C.; Pouly, J.-L.; Déchelotte, P.; Boucher, D.; Grizard, G. Human Ovarian Tissue from Cortex Surrounding Benign Cysts: A Model to Study Ovarian Tissue Cryopreservation. Hum. Reprod. 2005, 20, 1786–1792. [Google Scholar] [CrossRef]
- Alshehre, S.M.; Narice, B.F.; Fenwick, M.A.; Metwally, M. The Impact of Endometrioma on in Vitro Fertilisation/Intra-Cytoplasmic Injection IVF/ICSI Reproductive Outcomes: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2021, 303, 3–16. [Google Scholar] [CrossRef]
- Raffi, F.; Metwally, M.; Amer, S. The Impact of Excision of Ovarian Endometrioma on Ovarian Reserve: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2012, 97, 3146–3154. [Google Scholar] [CrossRef]
- Younis, J.S.; Shapso, N.; Ben-Sira, Y.; Nelson, S.M.; Izhaki, I. Endometrioma Surgery–a Systematic Review and Meta-Analysis of the Effect on Antral Follicle Count and Anti-Müllerian Hormone. Am. J. Obstet. Gynecol. 2022, 226, 33–51.e7. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; di Tucci, C.; di Feliciantonio, M.; Marchetti, C.; Perniola, G.; Panici, P.B. The Effect of Surgery for Endometrioma on Ovarian Reserve Evaluated by Antral Follicle Count: A Systematic Review and Meta-Analysis. Hum. Reprod. 2014, 29, 2190–2198. [Google Scholar] [CrossRef]
- Muzii, L.; Achilli, C.; Bergamini, V.; Candiani, M.; Garavaglia, E.; Lazzeri, L.; Lecce, F.; Maiorana, A.; Maneschi, F.; Marana, R.; et al. Comparison between the Stripping Technique and the Combined Excisional/Ablative Technique for the Treatment of Bilateral Ovarian Endometriomas: A Multicentre RCT. Hum. Reprod. 2016, 31, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Marana, R.; Angioli, R.; Bianchi, A.; Cucinella, G.; Vignali, M.; Benedetti Panici, P.; Busacca, M. Histologic Analysis of Specimens from Laparoscopic Endometrioma Excision Performed by Different Surgeons: Does the Surgeon Matter? Fertil. Steril. 2011, 95, 2116–2119. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, X.; Lu, D.; Sheng, J.; Mueck, A.O. Effect of Laparoscopic Endometrioma Cystectomy on Anti-Müllerian Hormone (AMH) Levels. Gynecol. Endocrinol. 2019, 35, 494–497. [Google Scholar] [CrossRef]
- Wu, C.Q.; Albert, A.; Alfaraj, S.; Taskin, O.; Alkusayer, G.M.; Havelock, J.; Yong, P.; Allaire, C.; Bedaiwy, M.A. Live Birth Rate after Surgical and Expectant Management of Endometriomas after In Vitro Fertilization: A Systematic Review, Meta-Analysis, and Critical Appraisal of Current Guidelines and Previous Meta-Analyses. J. Minim. Invasive Gynecol. 2019, 26, 299–311.e3. [Google Scholar] [CrossRef]
- Tao, X.; Chen, L.; Ge, S.; Cai, L. Weigh the Pros and Cons to Ovarian Reserve before Stripping Ovarian Endometriomas Prior to IVF/ICSI: A Meta-Analysis. PLoS ONE 2017, 12, e0177426. [Google Scholar] [CrossRef] [PubMed]
- Nickkho-Amiry, M.; Savant, R.; Majumder, K.; Edi-O’sagie, E.; Akhtar, M. The Effect of Surgical Management of Endometrioma on the IVF/ICSI Outcomes When Compared with No Treatment? A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2018, 297, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Opøien, H.K.; Fedorcsak, P.; Åbyholm, T.; Tanbo, T. Complete Surgical Removal of Minimal and Mild Endometriosis Improves Outcome of Subsequent IVF/ICSI Treatment. Reprod. Biomed. Online 2011, 23, 389–395. [Google Scholar] [CrossRef]
- Ban Frangež, H.; Vrtacnik Bokal, E.; Štimpfel, M.; Divjak Budihna, T.; Gulino, F.A.; Garzon, S.; Ghezzi, F.; Alkatout, I.; Gitas, G.; Laganà, A.S. Reproductive Outcomes after Laparoscopic Surgery in Infertile Women Affected by Ovarian Endometriomas, with or without in Vitro Fertilisation: Results from the SAFE (Surgery and ART for Endometriomas) Trial. J. Obstet. Gynaecol. 2022, 42, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Karadağ, C.; Yoldemir, T.; Demircan Karadağ, S.; Turgut, A. The Effects of Endometrioma Size and Bilaterality on Ovarian Reserve. J. Obstet. Gynaecol. 2020, 40, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Takae, S.; Kawamura, K.; Sato, Y.; Nishijima, C.; Yoshioka, N.; Sugishita, Y.; Horage, Y.; Tanaka, M.; Ishizuka, B.; Suzuki, N. Analysis of Late-Onset Ovarian Insufficiency after Ovarian Surgery: Retrospective Study with 75 Patients of Post-Surgical Ovarian Insufficiency. PLoS ONE 2014, 9, e98174. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.K.; Babu, N.K.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Upper Control Limit of Reactive Oxygen Species in Follicular Fluid beyond Which Viable Embryo Formation Is Not Favorable. Reprod. Toxicol. 2010, 29, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Barri, P.N.; Coroleu, B.; Tur, R.; Barri-Soldevila, P.N.; Rodríguez, I. Endometriosis-Associated Infertility: Surgery and IVF, a Comprehensive Therapeutic Approach. Reprod. Biomed. Online 2010, 21, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.; Gallos, I.; Chu, J.; Harb, M.; Coomarasamy, A. The Effect of Endometriosis on in Vitro Fertilisation Outcome: A Systematic Review and Meta-Analysis. BJOG 2013, 120, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 65) | OMA (n = 121) | OMA + S (n = 43) | p-Value | |
|---|---|---|---|---|
| Age (years) | 35.0 [33.0; 37.0] | 35.0 [33.0; 38.0] | 35.0 [32.5; 37.0] | 0.33 |
| Smoking habits | 14.5% (10) | 6.8% (8) | 10.0% (4) | 0.24 |
| Race | ||||
| Caucasian | 89.8% (53) | 99.0% (97) | 97.4% (37) | 0.019 |
| Non-Caucasian | 10.2% (10) | 1.0% (1) | 2.6% (1) | |
| Categorized | ||||
| <25 years | 3.1% (2) | 0.0% (0) | 0.0% (0) | 0.56 |
| 25–29 years | 9.2% (6) | 8.3% (10) | 4.7% (2) | |
| 30–34 years | 40.0% (26) | 43.0% (52) | 48.8% (21) | |
| 35–40 years | 47.7% (31) | 48.8% (59) | 46.5% (20) | |
| Weight (kg) | 66.5 [55.0; 74.2] | 62.0 [56.0; 67.0] | 62.0 [55.5; 72.0] | 0.26 |
| Height (m) | 1.65 [1.60; 1.69] | 1.65 [1.60; 1.69] | 1.65 [1.61; 1.70] | 0.82 |
| BMI (kg/m2) | 23.8 [21.8; 26.7] | 22.8 [20.7; 25.2] | 23.0 [20.9; 25.8] | 0.27 |
| Leucocytes (103/μL) | 6.34 [5.63; 7.89] | 6.60 [5.46; 7.82] | 6.16 [5.12; 7.42] | 0.34 |
| Neutrophils (103/μL) | 3.82 [3.04; 5.11] a | 5.19 [3.73; 6.04] b | 3.96 [2.95; 5.52] a | 0.001 |
| Control (n = 65) | OMA (n = 121) | OMA + S (n = 43) | p-Value | |
|---|---|---|---|---|
| AMH (ng/mL) | 3.10 [1.55; 4.70] a | 1.70 [0.87; 3.32] b | 1.20 [0.78; 1.97] b | <0.001 |
| Follicular count | 12.5 [9.0; 19.2] a | 7.0 [4.0; 10.0] b | 7.0 [5.8; 10.0] b | <0.001 |
| Number of cycles | 2.0 [1.0; 3.0] a | 1.0 [1.0; 2.0] b | 1.0 [1.0; 2.0] b | 0.001 |
| Number of follicles (>16 mm) | ||||
| 1st Cycle | 9.0 [3.0; 12.0] | 8.0 [4.0; 10.0] | 7.0 [3.0; 9.5] | 0.25 |
| 2nd Cycle | 0.0 [0.0; 11.0] | 0.0 [0.0; 7.0] | 0.0 [0.0; 5.5] | 0.33 |
| 3rd Cycle | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.0 [0.0; 1.0] | 0.17 |
| Follicles/cycle | 8.7 [6.0; 13.5] | 9.0 [5.0; 12.0] | 8.0 [3.50; 11.5] | 0.68 |
| Number of oocytes | ||||
| 1st Cycle | 9.0 [6.0; 14.0] a | 6.0 [4.0; 9.0] b | 5.0 [3.0; 8.0] b | <0.001 |
| 2nd Cycle | 4.0 [0.0; 11.0] a | 1.0 [0.0; 8.0] b | 0.0 [0.0;4.0] b | 0.021 |
| 3rd Cycle | 0.0 [0.0; 5.0] a | 0.0 [0.0; 0.0] b | 0.0 [0.0; 1.5] a,b | 0.024 |
| Oocytes/cycle | 9.0 [6.0; 15.0] a | 7.0 [5.0; 11.3] b | 6.0 [3.0; 12.5] b | 0.014 |
| 1st Embryo transfer | ||||
| No Transfer | 32.3% (21) | 41.3% (50) | 30.2% (13) | 0.19 |
| Fresh | 23.1% (15) | 10.7% (13) | 18.6% (8) | |
| Cryotransfer | 44.6% (29) | 47.9% (58) | 51.2% (22) | |
| 2nd Embryo transfer | ||||
| No Transfer | 22.5% (9) | 38.1% (24) | 45.5% (10) | 0.37 |
| Fresh | 17.5% (7) | 14.3% (9) | 13.6% (3) | |
| Cryotransfer | 60.0% (24) | 47.6% (30) | 40.9% (9) | |
| 3rd Embryo transfer | ||||
| No Transfer | 20.8% (5) | 27.3% (6) | 41.7% (5) | 0.28 |
| Fresh | 8.3% (2) | 18.2% (4) | 25.0% (3) | |
| Cryotransfer | 70.8% (17) | 54.5% (12) | 33.3% (4) | |
| Number of embryos (A + B category) | ||||
| 1st Cycle | 0.0 [0.0; 1.0] | 0.0 [0.0; 1.0] | 0.0 [0.0; 1.0] | 0.89 |
| 2nd Cycle | 0.0 [0.0; 1.0] | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.16 |
| 3rd Cycle | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.58 |
| Embryos A + B/cycle | 0.3 [0.0; 1.0] | 0.5 [0.0; 1.0] | 0.3 [0.0; 1.0] | 0.92 |
| Number of embryos (C + D category) | ||||
| 1st Cycle | 3.0 [1.0; 7.0] a | 1.0 [0.0; 3.0] b | 1.0 [0.0; 3.0] b | <0.001 |
| 2nd Cycle | 1.0 [0.0; 4.0] a | 0.0 [0.0; 2.0] b | 0.0 [0.0; 1.0] b | 0.001 |
| 3rd Cycle | 0.0 [0.0; 1.0] a | 0.0 [0.0; 0.0] b | 0.0 [0.0; 0.0] a,b | 0.009 |
| Embryos C + D/cycle | 4.0 [2.7; 7.0] a | 2.0 [1.0; 3.5] b | 2.0 [0.4; 3.0] b | <0.001 |
| Control (n = 65) | OMA (n = 121) | OMA + S (n = 43) | p-Value | |
|---|---|---|---|---|
| βCH (mIU/mL) | 590 [42.0; 1410] | 668 [172.0; 1764] | 860 [358.0; 1616] | 0.40 |
| Biochemical pregnancy (Positive βCH) | 63.1% (37) | 43.6% (44) | 55.3% (21) | 0.044 |
| Positive FHR at 6 weeks | 95.5% (42) | 79.4% (54) | 92.6% (25) | 0.034 |
| Positive FHR at 12 weeks | 97.0% (32) | 80.0% (44) | 88.9% (16) | 0.05 |
| Miscarriage (≤12 weeks) | 13.5% (5) | 22.4% (13) | 5.3% (1) | 0.22 |
| Abortion (>12 weeks) | 0.0% (0) | 13.6% (6) | 11.8% (2) | >0.99 |
| Reproductive Variables | OMA | p-Value | OMA + S | p-Value |
| AMH (ng/mL) | −1.61 ± 0.40 | <0.001 | −2.34 ± 0.47 | <0.001 |
| Follicular count | −7.20 ± 1.11 | <0.001 | −7.51 ± 1.33 | <0.001 |
| Number of cycles | −0.68 ± 0.14 | <0.001 | −0.65 ± 0.16 | <0.001 |
| Oocytes/cycle | −1.86 ± 1.41 | 0.19 | −3.43 ± 1.66 | 0.040 |
| Embryos C + D/cycle | −2.49 ± 0.72 | 0.001 | −2.26 ± 0.84 | <0.001 |
| Pregnancy Outcomes | OMA | p-Value | OMA + S | p-Value |
| Biochemical pregnancy (Positive βCH) | 2.19 [0.94; 5.24] | 0.07 | 2.22 [0.81; 6.24] | 0.12 |
| Positive FHR at 6 weeks | 0.08 [0.01; 0.50] | 0.025 | 0.72 [0.03; 19.6] | 0.82 |
| Reproductive Variables | OMA + S | p-Value |
| AMH (ng/mL) | −0.66 ± 0.28 | 0.020 |
| Follicular count | −0.27 ± 0.85 | 0.75 |
| Number of cycles | −0.01 ± 0.14 | 0.93 |
| Oocytes/cycle | −1.40 ± 1.57 | 0.37 |
| Embryos C + D/cycle | 0.30 ± 0.72 | 0.68 |
| Pregnancy outcomes | OMA + S | p-Value |
| Biochemical pregnancy (positive βCH) | 0.98 [0.39; 2.46] | 0.97 |
| Positive FHR at 6 weeks | 0.08 [0.001; 0.50] | 0.06 |
| Author, Year | Study Design | Women | Comparison Treatment before IVF | Results in IVF |
|---|---|---|---|---|
| Wu et al., 2019 [36] | Metanalysis | 2878 | Expectant management versus surgical treatment | No differences in pregnancy rate, miscarriage, and mature oocytes retrieved. However, a low number of oocytes was retrieved in the surgical group |
| Tao et al., 2017 [37] | Metanalysis | 2649 | Surgical versus non-surgical treatment | No differences in the duration of IVF, formed embryos, pregnancy rates, and live birth rates |
| Hamdam et al., 2015 [21] | Metanalysis | 2977 | Surgical versus non-surgical treatment | No differences in clinical pregnancy rate, live birth rate, and number of oocytes retrieved |
| Nickkho-Amiry et al., 2018 [38] | Metanalysis | - | Surgical versus non-surgical treatment | No differences in number of oocytes retrieved, pregnancy, and live birth rates |
| Opøien et al., 2011 [39] | Observational | 661 | Exploratory laparoscopy versus surgical removal of lesions | Improved implantation rate, pregnancy rate, and live birth rate in the group with complete excision of endometriosis lesions |
| Frangež et al., 2022 [40] | Observational | 436 | Cystectomy of endometriomas versus women with other infertility issues and without endometriosis | Differences in gonadotropins controlling ovarian stimulation (high doses in cystectomy group) and number of oocytes retrieved (low in endometriosis group). No differences in pregnancy rates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, A.; Sanz, A.; Spagnolo, E.; Lopez, A.; Martínez Jorge, P.; Iniesta, S.; Rodríguez, E.; Fernández Prada, S.; Ramiro-Cortijo, D. Impact of Ovarian Endometrioma and Surgery on Reproductive Outcomes: A Single-Center Spanish Cohort Study. Biomedicines 2023, 11, 844. https://doi.org/10.3390/biomedicines11030844
Hernández A, Sanz A, Spagnolo E, Lopez A, Martínez Jorge P, Iniesta S, Rodríguez E, Fernández Prada S, Ramiro-Cortijo D. Impact of Ovarian Endometrioma and Surgery on Reproductive Outcomes: A Single-Center Spanish Cohort Study. Biomedicines. 2023; 11(3):844. https://doi.org/10.3390/biomedicines11030844
Chicago/Turabian StyleHernández, Alicia, Angela Sanz, Emanuela Spagnolo, Ana Lopez, Paloma Martínez Jorge, Silvia Iniesta, Elena Rodríguez, Sara Fernández Prada, and David Ramiro-Cortijo. 2023. "Impact of Ovarian Endometrioma and Surgery on Reproductive Outcomes: A Single-Center Spanish Cohort Study" Biomedicines 11, no. 3: 844. https://doi.org/10.3390/biomedicines11030844
APA StyleHernández, A., Sanz, A., Spagnolo, E., Lopez, A., Martínez Jorge, P., Iniesta, S., Rodríguez, E., Fernández Prada, S., & Ramiro-Cortijo, D. (2023). Impact of Ovarian Endometrioma and Surgery on Reproductive Outcomes: A Single-Center Spanish Cohort Study. Biomedicines, 11(3), 844. https://doi.org/10.3390/biomedicines11030844







