Association of Antihistamine Use with Increased Risk of Esophageal Squamous Cell Carcinoma: A Nationwide, Long-Term Follow-Up Study Using Propensity Score Matching
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Study Covariates
2.3. AH Exposure
2.4. PSM and Covariates
2.5. Primary Endpoints
2.6. Sensitivity Analysis
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Association of Comorbidities and Concurrent Medications with ESCC Risk
3.3. IRs, IRRs, and aHRs for HCC among AH Users and Nonusers
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Napier, K.J.; Scheerer, M.; Misra, S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J. Gastrointest. Oncol. 2014, 6, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Ding, Y.F.; Hsu, H.L.; Chang, J.H.; Yuan, K.S.; Wu, A.T.H.; Chow, J.M.; Chang, C.L.; Chen, S.U.; Wu, S.Y. Value and application of trimodality therapy or definitive concurrent chemoradiotherapy in thoracic esophageal squamous cell carcinoma. Cancer 2017, 123, 3904–3915. [Google Scholar] [CrossRef]
- Yen, Y.C.; Chang, J.H.; Lin, W.C.; Chiou, J.F.; Chang, Y.C.; Chang, C.L.; Hsu, H.L.; Chow, J.M.; Yuan, K.S.; Wu, A.T.H.; et al. Effectiveness of esophagectomy in patients with thoracic esophageal squamous cell carcinoma receiving definitive radiotherapy or concurrent chemoradiotherapy through intensity-modulated radiation therapy techniques. Cancer 2017, 123, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Tsai, H.C.; Lin, W.C.; Chang, J.H.; Hsu, H.L.; Chow, J.M.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother. Oncol. 2017, 125, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Domper Arnal, M.J.; Ferrandez Arenas, A.; Lanas Arbeloa, A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 2015, 21, 7933–7943. [Google Scholar] [CrossRef]
- Oncology, N.C.P.G.I. NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf (accessed on 5 December 2022).
- Lin, W.C.; Chang, C.L.; Hsu, H.L.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Three-Dimensional Conformal Radiotherapy-Based or Intensity-Modulated Radiotherapy-Based Concurrent Chemoradiotherapy in Patients with Thoracic Esophageal Squamous Cell Carcinoma. Cancers 2019, 11, 1529. [Google Scholar] [CrossRef]
- Kalpaklioglu, F.; Baccioglu, A. Efficacy and safety of H1-antihistamines: An update. Antiinflamm Antiallergy Agents Med. Chem. 2012, 11, 230–237. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Y.; Li, Q.; Yao, J.; Yuan, X.; Zhang, Y.; Yin, X.; Saito, Y.; Fan, H.; Li, P.; et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell 2022, 40, 36–52.e9. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Chen, W.M.; Chen, Y.C.; Chiang, M.F.; Lee, M.C.; Soong, R.S. Effects of H1-Antihistamines on hepatocellular carcinoma risk in patients with type 2 diabetes mellitus. Diabetes Metab. 2022, 49, 101393. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Lee, W.H.; Wu, A.T.; Chow, J.M.; Chang, C.L.; Yuan, K.S.; Wu, S.Y. Cyproheptadine use in hepatocellular carcinoma. Am. J. Cancer Res. 2017, 7, 584–602. [Google Scholar]
- Shen, Y.C.; Hsu, H.C.; Lin, T.M.; Chang, Y.S.; Hu, L.F.; Chen, L.F.; Lin, S.H.; Kuo, P.I.; Chen, W.S.; Lin, Y.C.; et al. H1-Antihistamines Reduce the Risk of Hepatocellular Carcinoma in Patients With Hepatitis B Virus, Hepatitis C Virus, or Dual Hepatitis B Virus-Hepatitis C Virus Infection. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1206–1219. [Google Scholar] [CrossRef]
- Matsumoto, N.; Ebihara, M.; Oishi, S.; Fujimoto, Y.; Okada, T.; Imamura, T. Histamine H1 receptor antagonists selectively kill cisplatin-resistant human cancer cells. Sci. Rep. 2021, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Kacar, S.; Hacioglu, C.; Kar, F.; Sahinturk, V.; Kanbak, G. Cyproheptadine causes apoptosis and decreases inflammation by disrupting thiol/disulfide balance and enhancing the levels of SIRT1 in C6 glioblastoma cells. Toxicol Vitr. 2021, 73, 105135. [Google Scholar] [CrossRef]
- Chen, S.; Luster, A.D. Antihistamines for cancer immunotherapy: More than just treating allergies. Cancer Cell 2022, 40, 9–11. [Google Scholar] [CrossRef]
- Shi, Z.; Fultz, R.S.; Engevik, M.A.; Gao, C.; Hall, A.; Major, A.; Mori-Akiyama, Y.; Versalovic, J. Distinct roles of histamine H1- and H2-receptor signaling pathways in inflammation-associated colonic tumorigenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G205–G216. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Shen, C.H.; Lin, R.I.; Feng, Y.M.; Huang, S.Y.; Wang, Y.H.; Wu, S.F.; Hsu, C.D.; Chan, M.W. Cyproheptadine exhibits antitumor activity in urothelial carcinoma cells by targeting GSK3beta to suppress mTOR and beta-catenin signaling pathways. Cancer Lett. 2016, 370, 56–65. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Zhang, Y.; Dang, S.; He, S. Astemizole promotes the anti-tumor effect of vitamin D through inhibiting miR-125a-5p-meidated regulation of VDR in HCC. Biomed. Pharmacother. 2018, 107, 1682–1691. [Google Scholar] [CrossRef]
- Feng, Y.M.; Feng, C.W.; Chen, S.Y.; Hsieh, H.Y.; Chen, Y.H.; Hsu, C.D. Cyproheptadine, an antihistaminic drug, inhibits proliferation of hepatocellular carcinoma cells by blocking cell cycle progression through the activation of P38 MAP kinase. BMC Cancer 2015, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.S.; Lee, K.C.; Wang, J.L.; Tseng, L.L.; Chou, K.J.; Tang, K.Y.; Jan, C.R. Histamine-Induced increases in intracellular free Ca2+ levels in hepatoma cells. Chin. J. Physiol. 2000, 43, 165–169. [Google Scholar] [PubMed]
- Lampiasi, N.; Azzolina, A.; Montalto, G.; Cervello, M. Histamine and spontaneously released mast cell granules affect the cell growth of human hepatocellular carcinoma cells. Exp. Mol. Med. 2007, 39, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Chang, C.C.; Sheu, M.T.; Lin, S.Y.; Chung, C.C.; Teng, C.T.; Suk, F.M. The Antihistamine Deptropine Induces Hepatoma Cell Death through Blocking Autophagosome-Lysosome Fusion. Cancers 2020, 12, 1610. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.M.; Feng, C.W.; Lu, C.L.; Lee, M.Y.; Chen, C.Y.; Chen, S.C. Cyproheptadine significantly improves the overall and progression-free survival of sorafenib-treated advanced HCC patients. Jpn. J. Clin. Oncol. 2015, 45, 336–342. [Google Scholar] [CrossRef]
- Feng, Y.M.; Feng, C.W.; Chen, S.C.; Hsu, C.D. Unexpected remission of hepatocellular carcinoma (HCC) with lung metastasis to the combination therapy of thalidomide and cyproheptadine: Report of two cases and a preliminary HCC cell line study. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef]
- He, S.; Lin, B.; Chu, V.; Hu, Z.; Hu, X.; Xiao, J.; Wang, A.Q.; Schweitzer, C.J.; Li, Q.; Imamura, M.; et al. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci. Transl. Med. 2015, 7, 282ra249. [Google Scholar] [CrossRef]
- Wen, C.P.; Tsai, S.P.; Chung, W.S. A 10-year experience with universal health insurance in Taiwan: Measuring changes in health and health disparity. Ann. Intern. Med. 2008, 148, 258–267. [Google Scholar] [CrossRef]
- Sun, M.; Chen, W.M.; Wu, S.Y.; Zhang, J. Dementia risk after major elective surgery based on the route of anaesthesia: A propensity score-matched population-based cohort study. Eclinicalmedicine 2023, 55, 101727. [Google Scholar] [CrossRef]
- Sun, M.Y.; Chang, C.L.; Lu, C.Y.; Wu, S.Y.; Zhang, J.Q. Sarcopenia as an Independent Risk Factor for Specific Cancers: A Propensity Score-Matched Asian Population-Based Cohort Study. Nutrients 2022, 14, 1910. [Google Scholar] [CrossRef]
- Sun, M.; Lin, J.A.; Chang, C.L.; Wu, S.Y.; Zhang, J. Association between long-term opioid use and cancer risk in patients with chronic pain: A propensity score-matched cohort study. Br. J. Anaesth. 2022, 129, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chang, C.L.; Lu, C.Y.; Zhang, J.; Wu, S.Y. Effect of opioids on cancer survival in patients with chronic pain: A propensity score-matched population-based cohort study. Br. J. Anaesth. 2022, 128, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Shao, Y.J.; Chan, T.S.; Tsai, K.; Wu, S.Y. Association between proton pump inhibitors and the risk of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2018, 48, 460–468. [Google Scholar] [CrossRef]
- Bakhtiari, E.; Moazzen, N.; Amirabadi, A.; Ahanchian, H. The clinical relationship between histamine-1 receptor antagonists and risk of cancer: A systematic review and meta-analysis. Expert Rev. Anticancer. Ther. 2022, 23, 87–94. [Google Scholar] [CrossRef]
- Fortuny, J.; Johnson, C.C.; Bohlke, K.; Chow, W.H.; Hart, G.; Kucera, G.; Mujumdar, U.; Ownby, D.; Wells, K.; Yood, M.U.; et al. Use of anti-inflammatory drugs and lower esophageal sphincter-relaxing drugs and risk of esophageal and gastric cancers. Clin. Gastroenterol. Hepatol. 2007, 5, 1154–1159.e3. [Google Scholar] [CrossRef]
- Simons, F.E.; Simons, K.J. H1 antihistamines: Current status and future directions. World Allergy Organ. J. 2008, 1, 145–155. [Google Scholar] [CrossRef]
- Chen, G.; Kashiwagi, H.; Omura, N.; Aoki, T. Effect of a histamine H1 receptor antagonist on gastric endocrine cell proliferation induced by chronic acid suppression in rats. J. Gastroenterol. 2000, 35, 742–747. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, H.K.; Kang, H.S.; Lim, H.; Kim, J.H.; Kim, J.H.; Kim, N.Y.; Cho, S.J.; Nam, E.S.; Min, K.W.; et al. Possible Association between the Use of Proton Pump Inhibitors and H(2) Receptor Antagonists, and Esophageal Cancer: A Nested Case-Control Study Using a Korean National Health Screening Cohort. Pharmaceuticals 2022, 15, 517. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Cao, M.; Yu, Y.; Zhou, J.; Zhang, S.; Tong, F.; Gong, J.; Wang, H.; Yang, F.; et al. Long-term Incidence Rates of Esophageal Squamous Cell Carcinoma in Chinese Patients With Low-grade Intraepithelial Neoplasia and Association of Surveillance Endoscopy with Incidence. JAMA Netw. Open 2022, 5, e2247415. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, X.; Zhuang, M.; Yuan, Z.; Nie, S.; Lu, M.; Jin, L.; Ye, W. Smoking and alcohol drinking in relation to the risk of esophageal squamous cell carcinoma: A population-based case-control study in China. Sci. Rep. 2017, 7, 17249. [Google Scholar] [CrossRef] [PubMed]
- Gammon, M.D.; Schoenberg, J.B.; Ahsan, H.; Risch, H.A.; Vaughan, T.L.; Chow, W.H.; Rotterdam, H.; West, A.B.; Dubrow, R.; Stanford, J.L.; et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J. Natl. Cancer Inst. 1997, 89, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Santoni, G.; Ness-Jensen, E.; Lagergren, J.; Xie, S.H. Association Between Metformin Use and Risk of Esophageal Squamous Cell Carcinoma in a Population-Based Cohort Study. Am. J. Gastroenterol. 2020, 115, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Funkhouser, E.M.; Sharp, G.B. Aspirin and reduced risk of esophageal carcinoma. Cancer 1995, 76, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, A.G.; Singh, P.P.; Murad, M.H.; Iyer, P.G. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 620–629. [Google Scholar] [CrossRef]
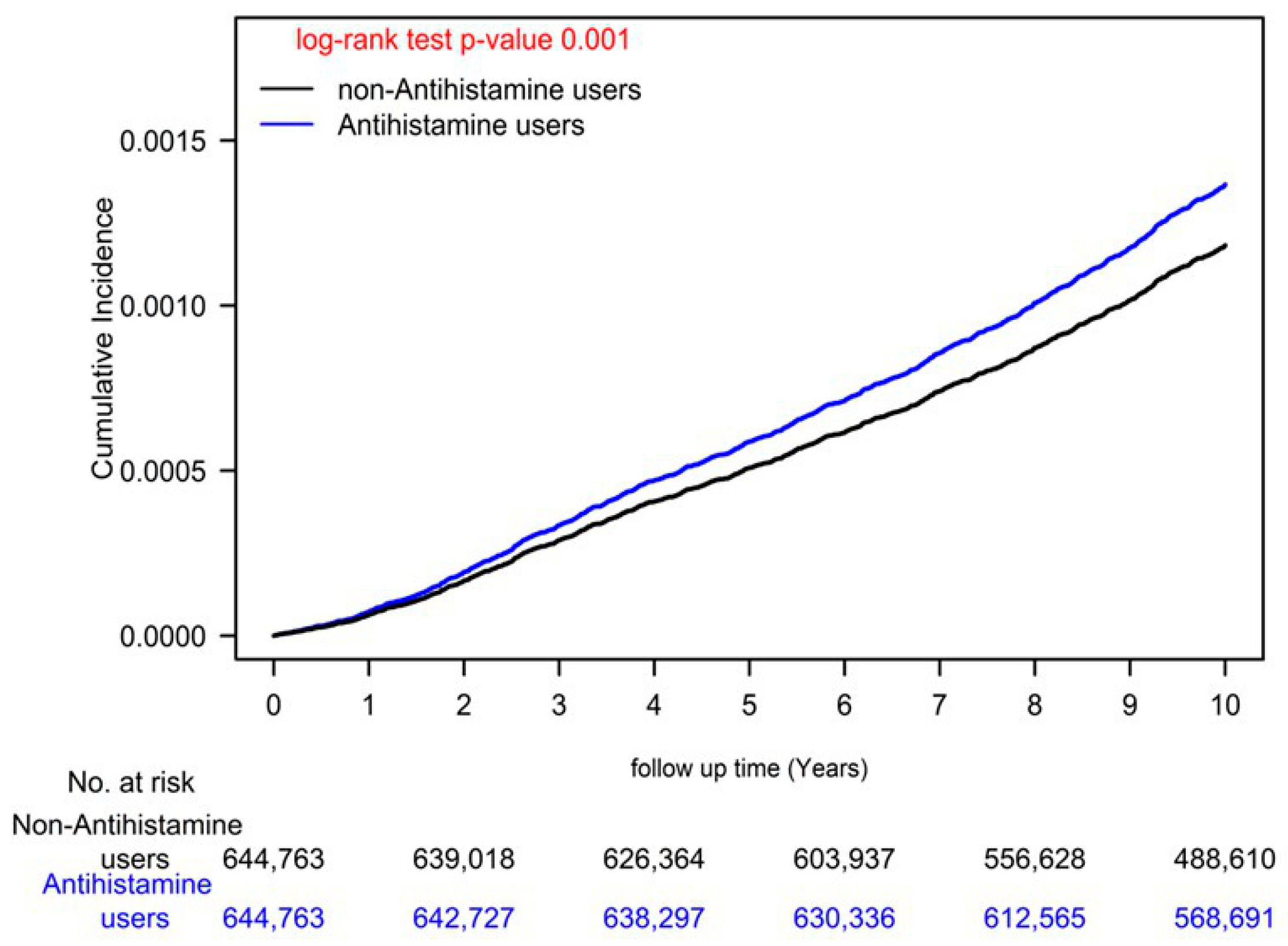

| AH Nonusers | AH Users | ASMD | |||
|---|---|---|---|---|---|
| N = 644,763 | N = 644,763 | ||||
| N | % | N | % | ||
| Age (mean ± SD), years-old | 54.86 ± 13.17 | 54.52 ± 13.06 | 0.001 | ||
| Age, median (IQR, Q1, Q3), years-old | 54.00 (43.00,66.00) | 54.00 (43.00,67.00) | |||
| Age group, years | 0.000 | ||||
| ≤50 | 286,941 | 44.50% | 286,941 | 44.50% | |
| 51–60 | 110,492 | 17.14% | 110,492 | 17.14% | |
| 61–70 | 101,162 | 15.69% | 101,162 | 15.69% | |
| >70 | 146,168 | 22.67% | 146,168 | 22.67% | |
| Sex | 0.000 | ||||
| Female | 308,603 | 47.86% | 308,603 | 47.86% | |
| Male | 336,160 | 52.14% | 336,160 | 52.14% | |
| Income (NTD) | 0.002 | ||||
| Low income | 3458 | 0.54% | 3631 | 0.56% | |
| ≤10,000 | 304,981 | 47.30% | 298,908 | 46.36% | |
| 10,001–20,000 | 180,082 | 28.93% | 177,490 | 27.53% | |
| 20,001–30,000 | 67,170 | 10.42% | 70,101 | 10.87% | |
| 30,001–45,000 | 52,677 | 8.17% | 57,260 | 8.88% | |
| >45,000 | 35,295 | 5.47% | 37,373 | 5.80% | |
| Urbanization | 0.002 | ||||
| Rural | 153,647 | 23.83% | 158,989 | 24.66% | |
| Urban | 491,115 | 76.17% | 485,772 | 75.34% | |
| Cigarettes smoking | 8575 | 1.33% | 8359 | 1.30% | 0.001 |
| Alcoholic related diseases | 1418 | 0.22% | 1321 | 0.20% | 0.001 |
| Comorbidities | |||||
| Diabetes | 9993 | 1.55% | 10,062 | 1.56% | 0.001 |
| Hypertension | 21,844 | 3.39% | 21,796 | 3.38% | 0.001 |
| Hyperlipidemia | 8188 | 1.27% | 8186 | 1.27% | 0.000 |
| Chronic Obstructive Pulmonary Disease | 8314 | 1.29% | 8359 | 1.30% | 0.000 |
| Gastroesophageal reflux disease | 322 | 0.05% | 429 | 0.07% | 0.001 |
| Barrett’s esophagus | 293 | 0.05% | 340 | 0.05% | 0.000 |
| Obesity | 292 | 0.05% | 343 | 0.05% | 0.000 |
| Achalasia | 46 | 0.01% | 47 | 0.01% | 0.000 |
| Tylosis (Howel-Evans syndrome) | 320 | 0.05% | 352 | 0.05% | 0.000 |
| Plummer–Vinson syndrome | 22 | 0.00% | 23 | 0.00% | 0.000 |
| Medication Use | |||||
| Aspirin | 54,077 | 8.39% | 91,324 | 14.16% | |
| Metformin | 34,178 | 5.30% | 50,840 | 7.89% | |
| Statin | 47,681 | 7.40% | 82,266 | 12.76% | |
| PPI | 52,381 | 8.12% | 95,553 | 14.82% | |
| CCI Scores | |||||
| Mean (SD) | 0.09 ± 0.31 | 0.09 ± 0.42 | 0.001 | ||
| Median (IQR, Q1-Q3) | 0.00 (0.00,0.00) | 0.00 (0.00,0.00) | |||
| CCI Scores | 0.001 | ||||
| 0 | 611,236 | 94.80% | 611,866 | 94.90% | |
| ≥1 | 33,527 | 5.20% | 32,897 | 5.10% | |
| AH use | |||||
| cDDD | |||||
| Nonuse | 644,763 | 100.00% | 0 | 0.00% | |
| Q1 | 0 | 0.00% | 162,162 | 25.15% | |
| Q2 | 0 | 0.00% | 160,152 | 24.84% | |
| Q3 | 0 | 0.00% | 161,235 | 25.01% | |
| Q4 | 0 | 0.00% | 161,214 | 25.00% | |
| daily DDD | |||||
| ≤1 | 0 | 0.00% | 468,111 | 72.60% | |
| >1 | 0 | 0.00% | 176,652 | 27.40% | |
| pValue | |||||
| Mean (+/- SD) follow-up, years | 12.59 ± 3.66 | 13.85 ± 2.89 | 0.274 | ||
| Median (IQR, Q1, Q3) follow-up, years | 13.79 (10.15,15.55) | 13.76 (12.62,15.93) | 0.629 | ||
| Primary Outcome | |||||
| ESCC | 0.001 | ||||
| No | 643,661 | 99.83% | 643,542 | 99.81% | |
| Yes | 1102 | 0.17% | 1321 | 0.20% | |
| Crude HR | (95% CI) | p | aHR * | (95% CI) | p | |
|---|---|---|---|---|---|---|
| AH use (ref. nonuser) | ||||||
| AH user | 0.99 | (0.91, 1.07) | 0.725 | 1.22 | (1.12, 1.33) | <0.001 |
| Sex (ref. female) | ||||||
| Male | 2.04 | (1.87, 2.23) | <0.001 | 2.31 | (2.11, 2.53) | <0.001 |
| Age group, years-old (ref. 18–50) | ||||||
| 51–60 | 1.47 | (1.13, 1.95) | <0.001 | 1.45 | (1.41, 1.88) | <0.001 |
| 61–70 | 1.76 | (1.07, 1.91) | <0.001 | 1.52 | (1.04, 1.77) | <0.001 |
| >70 | 2.35 | (1.44, 2.83) | <0.001 | 2.01 | (1.20, 2.18) | <0.001 |
| Income (Ref. Low income, NTD) | ||||||
| ≤10 000 | 1.03 | (0.74, 1.76) | 0.583 | 1.11 | (0.88, 1.15) | 0.516 |
| 10 001–20 000 | 1.36 | (0.77, 2.4) | 0.2915 | 1.16 | (0.65, 2.04) | 0.620 |
| 20 001–30 000 | 1.33 | (0.75, 2.37) | 0.327 | 1.04 | (0.59, 1.86) | 0.884 |
| 30,001–45 000 | 1.27 | (0.71, 2.27) | 0.414 | 0.88 | (0.49, 1.57) | 0.659 |
| >45 000 | 1.01 | (0.56, 1.81) | 0.985 | 0.59 | (0.33, 1.07) | 0.082 |
| Urbanization (Ref. rural) | ||||||
| Urban | 0.79 | (0.72, 0.86) | <0.001 | 0.99 | (0.9, 1.09) | 0.833 |
| Cigarettes smoking (Ref. non-smoker) | 1.37 | (1.16, 1.63) | 0.0003 | 1.22 | (1.13, 1.66) | 0.020 |
| Alcoholic related diseases (Ref. no Alcoholic related diseases) | 2.19 | (1.35, 2.91) | <0.001 | 2.14 | (1.33, 2.35) | <0.01 |
| Comorbidities | ||||||
| Diabetes | 4.29 | (3.49, 5.27) | <0.001 | 1.90 | (0.48, 2.43) | 0.163 |
| Hypertension | 3.37 | (2.87, 3.96) | <0.001 | 1.29 | (0.87, 1.54) | 0.257 |
| Hyperlipidemia | 2.02 | (1.67, 2.45) | <0.001 | 1.80 | (0.46, 2.22) | 0.366 |
| Chronic Obstructive Pulmonary Disease | 2.41 | (1.79, 3.25) | <0.001 | 0.85 | (0.61, 1.16) | 0.302 |
| Gastroesophageal reflux disease | 5.03 | (1.26, 20.09) | 0.0221 | 1.16 | (0.76, 4.34) | 0.433 |
| Barrett’s esophagus | 10.03 | (4.78, 21.04) | <0.001 | 2.20 | (0.73, 4.72) | 0.442 |
| Obesity | 1.24 | (0.18, 8.84) | 0.8269 | 0.69 | (0.1, 4.91) | 0.710 |
| Achalasia | 11.43 | (1.61, 80.91) | 0.0147 | 8.98 | (0.86, 14.13) | 0.329 |
| Tylosis (Howel-Evans syndrome) | 1.19 | (0.17, 8.32) | 0.8633 | 0.44 | (0.06, 3.16) | 0.416 |
| Plummer–Vinson syndrome | 1.00 | (0.43, 5.11) | 0.9429 | 1.00 | (0.66, 1.43) | 0.918 |
| Medication Use | ||||||
| Aspirin | 1.61 | (1.44, 1.79) | <0.001 | 0.63 | (0.56, 0.71) | <0.001 |
| Metformin | 1.46 | (1.27, 1.67) | <0.001 | 0.77 | (0.66, 0.9) | 0.001 |
| Statin | 0.88 | (0.77, 1.01) | 0.0738 | 0.38 | (0.33, 0.44) | <0.001 |
| PPI | 1.44 | (1.29, 1.61) | <0.001 | 1.22 | (1.65, 1.81) | <0.001 |
| CCI (Ref. 0) | ||||||
| CCI ≥ 1 | 2.95 | (2.56, 3.4) | <0.001 | 1.23 | (0.84, 1.46) | 0.316 |
| Events | Person-Years | IR, 10, 000 Person-Year (per 100,000 Person-Year) | IRR | 95%CI for IRR | aHR * | 95%CI for HR | p | |
|---|---|---|---|---|---|---|---|---|
| AH use | ||||||||
| Nonuse (≤28 cDDD) | 1102 | 8,116,205.0 | 1.36 | Ref. | Ref. | |||
| >28 | 1221 | 8,930,408.0 | 1.47 | 1.18 | (1.08, 1.28) | 1.22 | (1.12, 1.33) | <0.001 |
| AH use (cDDD) | ||||||||
| Nonuse (<28 cDDD) | 1102 | 8,116,205.0 | 1.36 | Ref. | Ref. | |||
| Q1, 182 cDDD | 335 | 2,112,942.0 | 1.49 | 1.08 | (0.95, 1.22) | 1.12 | (0.99, 1.27) | 0.084 |
| Q2, 488 cDDD | 334 | 2,224,884.0 | 1.37 | 1.15 | (1.01, 1.31) | 1.20 | (1.05, 1.36) | 0.006 |
| Q3, 1043 cDDD | 318 | 2,291,045.0 | 1.28 | 1.20 | (1.06, 1.37) | 1.25 | (1.1, 1.43) | 0.001 |
| Q4, 10,936 cDDD | 334 | 2,301,536.0 | 1.34 | 1.32 | (1.16, 1.5) | 1.37 | (1.2, 1.56) | <0.001 |
| Subpopulation or Exposure | No. of Patients | ESCC Risk | |||
|---|---|---|---|---|---|
| No. of ESCC | aHR * | 95% CI | p | ||
| Age group, years | |||||
| ≤50 | 573,882 | 44 | 1.08 | (0.36, 1.30) | 0.246 |
| 51–60 | 220,984 | 307 | 1.10 | (0.82, 1.31) | 0.753 |
| 61–70 | 202,324 | 616 | 1.11 | (0.94, 1.30) | 0.219 |
| ≥71 | 292,336 | 1356 | 1.24 | (1.11, 1.38) | <0.001 |
| Sex | |||||
| Female | 617,206 | 718 | 1.75 | (1.50, 2.03) | <0.001 |
| Male | 672,320 | 1605 | 0.97 | (0.87, 1.07) | 0.498 |
| Cigarettes smoking | 16,934 | 3377 | 1·27 | (1·05, 1·77) | <0.001 |
| Alcoholic related diseases | 2739 | 493 | 1·18 | (1·07, 2·29) | <0.001 |
| DDD | |||||
| ≤1 | 986,911 | 1765 | 0.97 | (0.81, 1.14) | 0.685 |
| >1 | 302,615 | 558 | 1.22 | (1.10, 1.34) | <0.001 |
| Medication use | |||||
| Aspirin | 145,401 | 409 | 1.06 | (0.86, 1.31) | 0.571 |
| Metformin | 85,018 | 227 | 0.89 | (0.68, 1.17) | 0.402 |
| Statin | 129,947 | 228 | 1.15 | (0.87, 1.52) | 0.336 |
| PPI | 147,934 | 386 | 1.07 | (0.63, 1.95) | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.-Y.; Yu, Y.-H.; Chen, W.-M.; Shia, B.-C.; Chen, M.; Wu, S.-Y. Association of Antihistamine Use with Increased Risk of Esophageal Squamous Cell Carcinoma: A Nationwide, Long-Term Follow-Up Study Using Propensity Score Matching. Biomedicines 2023, 11, 578. https://doi.org/10.3390/biomedicines11020578
Peng J-Y, Yu Y-H, Chen W-M, Shia B-C, Chen M, Wu S-Y. Association of Antihistamine Use with Increased Risk of Esophageal Squamous Cell Carcinoma: A Nationwide, Long-Term Follow-Up Study Using Propensity Score Matching. Biomedicines. 2023; 11(2):578. https://doi.org/10.3390/biomedicines11020578
Chicago/Turabian StylePeng, Jhao-Yang, Ying-Hui Yu, Wan-Ming Chen, Ben-Chang Shia, Mingchih Chen, and Szu-Yuan Wu. 2023. "Association of Antihistamine Use with Increased Risk of Esophageal Squamous Cell Carcinoma: A Nationwide, Long-Term Follow-Up Study Using Propensity Score Matching" Biomedicines 11, no. 2: 578. https://doi.org/10.3390/biomedicines11020578
APA StylePeng, J.-Y., Yu, Y.-H., Chen, W.-M., Shia, B.-C., Chen, M., & Wu, S.-Y. (2023). Association of Antihistamine Use with Increased Risk of Esophageal Squamous Cell Carcinoma: A Nationwide, Long-Term Follow-Up Study Using Propensity Score Matching. Biomedicines, 11(2), 578. https://doi.org/10.3390/biomedicines11020578






