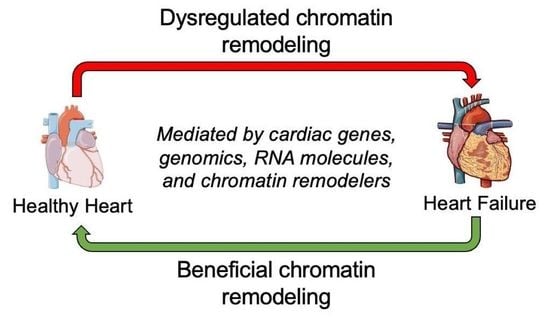
The Current Therapeutic Role of Chromatin Remodeling for the Prognosis and Treatment of Heart Failure
Abstract
1. Introduction
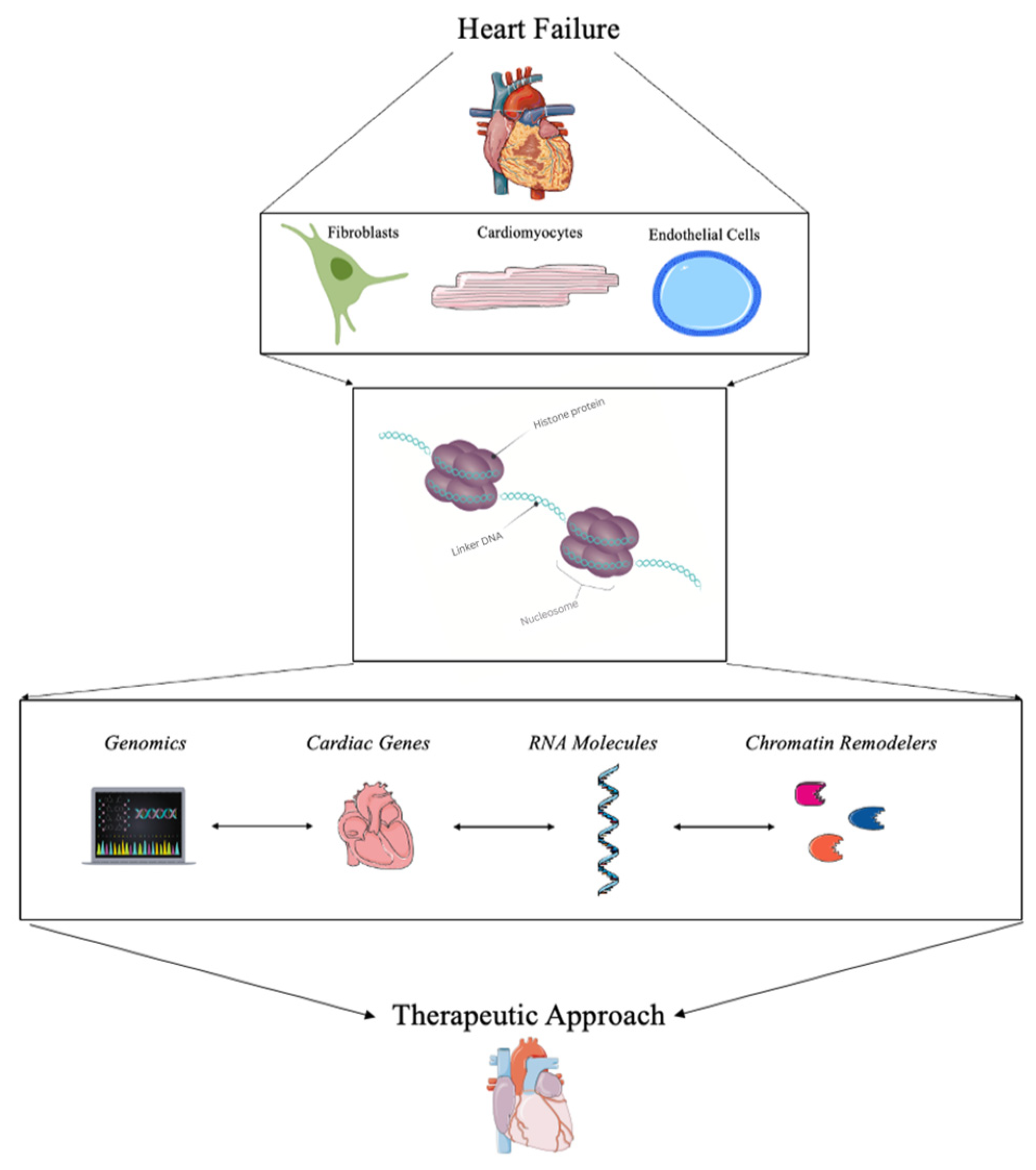
2. Current Strategies for Detecting Chromatin Remodeling in Heart Failure
3. Therapeutic Approaches Using Chromatin Remodeling in Heart Failure
3.1. Genomics and Bioinformatics
3.2. Targeting Cardiac Genes for Transcriptional Regulation
3.3. RNA Molecules
3.4. Chromatin Remodelers and Complexes
| Chromatin Alterations | Summary of Function | Source |
|---|---|---|
| Target Genes Med1 Gata4 Nxk2.5 CTCF AP-1 MEF2c Tbx5 BRG1 BHB | Transcriptional regulation, caridac development, metabolism, cardiac fibrosis, cell death regulation, epigenetic regulation, chromatin remodeling | [51,52,53,54,55,56,57,58,59,60,61,62,63] |
| RNA Molecules lncRNAs AHIT CHAIR MEG3 circRNA circNCX1 circHIPK3 miRNA miR-17-3p miR-193b | Regulating epigenetic marks, cardiac gene expression, chromatin remodeling, apoptoic regulation, redox regulation | [64,65,66,67,68,70,72,73,74,75,76,77,78,79,80,81] |
| Chromatin Remodelers BRG1 P300 SETD7 G9a Znhit1 Baf60c HDACs HATs BETs/BRD4 | Direct chromatin architecture regulation, adding and removing epigenetic modifications, cardiac disease regulation, DNA damage respones, pro-inflammatory response regulation, aging mechanisms | [28,83,84,85,86,87,88,89,90,91,92,93,94,95,96,98,99,100,101] |
4. Current Clinical Application of Chromatin Remodeling for the Treatment of Heart Failure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mc Namara, K.; Alzubaidi, H.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Res. Pract. 2019, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 February 2020).
- Ciumărnean, L.; Milaciu, M.V.; Negrean, V.; Orășan, O.H.; Vesa, S.C.; Sălăgean, O.; Iluţ, S.; Vlaicu, S.I. Cardiovascular Risk Factors and Physical Activity for the Prevention of Cardiovascular Diseases in the Elderly. Int. J. Environ. Res. Public Health 2022, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Lifestyle Strategies for Risk Factor Reduction, Prevention and Treatme. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781315201108-2/lifestyle-strategies-risk-factor-reduction-prevention-treatment-cardiovascular-disease-james-rippe-theodore-angelopoulos (accessed on 6 January 2023).
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kurmani, S.; Squire, I. Acute Heart Failure: Definition, Classification and Epidemiology. Curr. Heart Fail. Rep. 2017, 14, 385–392. [Google Scholar] [CrossRef] [PubMed]
- CDC Heart Failure|cdc.gov. Centers for Disease Control and Prevention. 8 September 2020. Available online: https://www.cdc.gov/heartdisease/heart_failure.htm (accessed on 12 October 2020).
- Lippi, G.; Sanchis-Gomar, F. Global epidemiology and future trends of heart failure. AME Med. J. 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Rossignol, P.; Hernandez, A.F.; Solomon, S.D.; Zannad, F. Heart failure drug treatment. Lancet 2019, 393, 1034–1044. [Google Scholar] [CrossRef]
- Smits, J.M.; Samuel, U.; Laufer, G. Bridging the gap in heart transplantation. Curr. Opin. Organ. Transplant. 2017, 22, 221–224. [Google Scholar] [CrossRef]
- Rheault-Henry, M.; White, I.; Grover, D.; Atoui, R. Stem cell therapy for heart failure: Medical breakthrough, or dead end? World J. Stem Cells 2021, 13, 236–259. [Google Scholar] [CrossRef]
- Segers, V.F.M.; Lee, R.T. Stem-cell therapy for cardiac disease. Nature 2008, 451, 937–942. [Google Scholar] [CrossRef]
- Alexanian, M.; Padmanabhan, A.; McKinsey, T.A.; Haldar, S.M. Epigenetic therapies in heart failure. J. Mol. Cell Cardiol. 2019, 130, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Morales, C.; Gillette, T.G.; Hill, J.A. Epigenetic Regulation in Heart Failure. Curr. Opin. Cardiol. 2016, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Tyagi, S.C. Epigenetic mechanisms underlying cardiac degeneration and regeneration. Int. J. Cardiol. 2014, 173, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Tang, W.H.W. Epigenetics in Cardiac Hypertrophy and Heart Failure. J. Am. Coll. Cardiol. Basic Trans. Sci. 2019, 4, 976–993. [Google Scholar] [CrossRef]
- McKinsey, T.A.; Vondriska, T.M.; Wang, Y. Epigenomic regulation of heart failure: Integrating histone marks, long noncoding RNAs, and chromatin architecture. F1000Res 2018, 7, F1000. [Google Scholar] [CrossRef]
- Kimball, T.H.; Vondriska, T.M. Metabolism, Epigenetics, and Causal Inference in Heart Failure. Trends Endocrinol. Metab. 2020, 31, 181–191. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Sig. Transduct. Target. 2022, 7, 200. [Google Scholar] [CrossRef]
- McGinty, R.K.; Tan, S. Histone, Nucleosome, and Chromatin Structure. In Fundamentals of Chromatin; Workman, J.L., Abmayr, S.M., Eds.; Springer: New York, NY, USA, 2014; pp. 1–28. ISBN 978-1-4614-8624-4. [Google Scholar]
- Shah, M.; He, Z.; Rauf, A.; Beikoghli Kalkhoran, S.; Heiestad, C.M.; Stensløkken, K.-O.; Parish, C.R.; Soehnlein, O.; Arjun, S.; Davidson, S.M.; et al. Extracellular histones are a target in myocardial ischaemia–reperfusion injury. Cardiovasc. Res. 2021, 118, 1115–1125. [Google Scholar] [CrossRef]
- Gilsbach, R.; Schwaderer, M.; Preissl, S.; Grüning, B.A.; Kranzhöfer, D.; Schneider, P.; Nührenberg, T.G.; Mulero-Navarro, S.; Weichenhan, D.; Braun, C.; et al. Distinct epigenetic programs regulate cardiac myocyte development and disease in the human heart in vivo. Nat. Commun. 2018, 9, 391. [Google Scholar] [CrossRef]
- Zhang, Q.-J.; Liu, Z.-P. Histone methylations in heart development, congenital and adult heart diseases. Epigenomics 2015, 7, 321–330. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Kraus, L. Targeting Epigenetic Regulation of Cardiomyocytes through Development for Therapeutic Cardiac Regeneration after Heart Failure. Int. J. Mol. Sci. 2022, 23, 11878. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Hang, C.T.; Yang, J.; Chang, C.-P. Chromatin remodeling in cardiovascular development and physiology. Circ. Res. 2011, 108, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Yang, J.; Shang, C.; Chang, C.-P. Chromatin Remodeling in Heart Failure. In Epigenetics in Cardiac Disease; Backs, J., McKinsey, T.A., Eds.; Cardiac and Vascullar Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 103–124. ISBN 978-3-319-41457-7. [Google Scholar]
- Lorch, Y.; Maier-Davis, B.; Kornberg, R.D. Mechanism of chromatin remodeling. Proc. Natl. Acad. Sci. USA 2010, 107, 3458–3462. [Google Scholar] [CrossRef] [PubMed]
- Chromatin Remodeling in Eukaryotes|Learn Science at Scitable. Available online: http://www.nature.com/scitable/topicpage/chromatin-remodeling-in-eukaryotes-1082 (accessed on 6 January 2023).
- Pasipoularides, A. Implementing genome-driven personalized cardiology in clinical practice. J. Mol. Cell. Cardiol. 2018, 115, 142–157. [Google Scholar] [CrossRef]
- Khomtchouk, B.B.; Tran, D.-T.; Vand, K.A.; Might, M.; Gozani, O.; Assimes, T.L. Cardioinformatics: The nexus of bioinformatics and precision cardiology. Brief. Bioinform. 2020, 21, 2031–2051. [Google Scholar] [CrossRef]
- Hunt, C.; Montgomery, S.; Berkenpas, J.W.; Sigafoos, N.; Oakley, J.C.; Espinosa, J.; Justice, N.; Kishaba, K.; Hippe, K.; Si, D.; et al. Recent Progress of Machine Learning in Gene Therapy. Curr. Gene Ther. 2022, 22, 132–143. [Google Scholar] [CrossRef]
- Tsompana, M.; Buck, M.J. Chromatin accessibility: A window into the genome. Epigenet. Chromatin 2014, 7, 33. [Google Scholar] [CrossRef]
- Xu, W.; Wen, Y.; Liang, Y.; Xu, Q.; Wang, X.; Jin, W.; Chen, X. A plate-based single-cell ATAC-seq workflow for fast and robust profiling of chromatin accessibility. Nat. Protoc. 2021, 16, 4084–4107. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kollipara, R.K.; Orquera-Tornakian, G.; Goetsch, S.; Zhang, M.; Perry, C.; Li, B.; Shelton, J.M.; Bhakta, M.; Duan, J.; et al. Global chromatin landscapes identify candidate noncoding modifiers of cardiac rhythm. J. Clin. Investig. 2022, 133, 3. [Google Scholar] [CrossRef]
- Jia, G.; Preussner, J.; Chen, X.; Guenther, S.; Yuan, X.; Yekelchyk, M.; Kuenne, C.; Looso, M.; Zhou, Y.; Teichmann, S.; et al. Single cell RNA-seq and ATAC-seq analysis of cardiac progenitor cell transition states and lineage settlement. Nat. Commun. 2018, 9, 4877. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, T.A.; Olson, E.N. Toward transcriptional therapies for the failing heart: Chemical screens to modulate genes. J. Clin. Investig. 2005, 115, 538–546. [Google Scholar] [CrossRef]
- Centore, R.C.; Sandoval, G.J.; Soares, L.M.M.; Kadoch, C.; Chan, H.M. Mammalian SWI/SNF Chromatin Remodeling Complexes: Emerging Mechanisms and Therapeutic Strategies. Trends Genet. 2020, 36, 936–950. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Chang, C.-P. Long non-coding RNA and chromatin remodeling. RNA Biol. 2015, 12, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, W.; Lin, C.-H.; Yang, J.; Shang, C.; Nuernberg, S.T.; Jin, K.K.; Xu, W.; Lin, C.-Y.; Lin, C.-J.; et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 2014, 514, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kuppe, C.; Ramirez Flores, R.O.; Li, Z.; Hayat, S.; Levinson, R.T.; Liao, X.; Hannani, M.T.; Tanevski, J.; Wünnemann, F.; Nagai, J.S.; et al. Spatial multi-omic map of human myocardial infarction. Nature 2022, 608, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Chapski, D.J.; Cabaj, M.; Morselli, M.; Mason, R.J.; Soehalim, E.; Ren, S.; Pellegrini, M.; Wang, Y.; Vondriska, T.M.; Rosa-Garrido, M. Early adaptive chromatin remodeling events precede pathologic phenotypes and are reinforced in the failing heart. J. Mol. Cell. Cardiol. 2021, 160, 73–86. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Duan, Z.; Hu, W. Hypoxia-induced alterations of transcriptome and chromatin accessibility in HL-1 cells. IUBMB Life 2020, 72, 1737–1746. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, S.; Zhao, Y.; Wang, W.; Zhang, H. A multi-omics approach to identify molecular alterations in a mouse model of heart failure. Theranostics 2022, 12, 1607–1620. [Google Scholar] [CrossRef]
- Arvanitis, M.; Tampakakis, E.; Zhang, Y.; Wang, W.; Auton, A.; Dutta, D.; Glavaris, S.; Keramati, A.; Chatterjee, N.; Chi, N.C.; et al. Genome-wide association and multi-omic analyses reveal ACTN2 as a gene linked to heart failure. Nat. Commun. 2020, 11, 1122. [Google Scholar] [CrossRef]
- Rosa-Garrido, M.; Chapski, D.J.; Schmitt, A.D.; Kimball, T.H.; Karbassi, E.; Monte, E.; Balderas, E.; Pellegrini, M.; Shih, T.-T.; Soehalim, E.; et al. High-Resolution Mapping of Chromatin Conformation in Cardiac Myocytes Reveals Structural Remodeling of the Epigenome in Heart Failure. Circulation 2017, 136, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Meder, B.; Haas, J.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Frese, K.; Lai, A.; Nietsch, R.; Scheiner, C.; Mester, S.; Bordalo, D.M.; et al. Epigenome-Wide Association Study Identifies Cardiac Gene Patterning and a Novel Class of Biomarkers for Heart Failure. Circulation 2017, 136, 1528–1544. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.S.; Chaturvedi, R.R.; Liston, E.; Manshaei, R.; Aul, R.B.; Bowdin, S.; Cohn, I.; Curtis, M.; Dhir, P.; Hayeems, R.Z.; et al. The Cardiac Genome Clinic: Implementing genome sequencing in pediatric heart disease. Genet. Med. 2020, 22, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.D.; Spitler, K.M.; Grueter, C.E. Disruption of cardiac Med1 inhibits RNA polymerase II promoter occupancy and promotes chromatin remodeling. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H314–H325. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Gu, F.; Hu, Y.; Ma, Q.; Yi Ye, L.; Akiyama, J.A.; Visel, A.; Pennacchio, L.A.; Pu, W.T. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat. Commun. 2014, 5, 4907. [Google Scholar] [CrossRef]
- Beisaw, A.; Kuenne, C.; Guenther, S.; Dallmann, J.; Wu, C.-C.; Bentsen, M.; Looso, M.; Stainier, D.Y.R. AP-1 Contributes to Chromatin Accessibility to Promote Sarcomere Disassembly and Cardiomyocyte Protrusion During Zebrafish Heart Regeneration. Circ. Res. 2020, 126, 1760–1778. [Google Scholar] [CrossRef]
- de Pater, E.; Clijsters, L.; Marques, S.R.; Lin, Y.-F.; Garavito-Aguilar, Z.V.; Yelon, D.; Bakkers, J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 2009, 136, 1633–1641. [Google Scholar] [CrossRef]
- Jiang, W.; Xiong, Y.; Li, X.; Yang, Y. Cardiac Fibrosis: Cellular Effectors, Molecular Pathways, and Exosomal Roles. Front. Cardiovasc. Med. 2021, 8, 715258. [Google Scholar] [CrossRef]
- Mathison, M.; Singh, V.P.; Sanagasetti, D.; Yang, L.; Pinnamaneni, J.P.; Yang, J.; Rosengart, T.K. Cardiac Reprogramming Factor Gata4 Reduces Post-Infarct Cardiac Fibrosis through Direct Repression of the Pro-Fibrotic Mediator Snail. J. Thorac. Cardiovasc. Surg. 2017, 154, 1601–1610. [Google Scholar] [CrossRef]
- Ieda, M. Key Regulators of Cardiovascular Differentiation and Regeneration: Harnessing the Potential of Direct Reprogramming to Treat Heart Failure. J. Card. Fail. 2020, 26, 80–84. [Google Scholar] [CrossRef]
- Yamakawa, H.; Ieda, M. Cardiac regeneration by direct reprogramming in this decade and beyond. Inflamm. Regen. 2021, 41, 20. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, T.A.; Foo, R.; Anene-Nzelu, C.G.; Travers, J.G.; Vagnozzi, R.J.; Weber, N.; Thum, T. Emerging epigenetic therapies of cardiac fibrosis and remodeling in heart failure: From basic mechanisms to early clinical development. Cardiovasc. Res. 2022, 118, cvac142. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Zhao, Z.-A.; Shen, Z. Direct reprogramming of fibroblasts into cardiomyocytes. Stem Cell Res. Ther. 2017, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Vondriska, T.M. How chromatin stiffens fibroblasts. Curr. Opin. Physiol. 2022, 26, 100537. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Seldin, M.M.; Fu, K.; Li, S.; Lam, L.; Wang, P.; Wang, Y.; Huang, D.; Nguyen, T.L.; Wei, B.; et al. Topological Arrangement of Cardiac Fibroblasts Regulates Cellular Plasticity. Circ. Res. 2018, 123, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kong, X.; Zhang, Y.; Zhang, Y.; Yu, L.; Guo, J.; Xu, Y. Dual roles of chromatin remodeling protein BRG1 in angiotensin II-induced endothelial–mesenchymal transition. Cell Death Dis. 2020, 11, 549. [Google Scholar] [CrossRef]
- Santulli, G.; Jankauskas, S.; Morelli, M.; Gambardella, J. Chromatin remodeling and mitochondrial biogenesis underlie the improved cardiac function in heart failure induced by ketogenic diet and beta-hydroxibutiyrate supplementation. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Lu, P.; Ding, F.; Xiang, Y.K.; Hao, L.; Zhao, M. Noncoding RNAs in Cardiac Hypertrophy and Heart Failure. Cells 2022, 11, 777. [Google Scholar] [CrossRef]
- Li, M.; Duan, L.; Li, Y.; Liu, B. Long noncoding RNA/circular noncoding RNA–miRNA–mRNA axes in cardiovascular diseases. Life Sci. 2019, 233, 116440. [Google Scholar] [CrossRef]
- Gong, C.; Zhou, X.; Lai, S.; Wang, L.; Liu, J. Long Noncoding RNA/Circular RNA-miRNA-mRNA Axes in Ischemia-Reperfusion Injury. Biomed. Res. Int. 2020, 2020, 8838524. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Zhong, L.-F.; Hong, X.; Zhu, Q.-L.; Wang, S.-J.; Han, J.-B.; Huang, W.-J.; Ye, B.-Z. Integrated Analysis of circRNA-miRNA-mRNA ceRNA Network in Cardiac Hypertrophy. Front. Genet. 2022, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Dou, L.; Chen, Y. Association of long-chain non-coding RNA MHRT gene single nucleotide polymorphism with risk and prognosis of chronic heart failure. Medicine 2020, 99, e19703. [Google Scholar] [CrossRef] [PubMed]
- Hobuß, L.; Bär, C.; Thum, T. Long Non-coding RNAs: At the Heart of Cardiac Dysfunction? Front. Physiol. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wan, J.; Liu, X.; Zhang, W. Strategies and technologies for exploring long noncoding RNAs in heart failure. Biomed. Pharmacother. 2020, 131, 110572. [Google Scholar] [CrossRef]
- Hermans-Beijnsberger, S.; van Bilsen, M.; Schroen, B. Long non-coding RNAs in the failing heart and vasculature. Noncoding RNA Res. 2018, 3, 118–130. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Xu, Z.; Lan, C.; Chen, C.; Li, C.; Chen, Z.; Yu, C.; Xia, X.; Liao, Q.; et al. Long Noncoding RNA Ahit Protects Against Cardiac Hypertrophy Through SUZ12 (Suppressor of Zeste 12 Protein Homolog)-Mediated Downregulation of MEF2A (Myocyte Enhancer Factor 2A). Circ. Heart Fail. 2020, 13, e006525. [Google Scholar] [CrossRef]
- Ge, Z.; Yin, C.; Li, Y.; Tian, D.; Xiang, Y.; Li, Q.; Tang, Y.; Zhang, Y. Long noncoding RNA NEAT1 promotes cardiac fibrosis in heart failure through increased recruitment of EZH2 to the Smad7 promoter region. J. Transl. Med. 2022, 20, 7. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, M.; Zhou, N.; Xu, X.; Zhang, J.; Ding, Q.; Wang, J. A long noncoding RNA CHAIR protects the heart from pathological stress. Clin. Sci. 2020, 134, 1843–1857. [Google Scholar] [CrossRef]
- Piccoli, M.-T.; Gupta, S.K.; Viereck, J.; Foinquinos, A.; Samolovac, S.; Kramer, F.L.; Garg, A.; Remke, J.; Zimmer, K.; Batkai, S.; et al. Inhibition of the Cardiac Fibroblast–Enriched lncRNA Meg3 Prevents Cardiac Fibrosis and Diastolic Dysfunction. Circ. Res. 2017, 121, 575–583. [Google Scholar] [CrossRef]
- Tan, W.L.W.; Lim, B.T.S.; Anene-Nzelu, C.G.O.; Ackers-Johnson, M.; Dashi, A.; See, K.; Tiang, Z.; Lee, D.P.; Chua, W.W.; Luu, T.D.A.; et al. A landscape of circular RNA expression in the human heart. Cardiovasc. Res. 2017, 113, 298–309. [Google Scholar] [CrossRef]
- Sun, C.; Ni, M.; Song, B.; Cao, L. Circulating Circular RNAs: Novel Biomarkers for Heart Failure. Front. Pharmacol. 2020, 11, 560537. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, W.; Tariq, M.A.; Chang, W.; Zhang, X.; Xu, W.; Hou, L.; Wang, Y.; Wang, J. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics 2018, 8, 5855–5869. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, J.; Xie, G.; Zeng, X.; Li, H. Circ-HIPK3 Strengthens the Effects of Adrenaline in Heart Failure by MiR-17-3p-ADCY6 Axis. Int. J. Biol. Sci. 2019, 15, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, R.M.W.; Calore, M. Epigenetics and microRNAs in cardiovascular diseases. Genomics 2021, 113, 540–551. [Google Scholar] [CrossRef]
- Satoh, T.; Wang, L.; Espinosa-Diez, C.; Wang, B.; Hahn, S.A.; Noda, K.; Rochon, E.R.; Dent, M.R.; Levine, A.R.; Baust, J.J.; et al. Metabolic Syndrome Mediates ROS-miR-193b-NFYA–Dependent Downregulation of Soluble Guanylate Cyclase and Contributes to Exercise-Induced Pulmonary Hypertension in Heart Failure With Preserved Ejection Fraction. Circulation 2021, 144, 615–637. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Zucker, I.H. Regulation of Nrf2 signaling pathway in heart failure: Role of extracellular vesicles and non-coding RNAs. Free Radic. Biol. Med. 2021, 167, 218–231. [Google Scholar] [CrossRef]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Shimizu, K.; Miyazaki, Y.; Sari, N.; Shimizu, S.; Mori, K.; Wada, H.; Hasegawa, K.; et al. Histone Acetylation Domains Are Differentially Induced during Development of Heart Failure in Dahl Salt-Sensitive Rats. Int. J. Mol. Sci. 2021, 22, 1771. [Google Scholar] [CrossRef]
- Costantino, S.; Ambrosini, S.; Mohammed, S.A.; Gorica, E.; Akhmedov, A.; Cosentino, F.; Ruschitzka, F.; Hamdani, N.; Paneni, F. A chromatin mark by SETD7 regulates myocardial inflammation in obesity-related heart failure with preserved ejection fraction. Eur. Heart J. 2022, 43, ehac544.2883. [Google Scholar] [CrossRef]
- Yan, F.; Chen, Z.; Cui, W. H3K9me2 regulation of BDNF expression via G9a partakes in the progression of heart failure. BMC Cardiovasc. Disord. 2022, 22, 182. [Google Scholar] [CrossRef]
- Shi, Y.; Fan, W.; Xu, M.; Lin, X.; Zhao, W.; Yang, Z. Critical role of Znhit1 for postnatal heart function and vacuolar cardiomyopathy. JCI Insight 2022, 7, e148752. [Google Scholar] [CrossRef]
- Sun, X.; Hota, S.K.; Zhou, Y.-Q.; Novak, S.; Miguel-Perez, D.; Christodoulou, D.; Seidman, C.E.; Seidman, J.G.; Gregorio, C.C.; Henkelman, R.M.; et al. Cardiac-enriched BAF chromatin-remodeling complex subunit Baf60c regulates gene expression programs essential for heart development and function. Biol. Open 2018, 7, bio029512. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Seto, E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, X.; Liu, Y.; Li, F.; Liu, Q.; Sun, J.; Cai, L. Dysregulation of Histone Acetyltransferases and Deacetylases in Cardiovascular Diseases. Oxid. Med. Cell Longev. 2014, 2014, 641979. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Y.; Ren, J. Acetylation in cardiovascular diseases: Molecular mechanisms and clinical implications. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165836. [Google Scholar] [CrossRef]
- Cao, D.J.; Wang, Z.V.; Battiprolu, P.K.; Jiang, N.; Morales, C.R.; Kong, Y.; Rothermel, B.A.; Gillette, T.G.; Hill, J.A. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc. Natl. Acad. Sci. USA 2011, 108, 4123–4128. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, R.A.; Weeks, K.L. Histone deacetylases in cardiovascular and metabolic diseases. J. Mol. Cell. Cardiol. 2019, 130, 151–159. [Google Scholar] [CrossRef]
- Jiménez-Uribe, A.P.; Gómez-Sierra, T.; Aparicio-Trejo, O.E.; Orozco-Ibarra, M.; Pedraza-Chaverri, J. Backstage players of fibrosis: NOX4, mTOR, HDAC, and S1P; companions of TGF-β. Cell. Signal. 2021, 87, 110123. [Google Scholar] [CrossRef]
- Gillette, T.G. HDAC Inhibition in the Heart: Erasing Hidden Fibrosis. Circulation 2021, 143, 1891–1893. [Google Scholar] [CrossRef]
- Travers, J.G.; Wennersten, S.A.; Peña, B.; Bagchi, R.A.; Smith, H.E.; Hirsch, R.A.; Vanderlinden, L.A.; Lin, Y.-H.; Dobrinskikh, E.; Demos-Davies, K.M.; et al. HDAC Inhibition Reverses Preexisting Diastolic Dysfunction and Blocks Covert Extracellular Matrix Remodeling. Circulation 2021, 143, 1874–1890. [Google Scholar] [CrossRef]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Sirt1 Regulates Aging and Resistance to Oxidative Stress in the Heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef]
- Ijaz, T.; Burke, M.A. BET Protein-Mediated Transcriptional Regulation in Heart Failure. Int. J. Mol. Sci. 2021, 22, 6059. [Google Scholar] [CrossRef]
- Haldar, S.M.; McKinsey, T.A. BET-ting on chromatin-based therapeutics for heart failure. J. Mol. Cell. Cardiol. 2014, 74, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, L.M.; Kharenko, O.A.; Stotz, S.C.; Rakai, B.D.; Sarsons, C.D.; Gilham, D.; Wasiak, S.; Fu, L.; Sweeney, M.; Johansson, J.O.; et al. Breaking boundaries: Pan BETi disrupt 3D chromatin structure, BD2-selective BETi are strictly epigenetic transcriptional regulators. Biomed. Pharmacother. 2022, 152, 113230. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, K.; Cai, W.; Liu, X.; Zhang, Y.; Orkin, S.H.; Xu, J.; Yuan, G.-C. Dissecting super-enhancer hierarchy based on chromatin interactions. Nat. Commun. 2018, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Abnousi, A.; Bazeley, P.; Ni, Y.; Morley, M.; Moravec, C.S.; Hu, M.; Tang, W.H.W. Global analysis of histone modifications and long-range chromatin interactions revealed the differential cistrome changes and novel transcriptional players in human dilated cardiomyopathy. J. Mol. Cell. Cardiol. 2020, 145, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Crosswhite, P.L. ATP-dependent chromatin remodeling complexes in embryonic vascular development and hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2019, 317, H575–H580. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Sig. Transduct. Target. 2022, 7, 374. [Google Scholar] [CrossRef]
- Soler-Botija, C.; Gálvez-Montón, C.; Bayés-Genís, A. Epigenetic Biomarkers in Cardiovascular Diseases. Front. Genet. 2019, 10, 950. [Google Scholar] [CrossRef]
- Gatsiou, A.; Stellos, K. Dawn of Epitranscriptomic Medicine. Circ. Genom. Precis. Med. 2018, 11, e001927. [Google Scholar] [CrossRef]
- Napoli, C.; Benincasa, G.; Donatelli, F.; Ambrosio, G. Precision medicine in distinct heart failure phenotypes: Focus on clinical epigenetics. Am. Heart J. 2020, 224, 113–128. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Verdonschot, J.; Collier, T.; Wang, P.; Pizard, A.; Bär, C.; Björkman, J.; Boccanelli, A.; Butler, J.; Clark, A.; et al. Proteomic Bioprofiles and Mechanistic Pathways of Progression to Heart Failure. Circ. Heart Fail. 2019, 12, e005897. [Google Scholar] [CrossRef] [PubMed]
- Schiano, C.; Costa, V.; Aprile, M.; Grimaldi, V.; Maiello, C.; Esposito, R.; Soricelli, A.; Colantuoni, V.; Donatelli, F.; Ciccodicola, A.; et al. Heart failure: Pilot transcriptomic analysis of cardiac tissue by RNA-sequencing. Cardiol. J. 2017, 24, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W.; Khouri, M.G.; Craig, D.; Haynes, C.; Ilkayeva, O.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure With Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J. Am. Heart Assoc. 2016, 5, e003190. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; Costantino, S.; Mügge, A.; Lebeche, D.; Tschöpe, C.; Thum, T.; Paneni, F. Leveraging clinical epigenetics in heart failure with preserved ejection fraction: A call for individualized therapies. Eur. Heart J. 2021, 42, 1940–1958. [Google Scholar] [CrossRef]
- Gorica, E.; Mohammed, S.A.; Ambrosini, S.; Calderone, V.; Costantino, S.; Paneni, F. Epi-Drugs in Heart Failure. Front. Cardiovasc. Med. 2022, 9, 923014. [Google Scholar] [CrossRef]
- Miranda Furtado, C.L.; Dos Santos Luciano, M.C.; Silva Santos, R.D.; Furtado, G.P.; Moraes, M.O.; Pessoa, C. Epidrugs: Targeting epigenetic marks in cancer treatment. Epigenetics 2019, 14, 1164–1176. [Google Scholar] [CrossRef]
- Iyer, A.; Fenning, A.; Lim, J.; Le, G.T.; Reid, R.C.; Halili, M.A.; Fairlie, D.P.; Brown, L. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Br. J. Pharmacol. 2010, 159, 1408–1417. [Google Scholar] [CrossRef]
- Ngo, V.; Fleischmann, B.K.; Jung, M.; Hein, L.; Lother, A. Histone Deacetylase 6 Inhibitor JS28 Prevents Pathological Gene Expression in Cardiac Myocytes. J. Am. Heart Assoc. 2022, 11, e025857. [Google Scholar] [CrossRef]
- McKinsey, T.A. Targeting Inflammation in Heart Failure with Histone Deacetylase Inhibitors. Mol. Med. 2011, 17, 434–441. [Google Scholar] [CrossRef]
- Cash, H.L.; McGarvey, S.T.; Houseman, E.A.; Marsit, C.J.; Hawley, N.L.; Lambert-Messerlian, G.M.; Viali, S.; Tuitele, J.; Kelsey, K.T. Cardiovascular disease risk factors and DNA methylation at the LINE-1 repeat region in peripheral blood from Samoan Islanders. Epigenetics 2011, 6, 1257–1264. [Google Scholar] [CrossRef]
- Ma, J.; Rebholz, C.M.; Braun, K.V.E.; Reynolds, L.M.; Aslibekyan, S.; Xia, R.; Biligowda, N.G.; Huan, T.; Liu, C.; Mendelson, M.M.; et al. Whole Blood DNA Methylation Signatures of Diet Are Associated With Cardiovascular Disease Risk Factors and All-Cause Mortality. Circ. Genom. Precis. Med. 2020, 13, e002766. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; McMahon, S.; Anand, P.; Shah, H.; Thomas, S.; Salunga, H.T.; Huang, Y.; Zhang, R.; Sahadevan, A.; Lemieux, M.E.; et al. BET bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Sci. Transl. Med. 2017, 9, eaah5084. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Poller, W.; Tsimikas, S.; Most, P.; Paneni, F.; Lüscher, T.F. From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases. Eur. Heart J. 2020, 41, 3884–3899. [Google Scholar] [CrossRef] [PubMed]
- Minerath, R.A.; Hall, D.D.; Grueter, C.E. Targeting transcriptional machinery to inhibit enhancer-driven gene expression in heart failure. Heart Fail Rev. 2019, 24, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hashimoto-Hill, S.; Woo, V.; Eshleman, E.M.; Whitt, J.; Engleman, L.; Karns, R.; Denson, L.A.; Haslam, D.B.; Alenghat, T. Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature 2020, 586, 108–112. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef] [PubMed]
- Viereck, J.; Thum, T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ. Res. 2017, 120, 381–399. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, L.; Beavens, B. The Current Therapeutic Role of Chromatin Remodeling for the Prognosis and Treatment of Heart Failure. Biomedicines 2023, 11, 579. https://doi.org/10.3390/biomedicines11020579
Kraus L, Beavens B. The Current Therapeutic Role of Chromatin Remodeling for the Prognosis and Treatment of Heart Failure. Biomedicines. 2023; 11(2):579. https://doi.org/10.3390/biomedicines11020579
Chicago/Turabian StyleKraus, Lindsay, and Brianna Beavens. 2023. "The Current Therapeutic Role of Chromatin Remodeling for the Prognosis and Treatment of Heart Failure" Biomedicines 11, no. 2: 579. https://doi.org/10.3390/biomedicines11020579
APA StyleKraus, L., & Beavens, B. (2023). The Current Therapeutic Role of Chromatin Remodeling for the Prognosis and Treatment of Heart Failure. Biomedicines, 11(2), 579. https://doi.org/10.3390/biomedicines11020579