1. Introduction
Directional cell migration of phagocytes during inflammation allows for their rapid accumulation at sites of injury and infection. Neutrophils and monocytes can migrate exceptionally fast and are the first immigrating cells after the onset of inflammation. In order to transmigrate, these cells have to permanently remodel their cytoskeleton. This process involves the orchestrated interplay of intracellular signaling pathways, which leads to the activation of specific protein kinases and the transient elevation of intracellular calcium concentrations [
1,
2,
3,
4].
The important role of the actin-based cytoskeleton and its regulation during adhesion and migration is well-characterized [
5,
6], but much less is known about the involvement of the other two major cytoskeletal components (intermediate filaments and microtubules (MTs)) and their interplay with the actin-based cytoskeleton. Phagocytes are characterized by a high turnover of MTs during transmigration [
1,
7], and reorganization of MTs is controlled by the phosphorylation of specific MT-associated proteins and by the modulation of intracellular calcium levels [
8,
9]. The elevation of intracellular calcium concentrations usually induces conformational changes in the calcium-binding proteins, which in turn allows them to interact with distinct intracellular targets.
The major calcium-binding molecules expressed in neutrophils and monocytes are two members of the S100 protein family: S100A8 (also known as MRP8) and S100A9 (also known as MRP14) [
10]. The heterodimer S100A8/S100A9 is the predominant intracellular form, and it constitutes up to 40% of neutrophils and 4–5% of monocyte cytosolic proteins [
11]. Increased local and systemic levels of released S100A8/S100A9 occur in many inflammatory disorders, and it is well-accepted that secreted S100A8/S100A9 exerts proinflammatory effects and contributes to disease progression [
10,
12,
13,
14,
15].
To date, most studies have focused on the extracellular role of S100A8/S100A9, and much less is known about the intracellular function(s) of this protein complex. It was recently reported that S100A9 was associated with cortical F-actin in human neutrophils, and calcium-induced tetramers of S100A8/S100A9 were shown to induce tubulin polymerization, bind MTs in vitro, and co-localize with MTs during the activation of monocytes [
16,
17,
18,
19,
20]. Furthermore, it was reported that phosphorylation of S100A9 at Thr113 by the p38 mitogen-activated protein kinase (MAPK) played a pivotal role in modulating this interaction [
16]. However, the functional consequences of S100-MT/S100-actin interactions and their impact on cytoskeletal properties, transmigration, and adhesion are still unclear.
Migratory processes require tightly controlled and regulated dynamic interactions between the cell and its surrounding substrate, which are usually mediated by specialized adhesion receptors such as selectins and integrins. These adhesion receptors link the cell to the extracellular matrix and transmit signals necessary for proper cell polarization and finally locomotion [
21,
22]. Although much is already known about the “leukocyte adhesion and transmigration cascade” that enables extravasation and even reverse migration [
23,
24], the role of the cytoskeleton in maintaining the resting state of phagocytes to prevent uncontrolled and detrimental extravasation is still unknown.
In this study, we assessed how intracellular S100A8/S100A9 influences the stability and repolymerization of MTs in living cells. We analyzed the function of S100A8/S100A9 complexes and investigated how S100A8/S100A9 deficiency affects cell polarization, adhesion, and the migration of phagocytes.
2. Materials and Methods
2.1. Cells, Transfection, Stimulation, and Mice
For the migration and adhesion assays, we used freshly isolated bone marrow cells (BMCs) from the femur and tibia of wildtype and S100A9 -/- mice (C57BL/6 strain). Mice were bred and housed under specific pathogen-free conditions and used at the age of 8–12 weeks. S100A9 -/- mice were generated by targeted gene disruption, as described previously [
25]. Expression analyses revealed that these mice are also deficient in S100A8 at the protein level, although the S100A8-mRNA levels are not altered. Therefore, these mice and cells are considered to be functional S100A8 and S100A9 double knockouts. The isolation of BMCs was carried out as described elsewhere and were cultured in Dulbecco’s MEM with 20% L-cell SN, 2% L-glutamine, 1% Pen/Strep, and 10% FCS (
v/
v, heat inactivated) [
25]. We subsequently characterized isolated bone marrow cells by fluorescence activated cell sorting (FACS) analyses using anti-Gr-1 (BD Biosciences, Heidelberg, Germany), anti-Ly6C (BioLegend, Fell, Germany), and anti-Ly6G (eBioscience, Frankfurt, Germany) antibodies. About 65% of the whole BMC population was Gr-1 positive. No differences in Ly6C (69 ± 3%) and Ly6G (76 ± 2%) surface expression between S100A9 -/- and wildtype BMC were detected. For the transendothelial migration experiments, we used murine bEND5 cells to provide a confluent endothelial cell layer.
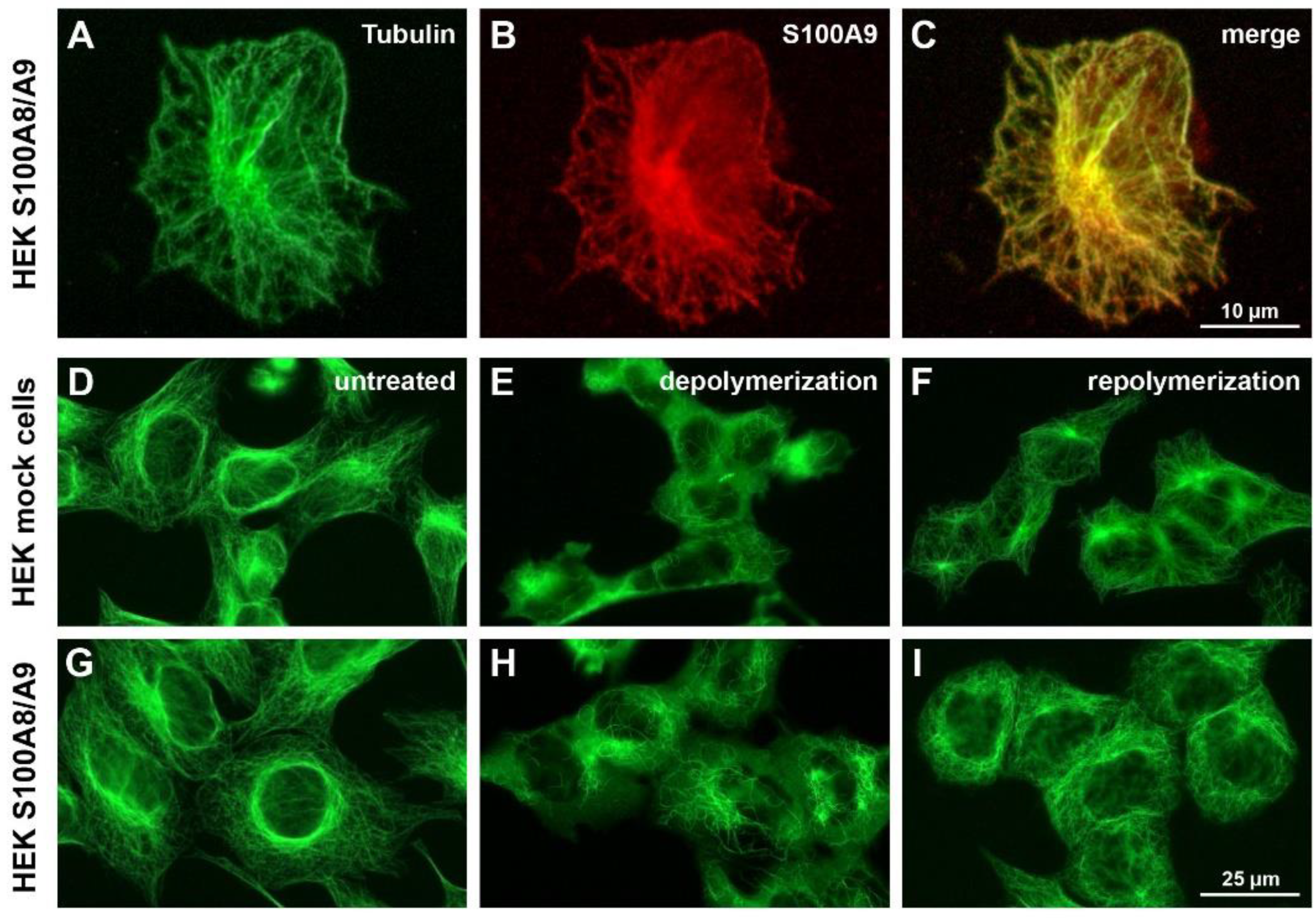
To analyze the intracellular function and impact of S100A8/S100A9 on the cytoskeletal dynamics, we stably transfected HEK293 and NIH 3T3 cells with a pVITRO2mcs (InvivoGen, San Diego, CA, USA) mammalian expression vector construct containing the coding sequences of human S100A8 (5′AgeI; 3′BamHI) and S100A9 (5′Xho; 3′Nhe1). HEK293 and NIH 3T3 were chosen for these type of experiments because they are large and flat, and therefore it is much easier to display submolecular structures compared to much smaller primary bone marrow cells. Both cell lines were cultured in Dulbecco’s MEM with 2% L-glutamine, 1% Pen/Strep, and 10% FCS (v/v, heat inactivated) in 20 cm2 culture flasks at 37 °C. A total of 50 μg/mL hygromycin was strictly used for all cultures. Transfections were carried out using the PolyFect® (Qiagen, Hilden, Germany) transfection reagent according to the manufacturer’s instructions. Untransfected or empty vector mock-transfected cells served as the controls and hygromycin was used for the selection of positive clones.
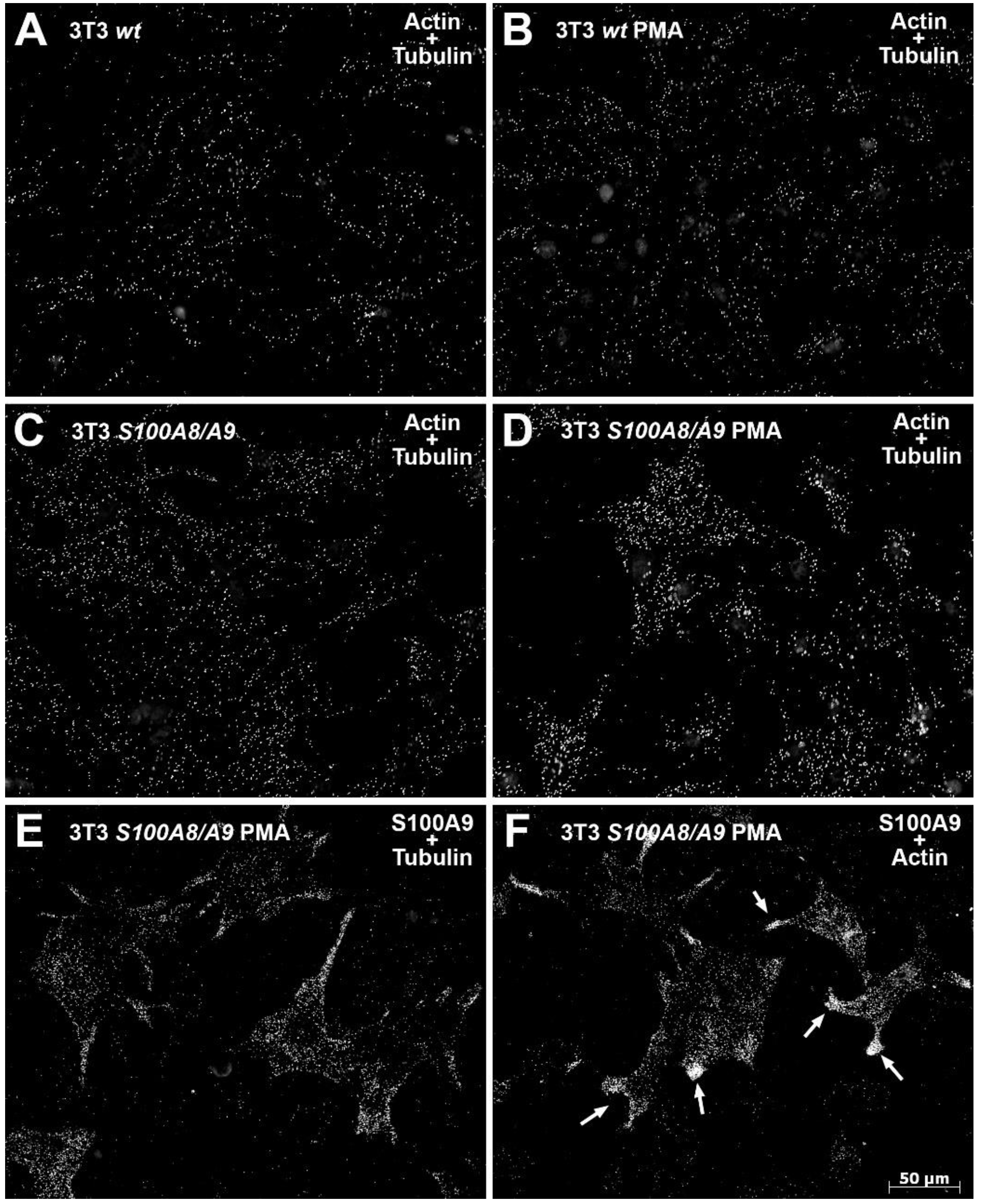
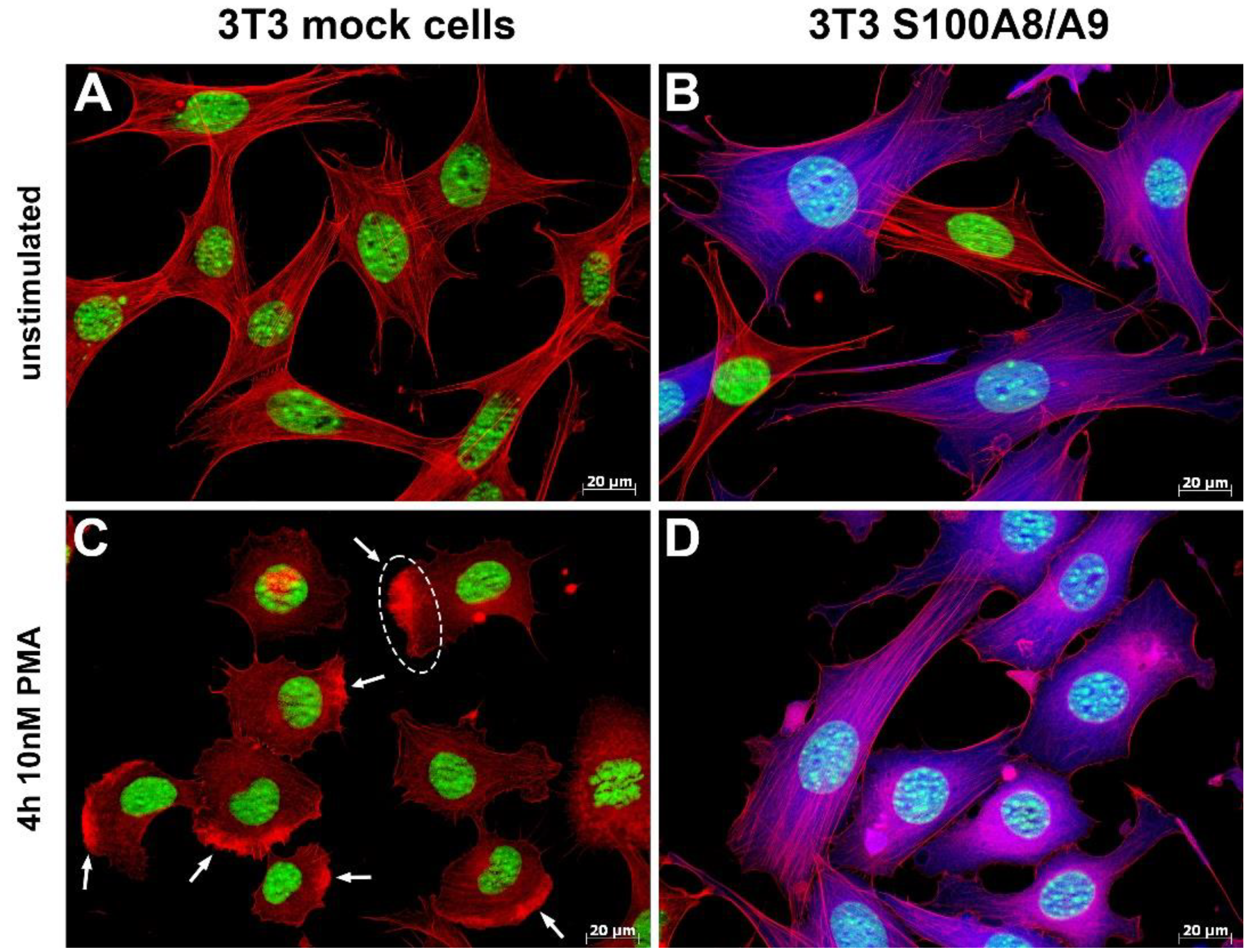
In some experiments, we stimulated cells for 4 h with 10 nM phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, Munich, Germany) to induce cell activation and membrane ruffling.
2.2. Immunofluorescence Microscopy
Cells were cultured overnight on fibronectin-coated Lab-Tek™ chamber slides (Nunc, Wiesbaden, Germany) and fixed in 4% paraformaldehyde for 30 min at room temperature. After cell permeabilization with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 25 min at room temperature, the unspecific binding of antibodies was blocked with 3% bovine serum albumin (BSA) in PBS for 2 h at room temperature prior to adding primary antibodies at 4 °C overnight. We used the following antibodies for the different stainings: mouse anti-α-tubulin [DM-1A] (MP Biomedicals, Illkirch, France), rabbit anti-actin22-33 (Sigma-Aldrich), rabbit anti-paxillin [Y113] (Abcam, Cambridge, UK), mouse anti-β1-integrin [HUTS-4] for the detection of activated β1-integrin (Millipore, Schwalbach Germany), rabbit anti-total-β1-integrin (a kind gift from J. Eble, University of Münster, Germany), and rabbit anti-S100A9 (generated in our institute). F-actin was visualized via staining with fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma-Aldrich).
Primary antibody incubation was followed by 2 h incubation at room temperature with corresponding Cy3-, FITC-, TRITC-, or DyLight649-conjugated secondary antibodies (all from Dianova, Hamburg, Germany), as indicated in the figures. Subsequently, slides were washed thoroughly in PBS and mounted with Dako® Fluorescent Mounting Medium (Dako, Hamburg, Germany).
We analyzed specimens under a Zeiss AxioObserver Z1 inverted microscope using an EC Plan-Neofluar 40×/1.3 or a Plan-Apochromat 63×/1.4 oil immersion objective (Carl Zeiss MicroImaging, Göttingen, Germany). Optical sections were acquired using the Zeiss ApoTome structured illumination system. Images were recorded with an AxioCAM MRm and processed with Zeiss AxioVision 4.8.1.
2.3. CS-T/CS Fixation
S100A8/S100A9-expressing HEK293 cells were cultured as described above and stimulated for 4 h with 10 nM PMA prior to fixation. Cells were washed with PBS, permeabilized for 1 min in CS buffer (10 mM Hepes, pH 6.8, 100 mM KCl, 3 mM MgCl2, 200 mM saccharose, and 1 mM phenylmethylsulfonyl fluoride) containing 0.5% Triton X-100 (to make CS-T buffer) and 20 µM taxol for microtubule stabilization, and washed twice in CS buffer for 5 min each time. After fixation in 4% formaldehyde in PBS for 4 min and methanol for 6 min at −20 °C, we processed the slides for double-labeling immunofluorescence, as described above using antibodies against S100A9 and α-tubulin.
2.4. MT de-/Repolymerization Assay
We treated S100A8/S100A9 and mock-transfected HEK293 cells with 10 µM nocodazole (Sigma-Aldrich) in the cell culture medium for 0, 10, 20, and 30 min, respectively, to assess the MT stability. After the incubation periods, cells were immediately fixed with 4% paraformaldehyde and stained for α-tubulin, as described above. To analyze the influence of S100A8/S100A9 on MT reformation, cells were pretreated with 10 µM of nocodazole in cell culture medium for 1 h to completely depolymerize MTs. Repolymerization was induced by nocodazole wash out with fresh nocodazole-free cell culture medium, and documented via α-tubulin antibody staining (see above) after 0, 5, 10, and 15 min of repolymerization.
2.5. MT/F-Actin Spin-Down Binding Assay
MTs and actin filaments were preassembled according to the manufacturer’s instructions for the Fluorescent Microtubule Biochem Kit and Actin Binding Protein Biochem Kit (both from Cytoskeleton, Denver, CO, USA), respectively. We investigated binding to F-actin by adding S100A8/S100A9 or S100A8/phospho-A9 complexes in the absence (addition of 1 mM EGTA) or presence of 100 µM calcium. After incubation for 30 min at room temperature, samples were centrifuged at 100,000× g for 1 h and further processed for analysis (see below).
We assessed the cross-linking activity by mixing equal volumes of MTs and F-actin followed by the addition S100A8/S100A9 or S100A8/phospho-S100A9 complexes in the absence (addition of EGTA) or presence of 100 µM calcium. Samples were loaded carefully onto a cushion buffer (5 mM Tris pH8, 30% glycerol) after incubation for 30 min at room temperature and centrifuged at 10,000× g for 1 h. We carefully adjusted the composition of the cushion buffer and the rotational speed to spin-down MTs and attached molecules while keeping non-bound smaller proteins and actin filaments in the supernatant fraction. Pellets and supernatants of both assay types (actin binding and cross-linking activity) were analyzed by SDS-PAGE, followed by Coomassie staining. Bands were quantified using the software Lumi-Analyst 3.0 of the Lumi-Imager F1 (Boehringer, Mannheim, Germany).
2.6. MT/Actin Fluorescence In Vitro Assay
Fluorescently labeled MTs were preassembled for 30 min at 37 °C under tubulin-polymerizing conditions (tubulin polymerization buffer (G-PEM): 80 mM PIPES, 2 mM MgCl2, 1.6 M glycerol, 1 mM GTP, pH 6.9). Subsequently, the formed microtubules were stabilized with 20 µM taxol according to the manufacturer’s instructions for the Fluorescent Microtubule Biochem Kit (Cytoskeleton) with the modification of using EGTA-free buffers. F-actin was assembled according to the manufacturer’s instructions for the Actin Binding Protein Biochem Kit (Cytoskeleton). Polymerized F-actin was subsequently stabilized and fluorescently labeled by adding 1 µM FITC-phalloidin (Sigma-Aldrich). We investigated binding to and cross-linking of MTs and F-actin using mixtures of both filaments after the addition of S100A8/S100A9 or S100A8/phospho-A9 complexes in the absence (addition of EGTA) or presence of 100 µM calcium. BSA served as the negative control, and α-actinin served as the positive control. Samples were incubated for 30 min at room temperature, diluted, and immediately analyzed by fluorescence microscopy.
2.7. Duolink® Assay
To identify protein–protein interactions, we conducted proximity ligation assays (PLA, DuoLink II®; Olink Bioscience, Uppsala, Sweden) according to the manufacturer’s instructions. Briefly, cells were cultured, treated, and fixed as described above or in the figures. To detect possible protein–protein interactions, we used the following antibody combinations in the PLA: mouse anti-α-tubulin/rabbit anti-S100A9, mouse anti-β-actin/rabbit anti-S100A9, and mouse anti-α-tubulin/rabbit anti-actin22-33. The antibody combination of mouse anti-human S100A8/rabbit anti-human S100A9 served as the negative control for mock-transfected NIH 3T3 cells and as the positive control for S100A8/S100A9-transfected NIH 3T3 cells. The PLA probes anti-mouse PLUS and anti-rabbit MINUS were used as secondary antibodies. To quantify unspecific background signals, we omitted primary antibodies in some experiments. Hybridization of the two PLA probes only occurs when the two proteins of interest are in close proximity (<40 nm). Hybridization yields a detectable spot-like signal after a subsequent amplification step, for which the Duolink® Detection Kit 563 was used. Specimens were mounted with Dako® Fluorescent Mounting Medium and analyzed under a Zeiss AxioObserver Z1 inverted microscope. Each visible spot represents protein–protein interactions.
2.8. Flow Cytometry
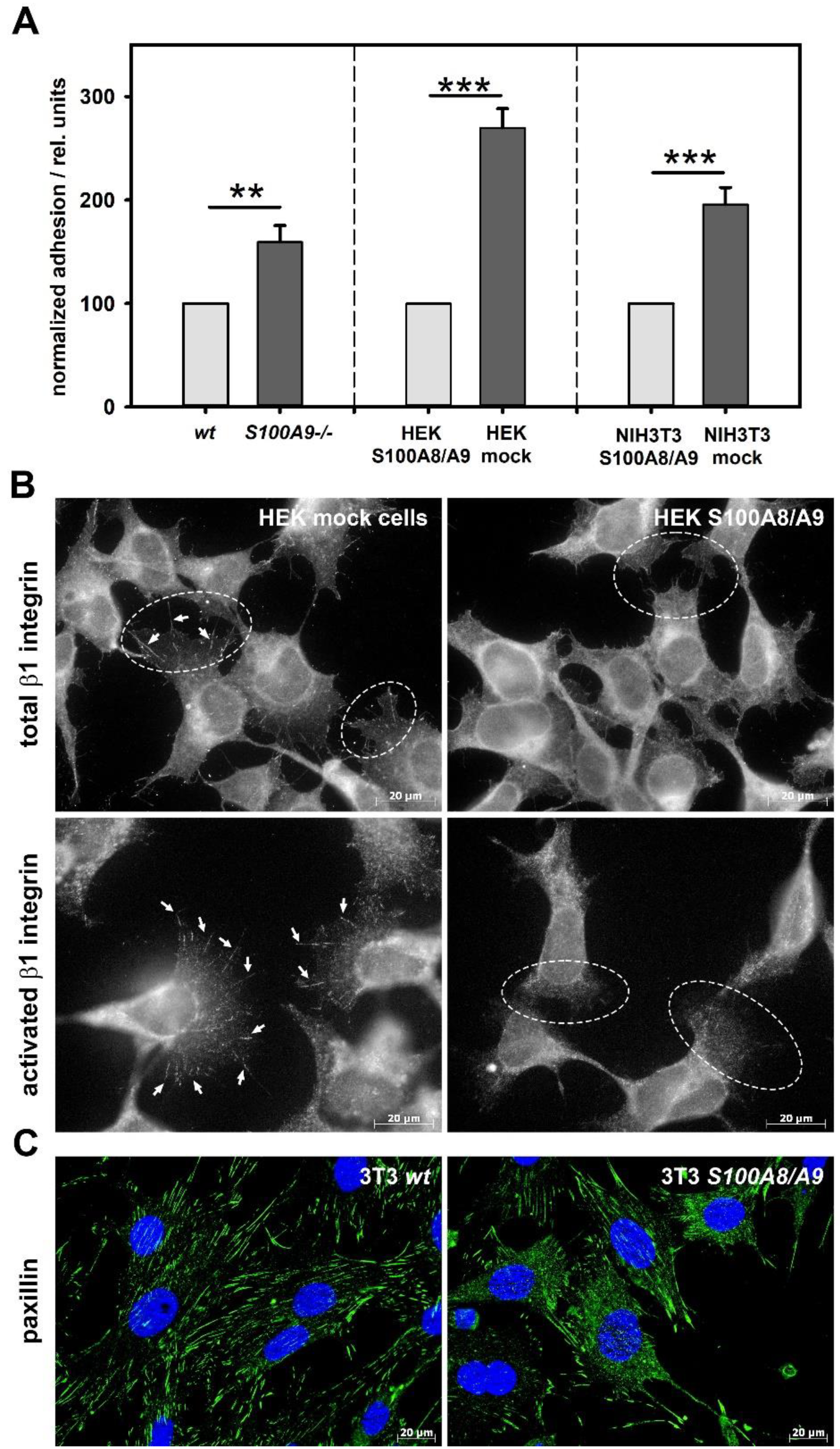
The cells (0.5 × 106 cells in 100 μL media) were centrifuged down at 1100× g rpm for 1 min at 4 °C. The supernatant was discarded and the cells were blocked with 1% BSA (in PBS) for 30 min on ice. The cells were centrifuged again at 1100× g rpm for 1 min at 4 °C. The supernatant was removed and the appropriate antibodies were added in 1:50 dilution in PBS and incubated on ice for 30 min. To quantify the surface expression of various integrins and selectins, we incubated HEK293 cells (S100A8/S100A9 as well as mock-transfected cells) or freshly isolated murine BMCs from the wildtype and S100A9 -/- mice with antibodies against CD11a (LFA-1), CD11b (Mac1), CD11c (αX), CD18 (β2), CD29 (β1), human or murine CD49d (VLA-4α) (ImmunoTools, Friesoythe, Germany), CD54 (ICAM-1), and CD106 (VCAM-1), respectively, followed by incubation with the corresponding FITC-, PE-, or APC-conjugated secondary antibodies (Dianova, Hamburg, Germany). Isotype matched polyclonal antibodies (Sigma-Aldrich) served as the controls. Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences), and data were evaluated using CellQuestPro and WinMDI software.
2.9. Adhesion Assay
Freshly isolated BMCs from wildtype and S100A9 -/- mice were seeded onto either uncoated or VCAM1-coated (1 µg/mL, 16 h, 37 °C) 24 well-plates (4 × 105 cells per well) and allowed to settle for 1 h. In additional experiments, we used S100A8/S100A9- or the mock-transfected HEK293 and NIH 3T3 cell lines. Cells were washed twice with PBS at 37 °C to remove non-adherent cells and fixed with 2% fresh glutaraldehyde for 10 min at room temperature. The fixed cells were stained with 0.5% crystal violet in 200 mM boric acid (pH 8.0) for 15 min at room temperature and then washed 3–4 times with deionized water. Cells were lysed with 10% acetic acid, and the optical density was measured at 560 nm using a Dynatech MRX microplate reader (Dynex Laboratories, Denkendorf, Germany). In additional experiments, adherent BMCs were resuspended and subsequently characterized by FACS analysis using anti-Gr-1, anti-Ly6C, and anti-Ly6G antibodies. Equal fractions of Ly6C- and Ly6G-positive adherent cells were observed by flow cytometry.
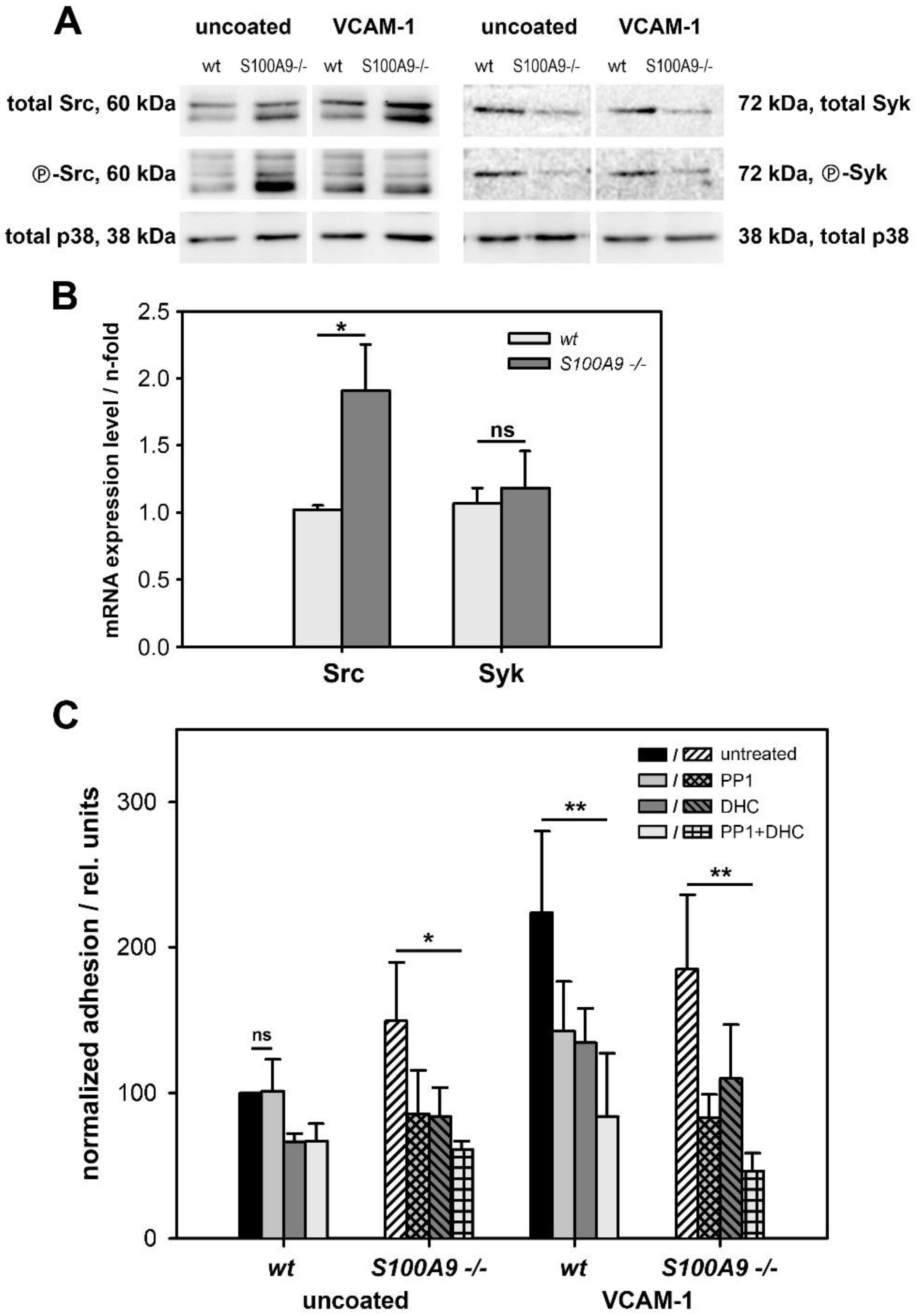
2.10. Src/Syk Expression and Western Blotting
Preparation of the whole cell extracts for Western blot analysis was performed as described earlier [
16]. Equal amounts of proteins (10 µg) per lane were separated on 12% polyacrylamide gels, transferred onto nitrocellulose membranes (Thermo Scientific, Waltham, MA, USA), and processed for antibody staining. We labeled membranes with antibodies against the total (Src Rabbit mAb) and phospho-Src (P-Src Family (Tyr416) Rabbit mAb) or total (Syk Rabbit Ab) and phospho-Syk (Tyr519/520, P-Syk Rabbit Ab), respectively, and the labeling of total p38 (p38 MAPK Rabbit Ab) served as an additional loading control (all from Cell Signaling Technology, Danvers, MA, USA). Finally, we stained the membranes with corresponding horseradish peroxidase-coupled secondary antibodies (Dianova, Hamburg, Germany), visualized them via enhanced chemiluminescence (Amersham Pharmacia Biotech), and documented the results using the Bio-Rad ChemiDoc™XRS+ system (Bio-Rad Laboratories, Munich, Germany).
2.11. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
For the RT-PCR analyses, we analyzed RNA isolated from the wildtype and S100A9 -/- BMCs in duplicate. cDNA was synthesized from 1 µg of total RNA using SuperScriptII RNase-H reverse transcriptase (Invitrogen, Waltham, MA, USA). The primers used for PCR analysis were as follows: Hck (Src Family Kinase; forward primer: GAC TTT GAC CCC CAG CAC GGA GAC; reverse primer: CAC ACT TCT CCA AAC TGC CCA) and Syk (forward primer: TCC ATG GCA ACA TCT CCA G; reverse primer: GAC ATG GTA CCG TGA GGA). RT-PCR was performed using the QuantiTect SYBR Green PCR Kit (Qiagen) according to the manufacturer’s instructions. Samples were subjected to initial denaturation at 95 °C for 15 min and 40 cycles of PCR (94 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s). We used GAPDH as the housekeeping gene (forward primer: CCA CCC CAG CAA GGA CAC T; reverse primer: TCC CTA GGC CCC TCC TGT TAT).
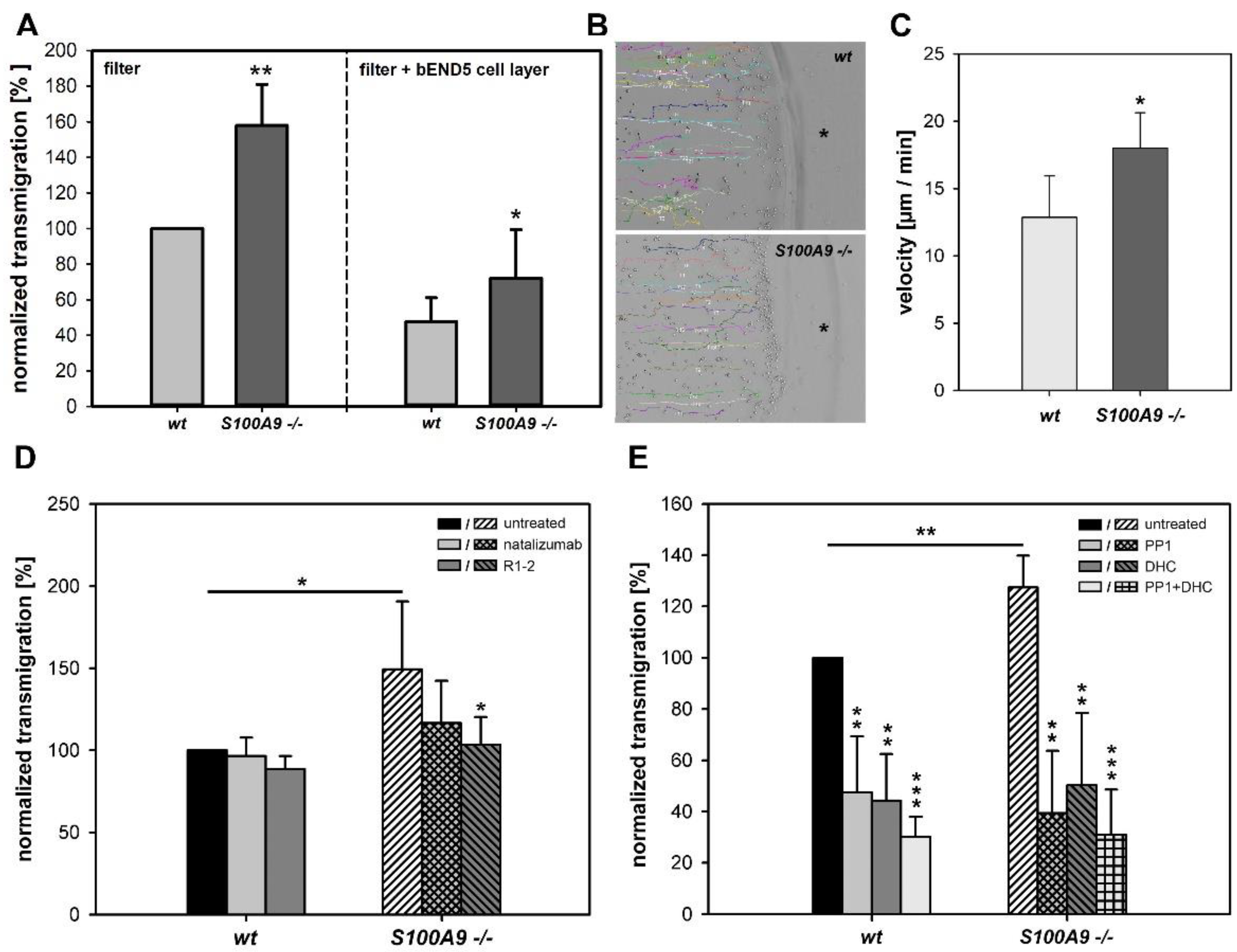
2.12. Transmigration Assay
We performed two chamber transmigration assays in the absence and presence of murine endothelial cells (bEND5) that were grown to confluency (~2–3 days) on 5 µm pore size Transwell filters (Costar, Bodenstein, Germany) as described elsewhere [
26]. Neither filters were coated by any adhesion molecules nor the endothelial monolayer pre-activated by any chemokines/cytokines before adding the cells. Freshly harvested BMCs from wildtype and S100A9 -/- mice were added to the upper chamber (1 × 10
6 cells/100 µL BMC medium) and allowed to transmigrate for 4 h at 37 °C. After incubation, the whole experimental plate was kept on ice for 20 min. Later, the chamber was rinsed with 200 μL of ice-cold PBS and the number of migrated cells counted using a CASY cell counter (Schärfe System, Reutlingen, Germany). Experiments were performed in quadruplicate. We verified the integrity of the bEND5-endothelial monolayer morphologically and by measuring transendothelial resistance using an EVOM
TM epithelial volt-ohmmeter (World Precision Instruments, Sarasota, FL, USA) before starting the experiment.
2.13. Blocking Experiments
To analyze the involvement of CD49d in adhesion and transmigration, freshly isolated BMCs from the wildtype and S100A9 -/- mice were preincubated for 30 min with 10 µg/mL of the CD49d blocking antibodies R1-2 (BD Biosciences) or natalizumab (a kind gift from L. Klotz, University of Münster, Germany) and used for the adhesion or transmigration studies as described above. Likewise, cells pretreated for 30 min with 10 µM of the Src inhibitor 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo-d-3,4-pyrimidine (PP1); Calbiochem, Darmstadt, Germany), 2 µM of the Syk inhibitor 2-(2-Aminoethylamino)-4-(3-trifluoromethylanilino)-pyrimidine-5-carboxamide (DHC), Calbiochem), or a combination of both were used to block Src and Syk kinase activation.
2.14. Two-Dimensional (2D) Migration Assay
We conducted 2D under agarose migration assays as described elsewhere [
27]. Briefly, 35 mm glass bottom Petri dishes (ibidi, Martinsried, Germany) were coated with 50 µg/mL of fibronectin (BD) for 1 h at 37 °C, washed twice with sterile water, and dried prior to casting the agarose gel. Next, 2% (
w/
v) agarose was boiled in plain RPMI1640 medium (Biochrom, Berlin, Germany) without any supplements and mixed with an equal amount of preheated (50 °C) complete RPMI1640 medium to yield a final agarose concentration of 1% (
w/
v). Finally, 2 mL of the warm and still fluid mixture were quickly added to the culture dish, covered with the dish lid, and allowed to solidify at room temperature.
To assess migration, two wells were punched 1.5 mm apart using a template and a 3 mm sterile skin punch. The prepared culture dishes were then equilibrated for 1 h at 37 °C and 5% CO2 prior to seeding of the cells. Freshly isolated BMCs (~2000 cells) were seeded into the first well and allowed to settle for 20 min. Subsequently, 10 µg/mL of the chemoattractant LTB4 (Cayman Chemical, Ann Arbor, MI, USA) was added to the second well, and the dish was carefully placed onto a heated microscope stage (35 °C) with additional CO2 gassing (5%). Movement of cells was recorded at 1 frame per minute with a Zeiss AxioCAM MRm for a total period of 90 min.
2.15. Statistical Analysis
Statistically significant differences between treatments were identified using the Mann–Whitney U test for values with nonparametric distribution or the Student’s t-test for normally distributed values. p < 0.05 was considered to be statistically significant. All results shown are the mean ± standard deviation (SD).
4. Discussion
The family of S100 proteins is the largest subgroup of calcium-binding proteins, and S100A8 and S100A9 are the major calcium-binding proteins of neutrophils and monocytes [
11]. Most previous S100A8/S100A9 studies were focused on elucidating the extracellular functions of these proteins [
13,
14,
40,
41,
42,
43], and much less is known about the intracellular role of this protein complex. One already described important function of S100A8/S100A9 is its direct interaction with MT to promote polymerization and bundling, but to date, this has only been shown in cell-free in vitro experiments using purified S100A8/S100A9 complexes and tubulin [
16].
Therefore, we established stable-transfected S100A8/S100A9-expressing HEK293 and NIH 3T3 cell lines to analyze the intracellular function of S100A8/S100A9 in more detail in a living cell system. Using HEK293 cells, we were able to confirm previous results reported for tubulin interaction with S100A8/S100A9 in human monocytes [
19]. Our data also indicate that S100A8/S100A9 not only promotes tubulin polymerization, but it also stabilizes MTs in vivo, thus affecting the overall MT dynamics. This result is of biological relevance, as both of these characteristics (MT polymerization and stability) have a significant impact on the overall dynamic remodeling of the cytoskeleton [
44,
45], which in turn is a prerequisite for locomotion [
30,
45,
46]. Furthermore, because the MT cytoskeleton of the resting S100A8/S100A9-expressing and non-expressing cells did not differ, our data suggest that S100A8/S100A9 predominantly affects dynamic MTs during phases of growth or shrinkage as opposed to “stationary” ones, and is thus not primarily involved in maintaining the steady-state of the MT cytoskeleton. This observation points to a function of S100A8/S100A9 in the coordination and modulation of the activation of cell dynamics. The stimulation of HEK293 cells or primary bone marrow cells by the phorbol ester PMA caused the cross-linking of microtubules and actin filaments via S100A8/S100A9 complexes. However, PMA is also well-known as an activator of protein kinase C, which in turn leads to the phosphorylation of integrins, resulting in changes in the integrin conformation and interaction with the cytoskeleton and downstream adaptor molecules. Therefore integrin phosphorylation provides a trigger and initiates rapid cell adhesion and signaling events [
47].
Because cell migration is mainly dependent on F-actin dynamics, we analyzed interactions of S100A8/S100A9 with MTs and actin. Co-sedimentation assays as well as in vitro and in vivo experiments revealed not only an interaction of S100A8/S100A9 complexes with F-actin, as reported earlier [
20], and with MTs [
16], but also a novel function of S100A8/S100A9 as a calcium-dependent MT/F-actin cross-linker that efficiently bridges both types of filaments. Additionally, our data show that the binding of S100A8/S100A9 to MTs and to F-actin is strictly calcium-dependent, whereas the phosphorylation of S100A9 alters the affinity toward F-actin, as suggested by Lominadze et al. (2005) [
20], and more importantly, modulates the cross-linking activity of the S100A8/S100A9 complex. This cross-linking activity was completely abrogated by phosphorylated S100A8/S100A9 complexes, which is analogous to previous findings about MT bundling [
16].
MTs also play an important role in the targeted delivery and transportation of numerous proteins and in the coordination of extending the lamellipodia of moving cells [
48,
49,
50,
51]. They are also involved in the formation and turnover of focal adhesions, as they are known to “contact” these structures, thereby leading to their disassembly [
52,
53]. Consequentially, we hypothesize that the cross-linking activity of S100A8/S100A9 described herein directly influences the targeted delivery and distribution of proteins that are critically involved in cell polarity or the composition of focal adhesions such as integrins, thereby also affecting overall integrin activation, distribution/clustering, and subsequent signaling. Thus, S100A8/S100A9 may promote the breakdown and recycling of these structures and prevent the maturation of nascent focal complexes to focal adhesions. This premise is supported by the results of the DuoLink
® PLA assays, which revealed the presence of S100A8/S100A9 in areas where membrane protrusions/lamellipodia were formed.
In summary, these data point to a local submembraneous mechanism that could help phagocytes maintain a non-adherent resting state until secondary signaling cascades activate kinases such as p38 MAPK, for which an interaction with S100A9 was already reported [
16,
20], and abrogate the cross-linking by phosphorylating S100A9 on threonine113. In the S100A8/S100A9-deficient BMCs, this accurate control of overall cell activation was markedly impaired, leading to increased cell dynamics, as indicated by disordered cell adhesion and uncontrolled migration rates. S100A8/S100A9 may also act as a formin-related protein that facilitates the guidance of MTs along actin filaments toward focal adhesions and supports their stabilization by linking them to the actin-rich substructure of focal adhesions or focal complexes [
54,
55,
56].
MTs have been shown to contact focal adhesion points, leading to their disassembly. It is therefore feasible to assume that the interplay of S100A8/S100A9 with the MT cytoskeleton has a measurable influence on cell adhesion, and the regulation and modulation of adhesion, in turn, affect directed cell migration. In fact, S100A8/S100A9-expressing cells showed a significant reduction in substrate adhesion that was caused by alterations in the surface expression and the activation of β1-integrins. They were further characterized by a redistribution of the integrin signaling downstream target paxillin and by altered regulation of the target kinases Src and Syk. In particular, we identified CD49d as being differentially regulated in S100A8/S100A9-expressing BMCs.
CD49d is the alpha subunit of the α4β1 lymphocyte homing receptor VLA-4, and it plays an important role in the adhesion of leukocytes to the endothelium prior to extravasation [
34,
57]. It supports lymphocyte rolling in vivo in venules of the central nervous system. Although CD49d is often described as playing only a minor role in neutrophil adhesion compared to CD11b/CD18 (Mac1), our data indicate that S100A8/S100A9 keeps the cells in a resting state via the modulation of CD49d signaling to prevent uncontrolled extravasation. However, we found that the regulation of CD49d surface expression was dependent on both S100A8/S100A9 expression and on the presence of the extracellular ligand VCAM-1 [
35]. This finding suggests that S100A8/S100A9 has a strong impact on the modulation of target-oriented integrin activation and signaling, whereas the loss of S100A8/S100A9 leads to impaired regulation and inappropriate cell activation, which finally results in the overall higher dynamic activity of S100A9 -/- phagocytes.
The proposed dysregulation of integrin signaling is supported by our finding that both expression and activation of the downstream target kinases Src and Syk were affected by the loss of S100A8/S100A9. Functional consequences of the observed alterations in Src/Syk expression and activity were in line with the results of subsequent blocking studies. We found that S100A9 -/- cells were prone to Src and Syk blocking, both in the presence and absence of VCAM-1, whereas the wildtype cells were only affected in the presence of the integrin ligand. The observed alterations in integrin-mediated adhesion had a direct impact on cell migration, as demonstrated by filter-based transmigration and 2D migration assays. This is a biologically relevant consequence that is important for the in vivo function of phagocytes to quickly reach sites of inflammation upon stimulation while otherwise remaining dormant. S100A9-/- BMCs exhibited an overall higher rate of spontaneous transmigration, whereas chemotactic orientation of both genotypes was comparable.
We also illustrated the significant influence of integrin (particularly CD49d) signaling on spontaneous transmigration via blocking studies using natalizumab or R1-2. Our new data support recent findings of a new extracellular function of S100A8/S100A9 on murine monocytes, whereby missing extracellular S100A8/S100A9 causes the preactivation of these cells. This effect is not linked to proinflammatory S100A8/S100A9 dimers, and is exclusively restricted to calcium-induced tetramers of S100A8/S100A9 binding and activating CD69 on monocytes [
58]. We also recently showed that the contact of neutrophils with the endothelium leads to a rapid S100A8/S100A9-dimer release, and that these dimers in turn bind in an autocrine manner to the neutrophil’s Toll-like receptor 4 and initiate the activation of β2-integrins from a low-affinity to an intermediate-affinity state. This process leads to the firm arrest of the cells and facilitates their extravasation [
42].
Clearly, intracellular and extracellular S100A8/S100A9 complexes have synergistic regulatory functions that prevent unwanted and/or uncontrolled phagocyte activation. Accordingly, the inhibition of Src and Syk activity was able to reduce the otherwise higher transmigration rate of knockout BMCs to the wildtype level. The function of integrins critically depends on their conformational state. During rolling along the endothelium, integrins undergo conformational changes from a low-affinity bent conformation to an intermediate, and finally to a high-affinity open conformation, which eventually allows for firm adhesion of the phagocytes and their extravasation without any changes in the overall expression levels of these molecules [
59]. In our study, we only detected differences in the CD49d expression levels between the S100A8/S100A9-expressing cells compared to the non-S100A8/S100A9-expressing cells. We currently cannot rule out the possibility that the functions of other β1- or β2-integrin molecules might also be affected by missing S100A8/S100A9, despite the lack of changes in their overall expression levels.
In summary, the results presented herein provide novel insights into the molecular mechanisms necessary to precisely control the regulation of phagocyte activation, adhesion, and migration. We showed that intracellular S100A8/S100A9, which was already known to integrate two major activation pathways (MAPK and calcium-dependent signaling), plays a pivotal role in cytoskeletal dynamics. Moreover, we demonstrated for the first time that S100A8/S100A9 cross-links MTs and actin filaments in a calcium- and phosphorylation-dependent manner. As a consequence, the modulation of cytoskeletal dynamics by S100A8/S100A9 affects the overall cell adhesion, proper cell polarization, and, as a result, transmigration. In contrast, the absence of intracellular S100A8/S100A9 leads to dysregulated and increased cell activation. Thus, S100A8/S100A9 promotes the maintenance of a resting/quiescent phagocyte state. The observed cross-linking activity of S100A8/S100A9 is strictly phosphorylation-dependent, so pharmacologic interference with this process may be an interesting and promising approach to restrict phagocyte activation in inflammatory diseases.