The Involvement of Endogenous Enkephalins in Glucose Homeostasis †
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet
2.3. The Role of Enkephalins in Body Weight and Food Intake
2.4. The Role of Enkephalins in Glucose Homeostasis
2.5. The Role of Enkephalins in Insulin Sensitivity
2.6. The Role of Enkephalin in Plasma Insulin Levels
2.7. The Role of Enkephalin in Plasma Leptin Levels
2.8. Statistical Analysis
3. Results
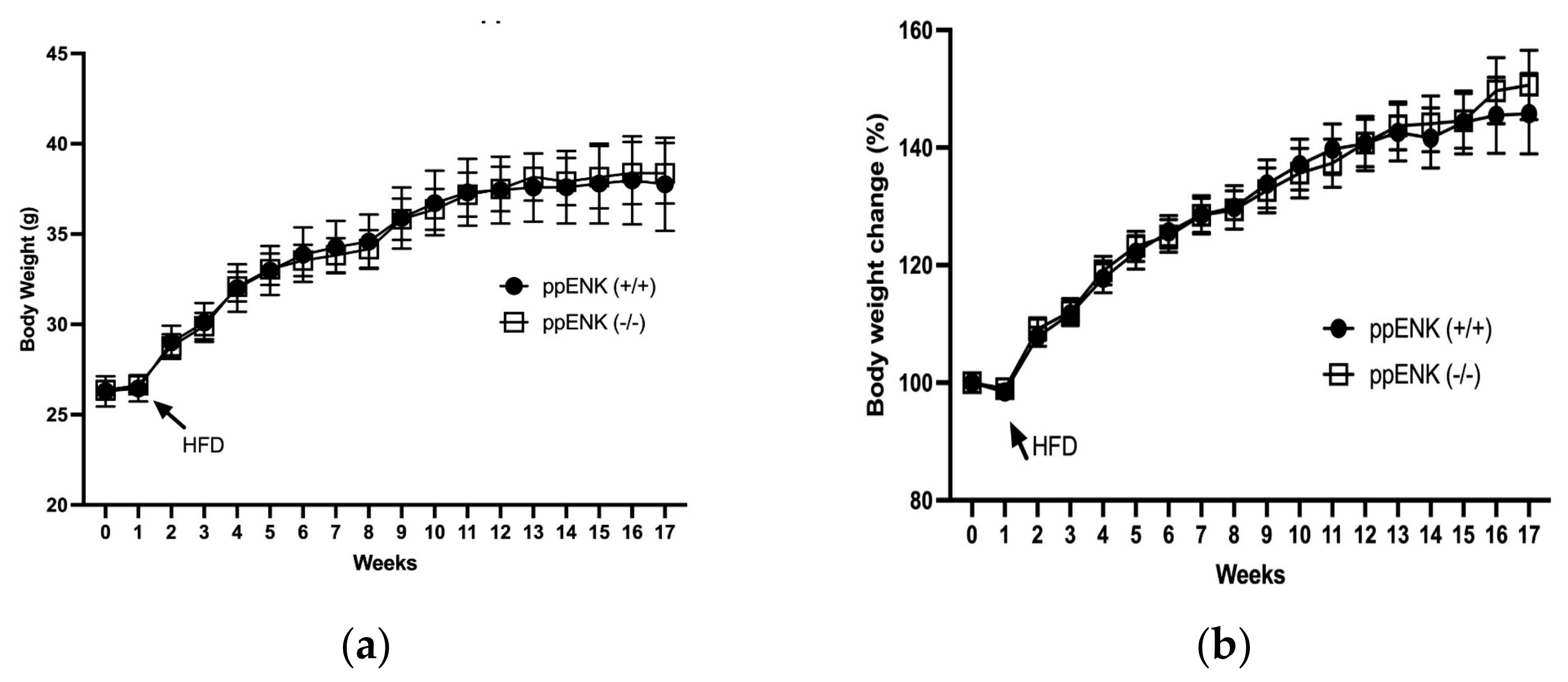
3.1. Body Wight Measurement in Wildtype and Knockout Mice
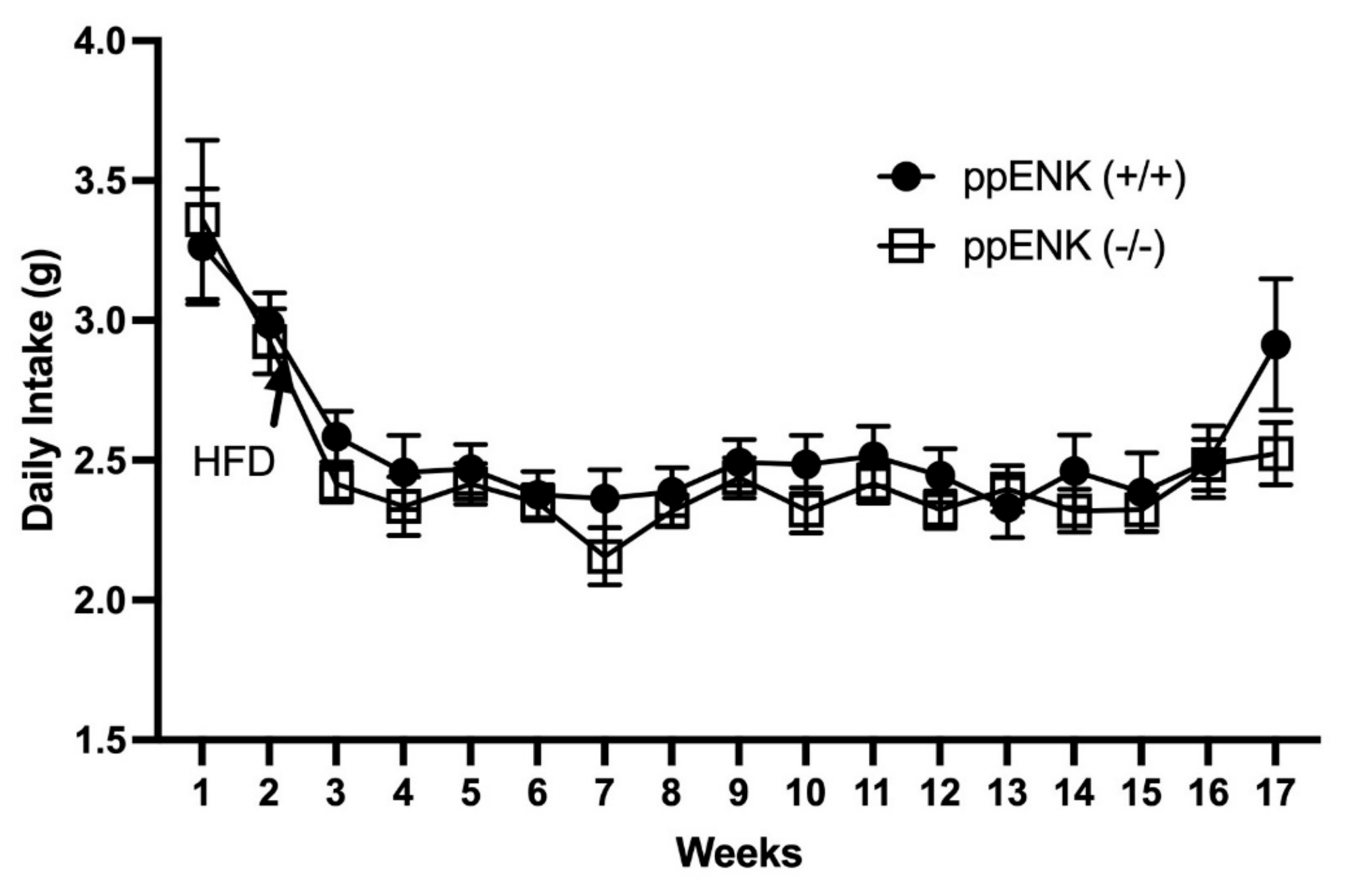
3.2. Food Intake Measurement in Wildtype and Knockout Mice
3.3. Oral Glucose Tolerance Test
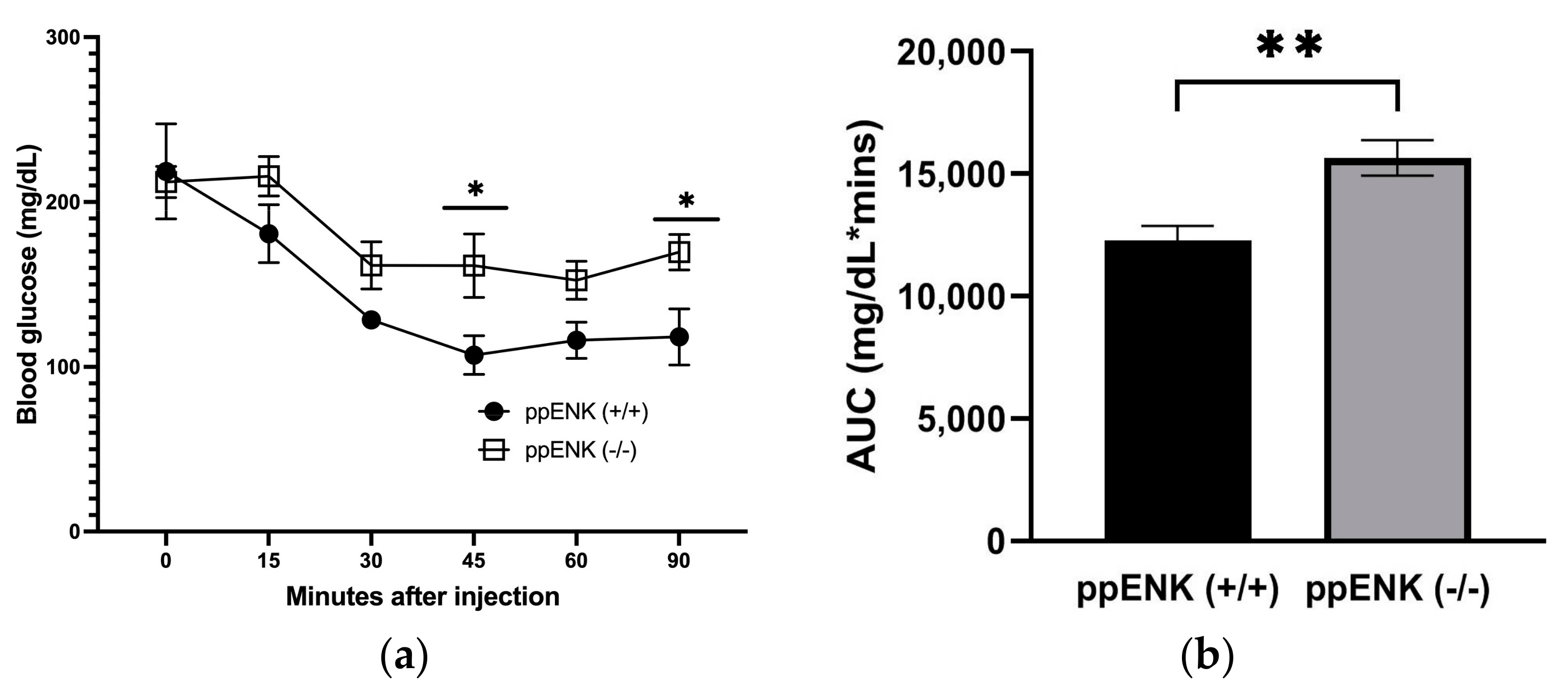
3.4. Insulin Tolerance Test
3.5. Insulin and Leptin Measurement in Wildtype and Knockout Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 June 2022).
- Volpi, F. World Obesity Day 2022—Accelerating Action to Stop Obesity. Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity#:~:text=WHO%20estimates%20that%20by%202025,predictable%20and%20preventable%20health%20crisis (accessed on 25 January 2023).
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. Natl. Health Stat. Rep. 2021, NHSR No. 158. [Google Scholar] [CrossRef]
- Bomberg, E.; Birch, L.; Endenburg, N.; German, A.J.; Neilson, J.; Seligman, H.; Takashima, G.; Day, M.J. The Financial Costs, Behaviour and Psychology of Obesity: A One Health Analysis. J. Comp. Pathol. 2017, 156, 310–325. [Google Scholar] [CrossRef]
- Bodnar, R.J. Endogenous opioid modulation of food intake and body weight: Implications for opioid influences upon motivation and addiction. Peptides 2019, 116, 42–62. [Google Scholar] [CrossRef]
- Pasternak, G.W.; Pan, Y.X. Mu opioids and their receptors: Evolution of a concept. Pharm. Rev. 2013, 65, 1257–1317. [Google Scholar] [CrossRef]
- Nogueiras, R.; Romero-Pico, A.; Vazquez, M.J.; Novelle, M.G.; Lopez, M.; Dieguez, C. The opioid system and food intake: Homeostatic and hedonic mechanisms. Obes. Facts 2012, 5, 196–207. [Google Scholar] [CrossRef]
- Giraudo, S.Q.; Billington, C.J.; Levine, A.S. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the central nucleus of the amygdala and in the paraventricular nucleus in the rat. Brain Res. 1998, 782, 18–23. [Google Scholar] [CrossRef]
- Spanagel, R.; Herz, A.; Shippenberg, T.S. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. USA 1992, 89, 2046–2050. [Google Scholar] [CrossRef]
- Levine, A.S.; Weldon, D.T.; Grace, M.; Cleary, J.P.; Billington, C.J. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am. J. Physiol. 1995, 268, R248–R252. [Google Scholar] [CrossRef]
- Kanarek, R.B.; Mathes, W.F.; Heisler, L.K.; Lima, R.P.; Monfared, L.S. Prior exposure to palatable solutions enhances the effects of naltrexone on food intake in rats. Pharm. Biochem. Behav. 1997, 57, 377–381. [Google Scholar] [CrossRef]
- Naleid, A.M.; Grace, M.K.; Chimukangara, M.; Billington, C.J.; Levine, A.S. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R99–R105. [Google Scholar] [CrossRef]
- Chang, G.Q.; Karatayev, O.; Barson, J.R.; Chang, S.Y.; Leibowitz, S.F. Increased enkephalin in brain of rats prone to overconsuming a fat-rich diet. Physiol. Behav. 2010, 101, 360–369. [Google Scholar] [CrossRef]
- Mendez, I.A.; Ostlund, S.B.; Maidment, N.T.; Murphy, N.P. Involvement of Endogenous Enkephalins and beta-Endorphin in Feeding and Diet-Induced Obesity. Neuropsychopharmacology 2015, 40, 2103–2112. [Google Scholar] [CrossRef]
- Denning, G.M.; Ackermann, L.W.; Barna, T.J.; Armstrong, J.G.; Stoll, L.L.; Weintraub, N.L.; Dickson, E.W. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides 2008, 29, 83–92. [Google Scholar] [CrossRef]
- Green, I.C.; Perrin, D.; Pedley, K.C.; Leslie, R.D.; Pyke, D.A. Effect of enkephalins and morphine on insulin secretion from isolated rat islets. Diabetologia 1980, 19, 158–161. [Google Scholar] [CrossRef]
- Schleicher, R.L. Beta-endorphin inhibits insulin secretion from isolated pancreatic islets. Endocrinology 1989, 124, 1254–1258. [Google Scholar] [CrossRef]
- Czyzyk, T.A.; Romero-Picó, A.; Pintar, J.; McKinzie, J.H.; Tschöp, M.H.; Statnick, M.A.; Nogueiras, R. Mice lacking δ-opioid receptors resist the development of diet-induced obesity. Faseb. J. 2012, 26, 3483–3492. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Kim, B.S.; Saenz, S.A.; Stine, R.R.; Monticelli, L.A.; Sonnenberg, G.F.; Thome, J.J.; Farber, D.L.; Lutfy, K.; Seale, P.; et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015, 519, 242–246. [Google Scholar] [CrossRef]
- Konig, M.; Zimmer, A.M.; Steiner, H.; Holmes, P.V.; Crawley, J.N.; Brownstein, M.J.; Zimmer, A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature 1996, 383, 535–538. [Google Scholar] [CrossRef]
- Marquez, P.; Baliram, R.; Gajawada, N.; Friedman, T.C.; Lutfy, K. Differential involvement of enkephalins in analgesic tolerance, locomotor sensitization, and conditioned place preference induced by morphine. Behav. Neurosci. 2006, 120, 10–15. [Google Scholar] [CrossRef]
- Tseng, A.; Nguyen, K.; Hamid, A.; Garg, M.; Marquez, P.; Lutfy, K. The role of endogenous beta-endorphin and enkephalins in ethanol reward. Neuropharmacology 2013, 73, 290–300. [Google Scholar] [CrossRef]
- Liu, Y.; Nakagawa, Y.; Wang, Y.; Liu, L.; Du, H.; Wang, W.; Ren, X.; Lutfy, K.; Friedman, T.C. Reduction of hepatic glucocorticoid receptor and hexose-6-phosphate dehydrogenase expression ameliorates diet-induced obesity and insulin resistance in mice. J. Mol. Endocrinol. 2008, 41, 53–64. [Google Scholar] [CrossRef]
- Murray, S.; Tulloch, A.; Gold, M.S.; Avena, N.M. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat. Rev. Endocrinol. 2014, 10, 540–552. [Google Scholar] [CrossRef]
- Richard, D. Cognitive and autonomic determinants of energy homeostasis in obesity. Nat. Rev. Endocrinol. 2015, 11, 489–501. [Google Scholar] [CrossRef]
- Czyzyk, T.A.; Nogueiras, R.; Lockwood, J.F.; McKinzie, J.H.; Coskun, T.; Pintar, J.E.; Hammond, C.; Tschöp, M.H.; Statnick, M.A. kappa-Opioid receptors control the metabolic response to a high-energy diet in mice. Faseb. J. 2010, 24, 1151–1159. [Google Scholar] [CrossRef]
- Tabarin, A.; Diz-Chaves, Y.; Carmona Mdel, C.; Catargi, B.; Zorrilla, E.P.; Roberts, A.J.; Coscina, D.V.; Rousset, S.; Redonnet, A.; Parker, G.C.; et al. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: Evidence for a “thrifty gene”. Diabetes 2005, 54, 3510–3516. [Google Scholar] [CrossRef]
- Wen, T.; Peng, B.; Pintar, J.E. The MOR-1 opioid receptor regulates glucose homeostasis by modulating insulin secretion. Mol. Endocrinol. 2009, 23, 671–678. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53 (Suppl. S3), S215–S219. [Google Scholar] [CrossRef]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escolero, V.; Tolentino, L.; Muhammad, A.B.; Hamid, A.; Lutfy, K. The Involvement of Endogenous Enkephalins in Glucose Homeostasis. Biomedicines 2023, 11, 671. https://doi.org/10.3390/biomedicines11030671
Escolero V, Tolentino L, Muhammad AB, Hamid A, Lutfy K. The Involvement of Endogenous Enkephalins in Glucose Homeostasis. Biomedicines. 2023; 11(3):671. https://doi.org/10.3390/biomedicines11030671
Chicago/Turabian StyleEscolero, Vanessa, Laica Tolentino, Abdul Bari Muhammad, Abdul Hamid, and Kabirullah Lutfy. 2023. "The Involvement of Endogenous Enkephalins in Glucose Homeostasis" Biomedicines 11, no. 3: 671. https://doi.org/10.3390/biomedicines11030671
APA StyleEscolero, V., Tolentino, L., Muhammad, A. B., Hamid, A., & Lutfy, K. (2023). The Involvement of Endogenous Enkephalins in Glucose Homeostasis. Biomedicines, 11(3), 671. https://doi.org/10.3390/biomedicines11030671







