Clinical and Pharmacotherapeutic Profile of Patients with Type 2 Diabetes Mellitus Admitted to a Hospital Emergency Department
Abstract
1. Introduction
2. Materials and Methods
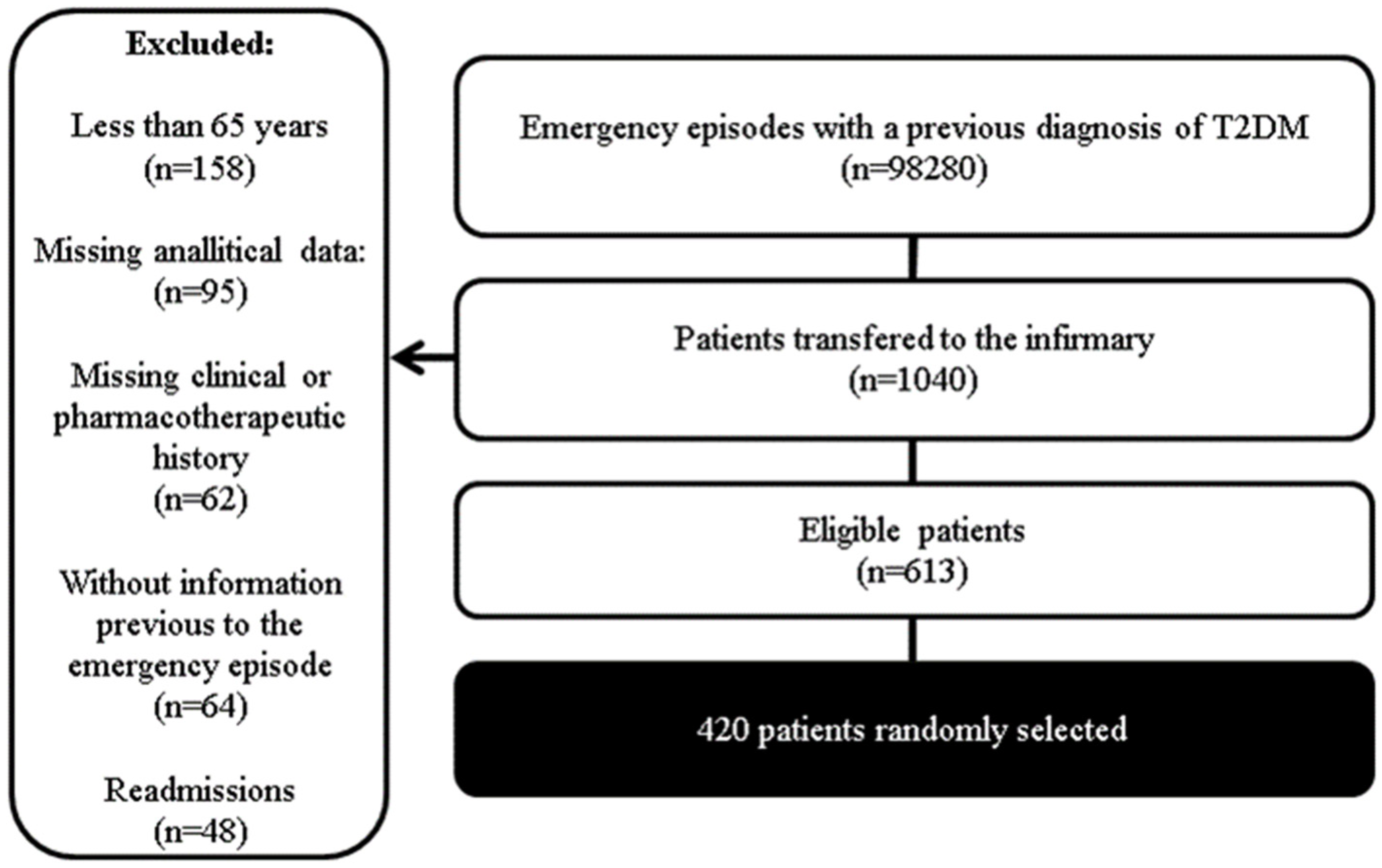
2.1. Study Design and Sample
- ✓
- Diagnosis of T2DM before admission to the emergency department;
- ✓
- At least 65 years of age;
- ✓
- At least one antidiabetic drug included in the chronic treatment plan before admission;
- ✓
- Complete and objective information described in the clinical diary regarding chronic medication, clinical history, and reason(s) for admission to the Emergency Department;
- ✓
- Complete information regarding the analytical parameters included in the study upon admission;
- ✓
- After applying these criteria, a sample of 613 patients was obtained, from which 420 were randomly selected.
2.2. Data Collection
- ✓
- Age, gender, and condition (autonomous, family support, or institutionalized);
- ✓
- Bioanalytical parameters at admission: blood glycemia; creatinine; hemoglobin; aspartate aminotransferase (AST), and alanine aminotransferase (ALT);
- ✓
- Personal pathological history: T2DM; high blood pressure (HBP); heart failure (HF); atrial fibrillation (AF); acute myocardial infarction (AMI); dyslipidemia; chronic kidney disease (CKD); hyperuricemia (HU); stroke, obesity; chronic liver disease (CLD); oncological disease (OD); chronic obstructive pulmonary disease (COPD); alcoholism; chronic anemia (CA);
- ✓
- Diagnosis that justifies admission: decompensated heart failure (DHF); acute chronic kidney disease (ACKD); acute kidney injury (AKI); urinary tract infection (UTI); pulmonary embolism (PE); stroke; AMI; respiratory tract infection (RTI); hydroelectrolytic disorders (HED); bleeding; gastroenteritis; acute chronic liver disease (ACLD); pancreatitis; hypoglycemia; respiratory failure; sepsis;
- ✓
- Antidiabetic drugs included in the therapeutic plan before admission: insulin; SGLT2i; DPP4i; GLP1 RA; metformin and sulfonylureas.
2.3. Statistical Analysis
3. Results
3.1. Sample Characterization
3.2. Relationship between Patient’s Condition before Admission, Age, and Gender with Glycemia Levels
3.3. Hyperuricemia, Hemoglobin, and AST/ALT Ratio
3.3.1. Hyperuricemia
3.3.2. Hemoglobin and T2DM
3.3.3. AST/ALT Ratio
3.4. Obesity, Dyslipidemia, and Acute Cardiovascular Disorders
3.5. Antidiabetic Therapy and Heart Failure
3.6. Antidiabetic Therapy and Kidney Function
3.7. Antidiabetic Therapy and Glycemia Levels
4. Discussion
4.1. Glycemia Levels and Patients’ Characteristics
4.2. Impact of Hyperuricemia on Kidney Function and Cardiovascular System
4.3. Relationship between Hemoglobin Levels, Gender, Age, and Kidney Function
4.4. Liver Function and Serum Glycemia Levels
4.5. Patients’ Pharmacotherapeutic Profile and Acute Cardiovascular Disease
4.6. Patients’ Pharmacotherapeutic Profile and Kidney Disease
4.7. Relationship between Patients’ Profile and Anti-Diabetic Drugs Used
4.8. Relationship between Glycemic Levels at Admission and Antidiabetic Drugs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Complications of Diabetes. Available online: https://www.mayoclinic.org/diseases-conditions/diabetes/symptoms-causes/syc-20371444 (accessed on 20 November 2022).
- Curtis, A.J.; Lee, W.A.A. Spatial patterns of diabetes related health problems for vulnerable populations in Los Angeles. Int. J. Health Geogr. 2010, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Agidew, E.; Wale, M.Z.; Kerebih, H.; Yirsaw, M.T.; Zewdie, T.H.; Girma, M.; Miskir, A. Adherence to diabetes self-care management and associated factors among people with diabetes in Gamo Gofa Zone public health hospitals. SAGE Open Med. 2021, 9, 20503121211053953. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.G.; Farfel, J.M.; Jaluul, O.; Queiroz, M.S.; Nery, M. Association between health literacy and glycemic control in elderly patients with type 2 diabetes and modifying effect of social support. Einstein 2020, 18, eAO5572. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Goryakin, Y.; Rocco, L.; Suhrcke, M. The contribution of urbanization to non-communicable diseases: Evidence from 173 countries from 1980 to 2008. Econ. Hum. Biol. 2017, 26, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef]
- Alqahtani, S.A.M.; Awan, Z.A.; Alasmary, M.Y.; Al Amoudi, S.M. Association between serum uric acid with diabetes and other biochemical markers. J. Family Med. Prim. Care 2022, 11, 1401–1409. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef]
- Maddatu, J.; Anderson-Baucum, E.; Evans-Molina, C. Smoking and the risk of type 2 diabetes. Transl. Res. 2017, 184, 101–107. [Google Scholar] [CrossRef]
- Darwish, L.; Beroncal, E.; Sison, M.V.; Swardfager, W. Depression in people with type 2 diabetes: Current perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 333–343. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, W.; Sa, R.; Liu, F. Prevalence and Risk Factors of Hypertension, Diabetes, and Dyslipidemia among Adults in Northwest China. Int. J. Hypertens. 2021, 2021, 5528007. [Google Scholar] [CrossRef]
- Cederberg, H.; Stančáková, A.; Kuusisto, J.; Laakso, M.; Smith, U. Family history of type 2 diabetes increases the risk of both obesity and its complications: Is type 2 diabetes a disease of inappropriate lipid storage? J. Intern. Med. 2015, 277, 540–551. [Google Scholar] [CrossRef]
- Nigussie, S.; Birhan, N.; Amare, F.; Mengistu, G.; Adem, F.; Abegaz, T.M. Rate of glycemic control and associated factors among type two diabetes mellitus patients in Ethiopia: A cross sectional study. PLoS ONE 2021, 16, e0251506. [Google Scholar] [CrossRef]
- Cao, H.; Liu, T.; Wang, L.; Ji, Q. Comparative efficacy of novel antidiabetic drugs on cardiovascular and renal outcomes in patients with diabetic kidney disease: A systematic review and network meta-analysis. Diabetes Obes. Metab. 2022, 24, 1448–1457. [Google Scholar] [CrossRef]
- Zanchi, A.; Lehmann, R.; Philippe, J. Antidiabetic drugs and kidney disease--recommendations of the Swiss Society for Endocrinology and Diabetology. Swiss Med. Wkly. 2012, 142, w13629. [Google Scholar] [CrossRef]
- Filippatos, T.; Tzavella, E.; Rizos, C.; Elisaf, M.; Liamis, G. Acid-base and electrolyte disorders associated with the use of antidiabetic drugs. Expert Opin. Drug. Saf. 2017, 16, 1121–1132. [Google Scholar] [CrossRef]
- Giugliano, D.; Longo, M.; Signoriello, S.; Maiorino, M.I.; Solerte, B.; Chiodini, P.; Esposito, K. The effect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: A network meta-analysis of 23 CVOTs. Cardiovasc. Diabetol. 2022, 21, 42. [Google Scholar] [CrossRef]
- Razavi, M.; Wei, Y.Y.; Rao, X.Q.; Zhong, J.X. DPP-4 inhibitors and GLP-1RAs: Cardiovascular safety and benefits. Military Med. Res. 2022, 9, 45. [Google Scholar] [CrossRef]
- Trevor, A.J.; Kruidering-Hall, M.; Katzung, B.G.; Tuan, R.L.; Vanderah, T.W. Katzung & Trevor’s Pharmacology: Examination & Board Review, 13th ed.; Mc Graw Hill: New York, NY, USA, 2021. [Google Scholar]
- Wells, B.; DiPiro, J.; Schwinghammer, T.; Dipiro, C. Pharmacotherapy Manual; McGraw Hill Eduaction: New York, NY, USA, 2016. [Google Scholar]
- Pape, E.; Nascimento, E.; Jordão, A. Fármacos na Diabetes; LIDEL: Lisboa, Portugal, 2019. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. 1), S83–S96. [Google Scholar] [CrossRef]
- Shahbaz, H.; Gupta, M. Creatinine Clearance; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK544228/ (accessed on 24 October 2022).
- Olagbemide, O.J.; Omosanya, O.E.; Ayodapo, A.O.; Agboola, S.M.; Adeagbo, A.O.; Olukokun, T.A. Family support and medication adherence among adult type 2 diabetes: Any meeting point? Ann. Afr. Med. 2021, 20, 282–287. [Google Scholar] [CrossRef] [PubMed]
- de Sousa-Munoz, R.L.; de Sá, A.D. Apoio social, funcionalidade familiar e controle glicêmico de pacientes diabéticos tipo 2. Revista De Medicina 2020, 99, 432–441. [Google Scholar] [CrossRef]
- Bennich, B.B.; Røder, M.E.; Overgaard, D.; Egerod, I.; Munch, L.; Knop, F.K.; Vilsbøll, T.; Konradsen, H. Supportive and non-supportive interactions in families with a type 2 diabetes patient: An integrative review. Diabetol. Metab. Syndr. 2017, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Pamungkas, R.A.; Chamroonsawasdi, K.; Vatanasomboon, P. A Systematic Review: Family Support Integrated with Diabetes Self-Management among Uncontrolled Type II Diabetes Mellitus Patients. Behav. Sci. 2017, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Zacarias, A.A.; Mavarez-Martinez, A.; Arias-Morales, C.E.; Stoicea, N.; Rogers, B. Impact of Demographic, Socioeconomic, and Psychological Factors on Glycemic Self-Management in Adults with Type 2 Diabetes Mellitus. Front. Public Health 2016, 4, 195. [Google Scholar] [CrossRef] [PubMed]
- Kushiyama, A.; Tanaka, K.; Hara, S.; Kawazu, S. Linking uric acid metabolism to diabetic complications. World J. Diabetes 2014, 5, 787–795. [Google Scholar] [CrossRef]
- Nishizawa, H.; Maeda, N.; Shimomura, I. Impact of hyperuricemia on chronic kidney disease and atherosclerotic cardiovascular disease. Hypertens. Res. 2022, 45, 635–640. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Singh, M.; Arya, V.; Garg, U.; Singh, V.P.; Jain, V. Prevalence of peripheral arterial disease in type 2 diabetes mellitus and its correlation with coronary artery disease and its risk factors. J. Assoc. Physicians. India 2012, 60, 28–32. [Google Scholar]
- Arersa, K.K.; Wondimnew, T.; Welde, M.; Husen, T.M. Prevalence and Determinants of Hyperuricemia in Type 2 Diabetes Mellitus Patients Attending Jimma Medical Center, Southwestern Ethiopia, 2019. Diabetes Metab. Syndr. Obes. 2020, 13, 2059–2067. [Google Scholar] [CrossRef]
- Li, G.X.; Jiao, X.H.; Cheng, X.B. Correlations between blood uric acid and the incidence and progression of type 2 diabetes nephropathy. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 506–511. [Google Scholar] [CrossRef]
- Ozsu, S.; Çoşar, A.M.; Aksoy, H.B.; Bülbül, Y.; Oztuna, F.; Karahan, S.C.; Ozlu, T. Prognostic Value of Uric Acid for Pulmonary Thromboembolism. Respir. Care 2017, 62, 1091–1096. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, M.; Lee, Y.H.; Lee, B.W.; Cha, B.S.; Kang, E.S. Uric Acid Variability as a Predictive Marker of Newly Developed Cardiovascular Events in Type 2 Diabetes. Front. Cardiovasc. Med. 2021, 8, 775753. [Google Scholar] [CrossRef]
- Gherghina, M.E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Doria, A.; Galecki, A.T.; Spino, C.; Pop-Busui, R.; Cherney, D.Z.; Lingvay, I.; Parsa, A.; Rossing, P.; Sigal, R.J.; Afkarian, M.; et al. Serum Urate Lowering with Allopurinol and Kidney Function in Type 1 Diabetes. N. Engl. J. Med. 2020, 382, 2493–2503. [Google Scholar] [CrossRef]
- Mauer, M.; Doria, A. Uric Acid and Diabetic Nephropathy Risk. Contrib. Nephrol. 2018, 192, 103–109. [Google Scholar] [CrossRef]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am. J. Kidney Dis. 2012, 60, 850–886. [Google Scholar] [CrossRef]
- La Grotta, R.; de Candia, P.; Olivieri, F.; Matacchione, G.; Giuliani, A.; Rippo, M.R.; Tagliabue, E.; Mancino, M.; Rispoli, F.; Ferroni, S.; et al. Anti-inflammatory effect of SGLT-2 inhibitors via uric acid and insulin. Cell Mol. Life Sci. 2022, 79, 273. [Google Scholar] [CrossRef]
- Taderegew, M.M.; Gebremariam, T.; Tareke, A.A.; Woldeamanuel, G.G. Anemia and Its Associated Factors Among Type 2 Diabetes Mellitus Patients Attending Debre Berhan Referral Hospital, North-East Ethiopia: A Cross-Sectional Study. J. Blood Med. 2020, 11, 47–58. [Google Scholar] [CrossRef]
- Solomon, D.; Bekele, K.; Atlaw, D.; Mamo, A.; Gezahegn, H.; Regasa, T.; Negash, G.; Nigussie, E.; Zenbaba, D.; Teferu, Z.; et al. Prevalence of anemia and associated factors among adult diabetic patients attending Bale zone hospitals, South-East Ethiopia. PLoS ONE 2022, 17, e0264007. [Google Scholar] [CrossRef]
- Fujita, Y.; Doi, Y.; Hamano, T.; Hatazaki, M.; Umayahara, Y.; Isaka, Y.; Tsubakihara, Y. Low erythropoietin levels predict faster renal function decline in diabetic patients with anemia: A prospective cohort study. Sci. Rep. 2019, 9, 14871. [Google Scholar] [CrossRef]
- AlDallal, S.M.; Jena, N. Prevalence of Anemia in Type 2 Diabetic Patients. J. Hematol. 2018, 7, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, S.H.; Park, K.S.; Kim, E.J.; Yeo, S.; Ha, I.H. Association between diabetes mellitus and anemia among Korean adults according to sex: A cross-sectional analysis of data from the Korea National Health and Nutrition Examination Survey (2010–2016). BMC Endocr. Disord. 2021, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, R.A.; Pais, S.; Reynolds, T.B.; Kaplowitz, N. Serum alanine aminotransferase in skeletal muscle diseases. Hepatology 2005, 41, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Malakouti, M.; Kataria, A.; Ali, S.K.; Schenker, S. Elevated Liver Enzymes in Asymptomatic Patients—What Should I Do? J. Clin. Transl. Hepatol. 2017, 5, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bhattarai, B.; Kafle, P.; Khalid, M.; Jonnadula, S.K.; Lamicchane, J.; Kanth, R.; Gayam, V. Elevated Liver Enzymes in Patients with Type 2 Diabetes Mellitus and Non-alcoholic Fatty Liver Disease. Cureus 2018, 10, e3626. [Google Scholar] [CrossRef]
- Muzurović, E.; van der Lely, A.J.; Gurnell, M. AST to ALT Ratio and Peripheral Arterial Disease in a Hypertensive Population—Is There a Link? Angiology 2021, 72, 905–907. [Google Scholar] [CrossRef]
- Niu, H.; Zhou, Y. Nonlinear Relationship Between AST-to-ALT Ratio and the Incidence of Type 2 Diabetes Mellitus: A Follow-Up Study. Int. J. Gen. Med. 2021, 14, 8373–8382. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Ma, C.X.; Ma, X.N.; Guan, C.H.; Li, Y.D.; Mauricio, D.; Fu, S.B. Cardiovascular disease in type 2 diabetes mellitus: Progress toward personalized management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Matheus, A.S.; Tannus, L.R.; Cobas, R.A.; Palma, C.C.; Negrato, C.A.; Gomes, M.B. Impact of diabetes on cardiovascular disease: An update. Int. J. Hypertens. 2013, 2013, 653789. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart. J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef]
- Erqou, S.; Lee, C.T.; Suffoletto, M.; Echouffo-Tcheugui, J.B.; de Boer, R.A.; van Melle, J.P.; Adler, A.I. Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: Systematic review and meta-analysis. Eur. J. Heart Fail. 2013, 15, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Solini, A.; Zoppini, G.; Orsi, E.; Fondelli, C.; Zerbini, G.; Morano, S.; Cavalot, F.; Lamacchia, O.; Trevisan, R.; et al. Hemoglobin A1c variability as an independent correlate of cardiovascular disease in patients with type 2 diabetes: A cross-sectional analysis of the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Cardiovasc. Diabetol. 2013, 12, 98. [Google Scholar] [CrossRef]
- Huri, H.; Ling, D.; Ahmad, W.A. Association between glycemic control and antidiabetic drugs in type 2 diabetes mellitus patients with cardiovascular complications. Drug Des. Devel. Ther. 2015, 9, 4735–4749. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Seferović, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 2099. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cardoso, J.; Andrade, A.; Brito, D.; Ferreira, J.; Fonseca, C.; Peres, M.; Franco, F.; Moura, B. SGLT-2 inhibitors: A step forward in the treatment of heart failure with reduced ejection fraction. Rev. Port. Cardiol. (Engl. Ed.) 2021, 40, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.L., Jr.; Hackstadt, A.J.; Hung, A.M.; Greevy, R.A.; Grijalva, C.G.; Griffin, M.R.; Elasy, T.A.; Roumie, C.L. Hospitalization for Heart Failure Among Patients With Diabetes Mellitus and Reduced Kidney Function Treated With Metformin Versus Sulfonylureas: A Retrospective Cohort Study. J. Am. Heart Assoc. 2021, 10, e019211. [Google Scholar] [CrossRef] [PubMed]
- Leiter, L.A. Latest Evidence on Sulfonylureas: What’s New? Diabetes Ther. 2020, 11 (Suppl. 1), 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wijnen, M.; Duschek, E.J.J.; Boom, H.; van Vliet, M. The effects of antidiabetic agents on heart failure. Neth. Heart J. 2022, 30, 65–75. [Google Scholar] [CrossRef]
- Gembillo, G.; Ingrasciotta, Y.; Crisafulli, S.; Luxi, N.; Siligato, R.; Santoro, D.; Trifirò, G. Kidney Disease in Diabetic Patients: From Pathophysiology to Pharmacological Aspects with a Focus on Therapeutic Inertia. Int. J. Mol. Sci. 2021, 22, 4824. [Google Scholar] [CrossRef]
- Xu, Y.; Surapaneni, A.; Alkas, J.; Evans, M.; Shin, J.I.; Selvin, E.; Chang, A.; Grams, M.E.; Carrero, J.J. Glycemic Control and the Risk of Acute Kidney Injury in Patients With Type 2 Diabetes and Chronic Kidney Disease: Parallel Population-Based Cohort Studies in U.S. and Swedish Routine Care. Diabetes Care 2020, 43, 2975–2982. [Google Scholar] [CrossRef]
- Vart, P.; Correa-Rotter, R.; Hou, F.F.; Jongs, N.; Chertow, G.M.; Langkilde, A.M.; McMurray, J.J.V.; Rossing p Sjöström, C.D.; Stefansson, B.V.; Toto, R.D.; et al. Efficacy and Safety of Dapagliflozin in Patients With CKD Across Major Geographic Regions. Kidney Int. Rep. 2022, 7, 699–707. [Google Scholar] [CrossRef]
- Song, A.; Zhang, C.; Meng, X. Mechanism and application of metformin in kidney diseases: An update. Biomed. Pharmacother. 2021, 138, 111454. [Google Scholar] [CrossRef]
- De Broe, M.E.; Kajbaf, F.; Lalau, J.D. Renoprotective Effects of Metformin. Nephron 2018, 138, 261–274. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Cianciolo, G.; De Pascalis, A.; Capelli, I.; Gasperoni, L.; Di Lullo, L.; Bellasi, A.; La Manna, G. Mineral and Electrolyte Disorders With SGLT2i Therapy. JBMR Plus 2019, 3, e10242. [Google Scholar] [CrossRef]
- Baker, C.; Retzik-Stahr, C.; Singh, V.; Plomondon, R.; Anderson, V.; Rasouli, N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018820980225. [Google Scholar] [CrossRef]
- Ober, S.K.; Watts, S.; Lawrence, R.H. Insulin use in elderly diabetic patients. Clin. Interv. Aging 2006, 1, 107–113. [Google Scholar] [CrossRef]
- Pedrosa, M.R.; Franco, D.R.; Gieremek, H.W.; Vidal, C.M.; Bronzeri, F.; de Cassia Rocha, A.; de Carvalho Cara, L.G.; Fogo, S.L.; Eliaschewitz, F.G. GLP-1 Agonist to Treat Obesity and Prevent Cardiovascular Disease: What Have We Achieved so Far? Curr. Atheroscler. Rep. 2022, 24, 867–884. [Google Scholar] [CrossRef]
- Dennis, J.M.; Henley, W.E.; Weedon, M.N.; Lonergan, M.; Rodgers, L.R.; Jones, A.G.; Hamilton, W.T.; Sattar, N.; Janmohamed, S.; Holman, R.R.; et al. Sex and BMI Alter the Benefits and Risks of Sulfonylureas and Thiazolidinediones in Type 2 Diabetes: A Framework for Evaluating Stratification Using Routine Clinical and Individual Trial Data. Diabetes Care 2018, 41, 1844–1853. [Google Scholar] [CrossRef]
- Custódio, J.S., Jr.; Roriz-Filho, J.; Cavalcanti, C.A.J.; Martins, A.; Salles, J.E.N. Use of SGLT2 Inhibitors in Older Adults: Scientific Evidence and Practical Aspects. Drugs Aging 2020, 37, 399–409. [Google Scholar] [CrossRef]
- Lee, W.C.; Balu, S.; Cobden, D.; Joshi, A.V.; Pashos, C.L. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: An analysis of third-party managed care claims data. Clin. Ther. 2006, 28, 1712–1725. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.F.; Purayidathil, F.W.; Bolge, S.C.; Williams, S.A. Patient-reported tolerability issues with oral antidiabetic agents: Associations with adherence; treatment satisfaction and health-related quality of life. Diabetes Res. Clin. Pract. 2010, 87, 204–210. [Google Scholar] [CrossRef]
- Jin, J.; Sklar, G.E.; Min Sen Oh, V.; Chuen Li, S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther. Clin. Risk Manag. 2008, 4, 269–286. [Google Scholar] [CrossRef]
- Wong, M.C.; Kong, A.P.; So, W.Y.; Jiang, J.Y.; Chan, J.C.; Griffiths, S.M. Adherence to oral hypoglycemic agents in 26,782 Chinese patients: A cohort study. J. Clin. Pharmacol. 2011, 51, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Akash, G.; Shwetha, B.; Basavaraj, C.K.; Chetan, A.C. Drug utilization pattern in type II diabetes mellitus patients attending non-communicable disease clinic in a tertiary care hospital. Int. J. Basic Clin. Pharmacol. 2019, 8, 539–544. [Google Scholar] [CrossRef]
- Orlando, V.; Guerriero, F.; Putignano, D.; Monetti, V.M.; Tari, D.U.; Farina, G.; Illario, M.; Iaccarino, G.; Menditto, E. Prescription Patterns of Antidiabetic Treatment in the Elderly. Results from Southern Italy. Curr. Diabetes Rev. 2015, 12, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, V.; Zhang, B.; Fleurence, R.L.; Krishnarajah, G.; Graham, J. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr. Med. Res. Opin. 2011, 27, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Bagonza, J.; Rutebemberwa, E.; Bazeyo, W. Adherence to anti diabetic medication among patients with diabetes in eastern Uganda; a cross sectional study. BMC Health Serv. Res. 2015, 15, 168. [Google Scholar] [CrossRef]
- Lunati, M.E.; Cimino, V.; Gandolfi, A.; Trevisan, M.; Montefusco, L.; Pastore, I.; Pace, C.; Betella, N.; Favacchio, G.; Bulgheroni, M.; et al. SGLT2-inhibitors are effective and safe in the elderly: The SOLD study. Pharmacol. Res. 2022, 183, 106396. [Google Scholar] [CrossRef]
- Fang, H.S.A.; Gao, Q.; Tan, W.Y.; Lee, M.L.; Hsu, W.; Tan, N.C. The effect of oral diabetes medications on glycated haemoglobin (HbA1c) in Asians in primary care: A retrospective cohort real-world data study. BMC Med. 2022, 20, 22. [Google Scholar] [CrossRef]
- Palanisamy, S.; Yien, E.L.H.; Shi, L.W.; Si, L.Y.; Qi, S.H.; Ling, L.S.C.; Lun, T.W.; Chen, Y.N. Systematic Review of Efficacy and Safety of Newer Antidiabetic Drugs Approved from 2013 to 2017 in Controlling HbA1c in Diabetes Patients. Pharmacy 2018, 6, 57. [Google Scholar] [CrossRef]
- Mearns, E.S.; Sobieraj, D.M.; White, C.M.; Saulsberry, W.J.; Kohn, C.G.; Doleh, Y.; Zaccaro, E.; Coleman, C.I. Comparative efficacy and safety of antidiabetic drug regimens added to metformin monotherapy in patients with type 2 diabetes: A network meta-analysis. PLoS ONE 2015, 10, e0125879. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, S.H.; Liu, X.N.; Sun, Q.Y. Efficacy of different antidiabetic drugs based on metformin in the treatment of type 2 diabetes mellitus: A network meta-analysis involving eight eligible randomized-controlled trials. J. Cell Physiol. 2019, 234, 2795–2806. [Google Scholar] [CrossRef]
- Chamberlain, J.J.; Rhinehart, A.S.; Shaefer, C.F., Jr.; Neuman, A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann. Intern. Med. 2016, 164, 542–552. [Google Scholar] [CrossRef]
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in Antidiabetic Drug Discovery: FDA Approved Drugs, New Drugs in Clinical Trials and Global Sales. Front. Pharmacol. 2022, 12, 807548. [Google Scholar] [CrossRef]
- van den Boom, L.; Kaiser, M.; Kostev, K. Prevalence of insulin as a first-line therapy and associated factors in people with type 2 diabetes in German primary care practices. Diabetes Med. 2020, 37, 1333–1339. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Insulin: Potential negative consequences of early routine use in patients with type 2 diabetes. Diabetes Care 2011, 34 (Suppl. 2), S225–S230. [Google Scholar] [CrossRef]
- Piera-Mardemootoo, C.; Lambert, P.; Faillie, J.L. Efficacy of metformin on glycemic control and weight in drug-naive type 2 diabetes mellitus patients: A systematic review and meta-analysis of placebo-controlled randomized trials. Therapie 2021, 76, 647–656. [Google Scholar] [CrossRef]
- Hostalek, U.; Gwilt, M.; Hildemann, S. Therapeutic Use of Metformin in Prediabetes and Diabetes Prevention. Drugs 2015, 75, 1071–1094. [Google Scholar] [CrossRef]
- Sola, D.; Rossi, L.; Schianca, G.P.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef]
- Rao, A.D.; Kuhadiya, N.; Reynolds, K.; Fonseca, V.A. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality?: A meta-analysis of observational studies. Diabetes Care 2008, 31, 1672–1678. [Google Scholar] [CrossRef]
- Schramm, T.K.; Gislason, G.H.; Vaag, A.; Rasmussen, J.N.; Folke, F.; Hansen, M.L.; Fosbøl, E.L.; Køber, L.; Norgaard, M.L.; Madsen, M.; et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: A nationwide study. Eur. Heart J. 2011, 32, 1900–1908. [Google Scholar] [CrossRef]
- Pop, L.M.; Lingvay, I. The Infamous, Famous Sulfonylureas and Cardiovascular Safety: Much Ado About Nothing? Curr. Diab. Rep. 2017, 17, 124. [Google Scholar] [CrossRef]
- Chai, S.; Zhang, R.; Zhang, Y.; Carr, R.D.; Zheng, Y.; Rajpathak, S.; Yu, M. Influence of dipeptidyl peptidase-4 inhibitors on glycemic variability in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Front. Endocrinol. 2022, 13, 935039. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Park, J.H. Pleiotropic Benefits of DPP-4 Inhibitors Beyond Glycemic Control. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 11795514211051698. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R. Cardiovascular Safety and Benefits of Semaglutide in Patients With Type 2 Diabetes: Findings From SUSTAIN 6 and PIONEER 6. Front. Endocrinol. 2021, 12, 645566. [Google Scholar] [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Pirklbauer, M.; Schupart, R.; Fuchs, L.; Staudinger, P.; Corazza, U.; Sallaberger, S.; Leierer, J.; Mayer, G.; Schramek, H. Unraveling reno-protective effects of SGLT2 inhibition in human proximal tubular cells. Am. J. Physiol. Renal. Physiol. 2019, 316, F449–F462. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yuan, C.; Chen, G.; Zhang, C.; Wu, X. SGLT2i: Beyond the glucose-lowering effect. Cardiovasc. Diabetol. 2020, 19, 98. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Anjana, R.M.; Deepa, M.; Pradeepa, R.; Joshi, S.R.; Bhansali, A.; Dhandania, V.K.; Joshi, P.P.; Madhu, S.V.; Rao, P.V.; et al. Glycemic control among individuals with self-reported diabetes in India--the ICMR-INDIAB Study. Diabetes Technol. Ther. 2014, 16, 596–603. [Google Scholar] [CrossRef]
- Gebrie, D.; Manyazewal, T.; AEjigu, D.; Makonnen, E. Metformin-Insulin versus Metformin-Sulfonylurea Combination Therapies in Type 2 Diabetes: A Comparative Study of Glycemic Control and Risk of Cardiovascular Diseases in Addis Ababa, Ethiopia. Diabetes Metab. Syndr. Obes. 2021, 14, 3345–3359. [Google Scholar] [CrossRef]
- Ling, S.; Zaccardi, F.; Lawson, C.; Seidu, S.I.; Davies, M.J.; Khunti, K. Glucose Control, Sulfonylureas, and Insulin Treatment in Elderly People With Type 2 Diabetes and Risk of Severe Hypoglycemia and Death: An Observational Study. Diabetes Care 2021, 44, 915–924. [Google Scholar] [CrossRef]
- Vedantam, D.; Poman, D.S.; Motwani, L.; Asif, N.; Patel, A.; Anne, K.K. Stress-Induced Hyperglycemia: Consequences and Management. Cureus 2022, 14, e26714. [Google Scholar] [CrossRef]
- Xiu, F.; Stanojcic, M.; Diao, L.; Jeschke, M.G. Stress Hyperglycemia, Insulin Treatment, and Innate Immune Cells. Int. J. Endocrinol. 2014, 2014, 486403. [Google Scholar] [CrossRef]
| Age | |||||
|---|---|---|---|---|---|
| Mean | 80.59 | Standard Deviation | 7.92 | Min-Max | 65–99 |
| Gender | |||||
| Male (n = 204) | Female (n = 216) | ||||
| Autonomous | 94 (22.4%) | 61 (14.5%) | |||
| Family support | 49 (11.7%) | 42 (10.0%) | |||
| Institutionalized | 61 (14.5%) | 113 (26.9%) | |||
| Pathological history previous to admission | |||||
| Type 2 Diabetes Mellitus | 204 (48.6%) | 216 (51.4%) | |||
| High blood pressure | 173 (41.2%) | 181 (43.1%) | |||
| Heart failure | 77 (18.3%) | 98 (23.3%) | |||
| Atrial fibrillation | 38 (9.0%) | 45 (10.7%) | |||
| Acute myocardial infarction | 14 (3.3%) | 17 (4.0%) | |||
| Dyslipidemia | 82 (19.5%) | 98 (23.3%) | |||
| Chronic kidney disease | 65 (15.5%) | 91 (21.7%) | |||
| Hyperuricemia | 30 (7.1%) | 42 (10.0%) | |||
| Stroke | 35 (8.3%) | 39 (9.3%) | |||
| Obesity | 27 (6.4%) | 44 (10.5%) | |||
| Chronic liver disease | 17 (4.0%) | 6 (1.4%) | |||
| Oncological disease | 25 (6.0%) | 11 (2.6%) | |||
| Chronic obstructive pulmonary disease | 19 (4.5%) | 17 (4.0%) | |||
| Diagnosis at admission | |||||
| Decompensated heart failure | 43 (10.2%) | 66 (15.7%) | |||
| Acute chronic kidney disease | 46 (11.0%) | 67 (16.0%) | |||
| Acute kidney injury | 66 (15.7%) | 51 (12.1%) | |||
| Urinary tract infection | 14 (3.3%) | 28 (6.7%) | |||
| Pulmonary embolism | 12 (2.9%) | 9 (2.1%) | |||
| Stroke | 18 (4.3%) | 19 (4.5%) | |||
| Acute myocardial infarction | 9 (2.1%) | 8 (1.9%) | |||
| Respiratory tract infection | 56 (12.9%) | 65 (15.5%) | |||
| Hydroelectrolytic disorders | 94 (22.4%) | 92 (21.9%) | |||
| Bleeding | 3 (0.7%) | 0 (0.0%) | |||
| Gastroenteritis | 4 (1.0%) | 2 (0.5%) | |||
| Acute chronic liver disease | 12 (2.9%) | 5 (1.2%) | |||
| Pancreatitis | 3 (0.7%) | 3 (0.7%) | |||
| Hypoglycemia | 2 (0.5%) | 2 (0.5%) | |||
| Respiratory failure | 59 (14.0%) | 74 (17.6%) | |||
| Sepsis | 5 (1.2%) | 11 (2.6%) | |||
| Antidiabetic drugs included in the therapeutic plan before admission | |||||
| Insulin | 100 (23.8%) | 127 (30.2%) | |||
| Sodium-glucose cotransporter inhibitors | 54 (12.9%) | 54 (12.9%) | |||
| Dipeptidyl peptidase inhibitors | 111 (26.4%) | 107 (25.5%) | |||
| Glucagon-like peptide-1 receptor agonists | 10 (2.4%) | 17 (4.0%) | |||
| Metformin | 107 (25.5%) | 96 (22.9%) | |||
| Sulfonylureas | 36 (8.6%) | 22 (5.2%) | |||
| Laboratory parameters at admission | |||||
| Glycemia | Blood creatinine | ||||
| <180 mg/dL | ≥180 mg/dL | <1.2 mg/dL | ≥1.2 mg/dL | ||
| 183 (43.6%) | 237 (56.4%) | 170 (40.5%) | 250 (59.5%) | ||
| Condition | |||||
|---|---|---|---|---|---|
| Glycemia | Autonomous | Family Support | Institutionalized | Total | p-Value |
| <180 mg/dL | 58 (13.8%) | 51 (12.1%) | 74 (7.6%) | 183 (43.6%) | 0.016 * |
| ≥180 mg/dL | 97 (23.1%) | 40 (9.5%) | 100 (23.8%) | 237 (56.4%) | |
| Total | 155 (36.9%) | 91 (21.7%) | 174 (41.4%) | 420 (100%) | |
| Previously Diagnosed Heart Failure | Decompensated Heart Failure at Admission | ||||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| 175 (41.7%) | 245 (58.3%) | 109 (26.0%) | 311 (74.0%) | ||
| Antidiabetic Drugs | Decompensated HF (No/Yes) | p-Value | OR | Confidence Interval 95% | |
| Insulin | 155 (49.8%) | 72 (66.1%) | p = 0.003 * | 1.959 | 1.243–3.085 |
| GLP1 RA | 15 (4.8%) | 12 (11.0%) | p = 0.023 * | 2.441 | 1.105–5.395 |
| Sulfonylureas | 51 (16.4%) | 7 (6.4%) | p = 0.009 * | 0.350 | 0.154–0.796 |
| Insulin + GLP1 RA | 9 (2.9%) | 10 (9.2%) | p = 0.007 * | 3.389 | 1.339–8.579 |
| Metformin + DPP4i | 100 (32.2%) | 23 (21.1%) | p = 0.029 * | 0.564 | 0.336–0.947 |
| Metformin + Sulfonylurea | 38 (12.2%) | 3 (2.8%) | p = 0.003 ** | 0.203 | 0.061–0.673 |
| Antidiabetic Drugs | AKI or ACKD (n = 230) | p-Value | OR | Confidence Interval 95% |
|---|---|---|---|---|
| Metformin | 99 (43.0%) | 0.017 * | 0.625 | 0.424–0.920 |
| Metformin + DPP4i | 56 (24.3%) | 0.014 * | 0.521 | 0.387–0.902 |
| Hydroelectrolytic disorders | ||||
| SGLT2i | 64 (34.4%) | 0.0003 * | 2.265 | 1.450–3.539 |
| GLP1 RA | 6 (3.2%) | 0.017 * | 0.338 | 0.134–0.856 |
| Insulin + DPP4i | 38 (20.4%) | 0.034 * | 0.614 | 0.390–0.967 |
| Insulin + Sulfonylurea | 8 (4.3%) | 0.026 ** | 5.213 | 1.094–24.853 |
| Metformin + SGLT2i | 34 (18.3%) | 0.026 * | 1.870 | 1.071–3.264 |
| DPP4i + SGLT2i | 29 (15.6%) | 0.007 * | 2.358 | 1.252–4.440 |
| DPP4i + GLP1 RA | 0 (0.0%) | 0.003 ** | 0.957 | 0.932–0.984 |
| Antidiabetic Drugs | Glycemia ≥ 180 mg/dL (No/Yes) | p-Value | OR | Confidence Interval 95% | |
|---|---|---|---|---|---|
| Sulfonylureas | 16 (8.7%) | 42 (17.7%) | p = 0.008 * | 2.248 | 1.219–4.145 |
| Insulin + Metformin | 20 (10.9%) | 43 (18.1%) | p = 0.040 * | 1.806 | 1.022–3.194 |
| Insulin + Sulfonylurea | 1 (0.5%) | 9 (3.8%) | p = 0.048 ** | 7.184 | 0.902–57.227 |
| DPP4i + Sulfonylurea | 11 (6.0%) | 29 (12.2%) | p = 0.031 * | 4.644 | 1.058–4.492 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.C.; Lourenço, O.; Roque, F.; Morgado, M. Clinical and Pharmacotherapeutic Profile of Patients with Type 2 Diabetes Mellitus Admitted to a Hospital Emergency Department. Biomedicines 2023, 11, 256. https://doi.org/10.3390/biomedicines11020256
Lopes AC, Lourenço O, Roque F, Morgado M. Clinical and Pharmacotherapeutic Profile of Patients with Type 2 Diabetes Mellitus Admitted to a Hospital Emergency Department. Biomedicines. 2023; 11(2):256. https://doi.org/10.3390/biomedicines11020256
Chicago/Turabian StyleLopes, António Cabral, Olga Lourenço, Fátima Roque, and Manuel Morgado. 2023. "Clinical and Pharmacotherapeutic Profile of Patients with Type 2 Diabetes Mellitus Admitted to a Hospital Emergency Department" Biomedicines 11, no. 2: 256. https://doi.org/10.3390/biomedicines11020256
APA StyleLopes, A. C., Lourenço, O., Roque, F., & Morgado, M. (2023). Clinical and Pharmacotherapeutic Profile of Patients with Type 2 Diabetes Mellitus Admitted to a Hospital Emergency Department. Biomedicines, 11(2), 256. https://doi.org/10.3390/biomedicines11020256







