Training vs. Tolerance: The Yin/Yang of the Innate Immune System
Abstract
1. Introduction
2. Trained Immunity
2.1. Stressor-Dependent Induction of Trained Innate Memory
2.2. Dose-Dependent Induction of Trained Innate Memory and Immune Tolerance
2.3. Ageing, Hormones and Dietary Restrictions as Decisive Modifiers of Innate Memory
3. Endotoxin Tolerance
4. Role of Trained Immunity and Endotoxin Tolerance in Inflammatory Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An Introduction to Immunology and Immunopathology. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. 2), 49. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.A.; Oettgen, H.C. Adaptive Immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Turvey, S.E.; Broide, D.H. Innate Immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Toll-like Receptors and Innate Immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Kurtz, J. Specific Memory within Innate Immune Systems. Trends Immunol. 2005, 26, 186–192. [Google Scholar] [CrossRef]
- Wenig, M.; Ghirardo, A.; Sales, J.H.; Pabst, E.S.; Breitenbach, H.H.; Antritter, F.; Weber, B.; Lange, B.; Lenk, M.; Cameron, R.K.; et al. Systemic Acquired Resistance Networks Amplify Airborne Defense Cues. Nat. Commun. 2019, 10, 3813. [Google Scholar] [CrossRef]
- Gourbal, B.; Pinaud, S.; Beckers, G.J.M.; Van Der Meer, J.W.M.; Conrath, U.; Netea, M.G. Innate Immune Memory: An Evolutionary Perspective. Immunol. Rev. 2018, 283, 21–40. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The Physiology and Habitat of the Last Universal Common Ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef]
- Purvis, A.; Hector, A. Getting the Measure of Biodiversity. Nature 2000, 405, 212–219. [Google Scholar] [CrossRef]
- Titley, M.A.; Snaddon, J.L.; Turner, E.C. Scientific Research on Animal Biodiversity Is Systematically Biased towards Vertebrates and Temperate Regions. PLoS ONE 2017, 12, e0189577. [Google Scholar] [CrossRef]
- Naeslund, C. Expérience de Vaccination Par Le BCG Dans La Province Du Norrbotten (Suède). Rev. Tuberc. 1931, 12, 617–636. [Google Scholar]
- Mackaness, G.B. The Immunology of Antituberculous Immunity. Am. Rev. Respir. Dis. 1968, 97, 337–344. [Google Scholar] [PubMed]
- Blanden, R.V.; Lefford, M.J.; Mackaness, G.B. The Host Response to Calmette-Guérin Bacillus Infection in Mice. J. Exp. Med. 1969, 129, 1079–1107. [Google Scholar] [CrossRef] [PubMed]
- Garly, M.L.; Martins, C.L.; Balé, C.; Baldé, M.A.; Hedegaard, K.L.; Gustafson, P.; Lisse, I.M.; Whittle, H.C.; Aaby, P. BCG Scar and Positive Tuberculin Reaction Associated with Reduced Child Mortality in West Africa: A Non-Specific Beneficial Effect of BCG? Vaccine 2003, 21, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized Trial of BCG Vaccination at Birth to Low-Birth-Weight Children: Beneficial Nonspecific Effects in the Neonatal Period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Martins, C.L.; Garly, M.-L.; Balé, C.; Andersen, A.; Rodrigues, A.; Ravn, H.; Lisse, I.M.; Benn, C.S.; Whittle, H.C. Non-Specific Effects of Standard Measles Vaccine at 4.5 and 9 Months of Age on Childhood Mortality: Randomised Controlled Trial. BMJ 2010, 341, c6495. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, S.; Jensen, K.J.; Monterio, I.; Ravn, H.; Aaby, P.; Benn, C.S. Rapid Protective Effects of Early BCG on Neonatal Mortality Among Low Birth Weight Boys: Observations From Randomized Trials. J. Infect. Dis. 2018, 217, 759–766. [Google Scholar] [CrossRef]
- Escobar, L.E.; Molina-Cruz, A.; Barillas-Mury, C. BCG Vaccine Protection from Severe Coronavirus Disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA 2020, 117, 17720–17726. [Google Scholar] [CrossRef] [PubMed]
- Prentice, S.; Nassanga, B.; Webb, E.L.; Akello, F.; Kiwudhu, F.; Akurut, H.; Elliott, A.M.; Arts, R.J.W.; Netea, M.G.; Dockrell, H.M.; et al. BCG-Induced Non-Specific Effects on Heterologous Infectious Disease in Ugandan Neonates: An Investigator-Blind Randomised Controlled Trial. Lancet. Infect. Dis. 2021, 21, 993–1003. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W.M. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Ochando, J.; Fayad, Z.A.; Madsen, J.C.; Netea, M.G.; Mulder, W.J.M. Trained Immunity in Organ Transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Braza, M.S.; van Leent, M.M.T.; Lameijer, M.; Sanchez-Gaytan, B.L.; Arts, R.J.W.; Pérez-Medina, C.; Conde, P.; Garcia, M.R.; Gonzalez-Perez, M.; Brahmachary, M.; et al. Inhibiting Inflammation with Myeloid Cell-Specific Nanobiologics Promotes Organ Transplant Acceptance. Immunity 2018, 49, 819–828.e6. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained Immunity - Basic Concepts and Contributions to Immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Pan, Y.; Qu, J.; Xu, Y.; Dou, H.; Hou, Y. 17β-Estradiol Promotes Trained Immunity in Females Against Sepsis via Regulating Nucleus Translocation of RelB. Front. Immunol. 2020, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, C.D.C.C.; Keating, S.T.; Groh, L.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P. Aldosterone Induces Trained Immunity: The Role of Fatty Acid Synthesis. Cardiovasc. Res. 2020, 116, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.B. Trained Immunity and Local Innate Immune Memory in the Lung. Cell 2018, 175, 1463–1465. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; ONeill, L.A.J.; Xavier, R.J. Trained Immunity: A Program of Innate Immune Memory in Health and Disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- West, M.A.; Heagy, W. Endotoxin Tolerance: A Review. Crit. Care Med. 2002, 30, S64–S73. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Chen, J.; Ivashkiv, L.B. IFN-γ Abrogates Endotoxin Tolerance by Facilitating Toll-like Receptor-Induced Chromatin Remodeling. Proc. Natl. Acad. Sci. USA 2010, 107, 19438–19443. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-Inflammatory Effect of IL-10 Mediated by Metabolic Reprogramming of Macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Jentho, E.; Lajqi, T.; Yang, K.; Winkler, R.; Stojiljkovic, M.; Wetzker, R.; Bauer, M. Pathogen-Induced Hormetic Responses. In The Science of Hormesis in Health and Longevity; Rattan, S.I.S., Kyriazis, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 161–170. [Google Scholar]
- Foster, S.L.; Hargreaves, D.C.; Medzhitov, R. Gene-Specific Control of Inflammation by TLR-Induced Chromatin Modifications. Nature 2007, 447, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Li, L.; McCall, C.E.; Yoza, B.K. Endotoxin Tolerance Disrupts Chromatin Remodeling and NF-ΚB Transactivation at the IL-1β Promoter. J. Immunol. 2005, 175, 461–468. [Google Scholar] [CrossRef]
- Chen, X.; El Gazzar, M.; Yoza, B.K.; McCall, C.E. The NF-KB Factor RelB and Histone H3 Lysine Methyltransferase G9a Directly Interact to Generate Epigenetic Silencing in Endotoxin Tolerance. J. Biol. Chem. 2009, 284, 27857–27865. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Andrés, J.; Novakovic, B.; Li, Y.; Scicluna, B.P.; Gresnigt, M.S.; Arts, R.J.W.; Oosting, M.; Moorlag, S.J.C.F.M.; Groh, L.A.; Zwaag, J.; et al. The Itaconate Pathway Is a Central Regulatory Node Linking Innate Immune Tolerance and Trained Immunity. Cell Metab. 2019, 29, 211–220.e5. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Kieser, K.J.; Kagan, J.C. Multi-Receptor Detection of Individual Bacterial Products by the Innate Immune System. Nat. Rev. Immunol. 2017, 17, 376–390. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Agier, J.; Pastwińska, J.; Brzezińska-Błaszczyk, E. An Overview of Mast Cell Pattern Recognition Receptors. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2018, 67, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, J.; Bekkering, S.; Joosten, L.A.B.; Netea, M.G.; Burgner, D.P.; Riksen, N.P. Trained Innate Immunity as a Novel Mechanism Linking Infection and the Development of Atherosclerosis. Circ. Res. 2018, 122, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Stiekema, L.C.A.; Bernelot Moens, S.; Verweij, S.L.; Novakovic, B.; Prange, K.; Versloot, M.; Roeters van Lennep, J.E.; Stunnenberg, H.; de Winther, M.; et al. Treatment with Statins Does Not Revert Trained Immunity in Patients with Familial Hypercholesterolemia. Cell Metab. 2019, 30, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wendeln, A.-C.; Degenhardt, K.; Kaurani, L.; Gertig, M.; Ulas, T.; Jain, G.; Wagner, J.; Häsler, L.M.; Wild, K.; Skodras, A.; et al. Innate Immune Memory in the Brain Shapes Neurological Disease Hallmarks. Nature 2018, 556, 332–338. [Google Scholar] [CrossRef] [PubMed]
- López-Collazo, E.; del Fresno, C. Pathophysiology of Endotoxin Tolerance: Mechanisms and Clinical Consequences. Crit. Care 2013, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Gilliam, E.A.; Li, L. Innate Immune Programing by Endotoxin and Its Pathological Consequences. Front. Immunol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Rieckmann, A.; Villumsen, M.; Sørup, S.; Haugaard, L.K.; Ravn, H.; Roth, A.; Baker, J.L.; Benn, C.S.; Aaby, P. Vaccinations against Smallpox and Tuberculosis Are Associated with Better Long-Term Survival: A Danish Case-Cohort Study 1971-2010. Int. J. Epidemiol. 2017, 46, 695–705. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.-C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef]
- Chavakis, T.; Mitroulis, I.; Hajishengallis, G. Hematopoietic Progenitor Cells as Integrative Hubs for Adaptation to and Fine-Tuning of Inflammation. Nat. Immunol. 2019, 20, 802–811. [Google Scholar] [CrossRef]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.-S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 2018, 172, 147–161.e12. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Weis, S.; Netea, M.G.; Wetzker, R. Remembering Pathogen Dose: Long-Term Adaptation in Innate Immunity. Trends Immunol. 2018, 39, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Lajqi, T.; Pöschl, J.; Frommhold, D.; Hudalla, H. The Role of Microbiota in Neutrophil Regulation and Adaptation in Newborns. Front. Immunol. 2020, 11, 568685. [Google Scholar] [CrossRef] [PubMed]
- Shwartzman, G. Concerning the Specificity and Nature of the Phenomenon of Local Skin Reactivity to Various Bacterial Filtrates. J. Exp. Med. 1930, 51, 571–583. [Google Scholar] [CrossRef]
- Ifrim, D.C.; Quintin, J.; Joosten, L.A.B.; Jacobs, C.; Jansen, T.; Jacobs, L.; Gow, N.A.R.; Williams, D.L.; van der Meer, J.W.M.; Netea, M.G. Trained Immunity or Tolerance: Opposing Functional Programs Induced in Human Monocytes after Engagement of Various Pattern Recognition Receptors. Clin. Vaccine Immunol. 2014, 21, 534–545. [Google Scholar] [CrossRef]
- Netea, M.G.; van der Meer, J.W.M. Trained Immunity: An Ancient Way of Remembering. Cell Host Microbe 2017, 21, 297–300. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Khan, N.; Novakovic, B.; Kaufmann, E.; Jansen, T.; van Crevel, R.; Divangahi, M.; Netea, M.G. β-Glucan Induces Protective Trained Immunity against Mycobacterium Tuberculosis Infection: A Key Role for IL-1. Cell Rep. 2020, 31, 107634. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida Albicans Infection Affords Protection against Reinfection via Functional Reprogramming of Monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Moerings, B.G.J.; de Graaff, P.; Furber, M.; Witkamp, R.F.; Debets, R.; Mes, J.J.; van Bergenhenegouwen, J.; Govers, C. Continuous Exposure to Non-Soluble β-Glucans Induces Trained Immunity in M-CSF-Differentiated Macrophages. Front. Immunol. 2021, 12, 672796. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate Immune Training of Granulopoiesis Promotes Anti-Tumor Activity. Cell 2020, 183, 771–785. [Google Scholar] [CrossRef]
- dos Santos, J.C.; Barroso de Figueiredo, A.M.; Teodoro Silva, M.V.; Cirovic, B.; de Bree, L.C.J.; Damen, M.S.M.A.; Moorlag, S.J.C.F.M.; Gomes, R.S.; Helsen, M.M.; Oosting, M.; et al. β-Glucan-Induced Trained Immunity Protects against Leishmania Braziliensis Infection: A Crucial Role for IL-32. Cell Rep. 2019, 28, 2659–2672.e6. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.E.; Shrestha, R.; Woeste, M.R.; Guo, H.; Hu, X.; Ding, C.; Andreeva, K.; Chariker, J.H.; Zhou, M.; Tieri, D.; et al. The Induction of Peripheral Trained Immunity in the Pancreas Incites Anti-Tumor Activity to Control Pancreatic Cancer Progression. Nat. Commun. 2022, 13, 759. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Glucan Supplementation Enhances the Immune Response against an Influenza Challenge in Mice. Ann. Transl. Med. 2015, 3, 22. [Google Scholar] [PubMed]
- De Zuani, M.; Dal Secco, C.; Tonon, S.; Arzese, A.; Pucillo, C.E.M.; Frossi, B. LPS Guides Distinct Patterns of Training and Tolerance in Mast Cells. Front. Immunol. 2022, 13, 835348. [Google Scholar] [CrossRef]
- Priem, B.; van Leent, M.M.T.; Teunissen, A.J.P.; Sofias, A.M.; Mourits, V.P.; Willemsen, L.; Klein, E.D.; Oosterwijk, R.S.; Meerwaldt, A.E.; Munitz, J.; et al. Trained Immunity-Promoting Nanobiologic Therapy Suppresses Tumor Growth and Potentiates Checkpoint Inhibition. Cell 2020, 183, 786–801.e19. [Google Scholar] [CrossRef]
- Mourits, V.P.; Koeken, V.A.C.M.; de Bree, L.C.J.; Moorlag, S.J.C.F.M.; Chu, W.C.; Xu, X.; Dijkstra, H.; Lemmers, H.; Joosten, L.A.B.; Wang, Y.; et al. BCG-Induced Trained Immunity in Healthy Individuals: The Effect of Plasma Muramyl Dipeptide Concentrations. J. Immunol. Res. 2020, 2020, 5812743. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Prince, A. Airway Immunometabolites Fuel Pseudomonas Aeruginosa Infection. Respir. Res. 2020, 21, 326. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas Aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Bigot, J.; Guillot, L.; Guitard, J.; Ruffin, M.; Corvol, H.; Chignard, M.; Hennequin, C.; Balloy, V. Respiratory Epithelial Cells Can Remember Infection: A Proof-of-Concept Study. J. Infect. Dis. 2020, 221, 1000–1005. [Google Scholar] [CrossRef]
- Ciarlo, E.; Heinonen, T.; Théroude, C.; Asgari, F.; Le Roy, D.; Netea, M.G.; Roger, T. Trained Immunity Confers Broad-Spectrum Protection Against Bacterial Infections. J. Infect. Dis. 2020, 222, 1869–1881. [Google Scholar] [CrossRef]
- Théroude, C.; Reverte, M.; Heinonen, T.; Ciarlo, E.; Schrijver, I.T.; Antonakos, N.; Maillard, N.; Pralong, F.; Le Roy, D.; Roger, T. Trained Immunity Confers Prolonged Protection From Listeriosis. Front. Immunol. 2021, 12, 723393. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, S.A.; Liimatta, K.; Wong Fok Lung, T.; Fields, B.; Ahn, D.; Chen, D.; Lozano, C.; Sáenz, Y.; Uhlemann, A.-C.; Kahl, B.C.; et al. Pseudomonas Aeruginosa Utilizes Host-Derived Itaconate to Redirect Its Metabolism to Promote Biofilm Formation. Cell Metab. 2020, 31, 1091–1106.e6. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.K.; Edmond, K.M.; Fawzi, W.W.; Hurt, L.; Kirkwood, B.R.; Masanja, H.; Muhihi, A.J.; Newton, S.; Noor, R.A.; Williams, P.L.; et al. Non-Specific Effects of BCG and DTP Vaccination on Infant Mortality: An Analysis of Birth Cohorts in Ghana and Tanzania. Vaccine 2022, 40, 3737–3745. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, M.; Tanner, R.; Wright, D.; Lopez Ramon, R.; Beglov, J.; Riste, M.; Marshall, J.L.; Harris, S.A.; Bettencourt, P.J.G.; Hamidi, A.; et al. Functional In-Vitro Evaluation of the Non-Specific Effects of BCG Vaccination in a Randomised Controlled Clinical Study. Sci. Rep. 2022, 12, 7808. [Google Scholar] [CrossRef]
- Aaby, P.; Benn, C.S.; Flanagan, K.L.; Klein, S.L.; Kollmann, T.R.; Lynn, D.J.; Shann, F. The Non-Specific and Sex-Differential Effects of Vaccines. Nat. Rev. Immunol. 2020, 20, 464–470. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin Induces NOD2-Dependent Nonspecific Protection from Reinfection via Epigenetic Reprogramming of Monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.B.; Jacobs, C.; Van Loenhout, J.; Xavier, R.J.; Aaby, P.; Van Der Meer, J.W.M.; et al. Long-Lasting Effects of Bcg Vaccination on Both Heterologous Th1/Th17 Responses and Innate Trained Immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. Gammadelta T Cell Effector Functions: A Blend of Innate Programming and Acquired Plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Caron, J.; Ridgley, L.A.; Bodman-Smith, M. How to Train Your Dragon: Harnessing Gamma Delta T Cells Antiviral Functions and Trained Immunity in a Pandemic Era. Front. Immunol. 2021, 12, 666983. [Google Scholar] [CrossRef]
- Deetz, C.O.; Hebbeler, A.M.; Propp, N.A.; Cairo, C.; Tikhonov, I.; Pauza, C.D. Gamma Interferon Secretion by Human Vgamma2Vdelta2 T Cells after Stimulation with Antibody against the T-Cell Receptor plus the Toll-Like Receptor 2 Agonist Pam3Cys. Infect. Immun. 2006, 74, 4505–4511. [Google Scholar] [CrossRef]
- Lester, S.N.; Li, K. Toll-like Receptors in Antiviral Innate Immunity. J. Mol. Biol. 2014, 426, 1246–1264. [Google Scholar] [CrossRef] [PubMed]
- Wesch, D.; Beetz, S.; Oberg, H.-H.; Marget, M.; Krengel, K.; Kabelitz, D. Direct Costimulatory Effect of TLR3 Ligand Poly(I:C) on Human Gamma Delta T Lymphocytes. J. Immunol. 2006, 176, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- González, S.; López-Soto, A.; Suarez-Alvarez, B.; López-Vázquez, A.; López-Larrea, C. NKG2D Ligands: Key Targets of the Immune Response. Trends Immunol. 2008, 29, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Spada, F.M.; Grant, E.P.; Peters, P.J.; Sugita, M.; Melián, A.; Leslie, D.S.; Lee, H.K.; van Donselaar, E.; Hanson, D.A.; Krensky, A.M.; et al. Self-Recognition of CD1 by Gamma/Delta T Cells: Implications for Innate Immunity. J. Exp. Med. 2000, 191, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Röring, R.J.; Debisarun, P.A.; Botey-Bataller, J.; Suen, T.K.; Bulut, Ö.; Kilic, G.; Koeken, V.A.C.M.; Sarlea, A.; Bahrar, H.; Dijkstra, H.; et al. MMR Vaccination Induces a Trained Immunity Program Characterized by Functional and Metabolic Reprogramming of Γδ T Cells. bioRxiv 2022. (Preprint). [Google Scholar] [CrossRef]
- Mazzola, T.N.; Da Silva, M.T.N.; Moreno, Y.M.F.; Lima, S.C.B.S.; Carniel, E.F.; Morcillo, A.M.; Antonio, M.A.R.G.M.; Zanolli, M.L.; Netto, A.A.; Blotta, M.H.; et al. Robust Gammadelta+ T Cell Expansion in Infants Immunized at Birth with BCG Vaccine. Vaccine 2007, 25, 6313–6320. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, C.; Germano, S.; Dutta, B.; Ritz, N.; Curtis, N. The Contribution of Non-Conventional T Cells and NK Cells in the Mycobacterial-Specific IFNγ Response in Bacille Calmette-Guérin (BCG)-Immunized Infants. PLoS ONE 2013, 8, e77334. [Google Scholar] [CrossRef] [PubMed]
- Taştan, Y.; Arvas, A.; Demir, G.; Alikaşifoğlu, M.; Gür, E.; Kiray, E. Influence of Bacillus Calmette-Guèrin Vaccination at Birth and 2 Months Old Age on the Peripheral Blood T-Cell Subpopulations [Gamma/Delta and Alpha-Beta T Cell]. Pediatr. allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2005, 16, 624–629. [Google Scholar] [CrossRef]
- Bukowski, J.F.; Morita, C.T.; Brenner, M.B. Recognition and Destruction of Virus-Infected Cells by Human Gamma Delta CTL. J. Immunol. 1994, 153, 5133–5140. [Google Scholar] [CrossRef]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.-J.; et al. Induction of Trained Immunity by Influenza Vaccination - Impact on COVID-19. PLoS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef]
- Murphy, D.M.; Cox, D.J.; Connolly, S.A.; Breen, E.P.; Brugman, A.A.; Phelan, J.J.; Keane, J.; Basdeo, S.A. Trained Immunity Is Induced in Humans after Immunization with an Adenoviral Vector COVID-19 Vaccine. J. Clin. Investig. 2023, 133, e162581. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive Immune Features of Natural Killer Cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.G.; Goodarzi, M.; Drayton, D.L.; von Andrian, U.H. T Cell- and B Cell-Independent Adaptive Immunity Mediated by Natural Killer Cells. Nat. Immunol. 2006, 7, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Madera, S.; Bezman, N.A.; Beilke, J.N.; Kaplan, M.H.; Lanier, L.L. Proinflammatory Cytokine Signaling Required for the Generation of Natural Killer Cell Memory. J. Exp. Med. 2012, 209, 947–954. [Google Scholar] [CrossRef]
- Björkström, N.K.; Lindgren, T.; Stoltz, M.; Fauriat, C.; Braun, M.; Evander, M.; Michaëlsson, J.; Malmberg, K.-J.; Klingström, J.; Ahlm, C.; et al. Rapid Expansion and Long-Term Persistence of Elevated NK Cell Numbers in Humans Infected with Hantavirus. J. Exp. Med. 2011, 208, 13–21. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Falco, M.; Podestà, M.; Locatelli, F.; Moretta, L.; Frassoni, F.; Moretta, A. Phenotypic and Functional Heterogeneity of Human NK Cells Developing after Umbilical Cord Blood Transplantation: A Role for Human Cytomegalovirus? Blood 2012, 119, 399–410. [Google Scholar] [CrossRef]
- Tarannum, M.; Romee, R. Cytokine-Induced Memory-like Natural Killer Cells for Cancer Immunotherapy. Stem Cell Res. Ther. 2021, 12, 592. [Google Scholar] [CrossRef]
- Luetke-Eversloh, M.; Hammer, Q.; Durek, P.; Nordström, K.; Gasparoni, G.; Pink, M.; Hamann, A.; Walter, J.; Chang, H.-D.; Dong, J.; et al. Human Cytomegalovirus Drives Epigenetic Imprinting of the IFNG Locus in NKG2Chi Natural Killer Cells. PLoS Pathog. 2014, 10, e1004441. [Google Scholar] [CrossRef]
- Brown, M.G.; Dokun, A.O.; Heusel, J.W.; Smith, H.R.; Beckman, D.L.; Blattenberger, E.A.; Dubbelde, C.E.; Stone, L.R.; Scalzo, A.A.; Yokoyama, W.M. Vital Involvement of a Natural Killer Cell Activation Receptor in Resistance to Viral Infection. Science 2001, 292, 934–937. [Google Scholar] [CrossRef]
- Karo, J.M.; Schatz, D.G.; Sun, J.C. The RAG Recombinase Dictates Functional Heterogeneity and Cellular Fitness in Natural Killer Cells. Cell 2014, 159, 94–107. [Google Scholar] [CrossRef]
- Cooper, M.A.; Elliott, J.M.; Keyel, P.A.; Yang, L.; Carrero, J.A.; Yokoyama, W.M. Cytokine-Induced Memory-like Natural Killer Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 1915–1919. [Google Scholar] [CrossRef]
- Romee, R.; Schneider, S.E.; Leong, J.W.; Chase, J.M.; Keppel, C.R.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Cytokine Activation Induces Human Memory-like NK Cells. Blood 2012, 120, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramón, S.; Conejero, L.; Netea, M.G.; Sancho, D.; Palomares, Ó.; Subiza, J.L. Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-Infectious Formulations. Front. Immunol. 2018, 9, 2936. [Google Scholar] [CrossRef]
- Conejero, L.; Saz-Leal, P.; Subiza, J.L. Trained Immunity-Based Vaccines: A Ready-to-Act Strategy to Tackle Viral Outbreaks. In Current Perspectives on Viral Disease Outbreaks; Claborn, D., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- O’Neill, L.A.J.; Netea, M.G. BCG-Induced Trained Immunity: Can It Offer Protection against COVID-19? Nat. Rev. Immunol. 2020, 20, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cruz, L.; Sevilla-Ortega, C.; Angelina, A.; Domínguez-Andrés, J.; Netea, M.G.; Subiza, J.L.; Palomares, O. From Trained Immunity in Allergy to Trained Immunity-Based Allergen Vaccines. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2022, 53, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Benito-Villalvilla, C.; Pérez-Diego, M.; Angelina, A.; Kisand, K.; Rebane, A.; Subiza, J.L.; Palomares, O. Allergoid-Mannan Conjugates Reprogram Monocytes into Tolerogenic Dendritic Cells via Epigenetic and Metabolic Rewiring. J. Allergy Clin. Immunol. 2022, 149, 212–222.e9. [Google Scholar] [CrossRef] [PubMed]
- Hartung, F.; Esser-von Bieren, J. Trained Immunity in Type 2 Immune Responses. Mucosal Immunol. 2022, 15, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Tatematsu, M.; Fuchimukai, A.; Yamada, T.; Yamagata, K.; Takasuga, S.; Yamada, T. Trained Innate Lymphoid Cells in Allergic Diseases. Allergol. Int. Off. J. Japanese Soc. Allergol. 2021, 70, 174–180. [Google Scholar] [CrossRef]
- Eljaszewicz, A.; Ruchti, F.; Radzikowska, U.; Globinska, A.; Boonpiyathad, T.; Gschwend, A.; Morita, H.; Helbling, A.; Arasi, S.; Kahlert, H.; et al. Trained Immunity and Tolerance in Innate Lymphoid Cells, Monocytes, and Dendritic Cells during Allergen-Specific Immunotherapy. J. Allergy Clin. Immunol. 2021, 147, 1865–1877. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Lasaviciute, G.; Barz, M.; van der Heiden, M.; Arasa, C.; Tariq, K.; Quin, J.; Östlund Farrants, A.-K.; Sverremark-Ekström, E. Gut Commensal Limosilactobacillus Reuteri Induces Atypical Memory-like Phenotype in Human Dendritic Cells in Vitro. Gut Microbes 2022, 14, 2045046. [Google Scholar] [CrossRef] [PubMed]
- Lajqi, T.; Köstlin-Gille, N.; Hillmer, S.; Braun, M.; Kranig, S.A.; Dietz, S.; Krause, C.; Rühle, J.; Frommhold, D.; Pöschl, J.; et al. Gut Microbiota-Derived Small Extracellular Vesicles Endorse Memory-like Inflammatory Responses in Murine Neutrophils. Biomedicines 2022, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, L.; Brichacek, B.; Prashant, N.M.; Pushkarsky, T.; Mukhamedova, N.; Fleetwood, A.J.; Xu, Y.; Dragoljevic, D.; Fitzgerald, M.; Horvath, A.; et al. Extracellular Vesicles Carrying HIV-1 Nef Induce Long-Term Hyperreactivity of Myeloid Cells. Cell Rep. 2022, 41, 111674. [Google Scholar] [CrossRef]
- van Leent, M.M.T.; Priem, B.; Schrijver, D.P.; de Dreu, A.; Hofstraat, S.R.J.; Zwolsman, R.; Beldman, T.J.; Netea, M.G.; Mulder, W.J.M. Regulating Trained Immunity with Nanomedicine. Nat. Rev. Mater. 2022, 7, 465–481. [Google Scholar] [CrossRef]
- Kalafati, L.; Hatzioannou, A.; Hajishengallis, G.; Chavakis, T. The Role of Neutrophils in Trained Immunity. Immunol. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Dandapat, S.; Manikandan, R.; Dinesh, M.; Subbaiyan, A.; Mani, P.; Dhawan, M.; Tiwari, R.; Bilal, M.; Bin Emran, T.; et al. Prophylactic and Therapeutic Insights into Trained Immunity: A Renewed Concept of Innate Immune Memory. Hum. Vaccin. Immunother. 2022, 18, 2040238. [Google Scholar] [CrossRef]
- Azimzadeh, A.M.; Pfeiffer, S.; Wu, G.S.; Schröder, C.; Zhou, H.; Zorn, G.L., 3rd; Kehry, M.; Miller, G.G.; Rose, M.L.; Pierson, R.N. 3rd. Humoral Immunity to Vimentin Is Associated with Cardiac Allograft Injury in Nonhuman Primates. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2005, 5, 2349–2359. [Google Scholar]
- Huang, Y.; Yin, H.; Han, J.; Huang, B.; Xu, J.; Zheng, F.; Tan, Z.; Fang, M.; Rui, L.; Chen, D.; et al. Extracellular Hmgb1 Functions as an Innate Immune-Mediator Implicated in Murine Cardiac Allograft Acute Rejection. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2007, 7, 799–808. [Google Scholar] [CrossRef]
- Uhl, B.; Vadlau, Y.; Zuchtriegel, G.; Nekolla, K.; Sharaf, K.; Gaertner, F.; Massberg, S.; Krombach, F.; Reichel, C.A. Aged Neutrophils Contribute to the First Line of Defense in the Acute Inflammatory Response. Blood 2016, 128, 2327–2337. [Google Scholar] [CrossRef]
- Christen, T.; Nahrendorf, M.; Wildgruber, M.; Swirski, F.K.; Aikawa, E.; Waterman, P.; Shimizu, K.; Weissleder, R.; Libby, P. Molecular Imaging of Innate Immune Cell Function in Transplant Rejection. Circulation 2009, 119, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Scozzi, D.; Ibrahim, M.; Menna, C.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The Role of Neutrophils in Transplanted Organs. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2017, 17, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, J.L.; Holvoet, P.; Dauwe, S.E.; Ysebaert, D.K.; Chapelle, T.; Jürgens, A.; Kovacic, V.; Van Marck, E.A.; De Broe, M.E.; Verpooten, G.A. Oxidative Modification of Low-Density Lipoproteins and the Outcome of Renal Allografts at 1 1/2 Years. Kidney Int. 2001, 59, 2346–2356. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prasad, G.V.R. Post-Transplant Dyslipidemia: Mechanisms, Diagnosis and Management. World J. Transplant. 2016, 6, 125–134. [Google Scholar] [CrossRef]
- Regnström, J.; Nilsson, J.; Tornvall, P.; Landou, C.; Hamsten, A. Susceptibility to Low-Density Lipoprotein Oxidation and Coronary Atherosclerosis in Man. Lancet 1992, 339, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Samouilidou, E.C.; Karpouza, A.P.; Kostopoulos, V.; Bakirtzi, T.; Pantelias, K.; Petras, D.; Tzanatou-Exarchou, H.; Grapsa, E.J. Lipid Abnormalities and Oxidized LDL in Chronic Kidney Disease Patients on Hemodialysis and Peritoneal Dialysis. Ren. Fail. 2012, 34, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Diepeveen, S.H.A.; Verhoeven, G.H.W.E.; van der Palen, J.; Dikkeschei, B.L.D.; van Tits, L.J.; Kolsters, G.; Offerman, J.J.G.; Bilo, H.J.G.; Stalenhoef, A.F.H. Oxidative Stress in Patients with End-Stage Renal Disease Prior to the Start of Renal Replacement Therapy. Nephron. Clin. Pract. 2004, 98, c3–c7. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Seiler, S.; Zawada, A.M.; Reichart, B.; Herath, E.; Roth, D.; Ulrich, C.; Fliser, D.; Heine, G.H. CD14++CD16+ Monocytes and Cardiovascular Outcome in Patients with Chronic Kidney Disease. Eur. Heart J. 2011, 32, 84–92. [Google Scholar] [CrossRef]
- Pertosa, G.; Grandaliano, G.; Gesualdo, L.; Schena, F.P. Clinical Relevance of Cytokine Production in Hemodialysis. Kidney Int. Suppl. 2000, 76, S104–S111. [Google Scholar] [CrossRef]
- Bekkering, S.; Quintin, J.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Netea, M.G.; Riksen, N.P. Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Schnack, L.; Sohrabi, Y.; Lagache, S.M.M.; Kahles, F.; Bruemmer, D.; Waltenberger, J.; Findeisen, H.M. Mechanisms of Trained Innate Immunity in OxLDL Primed Human Coronary Smooth Muscle Cells. Front. Immunol. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-F.; Grainge, M.J.; Zhang, W.; Doherty, M. Global Epidemiology of Gout: Prevalence, Incidence and Risk Factors. Nat. Rev. Rheumatol. 2015, 11, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Dalbeth, N. Critical Appraisal of Serum Urate Targets in the Management of Gout. Nat. Rev. Rheumatol. 2022, 18, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Cabău, G.; Crișan, T.O.; Klück, V.; Popp, R.A.; Joosten, L.A.B. Urate-Induced Immune Programming: Consequences for Gouty Arthritis and Hyperuricemia. Immunol. Rev. 2020, 294, 92–105. [Google Scholar] [CrossRef]
- Crișan, T.O.; Cleophas, M.C.P.; Oosting, M.; Lemmers, H.; Toenhake-Dijkstra, H.; Netea, M.G.; Jansen, T.L.; Joosten, L.A.B. Soluble Uric Acid Primes TLR-Induced Proinflammatory Cytokine Production by Human Primary Cells via Inhibition of IL-1Ra. Ann. Rheum. Dis. 2016, 75, 755–762. [Google Scholar] [CrossRef]
- Crişan, T.O.; Cleophas, M.C.P.; Novakovic, B.; Erler, K.; van de Veerdonk, F.L.; Stunnenberg, H.G.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Uric Acid Priming in Human Monocytes Is Driven by the AKT-PRAS40 Autophagy Pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 5485–5490. [Google Scholar] [CrossRef]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Mercurio, S.; Tolosano, E. Heme in Pathophysiology: A Matter of Scavenging, Metabolism and Trafficking across Cell Membranes. Front. Pharmacol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Jentho, E.; Ruiz-Moreno, C.; Novakovic, B.; Kourtzelis, I.; Megchelenbrink, W.L.; Martins, R.; Chavakis, T.; Soares, M.P.; Kalafati, L.; Guerra, J.; et al. Trained Innate Immunity, Long-Lasting Epigenetic Modulation, and Skewed Myelopoiesis by Heme. Proc. Natl. Acad. Sci. USA 2021, 118, e2102698118. [Google Scholar] [CrossRef]
- Schlegel, C.; Liu, K.; Spring, B.; Dietz, S.; Poets, C.F.; Hudalla, H.; Lajqi, T.; Köstlin-Gille, N.; Gille, C. Decreased Expression of Hypoxia-Inducible Factor 1α (HIF-1α) in Cord Blood Monocytes under Anoxia. Pediatr. Res. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.A.; Rao, N.A.; Aghajanirefah, A.; et al. MTOR- and HIF-1α-Mediated Aerobic Glycolysis as Metabolic Basis for Trained Immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Lajqi, T.; Lang, G.-P.; Haas, F.; Williams, D.L.; Hudalla, H.; Bauer, M.; Groth, M.; Wetzker, R.; Bauer, R. Memory-Like Inflammatory Responses of Microglia to Rising Doses of LPS: Key Role of PI3Kγ. Front. Immunol. 2019, 10, 2492. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Li, Q.; Ding, J.; Liang, F.; Gusev, E.; Lapohos, O.; Fonseca, G.J.; Kaufmann, E.; Divangahi, M.; Petrof, B.J. TLR4 Is a Regulator of Trained Immunity in a Murine Model of Duchenne Muscular Dystrophy. Nat. Commun. 2022, 13, 879. [Google Scholar] [CrossRef] [PubMed]
- Lajqi, T.; Frommhold, D.; Braun, M.; Alexander Kranig, S.; Pöschl, J.; Hudalla, H. Gram-Positive Staphylococcus Aureus LTA Promotes Distinct Memory-like Effects in Murine Bone Marrow Neutrophils. Cell. Immunol. 2022, 376, 104535. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, R.; Forde, A.J.; Lohrmann, F.; Kolter, J.; Ramirez, N.J.; Zimmermann, J.; Gomez de Agüero, M.; Henneke, P. Resident Macrophages Acquire Innate Immune Memory in Staphylococcal Skin Infection. Elife 2020, 9, e55602. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.C.; Rossetti, M.; Miller, L.S.; Filler, S.G.; Johnson, C.W.; Lee, H.K.; Wang, H.; Gjertson, D.; Fowler, V.G.J.; Reed, E.F.; et al. Protective Immunity in Recurrent Staphylococcus Aureus Infection Reflects Localized Immune Signatures and Macrophage-Conferred Memory. Proc. Natl. Acad. Sci. USA 2018, 115, E11111–E11119. [Google Scholar] [CrossRef] [PubMed]
- Horn, C.M.; Kielian, T. Crosstalk Between Staphylococcus Aureus and Innate Immunity: Focus on Immunometabolism. Front. Immunol. 2020, 11, 621750. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Quintin, J.; Kerstens, H.H.D.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.-C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic Programming of Monocyte-to-Macrophage Differentiation and Trained Innate Immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Netea, M.G. Trained Innate Immunity, Epigenetics, and Covid-19. N. Engl. J. Med. 2020, 383, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Rasid, O.; Chevalier, C.; Camarasa, T.M.-N.; Fitting, C.; Cavaillon, J.-M.; Hamon, M.A. H3K4me1 Supports Memory-like NK Cells Induced by Systemic Inflammation. Cell Rep. 2019, 29, 3933–3945.e3. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Chen, Y.; Zhou, X. The Roles of MicroRNAs in Epigenetic Regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zawislak, C.L.; Beaulieu, A.M.; Loeb, G.B.; Karo, J.; Canner, D.; Bezman, N.A.; Lanier, L.L.; Rudensky, A.Y.; Sun, J.C. Stage-Specific Regulation of Natural Killer Cell Homeostasis and Response against Viral Infection by MicroRNA-155. Proc. Natl. Acad. Sci. USA 2013, 110, 6967–6972. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; He, J.; Gu, L.; Shahror, R.A.; Li, Y.; Cao, T.; Wang, S.; Zhu, J.; Huang, H.; Chen, F.; et al. Brain Innate Immune Response via MiRNA-TLR7 Sensing in Polymicrobial Sepsis. Brain. Behav. Immun. 2022, 100, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Curtale, G.; Rubino, M.; Locati, M. MicroRNAs as Molecular Switches in Macrophage Activation. Front. Immunol. 2019, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Liang, Z.; Weng, S.; Sun, C.; Huang, J.; Zhang, T.; Wang, X.; Wu, S.; Zhang, Z.; Zhang, Y.; et al. MiR-9-5p Regulates Immunometabolic and Epigenetic Pathways in β-Glucan-Trained Immunity via IDH3α. JCI Insight 2021, 6, e144260. [Google Scholar] [CrossRef]
- Saz-Leal, P.; Del Fresno, C.; Brandi, P.; Martínez-Cano, S.; Dungan, O.M.; Chisholm, J.D.; Kerr, W.G.; Sancho, D. Targeting SHIP-1 in Myeloid Cells Enhances Trained Immunity and Boosts Response to Infection. Cell Rep. 2018, 25, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Fanucchi, S.; Fok, E.T.; Dalla, E.; Shibayama, Y.; Börner, K.; Chang, E.Y.; Stoychev, S.; Imakaev, M.; Grimm, D.; Wang, K.C.; et al. Immune Genes Are Primed for Robust Transcription by Proximal Long Noncoding RNAs Located in Nuclear Compartments. Nat. Genet. 2019, 51, 138–150. [Google Scholar] [CrossRef]
- Das, J.; Verma, D.; Gustafsson, M.; Lerm, M. Identification of DNA Methylation Patterns Predisposing for an Efficient Response to BCG Vaccination in Healthy BCG-Naïve Subjects. Epigenetics 2019, 14, 589–601. [Google Scholar] [CrossRef]
- Ferreira, A.V.; Domiguéz-Andrés, J.; Netea, M.G. The Role of Cell Metabolism in Innate Immune Memory. J. Innate Immun. 2021, 13, 194. [Google Scholar] [CrossRef]
- Riksen, N.P.; Netea, M.G. Immunometabolic Control of Trained Immunity. Mol. Aspects Med. 2021, 77, 100897. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Joosten, L.A.B.; Netea, M.G. Immunometabolic Circuits in Trained Immunity. Semin. Immunol. 2016, 28, 425–430. [Google Scholar] [CrossRef]
- Groh, L.A.; Ferreira, A.V.; Helder, L.; van der Heijden, C.D.C.C.; Novakovic, B.; van de Westerlo, E.; Matzaraki, V.; Moorlag, S.J.C.F.M.; de Bree, L.C.; Koeken, V.A.C.M.; et al. OxLDL-Induced Trained Immunity Is Dependent on Mitochondrial Metabolic Reprogramming. Immunometabolism 2021, 3, e210025. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.T.; Groh, L.; van der Heijden, C.D.C.C.; Rodriguez, H.; Dos Santos, J.C.; Fanucchi, S.; Okabe, J.; Kaipananickal, H.; van Puffelen, J.H.; Helder, L.; et al. The Set7 Lysine Methyltransferase Regulates Plasticity in Oxidative Phosphorylation Necessary for Trained Immunity Induced by β-Glucan. Cell Rep. 2020, 31, 107548. [Google Scholar] [CrossRef] [PubMed]
- Koeken, V.A.C.M.; Qi, C.; Mourits, V.P.; de Bree, L.C.J.; Moorlag, S.J.C.F.M.; Sonawane, V.; Lemmers, H.; Dijkstra, H.; Joosten, L.A.B.; van Laarhoven, A.; et al. Plasma Metabolome Predicts Trained Immunity Responses after Antituberculosis BCG Vaccination. PLoS Biol. 2022, 20, e3001765. [Google Scholar] [CrossRef]
- Lajqi, T.; Marx, C.; Hudalla, H.; Haas, F.; Große, S.; Wang, Z.Q.; Heller, R.; Bauer, M.; Wetzker, R.; Bauer, R. The Role of the Pathogen Dose and PI3Kγ in Immunometabolic Reprogramming of Microglia for Innate Immune Memory. Int. J. Mol. Sci. 2021, 22, 2578. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Novakovic, B.; ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, C.A.; Kern, C.C.; Butkeviciute, E.; McCarthy, E.; Dockrell, H.M.; Moreno-Altamirano, M.M.B.; Aguilar-López, B.A.; Bhosale, G.; Wang, H.; Gems, D.; et al. Mitochondrial Signature in Human Monocytes and Resistance to Infection in C. Elegans During Fumarate-Induced Innate Immune Training. Front. Immunol. 2020, 11, 1715. [Google Scholar] [CrossRef]
- Bekkering, S.; Arts, R.J.W.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.D.C.C.; Li, Y.; Popa, C.D.; ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146.e9. [Google Scholar] [CrossRef]
- Ferreira, A.V.; Koeken, V.A.C.M.; Matzaraki, V.; Kostidis, S.; Alarcon-Barrera, J.C.; de Bree, L.C.J.; Moorlag, S.J.C.F.M.; Mourits, V.P.; Novakovic, B.; Giera, M.A.; et al. Glutathione Metabolism Contributes to the Induction of Trained Immunity. Cells 2021, 10, 971. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Gonçalves, L.G.; Belinha, A.; et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef]
- Vrieling, F.; van Dierendonck, X.A.M.H.; Jaeger, M.; Janssen, A.W.M.; Hijmans, A.; Netea, M.G.; Tack, C.J.; Stienstra, R. Glycolytic Activity in Human Immune Cells: Inter-Individual Variation and Functional Implications during Health and Diabetes. Immunometabolism 2022, 4, e00008. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte Partnership during the Onset and Resolution of Inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Shen, Q.; Xu, P.; Luo, J.J.; Tang, Y. Phagocytosis of Microglia in the Central Nervous System Diseases. Mol. Neurobiol. 2014, 49, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Andrés, J.; Dos Santos, J.C.; Bekkering, S.; Mulder, W.J.M.; van der Meer, J.W.M.; Riksen, N.P.; Joosten, L.A.B.; Netea, M.G. Trained Immunity: Adaptation within Innate Immune Mechanisms. Physiol. Rev. 2023, 103, 313–346. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef] [PubMed]
- Bickett, T.E.; McLean, J.; Creissen, E.; Izzo, L.; Hagan, C.; Izzo, A.J.; Silva Angulo, F.; Izzo, A.A. Characterizing the BCG Induced Macrophage and Neutrophil Mechanisms for Defense Against Mycobacterium Tuberculosis. Front. Immunol. 2020, 11, 1202. [Google Scholar] [CrossRef]
- Schaafsma, W.; Zhang, X.; van Zomeren, K.C.; Jacobs, S.; Georgieva, P.B.; Wolf, S.A.; Kettenmann, H.; Janova, H.; Saiepour, N.; Hanisch, U.K.; et al. Long-Lasting pro-Inflammatory Suppression of Microglia by LPS-Preconditioning Is Mediated by RelB-Dependent Epigenetic Silencing. Brain. Behav. Immun. 2015, 48, 205–221. [Google Scholar] [CrossRef]
- Lajqi, T.; Stojiljkovic, M.; Williams, D.L.; Hudalla, H.; Bauer, M.; Witte, O.W.; Wetzker, R.; Bauer, R.; Schmeer, C. Memory-Like Responses of Brain Microglia Are Controlled by Developmental State and Pathogen Dose. Front. Immunol. 2020, 11, 546415. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Di Genaro, M.S.; Kalergis, A.M. Trained Immunity Contribution to Autoimmune and Inflammatory Disorders. Front. Immunol. 2022, 13, 868343. [Google Scholar] [CrossRef]
- Katzmarski, N.; Domínguez-Andrés, J.; Cirovic, B.; Renieris, G.; Ciarlo, E.; Le Roy, D.; Lepikhov, K.; Kattler, K.; Gasparoni, G.; Händler, K.; et al. Transmission of Trained Immunity and Heterologous Resistance to Infections across Generations. Nat. Immunol. 2021, 22, 1382–1390. [Google Scholar] [CrossRef]
- Kaufmann, E.; Landekic, M.; Downey, J.; Chronopoulos, J.; Teimouri Nezhad, S.; Tran, K.; Vinh, D.C.; Barreiro, L.B.; Divangahi, M. Lack of Evidence for Intergenerational Inheritance of Immune Resistance to Infections. Nat. Immunol. 2022, 23, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Katzmarski, N.; Domínguez-Andrés, J.; Cirovic, B.; Renieris, G.; Ciarlo, E.; Le Roy, D.; Lepikhov, K.; Kattler, K.; Gasparoni, G.; Händler, K.; et al. Reply to: “Lack of Evidence for Intergenerational Inheritance of Immune Resistance to Infections”. Nat. Immunol. 2022, 23, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Defining Hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How Does Hormesis Impact Biology, Toxicology, and Medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef]
- Lajqi, T.; Stojiljkovic, M.; Wetzker, R. Toxin-Induced Hormesis May Restrain Aging. Biogerontology 2019, 20, 571–581. [Google Scholar] [CrossRef]
- Leak, R.K.; Calabrese, E.J.; Kozumbo, W.J.; Gidday, J.M.; Johnson, T.E.; Mitchell, J.R.; Ozaki, C.K.; Wetzker, R.; Bast, A.; Belz, R.G.; et al. Enhancing and Extending Biological Performance and Resilience. Dose Response 2018, 16, 1559325818784501. [Google Scholar] [CrossRef] [PubMed]
- Gifford, G.E.; Lohmann-Matthes, M.L. Gamma Interferon Priming of Mouse and Human Macrophages for Induction of Tumor Necrosis Factor Production by Bacterial Lipopolysaccharide. J. Natl. Cancer Inst. 1987, 78, 121–124. [Google Scholar] [CrossRef]
- Koerner, T.J.; Adams, D.O.; Hamilton, T.A. Regulation of Tumor Necrosis Factor (TNF) Expression: Interferon-Gamma Enhances the Accumulation of MRNA for TNF Induced by Lipopolysaccharide in Murine Peritoneal Macrophages. Cell. Immunol. 1987, 109, 437–443. [Google Scholar] [CrossRef]
- Hayes, M.P.; Zoon, K.C. Priming of Human Monocytes for Enhanced Lipopolysaccharide Responses: Expression of Alpha Interferon, Interferon Regulatory Factors, and Tumor Necrosis Factor. Infect. Immun. 1993, 61, 3222–3227. [Google Scholar] [CrossRef]
- Hayes, M.P.; Freeman, S.L.; Donnelly, R.P. IFN-Gamma Priming of Monocytes Enhances LPS-Induced TNF Production by Augmenting Both Transcription and MRNA Stability. Cytokine 1995, 7, 427–435. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Schroder, A.; Reyes-Reyes, E.M.; Franco, R. MTOR/AMPK Signaling in the Brain: Cell Metabolism, Proteostasis and Survival. Curr. Opin. Toxicol. 2018, 8, 102–110. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N.; Lin, S.-C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.P.; Gozzelino, R.; Weis, S. Tissue Damage Control in Disease Tolerance. Trends Immunol. 2014, 35, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwak, H.J.; Cha, J.Y.; Jeong, Y.S.; Rhee, S.D.; Kim, K.R.; Cheon, H.G. Metformin Suppresses Lipopolysaccharide (LPS)-Induced Inflammatory Response in Murine Macrophages via Activating Transcription Factor-3 (ATF-3) Induction. J. Biol. Chem. 2014, 289, 23246–23255. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Hengstschlager, M.; Linke, M. Regulation of Innate Immune Cell Function by MTOR. Nat. Rev. Immunol. 2015, 15, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Reiling, J.H.; Sabatini, D.M. Stress and MTORture Signaling. Oncogene 2006, 25, 6373–6383. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M.M. Regulation and Function of AMPK in Physiology and Diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Geng, S.; Yuan, R.; Diao, N.; Upchurch, Z.; Li, L. Super-Low Dose Endotoxin Pre-Conditioning Exacerbates Sepsis Mortality. EBioMedicine 2015, 2, 324–333. [Google Scholar] [CrossRef]
- Yuan, R.; Geng, S.; Li, L. Molecular Mechanisms That Underlie the Dynamic Adaptation of Innate Monocyte Memory to Varying Stimulant Strength of TLR Ligands. Front. Immunol. 2016, 7, 497. [Google Scholar] [CrossRef]
- Rahtes, A.; Li, L. Polarization of Low-Grade Inflammatory Monocytes Through TRAM-Mediated Up-Regulation of Keap1 by Super-Low Dose Endotoxin. Front. Immunol. 2020, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Maitra, U.; Morris, M.; Li, L. Molecular Mechanism Responsible for the Priming of Macrophage Activation. J. Biol. Chem. 2013, 288, 3897–3906. [Google Scholar] [CrossRef] [PubMed]
- Maitra, U.; Deng, H.; Glaros, T.; Baker, B.; Capelluto, D.G.S.; Li, Z.; Li, L. Molecular Mechanisms Responsible for the Selective and Low-Grade Induction of Proinflammatory Mediators in Murine Macrophages by Lipopolysaccharide. J. Immunol. 2012, 189, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Lajqi, T.; Braun, M.; Kranig, S.A.; Frommhold, D.; Johannes, P.; Hudalla, H. LPS Induces Opposing Memory-like Inflammatory Responses in Mouse Bone Marrow Neutrophils. Int. J. Mol. Sci. 2021, 22, 9803. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Gilliam, E.A.; Button, J.; Li, L. Dynamic Modulation of Innate Immune Response by Varying Dosages of Lipopolysaccharide (LPS) in Human Monocytic Cells. J. Biol. Chem. 2014, 289, 21584–21590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Gao, R.; Hu, J.; Gao, D.P.; Liao, Y.L.; Yang, J.J. Trained Innate Immunity by Repeated Low-Dose Lipopolysaccharide Injections Displays Long-Term Neuroprotective Effects. Mediat. Inflamm. 2020, 2020, 8191079. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.; Zhang, X.; Borggrewe, M.; van Weering, H.R.J.; Brummer, M.L.; Nijboer, T.W.; Joosten, L.A.B.; Netea, M.G.; Boddeke, E.W.G.M.; Laman, J.D.; et al. Systemic Administration of β-Glucan Induces Immune Training in Microglia. J. Neuroinflammation 2021, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kracht, L.; Lerario, A.M.; Dubbelaar, M.L.; Brouwer, N.; Wesseling, E.M.; Boddeke, E.W.G.M.; Eggen, B.J.L.; Kooistra, S.M. Epigenetic Regulation of Innate Immune Memory in Microglia. J. Neuroinflammation 2022, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Grondman, I.; Arts, R.J.W.; Koch, R.M.; Leijte, G.P.; Gerretsen, J.; Bruse, N.; Kempkes, R.W.M.; Ter Horst, R.; Kox, M.; Pickkers, P.; et al. Frontline Science: Endotoxin-Induced Immunotolerance Is Associated with Loss of Monocyte Metabolic Plasticity and Reduction of Oxidative Burst. J. Leukoc. Biol. 2019, 106, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.W.; Shears, A.L.; Hibbitt, K.G. Increased Antibacterial Activity against Escherichia Coli in Bovine Serum after the Induction of Endotoxin Tolerance. Infect. Immun. 1976, 14, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Lahni, P.M.; Denenberg, A.G.; Poynter, S.E.; Wong, H.R.; Cook, J.A.; Zingarelli, B. Induction of Endotoxin Tolerance Enhances Bacterial Clearance and Survival in Murine Polymicrobial Sepsis. Shock 2008, 30, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lehner, M.D.; Ittner, J.; Bundschuh, D.S.; Van Rooijen, N.; Wendel, A.; Hartung, T. Improved Innate Immunity of Endotoxin-Tolerant Mice Increases Resistance to Salmonella Enterica Serovar Typhimurium Infection despite Attenuated Cytokine Response Improved Innate Immunity of Endotoxin-Tolerant Mice Increases Resistance to Salmonella Enter. Infect. Immun. 2001, 69, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-D.; Kim, S.Y.; Kim, J.; Kim, J.E.; Hong, Y.S.; Han, B.; Tak, E.; Ryu, Y.-M.; Kim, S.-Y.; Kim, T.W. Dynamic Increase of M2 Macrophages Is Associated with Disease Progression of Colorectal Cancers Following Cetuximab-Based Treatment. Sci. Rep. 2022, 12, 1678. [Google Scholar] [CrossRef]
- Duan, Z.; Luo, Y. Targeting Macrophages in Cancer Immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 127. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Genard, G.; Lucas, S.; Michiels, C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front. Immunol. 2017, 8, 828. [Google Scholar] [CrossRef]
- Montecino-Rodriguez, E.; Berent-Maoz, B.; Dorshkind, K. Causes, Consequences, and Reversal of Immune System Aging. J. Clin. Investig. 2013, 123, 958–965. [Google Scholar] [CrossRef]
- Mogilenko, D.A.; Shchukina, I.; Artyomov, M.N. Immune Ageing at Single-Cell Resolution. Nat. Rev. Immunol. 2022, 22, 484–498. [Google Scholar] [CrossRef]
- Niraula, A.; Sheridan, J.F.; Godbout, J.P. Microglia Priming with Aging and Stress. Neuropsychopharmacology 2017, 42, 318–333. [Google Scholar] [CrossRef]
- Bulut, O.; Kilic, G.; Domínguez-Andrés, J. Immune Memory in Aging: A Wide Perspective Covering Microbiota, Brain, Metabolism, and Epigenetics. Clin. Rev. Allergy Immunol. 2022, 63, 499–529. [Google Scholar] [CrossRef]
- Chambers, E.S.; Vukmanovic-Stejic, M.; Shih, B.B.; Trahair, H.; Subramanian, P.; Devine, O.P.; Glanville, J.; Gilroy, D.; Rustin, M.H.A.; Freeman, T.C.; et al. Recruitment of Inflammatory Monocytes by Senescent Fibroblasts Inhibits Antigen-Specific Tissue Immunity during Human Aging. Nat. Aging 2021, 1, 101–113. [Google Scholar] [CrossRef]
- Kasler, H.; Verdin, E. How Inflammaging Diminishes Adaptive Immunity. Nat. Aging 2021, 1, 24–25. [Google Scholar] [CrossRef]
- Gill, P.S.; Ozment, T.R.; Lewis, N.H.; Sherwood, E.R.; Williams, D.L. Trained Immunity Enhances Human Monocyte Function in Aging and Sepsis. Front. Immunol. 2022, 13, 872652. [Google Scholar] [CrossRef]
- Funder, J.W. Mineralocorticoid Receptors: Distribution and Activation. Heart Fail. Rev. 2005, 10, 15–22. [Google Scholar] [CrossRef]
- Zhong, C.; Yang, X.; Feng, Y.; Yu, J. Trained Immunity: An Underlying Driver of Inflammatory Atherosclerosis. Front. Immunol. 2020, 11, 284. [Google Scholar] [CrossRef]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2020, 11, 604000. [Google Scholar] [CrossRef]
- Acevedo, O.A.; Berrios, R.V.; Rodríguez-Guilarte, L.; Lillo-Dapremont, B.; Kalergis, A.M. Molecular and Cellular Mechanisms Modulating Trained Immunity by Various Cell Types in Response to Pathogen Encounter. Front. Immunol. 2021, 12, 745332. [Google Scholar] [CrossRef]
- D’Avila, H.; Roque, N.R.; Cardoso, R.M.; Castro-Faria-Neto, H.C.; Melo, R.C.N.; Bozza, P.T. Neutrophils Recruited to the Site of Mycobacterium Bovis BCG Infection Undergo Apoptosis and Modulate Lipid Body Biogenesis and Prostaglandin E Production by Macrophages. Cell. Microbiol. 2008, 10, 2589–2604. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, O.; Youm, Y.; Shchukina, I.; Ryu, S.; Sidorov, S.; Ravussin, A.; Nguyen, K.; Aladyeva, E.; Predeus, A.N.; Smith, S.R.; et al. Caloric Restriction in Humans Reveals Immunometabolic Regulators of Health Span. Science 2022, 375, 671–677. [Google Scholar] [CrossRef]
- Meydani, S.N.; Das, S.K.; Pieper, C.F.; Lewis, M.R.; Klein, S.; Dixit, V.D.; Gupta, A.K.; Villareal, D.T.; Bhapkar, M.; Huang, M.; et al. Long-Term Moderate Calorie Restriction Inhibits Inflammation without Impairing Cell-Mediated Immunity: A Randomized Controlled Trial in Non-Obese Humans. Aging 2016, 8, 1416–1431. [Google Scholar] [CrossRef]
- Ma, S.; Sun, S.; Geng, L.; Song, M.; Wang, W.; Ye, Y.; Ji, Q.; Zou, Z.; Wang, S.; He, X.; et al. Caloric Restriction Reprograms the Single-Cell Transcriptional Landscape of Rattus Norvegicus Aging. Cell 2020, 180, 984–1001.e22. [Google Scholar] [CrossRef]
- Christ, A.; Günther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175.e14. [Google Scholar] [CrossRef]
- Seufert, A.L.; Hickman, J.W.; Traxler, S.K.; Peterson, R.M.; Waugh, T.A.; Lashley, S.J.; Shulzhenko, N.; Napier, R.J.; Napier, B.A. Enriched Dietary Saturated Fatty Acids Induce Trained Immunity via Ceramide Production That Enhances Severity of Endotoxemia and Clearance of Infection. Elife 2022, 11, e76744. [Google Scholar] [CrossRef]
- van Splunter, M.; van Osch, T.L.J.; Brugman, S.; Savelkoul, H.F.J.; Joosten, L.A.B.; Netea, M.G.; van Neerven, R.J.J. Induction of Trained Innate Immunity in Human Monocytes by Bovine Milk and Milk-Derived Immunoglobulin G. Nutrients 2018, 10, 1378. [Google Scholar] [CrossRef]
- Beeson, P.B. Tolerance to Bacterial Pyrogens: I. Factors Influencing Its Development. J. Exp. Med. 1947, 86, 29–38. [Google Scholar] [CrossRef]
- Szabó, C.; Thiemermann, C.; Wu, C.C.; Perretti, M.; Vane, J.R. Attenuation of the Induction of Nitric Oxide Synthase by Endogenous Glucocorticoids Accounts for Endotoxin Tolerance in Vivo. Proc. Natl. Acad. Sci. USA 1994, 91, 271–275. [Google Scholar] [CrossRef]
- Hesse, M.; Modolell, M.; La Flamme, A.C.; Schito, M.; Fuentes, J.M.; Cheever, A.W.; Pearce, E.J.; Wynn, T.A. Differential Regulation of Nitric Oxide Synthase-2 and Arginase-1 by Type 1/Type 2 Cytokines in Vivo: Granulomatous Pathology Is Shaped by the Pattern of L-Arginine Metabolism. J. Immunol. 2001, 167, 6533–6544. [Google Scholar] [CrossRef]
- Suwanpradid, J.; Shih, M.; Pontius, L.; Yang, B.; Birukova, A.; Guttman-Yassky, E.; Corcoran, D.L.; Que, L.G.; Tighe, R.M.; MacLeod, A.S. Arginase1 Deficiency in Monocytes/Macrophages Upregulates Inducible Nitric Oxide Synthase To Promote Cutaneous Contact Hypersensitivity. J. Immunol. 2017, 199, 1827–1834. [Google Scholar] [CrossRef]
- Xu, H.; Chen, J.; Si, X.; Chen, M.; Pei, F.; Qiu, C.; Wu, J.; Guan, X. PKR Inhibition Mediates Endotoxin Tolerance in Macrophages through Inactivation of PI3K/AKT Signaling. Mol. Med. Rep. 2018, 17, 8548–8556. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. SHIP, TGF-Beta, and Endotoxin Tolerance. Immunity 2004, 21, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Bist, P.; Dhillon, M.K.; Kajiji, T.; Del Fresno, C.; Yamamoto, M.; Lopez-Collazo, E.; Akira, S.; Tergaonkar, V. Role for MyD88-Independent, TRIF Pathway in Lipid A/TLR4-Induced Endotoxin Tolerance. J. Immunol. 2007, 179, 4083–4092. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Tergaonkar, V. Myeloid Differentiation Factor 88-Independent Toll-like Receptor Pathway: Sustaining Inflammation or Promoting Tolerance? Int. J. Biochem. Cell Biol. 2007, 39, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.E.; Kopydlowski, K.M.; Vogel, S.N. Inhibition of Lipopolysaccharide-Induced Signal Transduction in Endotoxin-Tolerized Mouse Macrophages: Dysregulation of Cytokine, Chemokine, and Toll-like Receptor 2 and 4 Gene Expression. J. Immunol. 2000, 164, 5564–5574. [Google Scholar] [CrossRef]
- Vergadi, E.; Vaporidi, K.; Tsatsanis, C. Regulation of Endotoxin Tolerance and Compensatory Anti-Inflammatory Response Syndrome by Non-Coding RNAs. Front. Immunol. 2018, 9, 2705. [Google Scholar] [CrossRef]
- Chu, C.-H.; Wang, S.; Li, C.-L.; Chen, S.-H.; Hu, C.-F.; Chung, Y.-L.; Chen, S.-L.; Wang, Q.; Lu, R.-B.; Gao, H.-M.; et al. Neurons and Astroglia Govern Microglial Endotoxin Tolerance through Macrophage Colony-Stimulating Factor Receptor-Mediated ERK1/2 Signals. Brain. Behav. Immun. 2016, 55, 260–272. [Google Scholar] [CrossRef]
- Andrade, M.M.C.; Ariga, S.S.K.; Barbeiro, D.F.; Barbeiro, H.V.; Pimentel, R.N.; Petroni, R.C.; Soriano, F.G. Endotoxin Tolerance Modulates TREG and TH17 Lymphocytes Protecting Septic Mice. Oncotarget 2019, 10, 3451–3461. [Google Scholar] [CrossRef]
- Ogawa, H.; Rafiee, P.; Heidemann, J.; Fisher, P.J.; Johnson, N.A.; Otterson, M.F.; Kalyanaraman, B.; Pritchard, K.A.J.; Binion, D.G. Mechanisms of Endotoxin Tolerance in Human Intestinal Microvascular Endothelial Cells. J. Immunol. 2003, 170, 5956–5964. [Google Scholar] [CrossRef]
- Cavaillon, J.M. The Nonspecific Nature of Endotoxin Tolerance. Trends Microbiol. 1995, 3, 320–324. [Google Scholar] [CrossRef]
- Sly, L.M.; Rauh, M.J.; Kalesnikoff, J.; Song, C.H.; Krystal, G. LPS-Induced Upregulation of SHIP Is Essential for Endotoxin Tolerance. Immunity 2004, 21, 227–239. [Google Scholar] [CrossRef]
- Chang, J.; Kunkel, S.L.; Chang, C.-H. Negative Regulation of MyD88-Dependent Signaling by IL-10 in Dendritic Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 18327–18332. [Google Scholar] [CrossRef]
- Gómez-Piña, V.; Martínez, E.; Fernández-Ruíz, I.; Del Fresno, C.; Soares-Schanoski, A.; Jurado, T.; Siliceo, M.; Toledano, V.; Fernández-Palomares, R.; García-Rio, F.; et al. Role of MMPs in Orchestrating Inflammatory Response in Human Monocytes via a TREM-1-PI3K-NF-ΚB Pathway. J. Leukoc. Biol. 2012, 91, 933–945. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Joosten, L.A.B.; van der Meer, J.W.M.; Netea, M.G. TREM-1: Intracellular Signaling Pathways and Interaction with Pattern Recognition Receptors. J. Leukoc. Biol. 2013, 93, 209–215. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hernandez, L.D.; Galán, J.E.; Janeway, C.A.J.; Medzhitov, R.; Flavell, R.A. IRAK-M Is a Negative Regulator of Toll-like Receptor Signaling. Cell 2002, 110, 191–202. [Google Scholar] [CrossRef]
- Kinjyo, I.; Hanada, T.; Inagaki-Ohara, K.; Mori, H.; Aki, D.; Ohishi, M.; Yoshida, H.; Kubo, M.; Yoshimura, A. SOCS1/JAB Is a Negative Regulator of LPS-Induced Macrophage Activation. Immunity 2002, 17, 583–591. [Google Scholar] [CrossRef]
- Xiong, Y.; Medvedev, A.E. Induction of Endotoxin Tolerance in Vivo Inhibits Activation of IRAK4 and Increases Negative Regulators IRAK-M, SHIP-1, and A20. J. Leukoc. Biol. 2011, 90, 1141–1148. [Google Scholar] [CrossRef]
- Chang, E.Y.; Guo, B.; Doyle, S.E.; Cheng, G. Cutting Edge: Involvement of the Type I IFN Production and Signaling Pathway in Lipopolysaccharide-Induced IL-10 Production. J. Immunol. 2007, 178, 6705–6709. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Hardie, D.G. Metabolism of Inflammation Limited by AMPK and Pseudo-Starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef]
- Wilson, A.G. Epigenetic Regulation of Gene Expression in the Inflammatory Response and Relevance to Common Diseases. J. Periodontol. 2008, 79, 1514–1519. [Google Scholar] [CrossRef]
- El Gazzar, M.; Yoza, B.K.; Chen, X.; Garcia, B.A.; Young, N.L.; McCall, C.E. Chromatin-Specific Remodeling by HMGB1 and Linker Histone H1 Silences Proinflammatory Genes during Endotoxin Tolerance. Mol. Cell. Biol. 2009, 29, 1959–1971. [Google Scholar] [CrossRef]
- Nahid, M.A.; Satoh, M.; Chan, E.K. MicroRNA in TLR Signaling and Endotoxin Tolerance. Cell. Mol. Immunol. 2011, 8, 388–403. [Google Scholar] [CrossRef]
- Quinn, E.M.; Wang, J.; Redmond, H.P. The Emerging Role of MicroRNA in Regulation of Endotoxin Tolerance. J. Leukoc. Biol. 2012, 91, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Baltimore, D.; Boldin, M.P.; O’Connell, R.M.; Rao, D.S.; Taganov, K.D. MicroRNAs: New Regulators of Immune Cell Development and Function. Nat. Immunol. 2008, 9, 839–845. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-KappaB-Dependent Induction of MicroRNA MiR-146, an Inhibitor Targeted to Signaling Proteins of Innate Immune Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.-J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Fabbri, M.; Alder, H.; Liu, C.G.; Calin, G.A.; et al. Modulation of MiR-155 and MiR-125b Levels Following Lipopolysaccharide/TNF-Alpha Stimulation and Their Possible Roles in Regulating the Response to Endotoxin Shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Splinter, P.L.; O’Hara, S.P.; LaRusso, N.F. A Cellular Micro-RNA, Let-7i, Regulates Toll-like Receptor 4 Expression and Contributes to Cholangiocyte Immune Responses against Cryptosporidium Parvum Infection. J. Biol. Chem. 2007, 282, 28929–28938. [Google Scholar] [CrossRef]
- Nahid, M.A.; Pauley, K.M.; Satoh, M.; Chan, E.K.L. MiR-146a Is Critical for Endotoxin-Induced Tolerance: Implication in Innate Immunity. J. Biol. Chem. 2009, 284, 34590–34599. [Google Scholar] [CrossRef]
- Chassin, C.; Kocur, M.; Pott, J.; Duerr, C.U.; Gütle, D.; Lotz, M.; Hornef, M.W. MiR-146a Mediates Protective Innate Immune Tolerance in the Neonate Intestine. Cell Host Microbe 2010, 8, 358–368. [Google Scholar] [CrossRef]
- Shaked, I.; Meerson, A.; Wolf, Y.; Avni, R.; Greenberg, D.; Gilboa-Geffen, A.; Soreq, H. MicroRNA-132 Potentiates Cholinergic Anti-Inflammatory Signaling by Targeting Acetylcholinesterase. Immunity 2009, 31, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Incoronato, M.; Garofalo, M.; Urso, L.; Romano, G.; Quintavalle, C.; Zanca, C.; Iaboni, M.; Nuovo, G.; Croce, C.M.; Condorelli, G. MiR-212 Increases Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Sensitivity in Non-Small Cell Lung Cancer by Targeting the Antiapoptotic Protein PED. Cancer Res. 2010, 70, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Nahid, M.A.; Yao, B.; Dominguez-Gutierrez, P.R.; Kesavalu, L.; Satoh, M.; Chan, E.K.L. Regulation of TLR2-Mediated Tolerance and Cross-Tolerance through IRAK4 Modulation by MiR-132 and MiR-212. J. Immunol. 2013, 190, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Seeley, J.J.; Baker, R.G.; Mohamed, G.; Bruns, T.; Hayden, M.S.; Deshmukh, S.D.; Freedberg, D.E.; Ghosh, S. Induction of Innate Immune Memory via MicroRNA Targeting of Chromatin Remodelling Factors. Nature 2018, 559, 114–119. [Google Scholar] [CrossRef] [PubMed]
- El Gazzar, M.; McCall, C.E. MicroRNAs Distinguish Translational from Transcriptional Silencing during Endotoxin Tolerance. J. Biol. Chem. 2010, 285, 20940–20951. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Song, Y.; Lai, L.; Wang, J.; Yu, H.; Cao, X.; Wang, Q. MicroRNA-98 Negatively Regulates IL-10 Production and Endotoxin Tolerance in Macrophages after LPS Stimulation. FEBS Lett. 2011, 585, 1963–1968. [Google Scholar] [CrossRef]
- Lang, C.H.; Spitzer, J.A. Glucose Kinetics and Development of Endotoxin Tolerance during Long-Term Continuous Endotoxin Infusion. Metabolism 1987, 36, 469–474. [Google Scholar] [CrossRef]
- Androulidaki, A.; Iliopoulos, D.; Arranz, A.; Doxaki, C.; Schworer, S.; Zacharioudaki, V.; Margioris, A.N.; Tsichlis, P.N.; Tsatsanis, C. The Kinase Akt1 Controls Macrophage Response to Lipopolysaccharide by Regulating MicroRNAs. Immunity 2009, 31, 220–231. [Google Scholar] [CrossRef]
- Liu, T.F.; Yoza, B.K.; El Gazzar, M.; Vachharajani, V.T.; McCall, C.E. NAD+-Dependent SIRT1 Deacetylase Participates in Epigenetic Reprogramming during Endotoxin Tolerance. J. Biol. Chem. 2011, 286, 9856–9864. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Pan, H.; Feldser, H.G.; Lainez, E.; Miller, C.; Leung, S.; Zhong, Z.; Zhao, H.; Sweitzer, S.; et al. SIRT1 Activators Suppress Inflammatory Responses through Promotion of P65 Deacetylation and Inhibition of NF-ΚB Activity. PLoS ONE 2012, 7, e46364. [Google Scholar] [CrossRef]
- van der Meer, A.J.; Scicluna, B.P.; Moerland, P.D.; Lin, J.; Jacobson, E.W.; Vlasuk, G.P.; van der Poll, T. The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Crit. Care Med. 2015, 43, e199–e202. [Google Scholar] [CrossRef] [PubMed]
- Gaber, T.; Strehl, C.; Buttgereit, F. Metabolic Regulation of Inflammation. Nat. Rev. Rheumatol. 2017, 13, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic Shifts in Immunity and Inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Targeting Immunometabolism as an Anti-Inflammatory Strategy. Cell Res. 2020, 30, 300–314. [Google Scholar] [CrossRef]
- Xu, J.; Gao, C.; He, Y.; Fang, X.; Sun, D.; Peng, Z.; Xiao, H.; Sun, M.; Zhang, P.; Zhou, T.; et al. NLRC3 Expression in Macrophage Impairs Glycolysis and Host Immune Defense by Modulating the NF-ΚB-NFAT5 Complex during Septic Immunosuppression. Mol. Ther. 2023, 31, 154–173. [Google Scholar] [CrossRef]
- Ratter, J.M.; Rooijackers, H.M.M.; Hooiveld, G.J.; Hijmans, A.G.M.; de Galan, B.E.; Tack, C.J.; Stienstra, R. In Vitro and in Vivo Effects of Lactate on Metabolism and Cytokine Production of Human Primary PBMCs and Monocytes. Front. Immunol. 2018, 9, 2564. [Google Scholar] [CrossRef]
- Caslin, H.L.; Abebayehu, D.; Abdul Qayum, A.; Haque, T.T.; Taruselli, M.T.; Paez, P.A.; Pondicherry, N.; Barnstein, B.O.; Hoeferlin, L.A.; Chalfant, C.E.; et al. Lactic Acid Inhibits Lipopolysaccharide-Induced Mast Cell Function by Limiting Glycolysis and ATP Availability. J. Immunol. 2019, 203, 453–464. [Google Scholar] [CrossRef]
- Abebayehu, D.; Spence, A.J.; Qayum, A.A.; Taruselli, M.T.; McLeod, J.J.A.; Caslin, H.L.; Chumanevich, A.P.; Kolawole, E.M.; Paranjape, A.; Baker, B.; et al. Lactic Acid Suppresses IL-33-Mediated Mast Cell Inflammatory Responses via Hypoxia-Inducible Factor-1α-Dependent MiR-155 Suppression. J. Immunol. 2016, 197, 2909–2917. [Google Scholar] [CrossRef]
- Yang, K.; Xu, J.; Fan, M.; Tu, F.; Wang, X.; Ha, T.; Williams, D.L.; Li, C. Lactate Suppresses Macrophage Pro-Inflammatory Response to LPS Stimulation by Inhibition of YAP and NF-ΚB Activation via GPR81-Mediated Signaling. Front. Immunol. 2020, 11, 587913. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Scicluna, B.P.; Arts, R.J.W.; Gresnigt, M.S.; Lachmandas, E.; Giamarellos-Bourboulis, E.J.; Kox, M.; Manjeri, G.R.; Wagenaars, J.A.L.; Cremer, O.L.; et al. Broad Defects in the Energy Metabolism of Leukocytes Underlie Immunoparalysis in Sepsis. Nat. Immunol. 2016, 17, 406–413. [Google Scholar] [CrossRef]
- Gillen, J.; Ondee, T.; Gurusamy, D.; Issara-Amphorn, J.; Manes, N.P.; Yoon, S.H.; Leelahavanichkul, A.; Nita-Lazar, A. LPS Tolerance Inhibits Cellular Respiration and Induces Global Changes in the Macrophage Secretome. Biomolecules 2021, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Morcelle, C.; Cheng, Z.-L.; Chen, X.; Xu, Y.; Gao, Y.; Song, J.; Li, Z.; Smith, M.D.; Shi, M.; et al. Itaconate Inhibits TET DNA Dioxygenases to Dampen Inflammatory Responses. Nat. Cell Biol. 2022, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Qin, K.; Zhang, Y.; Jia, W.; Chen, Y.; Cheng, B.; Peng, L.; Chen, N.; Liu, Y.; Zhou, W.; et al. S-Glycosylation-Based Cysteine Profiling Reveals Regulation of Glycolysis by Itaconate. Nat. Chem. Biol. 2019, 15, 983–991. [Google Scholar] [CrossRef]
- Zasłona, Z.; O’Neill, L.A.J. Cytokine-like Roles for Metabolites in Immunity. Mol. Cell 2020, 78, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.-C.; Chou, C.-H.; Vavakova, M.; et al. α-Ketoglutarate Orchestrates Macrophage Activation through Metabolic and Epigenetic Reprogramming. Nat. Immunol. 2017, 18, 985–994. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Wu, Z. The Regulatory Role of α-Ketoglutarate Metabolism in Macrophages. Mediators Inflamm. 2021, 2021, 5577577. [Google Scholar] [CrossRef]
- Nomura, M.; Liu, J.; Rovira, I.I.; Gonzalez-Hurtado, E.; Lee, J.; Wolfgang, M.J.; Finkel, T. Fatty Acid Oxidation in Macrophage Polarization. Nature Immunol. 2016, 17, 216–217. [Google Scholar] [CrossRef]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Wagner, R.A.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative Metabolism and PGC-1beta Attenuate Macrophage-Mediated Inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Jaroonwitchawan, T.; Visitchanakun, P.; Dang, P.C.; Ritprajak, P.; Palaga, T.; Leelahavanichkul, A. Dysregulation of Lipid Metabolism in Macrophages Is Responsible for Severe Endotoxin Tolerance in FcgRIIB-Deficient Lupus Mice. Front. Immunol. 2020, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Yu, X.; Saha, G.; Kalafati, L.; Ioannidis, C.; Mitroulis, I.; Netea, M.G.; Chavakis, T.; Hajishengallis, G. Maladaptive Innate Immune Training of Myelopoiesis Links Inflammatory Comorbidities. Cell 2022, 185, 1709–1727.e18. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis - an Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Libby, P. Targeting Inflammation in Atherosclerosis-from Experimental Insights to the Clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Edgar, L.; Akbar, N.; Braithwaite, A.T.; Krausgruber, T.; Gallart-Ayala, H.; Bailey, J.; Corbin, A.L.; Khoyratty, T.E.; Chai, J.T.; Alkhalil, M.; et al. Hyperglycemia Induces Trained Immunity in Macrophages and Their Precursors and Promotes Atherosclerosis. Circulation 2021, 144, 961–982. [Google Scholar] [CrossRef]
- Flores-Gomez, D.; Bekkering, S.; Netea, M.G.; Riksen, N.P. Trained Immunity in Atherosclerotic Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 62–69. [Google Scholar] [CrossRef]
- Bekkering, S.; van den Munckhof, I.; Nielen, T.; Lamfers, E.; Dinarello, C.; Rutten, J.; de Graaf, J.; Joosten, L.A.B.; Netea, M.G.; Gomes, M.E.R.; et al. Innate Immune Cell Activation and Epigenetic Remodeling in Symptomatic and Asymptomatic Atherosclerosis in Humans in Vivo. Atherosclerosis 2016, 254, 228–236. [Google Scholar] [CrossRef]
- Lamb, D.J.; Eales, L.J.; Ferns, G.A. Immunization with Bacillus Calmette-Guerin Vaccine Increases Aortic Atherosclerosis in the Cholesterol-Fed Rabbit. Atherosclerosis 1999, 143, 105–113. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Holmstrup, P.; Damgaard, C.; Olsen, I.; Klinge, B.; Flyvbjerg, A.; Nielsen, C.H.; Hansen, P.R. Comorbidity of Periodontal Disease: Two Sides of the Same Coin? An Introduction for the Clinician. J. Oral Microbiol. 2017, 9, 1332710. [Google Scholar] [CrossRef]
- Edilova, M.I.; Akram, A.; Abdul-Sater, A.A. Innate Immunity Drives Pathogenesis of Rheumatoid Arthritis. Biomed. J. 2021, 44, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Gierut, A.; Perlman, H.; Pope, R.M. Innate Immunity and Rheumatoid Arthritis. Rheum. Dis. Clin. North Am. 2010, 36, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid Arthritis. Lancet (Lond. Engl.) 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid Arthritis: Pathological Mechanisms and Modern Pharmacologic Therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- McGarry, T.; Hanlon, M.M.; Marzaioli, V.; Cunningham, C.C.; Krishna, V.; Murray, K.; Hurson, C.; Gallagher, P.; Nagpal, S.; Veale, D.J.; et al. Rheumatoid Arthritis CD14(+) Monocytes Display Metabolic and Inflammatory Dysfunction, a Phenotype That Precedes Clinical Manifestation of Disease. Clin. Transl. Immunol. 2021, 10, e1237. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Dai, X.; Gong, Z.; Yang, C.; Zeng, K.; Gong, F.-Y.; Zhong, Q.; Gao, X.-M. Disease-Specific Autoantibodies Induce Trained Immunity in RA Synovial Tissues and Its Gene Signature Correlates with the Response to Clinical Therapy. Mediat. Inflamm. 2020, 2020, 2109325. [Google Scholar] [CrossRef]
- Messemaker, T.C.; Mikkers, H.M.M.; Huizinga, T.W.; Toes, R.E.M.; Van Der Helm-Van Mil, A.H.M.; Kurreeman, F. Inflammatory Genes TNFα and IL6 Display No Signs of Increased H3K4me3 in Circulating Monocytes from Untreated Rheumatoid Arthritis Patients. Genes Immun. 2017, 18, 191–196. [Google Scholar] [CrossRef]
- Shao, P.; Ma, L.; Ren, Y.; Liu, H. Modulation of the Immune Response in Rheumatoid Arthritis with Strategically Released Rapamycin. Mol. Med. Rep. 2017, 16, 5257–5262. [Google Scholar] [CrossRef]
- Jeljeli, M.; Riccio, L.G.C.; Doridot, L.; Chêne, C.; Nicco, C.; Chouzenoux, S.; Deletang, Q.; Allanore, Y.; Kavian, N.; Batteux, F. Trained Immunity Modulates Inflammation-Induced Fibrosis. Nat. Commun. 2019, 10, 5670. [Google Scholar] [CrossRef]
- Pisetsky, D.S. The Role of Innate Immunity in the Induction of Autoimmunity. Autoimmun. Rev. 2008, 8, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yin, X.; Wen, L.; Yang, C.; Sheng, Y.; Lin, Y.; Zhu, Z.; Shen, C.; Shi, Y.; Zheng, Y.; et al. Several Critical Cell Types, Tissues, and Pathways Are Implicated in Genome-Wide Association Studies for Systemic Lupus Erythematosus. G3 (Bethesda) 2016, 6, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, P.Y.; Reeves, W.H. Monocyte and Macrophage Abnormalities in Systemic Lupus Erythematosus. Arch. Immunol. Ther. Exp. 2010, 58, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Herrada, A.A.; Escobedo, N.; Iruretagoyena, M.; Valenzuela, R.A.; Burgos, P.I.; Cuitino, L.; Llanos, C. Innate Immune Cells’ Contribution to Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Kavai, M.; Szegedi, G. Immune Complex Clearance by Monocytes and Macrophages in Systemic Lupus Erythematosus. Autoimmun. Rev. 2007, 6, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, F.; Henke, F.; Krause, B.; Thiele, B.; Burmester, G.R.; Hiepe, F. Monocytes from Systemic Lupus Erythematous Patients Are Severely Altered in Phenotype and Lineage Flexibility. Ann. Rheum. Dis. 2000, 59, 283–288. [Google Scholar] [CrossRef]
- Kaplan, M.J. Neutrophils in the Pathogenesis and Manifestations of SLE. Nat. Rev. Rheumatol. 2011, 7, 691–699. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, F.; Sun, X.; Zhang, X.; Zhu, L.; Liu, X.; Li, X.; Xu, L.; Shi, L.; Gan, Y.; et al. CD16(+) Monocyte Subset Was Enriched and Functionally Exacerbated in Driving T-Cell Activation and B-Cell Response in Systemic Lupus Erythematosus. Front. Immunol. 2016, 7, 512. [Google Scholar] [CrossRef]
- Yu, Y.; Su, K. Neutrophil Extracellular Traps and Systemic Lupus Erythematosus. J. Clin. Cell. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Saithong, S.; Saisorn, W.; Visitchanakun, P.; Sae-Khow, K.; Chiewchengchol, D.; Leelahavanichkul, A. A Synergy Between Endotoxin and (1→3)-Beta-D-Glucan Enhanced Neutrophil Extracellular Traps in Candida Administered Dextran Sulfate Solution Induced Colitis in FcGRIIB-/- Lupus Mice, an Impact of Intestinal Fungi in Lupus. J. Inflamm. Res. 2021, 14, 2333–2352. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.-G.; Liu, Z.-Z.; Ma, K.; Lin, X.-Q.; Zhang, J.-B.; Chen, W.; Yang, Y.-J. Extracellular Trap Can Be Trained as a Memory Response. Virulence 2022, 13, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Marone, G.; Mantovani, A. Cancer Inflammation and Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 Diabetes as an Inflammatory Disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Das, U.N. Is Obesity an Inflammatory Condition? Nutrition 2001, 17, 953–966. [Google Scholar] [CrossRef]
- Choudhury, R.P.; Edgar, L.; Rydén, M.; Fisher, E.A. Diabetes and Metabolic Drivers of Trained Immunity: New Therapeutic Targets Beyond Glucose. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1284–1290. [Google Scholar] [CrossRef]
- Bekkering, S.; Saner, C.; Riksen, N.P.; Netea, M.G.; Sabin, M.A.; Saffery, R.; Stienstra, R.; Burgner, D.P. Trained Immunity: Linking Obesity and Cardiovascular Disease across the Life-Course? Trends Endocrinol. Metab. 2020, 31, 378–389. [Google Scholar] [CrossRef]
- Van Tuijl, J.; Vreeken, D.; Broeders, W.; Stienstra, R.; Joosten, L.A.B.; Netea, M.G.; Hazebroek, E.J.; Kiliaan, A.J.; Bekkering, S.; Riksen, N.P. Adipose Tissue Induces Trained Innate Immunity in Patients with Obesity. Eur. Heart J. 2021, 42. [Google Scholar] [CrossRef]
- Kotlyarov, S. Involvement of the Innate Immune System in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2022, 23, 985. [Google Scholar] [CrossRef]
- McGrath, J.J.C.; Stampfli, M.R. The Immune System as a Victim and Aggressor in Chronic Obstructive Pulmonary Disease. J. Thorac. Dis. 2018, 10, S2011–S2017. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; Valk, P. van der. Inflammation in Neurodegenerative Diseases. Immunology 2010, 129, 154. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How Neuroinflammation Contributes to Neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Holmes, C. Microglial Priming in Neurodegenerative Disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Cunningham, C. Priming Microglia for Innate Immune Memory in the Brain. Trends Immunol. 2019, 40, 358–374. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Gradini, R.; Cummings, M.; Diaz, E.; Price, A.I.; Osorio, C. Rusty Microglia: Trainers of Innate Immunity in Alzheimer’s Disease. Front. Neurol. 2018, 9, 1062. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Garlanda, C.; Jaillon, S.; Marone, G.; Mantovani, A. Tumor Associated Macrophages and Neutrophils in Tumor Progression. J. Cell. Physiol. 2013, 228, 1404–1412. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-Associated Neutrophils in Patients with Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- del Fresno, C.; Otero, K.; Gómez-García, L.; González-León, M.C.; Soler-Ranger, L.; Fuentes-Prior, P.; Escoll, P.; Baos, R.; Caveda, L.; García, F.; et al. Tumor Cells Deactivate Human Monocytes by Up-Regulating IL-1 Receptor Associated Kinase-M Expression via CD44 and TLR4. J. Immunol. 2005, 174, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Soares-Schanoski, A.; Jurado, T.; Córdoba, R.; Siliceo, M.; Del Fresno, C.; Gómez-Piña, V.; Toledano, V.; Vallejo-Cremades, M.T.; Alfonso-Iñiguez, S.; Carballo-Palos, A.; et al. Impaired Antigen Presentation and Potent Phagocytic Activity Identifying Tumor-Tolerant Human Monocytes. Biochem. Biophys. Res. Commun. 2012, 423, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Nosari, A. Infectious Complications in Chronic Lymphocytic Leukemia. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012070. [Google Scholar] [CrossRef] [PubMed]
- Rolston, K.V.I. Infections in Patients with Acute Leukemia. In Infections in Hematology; Maschmeyer, G., Rolston, K.V.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–23. [Google Scholar]
- Logan, C.; Koura, D.; Taplitz, R. Updates in Infection Risk and Management in Acute Leukemia. Hematol. Am. Soc. Hematol. Educ. Progr. 2020, 2020, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Pena, O.M.; Pistolic, J.; Raj, D.; Fjell, C.D.; Hancock, R.E.W. Endotoxin Tolerance Represents a Distinctive State of Alternative Polarization (M2) in Human Mononuclear Cells. J. Immunol. 2011, 186, 7243–7254. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A. Macrophages, Innate Immunity and Cancer: Balance, Tolerance, and Diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The Immunopathology of Sepsis and Potential Therapeutic Targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and Septic Shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Cao, C.; Yu, M.; Chai, Y. Pathological Alteration and Therapeutic Implications of Sepsis-Induced Immune Cell Apoptosis. Cell Death Dis. 2019, 10, 782. [Google Scholar] [CrossRef]
- Gogos, C.; Kotsaki, A.; Pelekanou, A.; Giannikopoulos, G.; Vaki, I.; Maravitsa, P.; Adamis, S.; Alexiou, Z.; Andrianopoulos, G.; Antonopoulou, A.; et al. Early Alterations of the Innate and Adaptive Immune Statuses in Sepsis According to the Type of Underlying Infection. Crit. Care 2010, 14, R96. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.-M.; Adrie, C.; Fitting, C.; Adib-Conquy, M. Endotoxin Tolerance: Is There a Clinical Relevance? J. Endotoxin Res. 2003, 9, 101–107. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in Sepsis: A Novel Understanding of the Disorder and a New Therapeutic Approach. Lancet. Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Pena, O.M.; Hancock, D.G.; Lyle, N.H.; Linder, A.; Russell, J.A.; Xia, J.; Fjell, C.D.; Boyd, J.H.; Hancock, R.E.W. An Endotoxin Tolerance Signature Predicts Sepsis and Organ Dysfunction at Initial Clinical Presentation. EBioMedicine 2014, 1, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, A.J.; Garcia-Laorden, M.I.; van Vught, L.A.; Wiewel, M.A.; Belkasim-Bohoudi, H.; Duitman, J.; Horn, J.; Schultz, M.J.; Scicluna, B.P.; van ’t Veer, C.; et al. Sepsis Patients Display a Reduced Capacity to Activate Nuclear Factor-ΚB in Multiple Cell Types. Crit. Care Med. 2017, 45, e524–e531. [Google Scholar] [CrossRef] [PubMed]
- Dar, W.A.; Sullivan, E.; Bynon, J.S.; Eltzschig, H.; Ju, C. Ischaemia Reperfusion Injury in Liver Transplantation: Cellular and Molecular Mechanisms. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39, 788–801. [Google Scholar] [CrossRef]
- Jaeschke, H. Molecular Mechanisms of Hepatic Ischemia-Reperfusion Injury and Preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G15–G26. [Google Scholar] [CrossRef]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic Ischemia and Reperfusion Injury: Effects on the Liver Sinusoidal Milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef]
- Hirao, H.; Nakamura, K.; Kupiec-Weglinski, J.W. Liver Ischaemia-Reperfusion Injury: A New Understanding of the Role of Innate Immunity. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 239–256. [Google Scholar] [CrossRef]
- Fernández, E.D.; Flohé, S.; Siemers, F.; Nau, M.; Ackermann, M.; Ruwe, M.; Schade, F.U. Endotoxin Tolerance Protects against Local Hepatic Ischemia/Reperfusion Injury in the Rat. J. Endotoxin Res. 2000, 6, 321–328. [Google Scholar] [CrossRef]
- Li, J.; Lai, X.; Chen, Y.; Niu, B.; Gong, J. Endotoxin Tolerance Attenuates Liver Ischemia/Reperfusion Injury by down-Regulation of Interleukin-1 Receptor-Associated Kinase 4 in Kupffer Cells. Transplant. Proc. 2011, 43, 2531–2535. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Grosso, M.A.; Terada, L.S.; Whitman, G.J.; Banerjee, A.; White, C.W.; Harken, A.H.; Repine, J.E. Endotoxin Pretreatment Increases Endogenous Myocardial Catalase Activity and Decreases Ischemia-Reperfusion Injury of Isolated Rat Hearts. Proc. Natl. Acad. Sci. USA 1989, 86, 2516–2520. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, E.J.; Pellicane, J.V.; Lee, R.B. Hemorrhagic Shock in Endotoxin-Resistant Mice: Improved Survival Unrelated to Deficient Production of Tumor Necrosis Factor. J. Trauma 1993, 35, 720–725. [Google Scholar] [CrossRef]
- del Fresno, C.; Soler-Rangel, L.; Soares-Schanoski, A.; Gómez-Piña, V.; González-León, M.C.; Gómez-García, L.; Mendoza-Barberá, E.; Rodríguez-Rojas, A.; García, F.; Fuentes-Prior, P.; et al. Inflammatory Responses Associated with Acute Coronary Syndrome Up-Regulate IRAK-M and Induce Endotoxin Tolerance in Circulating Monocytes. J. Endotoxin Res. 2007, 13, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Nica, V.; Popp, R.A.; Crișan, T.O.; Joosten, L.A.B. The Future Clinical Implications of Trained Immunity. Expert Rev. Clin. Immunol. 2022, 18, 1125–1134. [Google Scholar] [CrossRef]
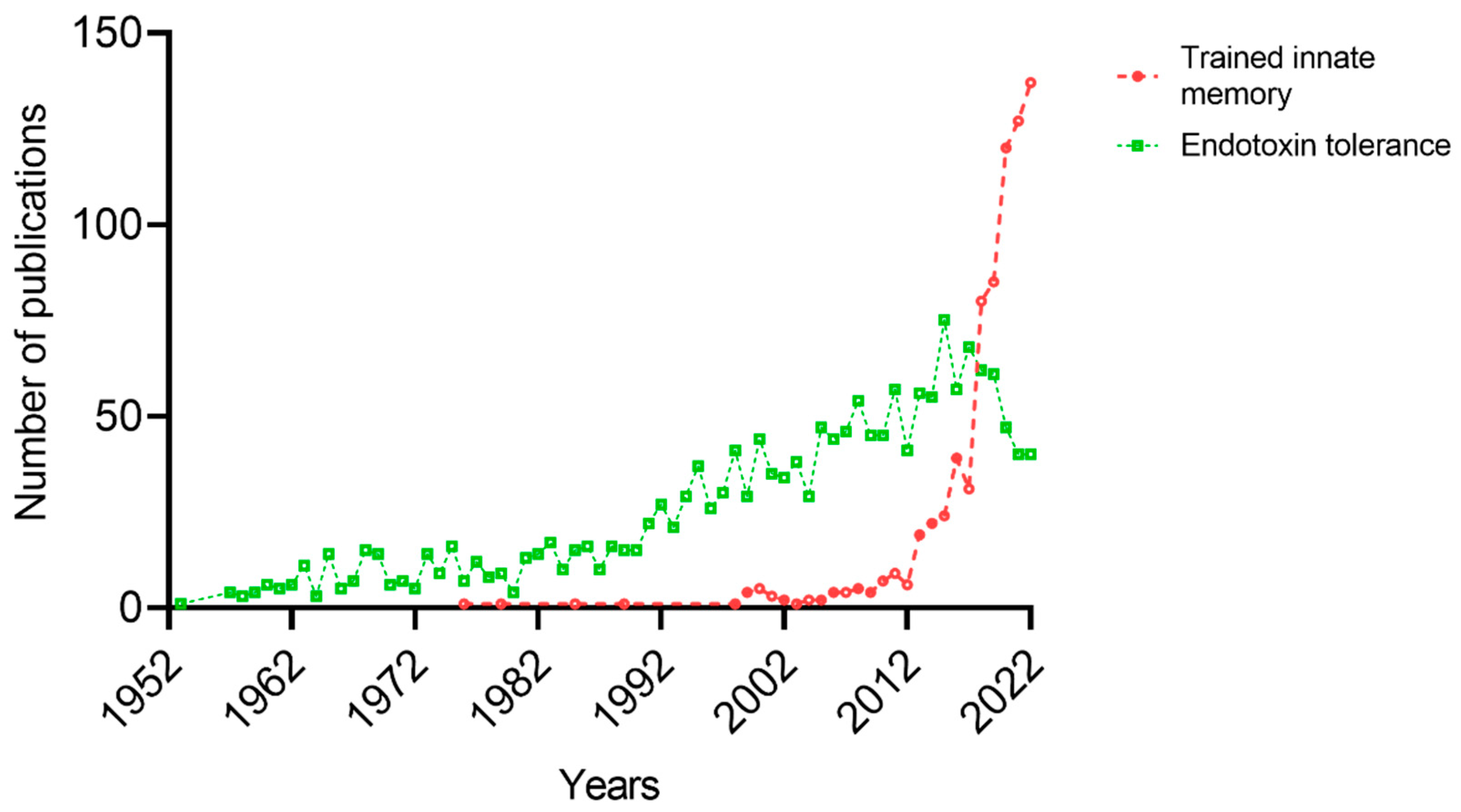
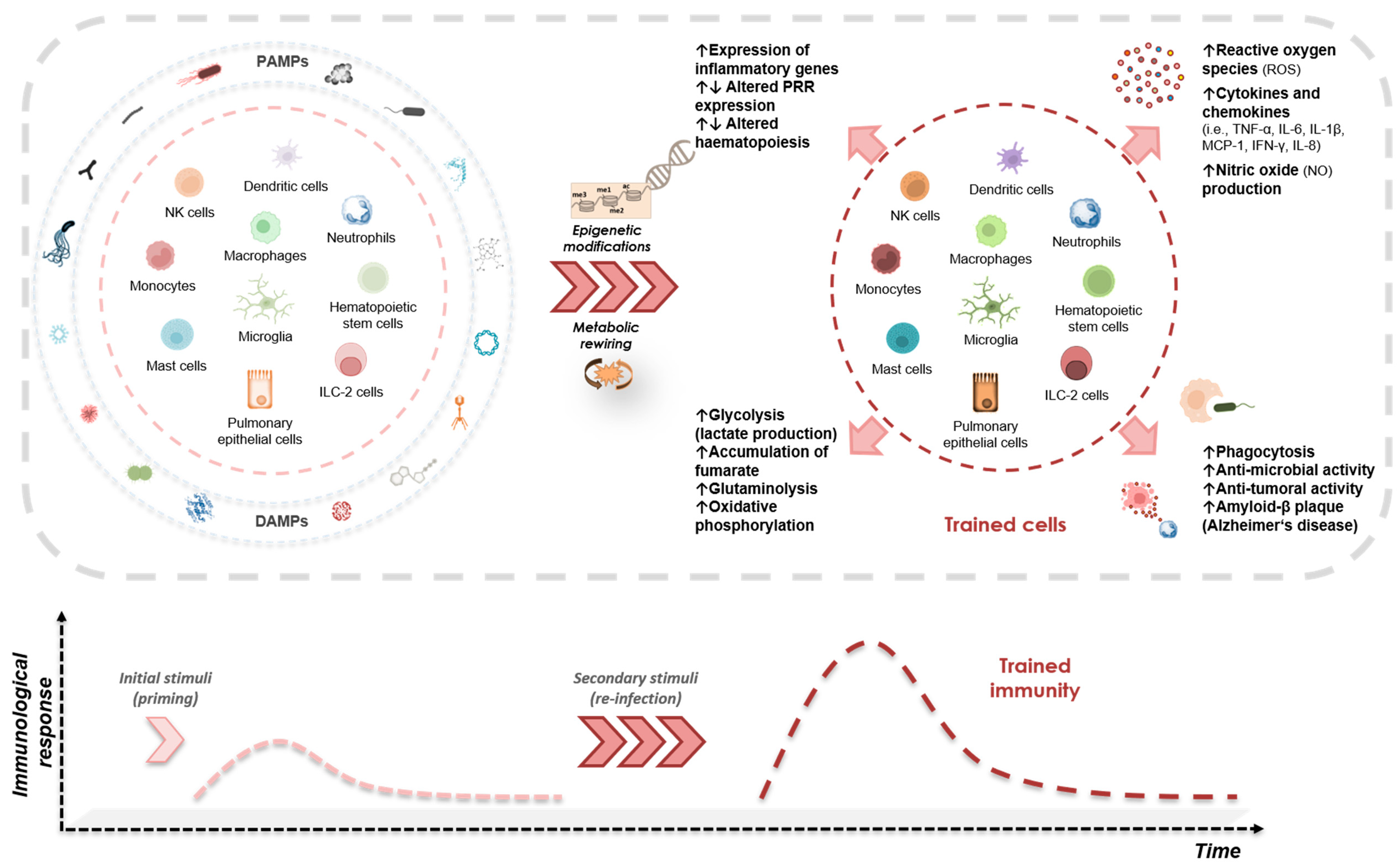
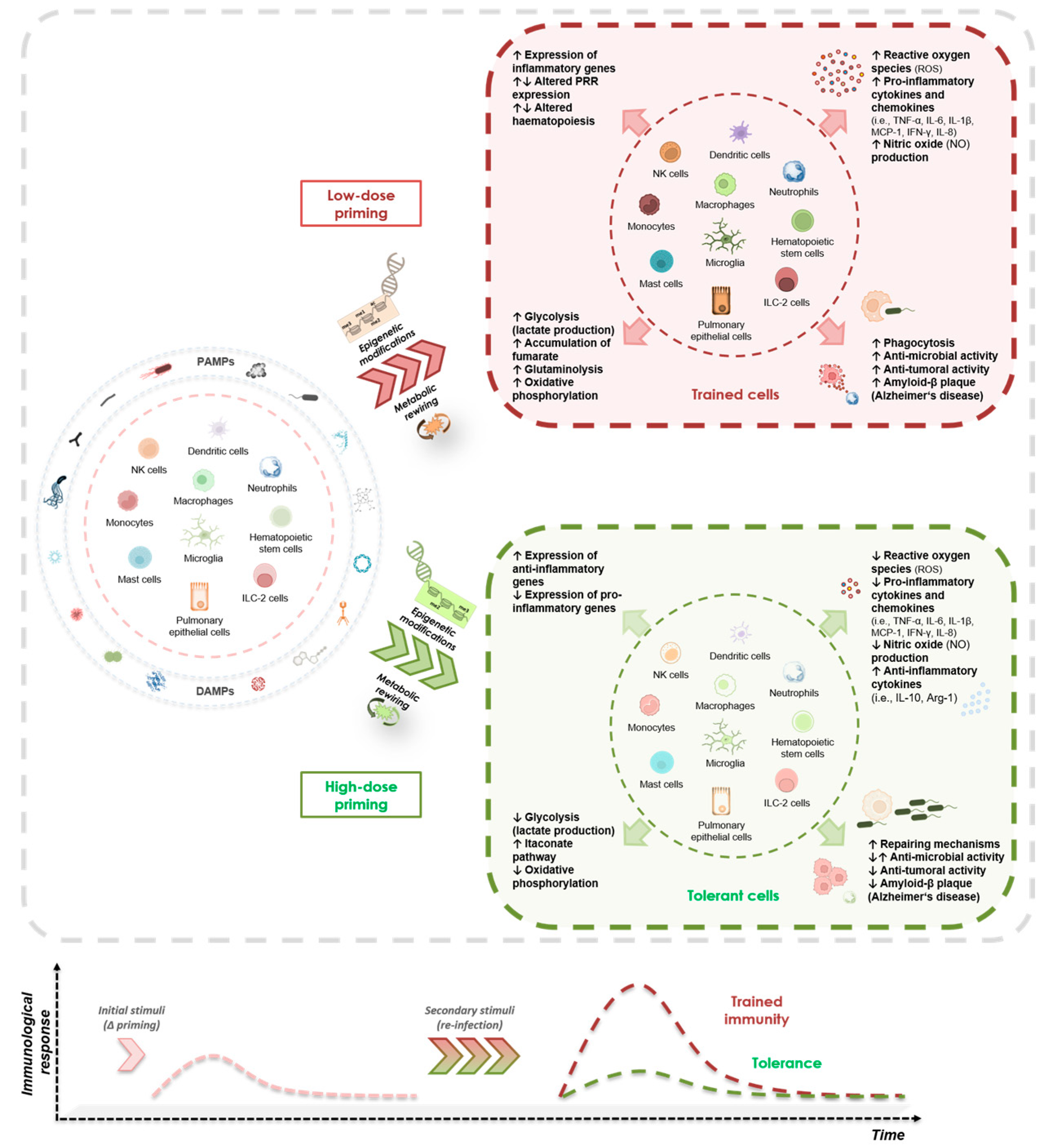

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajqi, T.; Köstlin-Gille, N.; Bauer, R.; Zarogiannis, S.G.; Lajqi, E.; Ajeti, V.; Dietz, S.; Kranig, S.A.; Rühle, J.; Demaj, A.; et al. Training vs. Tolerance: The Yin/Yang of the Innate Immune System. Biomedicines 2023, 11, 766. https://doi.org/10.3390/biomedicines11030766
Lajqi T, Köstlin-Gille N, Bauer R, Zarogiannis SG, Lajqi E, Ajeti V, Dietz S, Kranig SA, Rühle J, Demaj A, et al. Training vs. Tolerance: The Yin/Yang of the Innate Immune System. Biomedicines. 2023; 11(3):766. https://doi.org/10.3390/biomedicines11030766
Chicago/Turabian StyleLajqi, Trim, Natascha Köstlin-Gille, Reinhard Bauer, Sotirios G. Zarogiannis, Esra Lajqi, Valdrina Ajeti, Stefanie Dietz, Simon A. Kranig, Jessica Rühle, Ardian Demaj, and et al. 2023. "Training vs. Tolerance: The Yin/Yang of the Innate Immune System" Biomedicines 11, no. 3: 766. https://doi.org/10.3390/biomedicines11030766
APA StyleLajqi, T., Köstlin-Gille, N., Bauer, R., Zarogiannis, S. G., Lajqi, E., Ajeti, V., Dietz, S., Kranig, S. A., Rühle, J., Demaj, A., Hebel, J., Bartosova, M., Frommhold, D., Hudalla, H., & Gille, C. (2023). Training vs. Tolerance: The Yin/Yang of the Innate Immune System. Biomedicines, 11(3), 766. https://doi.org/10.3390/biomedicines11030766







