Targeted Therapy Development in Acute Myeloid Leukemia
Abstract
1. Introduction
2. Standard Treatment of AML and Resistance Mechanisms
3. History of Small Molecule Development in Acute Myeloid Leukemia
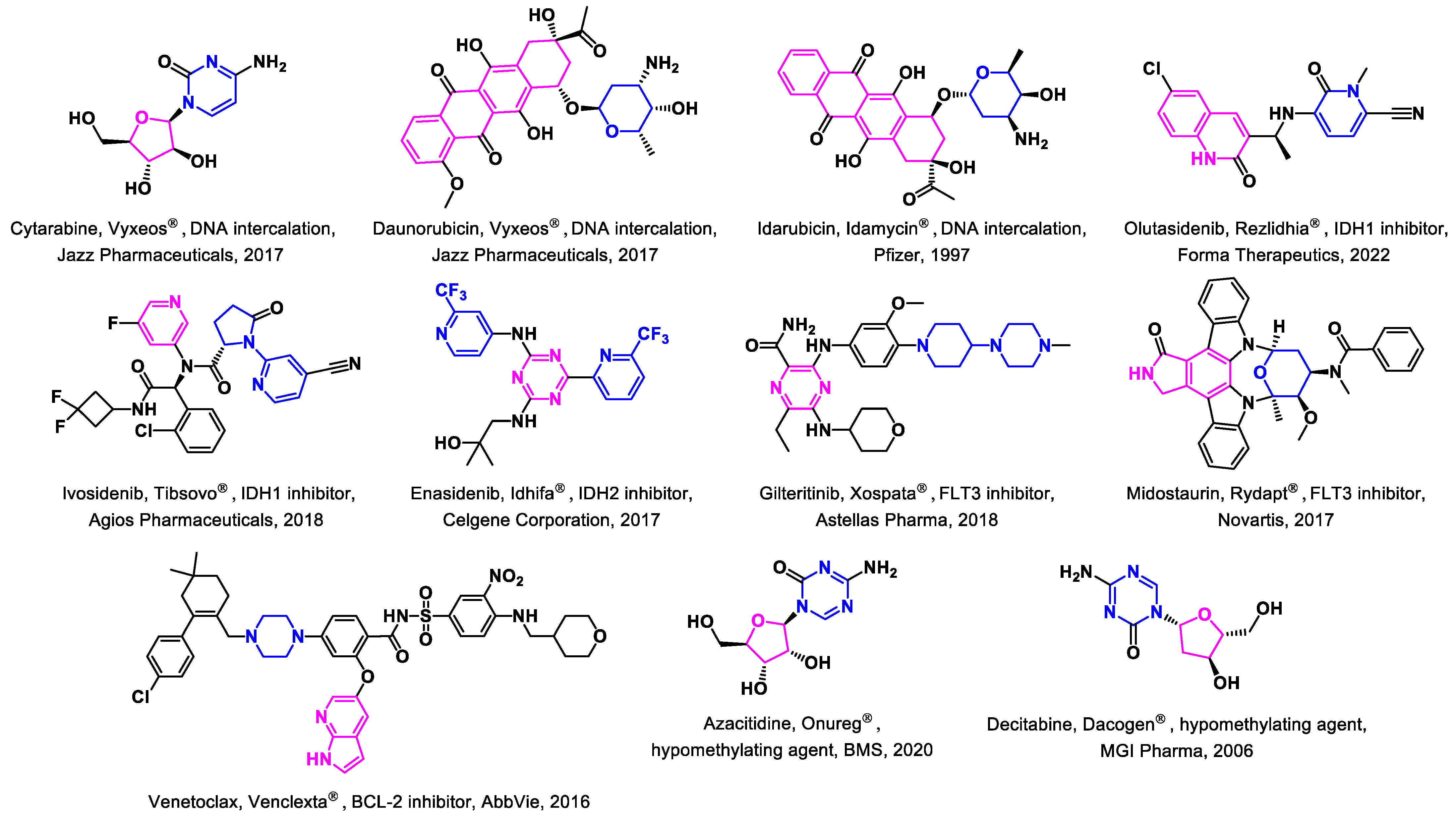
3.1. Approved Drugs
3.1.1. IDH Inhibitors
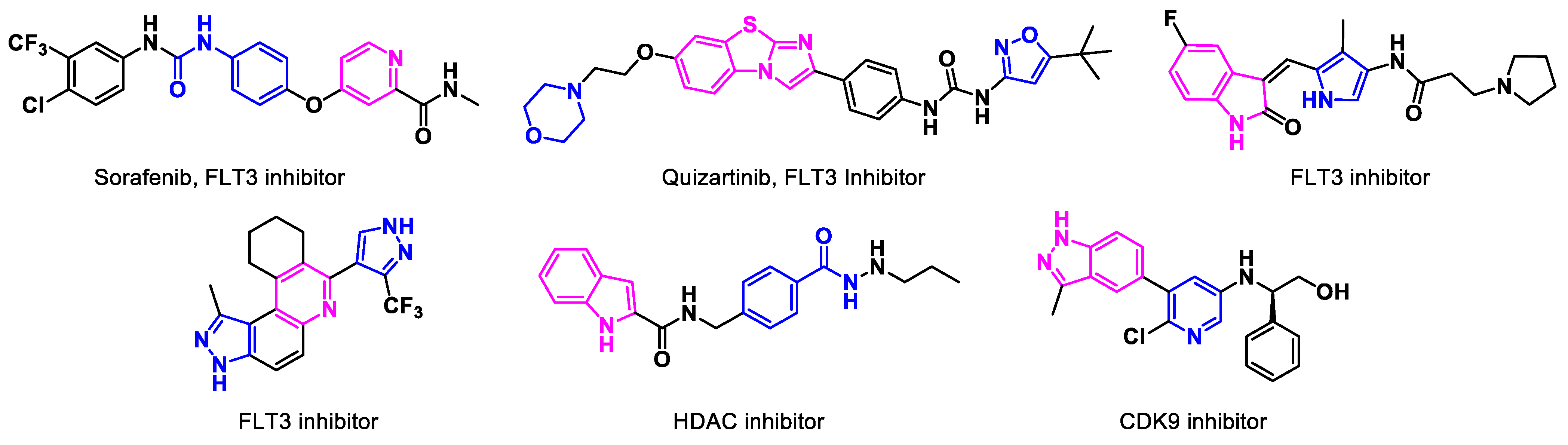
3.1.2. FLT3 Inhibitors
3.1.3. BCL2-Inhibitors
3.1.4. Hypomethylating Agents
3.2. Non-Approved Drugs
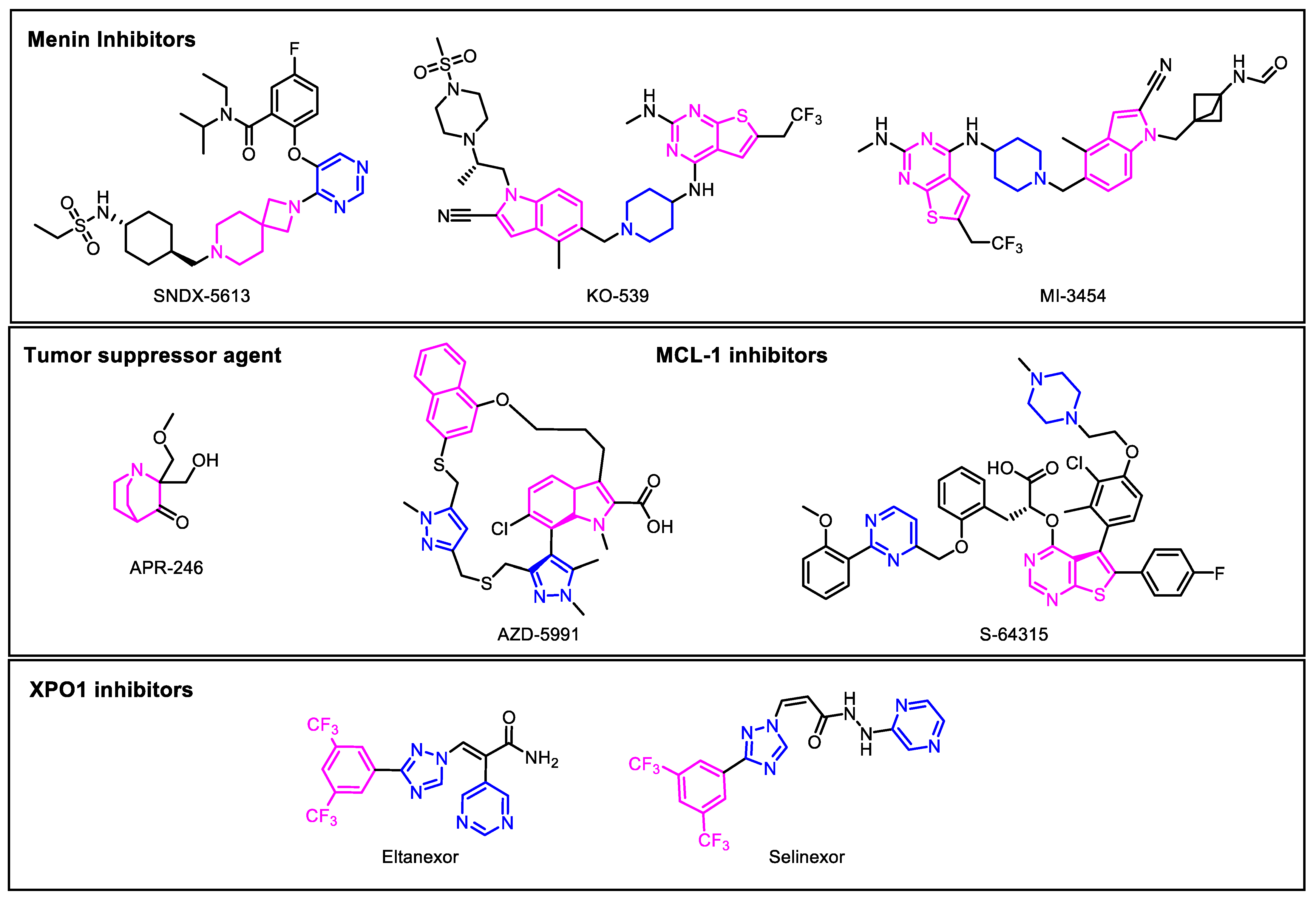
4. Novel Targeted Therapies in Development
4.1. Menin Inhibitors
4.2. Tumor Suppressor Targets
4.3. Apoptotic Inhibitors
MCL-1 Inhibitors
4.4. XPO1 Inhibitors
4.5. Immune Checkpoint Inhibitors
4.6. Combinatorial Therapies in Development
5. Translational Perspective
Selective vs. Non-Selective Drugs
6. Conclusions
7. Future Research Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schönherz, A.A.; Bødker, J.S.; Schmitz, A.; Brøndum, R.F.; Jakobsen, L.H.; Roug, A.S.; Severinsen, M.T.; El-Galaly, T.C.; Jensen, P.; Johnsen, H.E.; et al. Normal myeloid progenitor cell subset-associated gene signatures for acute myeloid leukaemia subtyping with prognostic impact. PLoS ONE 2020, 15, e0229593. [Google Scholar] [CrossRef]
- Ediriwickrema, A.; Gentles, A.J.; Majeti, R. Single cell genomics in AML: Extending the frontiers of AML research. Blood 2022, 141, 345–355. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, P.; Humphreys, M.; Hull, D.; McMullin, M.F. Transformation of cytogenetically normal chronic myelomonocytic leukaemia to an acute myeloid leukaemia and the emergence of a novel +13, +15 double trisomy resulting in an adverse outcome. Ulster Med. J. 2007, 76, 131–135. [Google Scholar] [PubMed]
- Lazarus, H.M.; Vogler, W.R.; Burns, C.P.; Winton, E.F. High-dose cytosine arabinoside and daunorubicin as primary therapy in elderly patients with acute myelogenous leukemia. A phase I-II study of the Southeastern Cancer Study Group. Cancer 1989, 63, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.R.; Wintrobe, M.M.; Cartwright, G.E. The acute leukemias. Analysis of 322 cases and review of the literature. Med. Baltim. 1962, 41, 163–225. [Google Scholar] [CrossRef]
- Thomas, D.; Majeti, R. Biology and relevance of human acute myeloid leukemia stem cells. Blood 2017, 129, 1577–1585. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Surveillance Research Program, National Cancer Institute. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 27 January 2023).
- Hirsch, P.; Tang, R.; Abermil, N.; Flandrin, P.; Moatti, H.; Favale, F.; Suner, L.; Lorre, F.; Marzac, C.; Fava, F.; et al. Precision and prognostic value of clone-specific minimal residual disease in acute myeloid leukemia. Haematologica 2017, 102, 1227–1237. [Google Scholar] [CrossRef]
- Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; Baty, J.D.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef]
- Kellaway, S.; Chin, P.S.; Barneh, F.; Bonifer, C.; Heidenreich, O. t(8;21) Acute Myeloid Leukemia as a Paradigm for the Understanding of Leukemogenesis at the Level of Gene Regulation and Chromatin Programming. Cells 2020, 9, 2681. [Google Scholar] [CrossRef]
- Hanekamp, D.; Cloos, J.; Schuurhuis, G.J. Leukemic stem cells: Identification and clinical application. Int. J. Hematol. 2017, 105, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Kadia, T.M.; DiNardo, C.D.; Welch, M.A.; Ravandi, F. Acute myeloid leukemia: Treatment and research outlook for 2021 and the MD Anderson approach. Cancer 2021, 127, 1186–1207. [Google Scholar] [CrossRef] [PubMed]
- Papuc, S.M.; Erbescu, A.; Cisleanu, D.; Ozunu, D.; Enache, C.; Dumitru, I.; Lupoaia Andrus, E.; Gaman, M.; Popov, V.M.; Dobre, M.; et al. Delineation of Molecular Lesions in Acute Myeloid Leukemia Patients at Diagnosis: Integrated Next Generation Sequencing and Cytogenomic Studies. Genes 2021, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Venugopal, S.; Ravandi, F. FLT3 mutated acute myeloid leukemia: 2021 treatment algorithm. Blood Cancer J. 2021, 11, 104. [Google Scholar] [CrossRef]
- Stasik, S.; Eckardt, J.N.; Kramer, M.; Röllig, C.; Krämer, A.; Scholl, S.; Hochhaus, A.; Crysandt, M.; Brümmendorf, T.H.; Naumann, R.; et al. Impact of PTPN11 mutations on clinical outcome analyzed in 1529 patients with acute myeloid leukemia. Blood Adv. 2021, 5, 3279–3289. [Google Scholar] [CrossRef]
- Thol, F.; Kade, S.; Schlarmann, C.; Löffeld, P.; Morgan, M.; Krauter, J.; Wlodarski, M.W.; Kölking, B.; Wichmann, M.; Görlich, K.; et al. Frequency and prognostic impact of mutations in SRSF2, U2AF1, and ZRSR2 in patients with myelodysplastic syndromes. Blood 2012, 119, 3578–3584. [Google Scholar] [CrossRef]
- Song, G.Y.; Kim, T.; Ahn, S.Y.; Jung, S.H.; Kim, M.; Yang, D.H.; Lee, J.J.; Choi, S.H.; Kim, M.Y.; Jung, C.W.; et al. Allogeneic hematopoietic cell transplantation can overcome the adverse prognosis indicated by secondary-type mutations in de novo acute myeloid leukemia. Bone Marrow Transpl. 2022, 57, 1810–1819. [Google Scholar] [CrossRef]
- Mitrea, D.M.; Grace, C.R.; Buljan, M.; Yun, M.K.; Pytel, N.J.; Satumba, J.; Nourse, A.; Park, C.G.; Madan Babu, M.; White, S.W.; et al. Structural polymorphism in the N-terminal oligomerization domain of NPM1. Proc. Natl. Acad. Sci. USA 2014, 111, 4466–4471. [Google Scholar] [CrossRef]
- McClure, R.F.; Ewalt, M.D.; Crow, J.; Temple-Smolkin, R.L.; Pullambhatla, M.; Sargent, R.; Kim, A.S. Clinical Significance of DNA Variants in Chronic Myeloid Neoplasms: A Report of the Association for Molecular Pathology. J. Mol. Diagn. 2018, 20, 717–737. [Google Scholar] [CrossRef]
- Granowicz, E.M.; Jonas, B.A. Targeting TP53-Mutated Acute Myeloid Leukemia: Research and Clinical Developments. OncoTargets Ther. 2022, 15, 423–436. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Cortes, J.E. Mutations in AML: Prognostic and therapeutic implications. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 348–355. [Google Scholar] [CrossRef]
- Borlenghi, E.; Cattaneo, C.; Cerqui, E.; Archetti, S.; Bertoli, D.; Bellotti, D.; Gramegna, D.; Soverini, G.; Oberti, M.; Schieppati, F.; et al. Postremission therapy with repeated courses of high-dose cytarabine, idarubicin, and limited autologous stem cell support achieves a very good long-term outcome in European leukemia net favorable and intermediate-risk acute myeloid leukemia. Hematol. Oncol. 2020, 38, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Tallman, M.S. Novel and emerging drugs for acute myeloid leukemia. Curr. Cancer Drug. Targets. 2012, 12, 522–530. [Google Scholar] [CrossRef]
- Backhaus, D.; Brauer, D.; Pointner, R.; Bischof, L.; Vucinic, V.; Franke, G.N.; Niederwieser, D.; Platzbecker, U.; Jentzsch, M.; Schwind, S. A high hematopoietic cell transplantation comorbidity Index (HCT-CI) does not impair outcomes after non-myeloablative allogeneic stem cell transplantation in acute myeloid leukemia patients 60 years or older. Bone Marrow Transpl. 2022, 58, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. NCCN clinical practice guidelines in oncology: Adult cancer pain. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf (accessed on 18 February 2023).
- Hilberink, J.; Hazenberg, C.; van den Berg, E.; Mulder, A.; Schuringa, J.J.; van der Helm, L.; de Groot, M.; Choi, G.; de Bock, G.H.; Vellenga, E.; et al. Not type of induction therapy but consolidation with allogeneic hematopoietic cell transplantation determines outcome in older AML patients: A single center experience of 355 consecutive patients. Leuk. Res. 2019, 80, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F. Relapsed acute myeloid leukemia: Why is there no standard of care? Best Pract. Res. Clin. Haematol. 2013, 26, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Kaufmann, S.H. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: The importance of sequence of administration. Cancer Res. 1997, 57, 3375–3380. [Google Scholar]
- Loncharich, M.F.; Anderson, C.W. Interferon Inhibition for Lupus with Anifrolumab: Critical Appraisal of the Evidence Leading to FDA Approval. ACR Open Rheumatol. 2022, 4, 486–491. [Google Scholar] [CrossRef]
- Nakazawa, Y. Current status and future perspective of CAR T-cell therapy for acute myeloid leukemia. Rinsho Ketsueki 2022, 63, 1446–1453. [Google Scholar] [CrossRef]
- Xie, G.; Ivica, N.A.; Jia, B.; Li, Y.; Dong, H.; Liang, Y.; Brown, D.; Romee, R.; Chen, J. CAR-T cells targeting a nucleophosmin neoepitope exhibit potent specific activity in mouse models of acute myeloid leukaemia. Nat. Biomed. Eng. 2021, 5, 399–413. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Barreca, M.; Spanò, V.; Raimondi, M.V.; Poma, P.; Notarbartolo, M.; Barraja, P.; Montalbano, A. Novel insights on [1,2]oxazolo[5,4-e]isoindoles on multidrug resistant acute myeloid leukemia cell line. Drug. Dev. Res. 2022, 83, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Qiu, R.Z.; Sun, S.L.; Zhao, C.; Fan, T.Y.; Chen, M.; Li, N.G.; Shi, Z.H. Small-Molecule Fms-like Tyrosine Kinase 3 Inhibitors: An Attractive and Efficient Method for the Treatment of Acute Myeloid Leukemia. J. Med. Chem. 2020, 63, 12403–12428. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Döhner, K. Impact of new prognostic markers in treatment decisions in acute myeloid leukemia. Curr. Opin. Hematol. 2009, 16, 98–104. [Google Scholar] [CrossRef]
- Bazinet, A.; Assouline, S. A review of FDA-approved acute myeloid leukemia therapies beyond ‘7 + 3’. Expert Rev. Hematol. 2021, 14, 185–197. [Google Scholar] [CrossRef]
- Walter, R.B.; Appelbaum, F.R.; Estey, E.H.; Bernstein, I.D. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood 2012, 119, 6198–6208. [Google Scholar] [CrossRef]
- Diesch, J.; Zwick, A.; Garz, A.-K.; Palau, A.; Buschbeck, M.; Götze, K.S. A clinical-molecular update on azanucleoside-based therapy for the treatment of hematologic cancers. Clin. Epigenetics 2016, 8, 71. [Google Scholar] [CrossRef]
- Larrosa-Garcia, M.; Baer, M.R. FLT3 Inhibitors in Acute Myeloid Leukemia: Current Status and Future Directions. Molecular Cancer Ther. 2017, 16, 991–1001. [Google Scholar] [CrossRef]
- Liu, X.; Gong, Y. Isocitrate dehydrogenase inhibitors in acute myeloid leukemia. Biomark. Res. 2019, 7, 22. [Google Scholar] [CrossRef]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef]
- Kim, H.P.; Gerhard, B.; Harasym, T.O.; Mayer, L.D.; Hogge, D.E. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp. Hematol. 2011, 39, 741–750. [Google Scholar] [CrossRef]
- Norsworthy, K.J.; By, K.; Subramaniam, S.; Zhuang, L.; Del Valle, P.L.; Przepiorka, D.; Shen, Y.-L.; Sheth, C.M.; Liu, C.; Leong, R.; et al. FDA Approval Summary: Glasdegib for Newly Diagnosed Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 6021–6025. [Google Scholar] [CrossRef]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Ravandi, F.; Agresta, S.; Konopleva, M.; Takahashi, K.; Kadia, T.; Routbort, M.; Patel, K.P.; Brandt, M.; Pierce, S.; et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am. J. Hematol. 2015, 90, 732–736. [Google Scholar] [CrossRef]
- Dhillon, S. Ivosidenib: First Global Approval. Drugs 2018, 78, 1509–1516. [Google Scholar] [CrossRef]
- Kim, E.S. Enasidenib: First Global Approval. Drugs 2017, 77, 1705–1711. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olutasidenib-relapsed-or-refractory-acute-myeloid-leukemia-susceptible-idh1-mutation (accessed on 18 February 2023).
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- McMurry, H.; Fletcher, L.; Traer, E. IDH Inhibitors in AML-Promise and Pitfalls. Curr. Hematol. Malig. Rep. 2021, 16, 207–217. [Google Scholar] [CrossRef]
- Majothi, S.; Adams, D.; Loke, J.; Stevens, S.P.; Wheatley, K.; Wilson, J.S. FLT3 inhibitors in acute myeloid leukaemia: Assessment of clinical effectiveness, adverse events and future research-a systematic review and meta-analysis. Syst. Rev. 2020, 9, 285. [Google Scholar] [CrossRef]
- Cerchione, C.; Peleteiro Raíndo, A.; Mosquera Orgueira, A.; Mosquera Torre, A.; Bao Pérez, L.; Marconi, G.; Isidori, A.; Pérez Encinas, M.M.; Martinelli, G. Safety of FLT3 inhibitors in patients with acute myeloid leukemia. Expert. Rev. Hematol. 2021, 14, 851–865. [Google Scholar] [CrossRef]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef]
- Zhang, H.; Nimmer, P.M.; Tahir, S.K.; Chen, J.; Fryer, R.M.; Hahn, K.R.; Iciek, L.A.; Morgan, S.J.; Nasarre, M.C.; Nelson, R.; et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007, 14, 943–951. [Google Scholar] [CrossRef]
- Leverson, J.D.; Phillips, D.C.; Mitten, M.J.; Boghaert, E.R.; Diaz, D.; Tahir, S.K.; Belmont, L.D.; Nimmer, P.; Xiao, Y.; Ma, X.M.; et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 2015, 7, 279ra240. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Juárez-Salcedo, L.M.; Desai, V.; Dalia, S. Venetoclax: Evidence to date and clinical potential. Drugs Context 2019, 8, 212574. [Google Scholar] [CrossRef]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Sorrentino, V.G.; Thota, S.; Gonzalez, E.A.; Rameshwar, P.; Chang, V.T.; Etchegaray, J.P. Hypomethylating Chemotherapeutic Agents as Therapy for Myelodysplastic Syndromes and Prevention of Acute Myeloid Leukemia. Pharmaceuticals 2021, 14, 641. [Google Scholar] [CrossRef]
- Stresemann, C.; Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer 2008, 123, 8–13. [Google Scholar] [CrossRef]
- Schwartsmann, G.; Schunemann, H.; Gorini, C.N.; Filho, A.F.; Garbino, C.; Sabini, G.; Muse, I.; DiLeone, L.; Mans, D.R. A phase I trial of cisplatin plus decitabine, a new DNA-hypomethylating agent, in patients with advanced solid tumors and a follow-up early phase II evaluation in patients with inoperable non-small cell lung cancer. Investig. New Drugs 2000, 18, 83–91. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Fenaux, P.; Gobbi, M.; Mayer, J.; Roboz, G.J.; Krauter, J.; Robak, T.; Kantarjian, H.M.; Novák, J.; Jedrzejczak, W.W.; et al. Prospective comparison of outcomes with azacitidine and decitabine in patients with AML ineligible for intensive chemotherapy. Blood 2022, 140, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.K.; Chow, S.; Hedley, D. Pharmacodynamic monitoring of BAY 43-9006 (Sorafenib) in phase I clinical trials involving solid tumor and AML/MDS patients, using flow cytometry to monitor activation of the ERK pathway in peripheral blood cells. Cytom. B Clin. Cytom. 2006, 70, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Kantarjian, H.; Foran, J.M.; Ghirdaladze, D.; Zodelava, M.; Borthakur, G.; Gammon, G.; Trone, D.; Armstrong, R.C.; James, J.; et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3–internal tandem duplication status. J. Clin. Oncol. 2013, 31, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/media/124896/download (accessed on 18 February 2023).
- Hu, N.; Wang, F.; Sun, T.; Xu, Z.; Zhang, J.; Bernard, D.; Xu, S.; Wang, S.; Kaminski, M.; Devata, S.; et al. Follicular Lymphoma–associated BTK Mutations are Inactivating Resulting in Augmented AKT Activation. Clin. Cancer Res. 2021, 27, 2301–2313. [Google Scholar] [CrossRef]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef]
- Dayal, N.; Řezníčková, E.; Hernandez, D.E.; Peřina, M.; Torregrosa-Allen, S.; Elzey, B.D.; Škerlová, J.; Ajani, H.; Djukic, S.; Vojáčková, V.; et al. 3H-Pyrazolo[4,3-f]quinoline-Based Kinase Inhibitors Inhibit the Proliferation of Acute Myeloid Leukemia Cells In Vivo. J. Med. Chem. 2021, 64, 10981–10996. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, J.; Yue, K.; Huang, C.; Qin, M.; Chi, D.; Yu, Q.; Zhu, Y.; Hou, X.; Xu, T.; et al. Potent Hydrazide-Based HDAC Inhibitors with a Superior Pharmacokinetic Profile for Efficient Treatment of Acute Myeloid Leukemia In Vivo. J. Med. Chem. 2022, 65, 285–302. [Google Scholar] [CrossRef]
- Han, X.; Song, N.; Saidahmatov, A.; Wang, P.; Wang, Y.; Hu, X.; Kan, W.; Zhu, W.; Gao, L.; Zeng, M.; et al. Rational Design and Development of Novel CDK9 Inhibitors for the Treatment of Acute Myeloid Leukemia. J. Med. Chem. 2021, 64, 14647–14663. [Google Scholar] [CrossRef]
- Levis, M. Quizartinib for the treatment of FLT3/ITD acute myeloid leukemia. Future Oncol. 2014, 10, 1571–1579. [Google Scholar] [CrossRef]
- Takahashi, T.; Usuki, K.; Matsue, K.; Ohno, H.; Sakura, T.; Imanaka, R.; Murakami, M.; Ohwada, S.; Takagi, T.; Sakajiri, S. Efficacy and safety of quizartinib in Japanese patients with FLT3-ITD positive relapsed or refractory acute myeloid leukemia in an open-label, phase 2 study. Int. J. Hematol. 2019, 110, 665–674. [Google Scholar] [CrossRef]
- Quek, L.; David, M.D.; Kennedy, A.; Metzner, M.; Amatangelo, M.; Shih, A.; Stoilova, B.; Quivoron, C.; Heiblig, M.; Willekens, C.; et al. Clonal heterogeneity of acute myeloid leukemia treated with the IDH2 inhibitor enasidenib. Nat. Med. 2018, 24, 1167–1177. [Google Scholar] [CrossRef]
- Swaminathan, M.; Bourgeois, W.; Armstrong, S.A.; Wang, E.S. Menin Inhibitors in Acute Myeloid Leukemia-What Does the Future Hold? Cancer J. 2022, 28, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Fiskus, W.; Boettcher, S.; Daver, N.; Mill, C.P.; Sasaki, K.; Birdwell, C.E.; Davis, J.A.; Takahashi, K.; Kadia, T.M.; DiNardo, C.D.; et al. Effective Menin inhibitor-based combinations against AML with MLL rearrangement or NPM1 mutation (NPM1c). Blood Cancer J. 2022, 12, 5. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Staunton, J.E.; Silverman, L.B.; Pieters, R.; den Boer, M.L.; Minden, M.D.; Sallan, S.E.; Lander, E.S.; Golub, T.R.; Korsmeyer, S.J. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat. Genet. 2002, 30, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Andreeff, M.; Ruvolo, V.; Gadgil, S.; Zeng, C.; Coombes, K.; Chen, W.; Kornblau, S.; Barón, A.E.; Drabkin, H.A. HOX expression patterns identify a common signature for favorable AML. Leukemia 2008, 22, 2041–2047. [Google Scholar] [CrossRef]
- Kühn, M.W.; Song, E.; Feng, Z.; Sinha, A.; Chen, C.W.; Deshpande, A.J.; Cusan, M.; Farnoud, N.; Mupo, A.; Grove, C.; et al. Targeting Chromatin Regulators Inhibits Leukemogenic Gene Expression in NPM1 Mutant Leukemia. Cancer Discov. 2016, 6, 1166–1181. [Google Scholar] [CrossRef]
- Fiskus, W.; Daver, N.; Boettcher, S.; Mill, C.P.; Sasaki, K.; Birdwell, C.E.; Davis, J.A.; Das, K.; Takahashi, K.; Kadia, T.M.; et al. Activity of menin inhibitor ziftomenib (KO-539) as monotherapy or in combinations against AML cells with MLL1 rearrangement or mutant NPM1. Leukemia 2022, 36, 2729–2733. [Google Scholar] [CrossRef]
- Boettcher, S.; Miller, P.G.; Sharma, R.; McConkey, M.; Leventhal, M.; Krivtsov, A.V.; Giacomelli, A.O.; Wong, W.; Kim, J.; Chao, S.; et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. Science 2019, 365, 599–604. [Google Scholar] [CrossRef]
- Oren, M.; Rotter, V. Mutant p53 gain-of-function in cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a001107. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.V.; Zhang, B.Z.; Fujihara, K.M.; Dawar, S.; Phillips, W.A.; Clemons, N.J. Transketolase regulates sensitivity to APR-246 in p53-null cells independently of oxidative stress modulation. Sci. Rep. 2021, 11, 4480. [Google Scholar] [CrossRef]
- Zhang, Q.; Bykov, V.J.N.; Wiman, K.G.; Zawacka-Pankau, J. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 2018, 9, 439. [Google Scholar] [CrossRef]
- Maslah, N.; Salomao, N.; Drevon, L.; Verger, E.; Partouche, N.; Ly, P.; Aubin, P.; Naoui, N.; Schlageter, M.H.; Bally, C.; et al. Synergistic effects of PRIMA-1(Met) (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 2020, 105, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Sanz, G.; Singh, M.; Peuget, S.; Selivanova, G. Inhibition of p53 inhibitors: Progress, challenges and perspectives. J. Mol. Cell Biol. 2019, 11, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Voltan, R.; Gonelli, A.; Secchiero, P.; Zauli, G. MDM2/X inhibitors under clinical evaluation: Perspectives for the management of hematological malignancies and pediatric cancer. J. Hematol. Oncol. 2017, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Bolomsky, A.; Vogler, M.; Köse, M.C.; Heckman, C.A.; Ehx, G.; Ludwig, H.; Caers, J. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J. Hematol. Oncol. 2020, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting MCL-1 in cancer: Current status and perspectives. J. Hematol. Oncol. 2021, 14, 67. [Google Scholar] [CrossRef]
- Taniai, M.; Grambihler, A.; Higuchi, H.; Werneburg, N.; Bronk, S.F.; Farrugia, D.J.; Kaufmann, S.H.; Gores, G.J. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004, 64, 3517–3524. [Google Scholar] [CrossRef]
- Wei, A.H.; Roberts, A.W.; Spencer, A.; Rosenberg, A.S.; Siegel, D.; Walter, R.B.; Caenepeel, S.; Hughes, P.; McIver, Z.; Mezzi, K.; et al. Targeting MCL-1 in hematologic malignancies: Rationale and progress. Blood Rev. 2020, 44, 100672. [Google Scholar] [CrossRef]
- Etchin, J.; Berezovskaya, A.; Conway, A.S.; Galinsky, I.A.; Stone, R.M.; Baloglu, E.; Senapedis, W.; Landesman, Y.; Kauffman, M.; Shacham, S.; et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia 2017, 31, 143–150. [Google Scholar] [CrossRef]
- Lee, S.; Mohan, S.; Knupp, J.; Chamoun, K.; de Jonge, A.; Yang, F.; Baloglu, E.; Shah, J.; Kauffman, M.G.; Shacham, S.; et al. Oral eltanexor treatment of patients with higher-risk myelodysplastic syndrome refractory to hypomethylating agents. J. Hematol. Oncol. 2022, 15, 103. [Google Scholar] [CrossRef]
- Taylor, J.; Mi, X.; Penson, A.V.; Paffenholz, S.V.; Alvarez, K.; Sigler, A.; Chung, S.S.; Rampal, R.K.; Park, J.H.; Stein, E.M.; et al. Safety and activity of selinexor in patients with myelodysplastic syndromes or oligoblastic acute myeloid leukaemia refractory to hypomethylating agents: A single-centre, single-arm, phase 2 trial. Lancet Haematol. 2020, 7, e566–e574. [Google Scholar] [CrossRef]
- Totiger, T.M.; Chaudhry, S.; Musi, E.; Afaghani, J.; Montoya, S.; Owusu-Ansah, F.; Lee, S.; Schwartz, G.; Klimek, V.; Taylor, J. Protein biomarkers for response to XPO1 inhibition in hematologic malignancies. medRxiv 2022. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.K.; Loken, M.; Lane, T.A.; Ball, E.D. CTLA-4 blockade by a human MAb enhances the capacity of AML-derived DC to induce T-cell responses against AML cells in an autologous culture system. Cytotherapy 2006, 8, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, F.S.; Krupka, C.; Haubner, S.; Köhnke, T.; Subklewe, M. Recent developments in immunotherapy of acute myeloid leukemia. J. Hematol. Oncol. 2017, 10, 142. [Google Scholar] [CrossRef]
- Montoya, S.; Soong, D.; Nguyen, N.; Affer, M.; Munamarty, S.P.; Taylor, J. Targeted Therapies in Cancer: To Be or Not to Be, Selective. Biomedicines 2021, 9, 1591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ly, C.; Ishizawa, J.; Mu, H.; Ruvolo, V.; Shacham, S.; Daver, N.; Andreeff, M. Combinatorial targeting of XPO1 and FLT3 exerts synergistic anti-leukemia effects through induction of differentiation and apoptosis in FLT3-mutated acute myeloid leukemias: From concept to clinical trial. Haematologica 2018, 103, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Chaudhry, S.; Totiger, T.M.; Diaz, R.; Roberts, E.; Montoya, S.; Pardo, G.; Pardo, A.; Afaghani, J.; Affer, M.; et al. Combination venetoclax and selinexor effective in relapsed refractory multiple myeloma with translocation t(11;14). NPJ Precis. Oncol. 2022, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]

| Selective | Target | MOA | Non-Selective | Target | MOA |
|---|---|---|---|---|---|
| Flavopiridol | CDK Inhibitor | Cell-cycle arrest and apoptosis | Daunorubicin | Anthracycline | Cytotoxic |
| CD33-Targeted ADCs | CD33 Target | Targeted delivery of toxic drug | Idarubicin | Anthracycline | Cytotoxic |
| Eltanexor | XPO1 Inhibitor | XPO1 inhibition | Mitoxantrone | Anthracycline | Topoisomerase inhibitor |
| Venetoclax | BCL-2 Inhibitor | Anti-apoptotic Protein inhibition | Cytarabine (CPX351) | Pyrimidine analog | DNA polymerase inhibition |
| Sorafenib | FLT3 Inhibitor | FLT3-ITD inhibition | Guadecitabine | Hypomethylation | DNA Methyltransferase inhibition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totiger, T.M.; Ghoshal, A.; Zabroski, J.; Sondhi, A.; Bucha, S.; Jahn, J.; Feng, Y.; Taylor, J. Targeted Therapy Development in Acute Myeloid Leukemia. Biomedicines 2023, 11, 641. https://doi.org/10.3390/biomedicines11020641
Totiger TM, Ghoshal A, Zabroski J, Sondhi A, Bucha S, Jahn J, Feng Y, Taylor J. Targeted Therapy Development in Acute Myeloid Leukemia. Biomedicines. 2023; 11(2):641. https://doi.org/10.3390/biomedicines11020641
Chicago/Turabian StyleTotiger, Tulasigeri M., Anirban Ghoshal, Jenna Zabroski, Anya Sondhi, Saanvi Bucha, Jacob Jahn, Yangbo Feng, and Justin Taylor. 2023. "Targeted Therapy Development in Acute Myeloid Leukemia" Biomedicines 11, no. 2: 641. https://doi.org/10.3390/biomedicines11020641
APA StyleTotiger, T. M., Ghoshal, A., Zabroski, J., Sondhi, A., Bucha, S., Jahn, J., Feng, Y., & Taylor, J. (2023). Targeted Therapy Development in Acute Myeloid Leukemia. Biomedicines, 11(2), 641. https://doi.org/10.3390/biomedicines11020641





