EGPA Phenotyping: Not Only ANCA, but Also Eosinophils
Abstract
1. Introduction
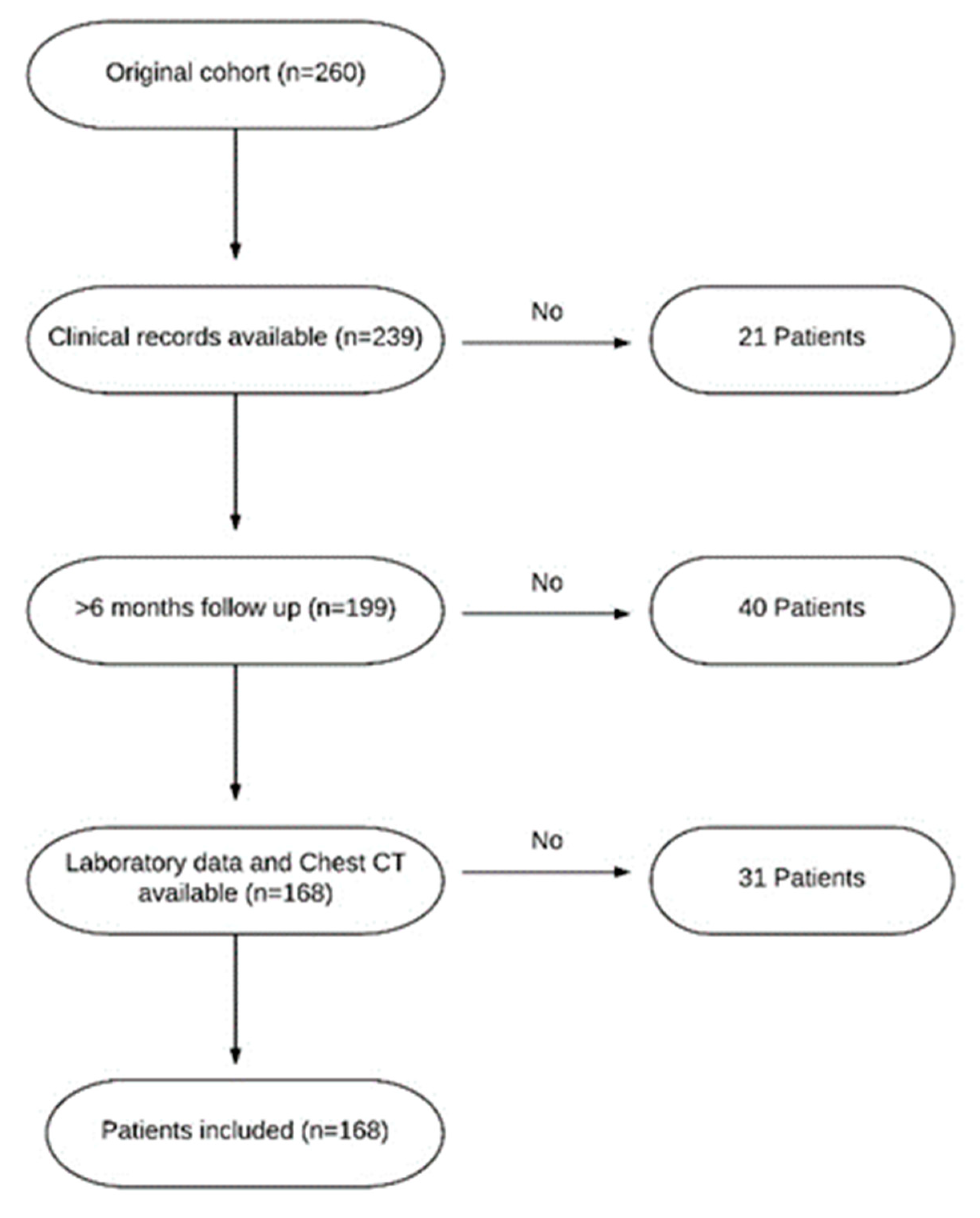
2. Materials and Methods
2.1. Study Population
2.2. Statistical Methodology
3. Results
3.1. Patients Characteristics and Clinical Phenotypes of EGPA
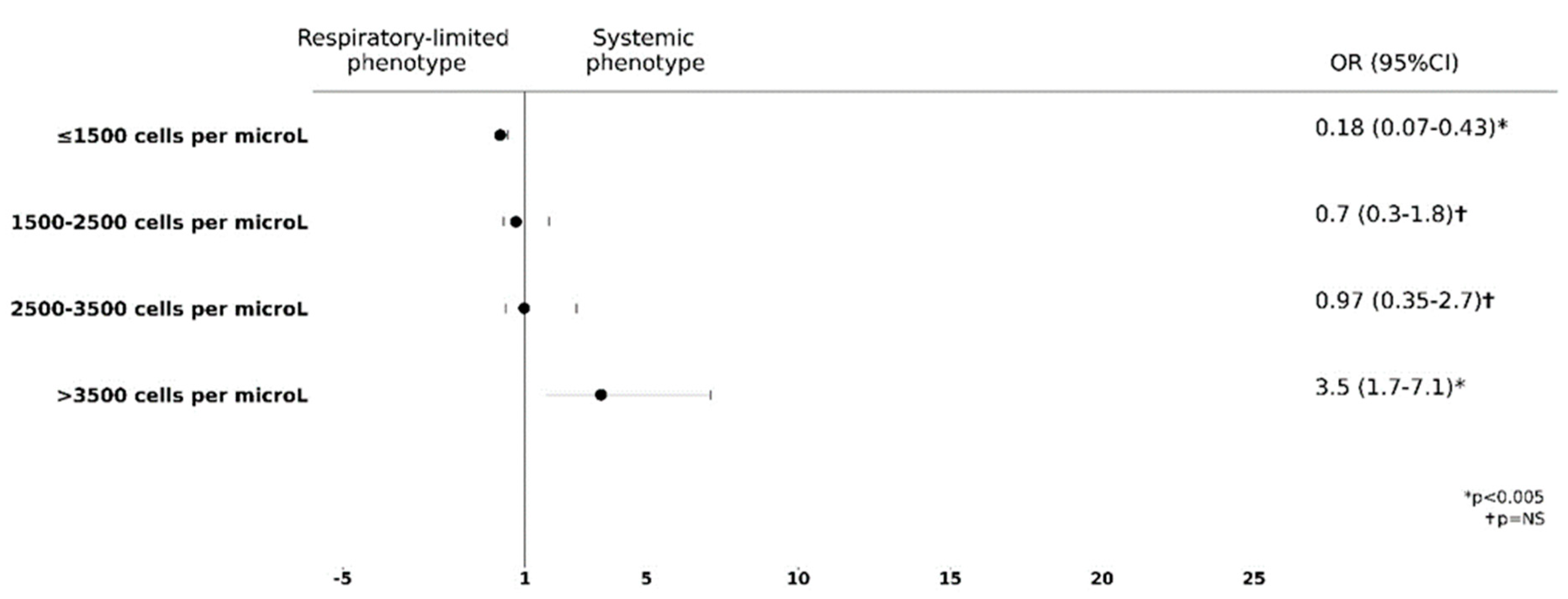
3.2. Blood Eosinophils Count Is Predictive of Extra Lung Involvement
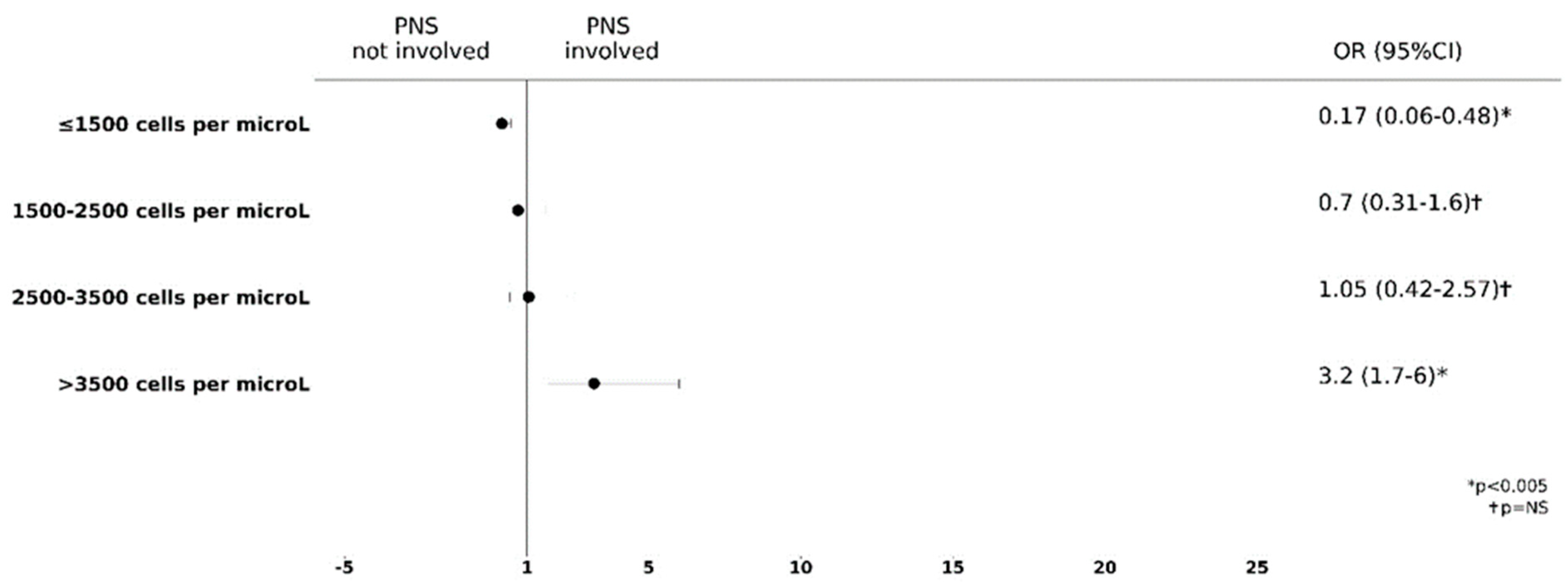
3.3. Higher Blood Eosinophils Count Is Predictive of PNS Involvement
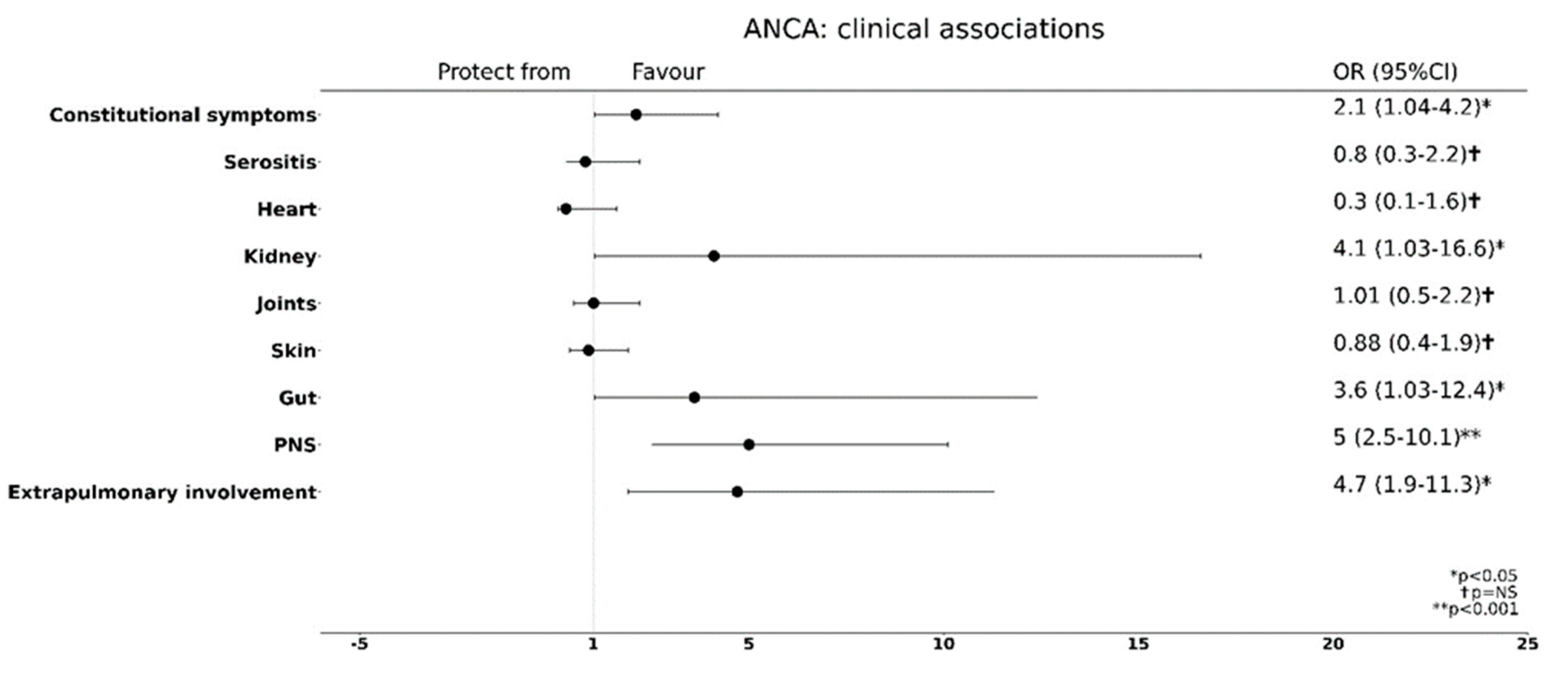
3.4. ANCA Positivity Is Predictive of a Preferential Extra-Pulmonary Organ Involvement
4. Discussion
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dispenza, M.C.; Bocher, B.S. Diagnosis and novel approaches to the treatment of hypereosinophilc syndrome. Curr. Hematol. Malig. Rep. 2018, 13, 191–201. [Google Scholar] [CrossRef]
- White, J.; Dubey, S. Eosinophilic granulomatosis with polyangiitis: A review. Autoimmun. Rev. 2023, 22, 103219. [Google Scholar] [CrossRef] [PubMed]
- Mouthon, L.; Dunogue, B.; Guillevin, L. Diagnosis and classification of eosinophilic granulomatosis with polyangiitis (former named Churg-Strauss syndrome). J. Autoimmun. 2014, 48–49, 91–93. [Google Scholar]
- Wu, E.Y.; Hernandez, M.L.; Jennette, J.C.; Falk, R.J. Eosinophilic Granulomatosis with Polyangiitis: Clinical Pathology Conference and Review. J. Allergy Clin. Immunol. Pract. 2018, 6, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Jakiela, B.; Sanak, M.; Szczeklik, W.; Sokolowska, B.; Plutecka, H.; Mastalerz, L.; Musial, J.; Szczeklik, A. Both Th2 and Th17 responses are involved in the pathogenesis of Churg-Strauss syndrome. Ann. Rheum. Dis. 2011, 29, S23–S34. [Google Scholar]
- Tsurikisawa, N.; Saito, H.; Oshikata, C.; Tsuburai, T.; Akiyama, K. Decreases in the Numbers of Peripheral Blood Regulatory T Cells, and Increases in the Levels of Memory and Activated B Cells, in Patients with Active Eosinophilic Granulomatosis and Polyangiitis. J. Clin. Immunol. 2013, 33, 965–976. [Google Scholar] [CrossRef]
- Masi, A.T.; Hunder, G.G.; Lie, J.T.; Michel, B.A.; Bloch, D.A.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990, 33, 1094–1100. [Google Scholar] [CrossRef]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef]
- Cottin, V.; Bel, E.; Bottero, P.; Dalhoff, K.; Humbert, M.; Lazor, R.; Sinico, R.A.; Sivasothy, P.; Wechsler, M.E.; Groh, M.; et al. Respiratory Manifestations of Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss). Eur. Respir. J. 2016, 48, 1429–1441. [Google Scholar] [CrossRef]
- Greco, A.; Rizzo, M.I.; De Virgilio, A.; Gallo, A.; Fusconi, M.; Ruoppolo, G.; Altissimi, G.; De Vincentiis, M. Churg-Strauss syndrome. Autoimmun. Rev. 2015, 14, 341–348. [Google Scholar] [CrossRef]
- Price, D.B.; Rigazio, A.; Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Thomas, M.; Wenzel, S.E.; Wilson, A.M.; Small, M.B.; Gopalan, G.; et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar] [CrossRef]
- Kronbichler, A.; Lee, K.H.; Denicolò, S.; Choi, D.; Lee, H.; Ahn, D.; Kim, K.H.; Lee, J.H.; Kim, H.; Hwang, M.; et al. Immunopathogenesis of ANCA-Associated Vasculitis. Int. J. Mol. Sci. 2020, 21, 7319. [Google Scholar] [CrossRef]
- Comarmond, C.; Pagnoux, C.; Khellaf, M.; Cordier, J.-F.; Hamidou, M.; Viallard, J.-F.; Maurier, F.; Jouneau, S.; Bienvenu, B.; Puéchal, X.; et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): Clinical characteristics and long-term follow-up of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013, 65, 270–281. [Google Scholar] [CrossRef]
- Mukherjee, M.; Nair, P. Autoimmune Responses in Severe Asthma. Allergy Asthma Immunol. Res. 2018, 10, 428–447. [Google Scholar] [CrossRef]
- Bond, M.; Fagni, F.; Moretti, M.; Bello, F.; Egan, A.; Vaglio, A.; Emmi, G.; Dejaco, C. At the Heart of Eosinophilic Granulomatosis with Polyangiitis: Into Cardiac and Vascular Involvement. Curr. Rheumatol. Rep. 2022, 24, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.; Janagan, S.; Wells, M.; Crawshaw, H.; McAdoo, S.; Robson, J.C. ANCA Associated Vasculitis Subtypes: Recent Insights and Future Perspectives. J. Inflamm. Res. 2022, 15, 2567–2582. [Google Scholar] [CrossRef] [PubMed]
- McBrian, C.N.; Menzies-Gow, A. The biology of eosinophils and their role in asthma. Front. Med. 2017, 4, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.-C.; Spry, C.; Olsen, E.; Ackerman, S.; Dunnette, S.; Gleich, G. Deposits of Eosinophil Granule Proteins in Cardiac Tissues of Patients with Eosinophilic Endomyocardial Disease. Lancet 1987, 329, 643–647. [Google Scholar] [CrossRef]
- Tajima, K.; Katagiri, T. Deposits of eosinophil granule proteins in eosinophilic cholecystitis and eosinophilic colitis associated with hypereosinophilic syndrome. Dig. Dis. Sci. 1996, 41, 282–288. [Google Scholar] [CrossRef]
- Motojima, S.; Frigas, E.; Loegering, D.A.; Gleich, G.J. Toxicity of Eosinophil Cationic Proteins for Guinea Pig Tracheal Epithelium In Vitro. Am. Rev. Respir. Dis. 1989, 139, 801–805. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ueki, S.; Kamide, Y.; Miyabe, Y.; Fukuchi, M.; Yokoyama, Y.; Furukawa, T.; Azuma, N.; Oka, N.; Takeuchi, H.; et al. Increased Circulating Cell-Free DNA in Eosinophilic Granulomatosis with Polyangiitis: Implications for Eosinophil Extracellular Traps and Immunothrombosis. Front. Immunol. 2022, 12, 801897. [Google Scholar] [CrossRef]
- Thompson-Souza, G.A.; Vasconcelos, C.R.I.; Neves, J.S. Eosinophils: Focus on DNA extracellular traps. Life Sci. 2022, 311, 121191. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Vivarelli, E.; Perlato, M.; Mecheri, V.; Accinno, M.; Cosmi, L.; Parronchi, P.; Rossi, O.; Vultaggio, A. Baseline Eosinophil Count as a Potential Clinical Biomarker for Clinical Complexity in EGPA: A Real-Life Experience. Biomedicines 2022, 10, 2688. [Google Scholar] [CrossRef] [PubMed]
- Konstantouli, A.M.; Lioulios, G.; Stai, S.; Moysidou, E.; Fylaktou, A.; Papagianni, A.; Stangou, M. Type of ANCA May Be Indispensable in Distinguishing Subphenotypes of Different Clinical Entities in ANCA-Associated Vasculitis. Life 2022, 12, 1467. [Google Scholar] [CrossRef]
- Osman, M.S.; Tervaert, J.W.C. Anti-neutrophil Cytoplasmic Antibodies (ANCA) as Disease Activity Biomarkers in a “Personalized Medicine Approach” in ANCA-Associated Vasculitis. Curr. Rheumatol. Rep. 2019, 21, 76. [Google Scholar] [CrossRef]
- Müller, A.; Krause, B.; Kerstein-Stähle, A.; Comdühr, S.; Klapa, S.; Ullrich, S.; Holl-Ulrich, K.; Lamprecht, P. Granulomatous Inflammation in ANCA-Associated Vasculitis. Int. J. Mol. Sci. 2021, 22, 6474. [Google Scholar] [CrossRef]
- Khoury, P.; Grayson, P.C.; Klion, A.D. Eosinophils in vasculitis: Characteristics and roles in pathogenesis. Nat. Rev. Rheumatol. 2014, 10, 474–483. [Google Scholar] [CrossRef]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Prim. 2020, 6, 71. [Google Scholar] [CrossRef]
- Folci, M.; Ramponi, G.; Arcari, I.; Zumbo, A.; Brunetta, E. Eosinophils as Major Player in Type 2 Inflammation: Autoimmunity and Beyond. Adv. Exp. Med. Biol. 2021, 1347, 197–219. [Google Scholar]
- Nishi, R.; Koike, H.; Ohyama, K.; Fukami, Y.; Ikeda, S.; Kawagashira, Y.; Iijima, M.; Katsuno, M.; Sobue, G. Differential clinicopathologic features of EGPA-associated neuropathy with and without ANCA. Neurology 2020, 94, e1726–e1737. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, Y.; Lee, H.; Mun, J.; Sim, S.; Lee, D.; Le Pham, D.; Kim, S.; Shin, Y.S.; Lee, S.; et al. Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy 2019, 75, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and Exacerbations of Refractory Eosinophilic Asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; Fitzgerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef]
- Charles, D.; Shanley, J.; Temple, S.N.; Rattu, A.; Khaleva, E.; Roberts, G. Real-world efficacy of treatment with benralizumab, dupilumab, mepolizumab and reslizumab for severe asthma: A systematic review and meta-analysis. Clin. Exp. Allergy 2022, 52, 616–627. [Google Scholar] [CrossRef]
- Kavanagh, J.E.; Hearn, A.P.; Dhariwal, J.; D’Ancona, G.; Douiri, A.; Roxas, C.; Fernandes, M.; Green, L.; Thomson, L.; Nanzer, A.M.; et al. Real-World Effectiveness of Benralizumab in Severe Eosinophilic Asthma. Chest 2020, 159, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Roufosse, F.; Kahn, J.-E.; Rothenberg, M.E.; Wardlaw, A.J.; Klion, A.D.; Kirby, S.Y.; Gilson, M.J.; Bentley, J.H.; Bradford, E.S.; Yancey, S.W.; et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 146, 1397–1405. [Google Scholar] [CrossRef]
- Harish, A.; Schwartz, S.A. Targeted Anti-IL-5 Therapies and Future Therapeutics for Hypereosinophilic Syndrome and Rare Eosinophilic Conditions. Clin. Rev. Allergy Immunol. 2020, 59, 231–247. [Google Scholar] [CrossRef]
- Moosig, F.; Gross, W.L.; Herrmann, K.; Bremer, J.P.; Hellmich, B. Targeting Interleukin-5 in Refractory and Relapsing Churg–Strauss Syndrome. Ann. Intern. Med. 2011, 155, 341–343. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef]
| Demographic, Clinical and Laboratory Features | Study Cohort (n = 168) |
|---|---|
| Female/Male | 101/67 |
| Age at diagnosis (y) | 50.1 ± 12.9 |
| Age at symptoms onset (y) | 46.8 ± 13.6 |
| Diagnostic delay (y) | 3.2 ± 6.4 |
| Atopy | 68 (40.5) |
| Asthma | 168 (100) |
| CRSwNP | 161 (95.8) |
| Arthralgia/Arthritis | 34 (20.2) |
| CNS involvement | 1 (0.5) |
| Constitutional symptoms | 45 (26.8) |
| Gut involvement | 12 (7.1) |
| Heart involvement | 11 (6.5) |
| Kidney involvement | 10 (5.9) |
| Lung opacities | 144 (85.7) |
| PNS involvement | 90 (53.6) |
| Serositis | 18 (10.7) |
| Skin involvement | 39 (23.2) |
| FEV1 (%) | 69.5 ± 22 |
| Blood eosinophils (cells/μL) | 5416 ± 4988 |
| Blood eosinophils (%) | 33.1 ± 15.3 |
| ECP (μg/L) | 75.7 ± 98.2 |
| Total serum IgE (kU/L) | 505.7 ± 667 |
| ANCA+ | 64 (38.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matucci, A.; Vivarelli, E.; Perlato, M.; Mecheri, V.; Accinno, M.; Cosmi, L.; Parronchi, P.; Rossi, O.; Vultaggio, A. EGPA Phenotyping: Not Only ANCA, but Also Eosinophils. Biomedicines 2023, 11, 776. https://doi.org/10.3390/biomedicines11030776
Matucci A, Vivarelli E, Perlato M, Mecheri V, Accinno M, Cosmi L, Parronchi P, Rossi O, Vultaggio A. EGPA Phenotyping: Not Only ANCA, but Also Eosinophils. Biomedicines. 2023; 11(3):776. https://doi.org/10.3390/biomedicines11030776
Chicago/Turabian StyleMatucci, Andrea, Emanuele Vivarelli, Margherita Perlato, Valentina Mecheri, Matteo Accinno, Lorenzo Cosmi, Paola Parronchi, Oliviero Rossi, and Alessandra Vultaggio. 2023. "EGPA Phenotyping: Not Only ANCA, but Also Eosinophils" Biomedicines 11, no. 3: 776. https://doi.org/10.3390/biomedicines11030776
APA StyleMatucci, A., Vivarelli, E., Perlato, M., Mecheri, V., Accinno, M., Cosmi, L., Parronchi, P., Rossi, O., & Vultaggio, A. (2023). EGPA Phenotyping: Not Only ANCA, but Also Eosinophils. Biomedicines, 11(3), 776. https://doi.org/10.3390/biomedicines11030776







