Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol
Abstract
1. Introduction
2. Phytochemical Overview
3. Methodology
4. Mechanisms Related to Macrophages’ and Other Immune Cells’ Activation
5. Arthritis
6. Lung Inflammation
7. Skin Inflammation
8. Neuroinflammation
9. Diabetes-Associated Inflammation
10. Cardiac Inflammation
11. Hepato- and Renal Inflammation
12. Obesity-Associated Inflammation
13. Endothelial Inflammation
14. Gut Inflammation
15. General Discussion
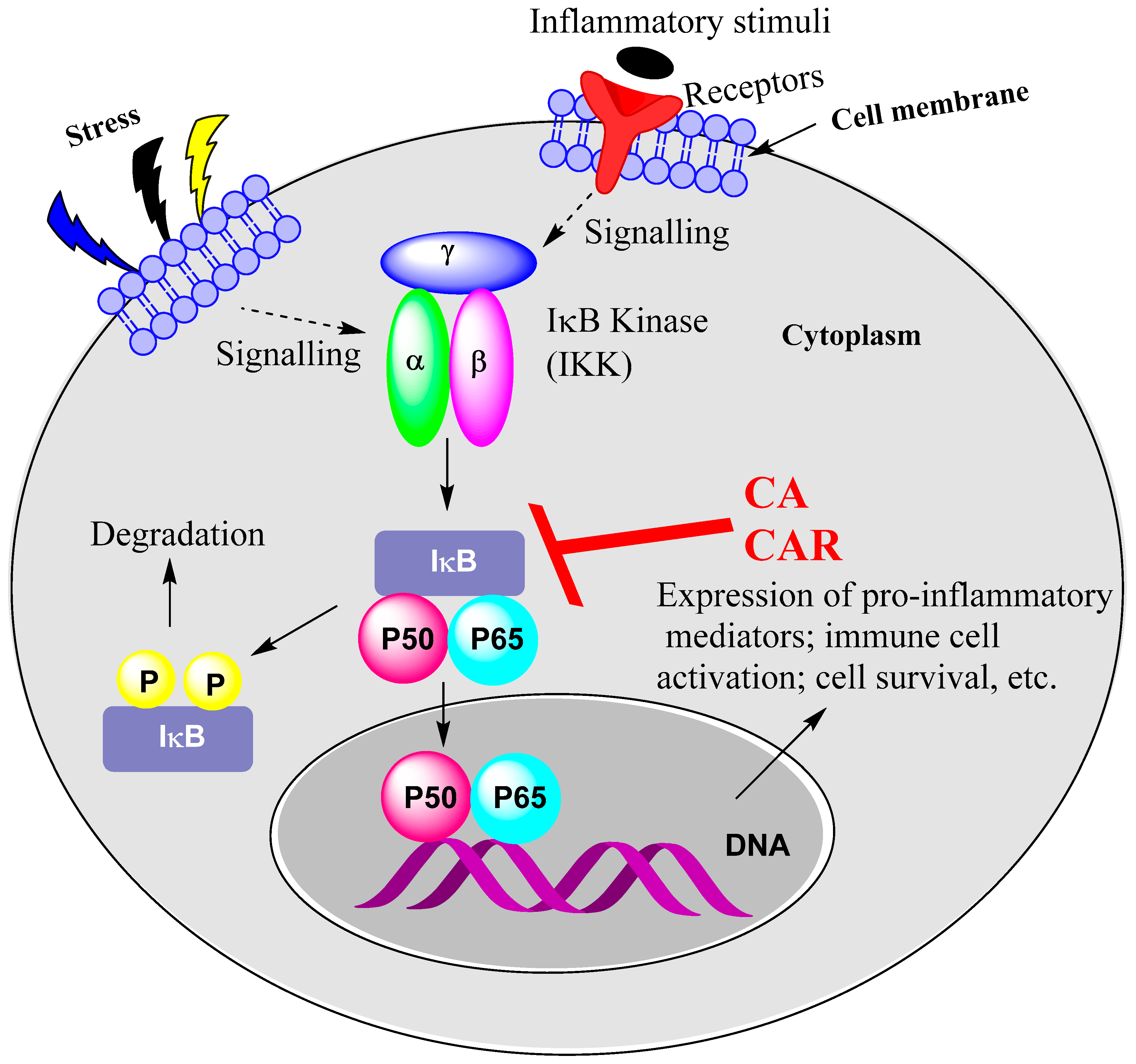
15.1. Rosemary Diterpenes Inhibit Activation of NF-κB
15.2. Rosemary Diterpenes Modulate the MAPK Pathways
15.3. Rosemary Diterpenes Modulate the SIRT1/SERT3 Pathways
15.4. Rosemary Diterpenes Activate the Nrf2/HO-1 Pathways of Cytoprotection
15.5. Rosemary Diterpenes Suppress the NLRP3 Inflammasome
16. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Sig. Transduct. Target Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, C.; McKernan, D.P. Anti-Viral Pattern Recognition Receptors as Therapeutic Targets. Cells 2021, 10, 2258. [Google Scholar] [CrossRef]
- Kimura, Y.; Tsukui, D.; Kono, H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 12394. [Google Scholar] [CrossRef]
- Kanneganti, T.D. The inflammasome: Firing up innate immunity. Immunol. Rev. 2015, 265, 1–5. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Kotsovilis, S.; Andreakos, E. Therapeutic human monoclonal antibodies in inflammatory diseases. Methods Mol. Biol. 2014, 1060, 37–59. [Google Scholar]
- Lai, Y.; Dong, C. Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases. Int. Immunol. 2016, 28, 181–188. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Kim, M.E.; Kim, D.H.; Lee, J.S. Transcription Factors as Targets of Natural Compounds in Age-Related Diseases and Cancer: Potential Therapeutic Applications. Int. J. Mol. Sci. 2022, 23, 13882. [Google Scholar] [CrossRef]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb. Perspect. Biol. 2019, 11, a028548. [Google Scholar] [CrossRef]
- Habtemariam, S. The Therapeutic Potential of Rosemary (Rosmarinus officinalis) Diterpenes for Alzheimer’s Disease. Evid. Based Complement. Alternat. Med. 2016, 2016, 2680409. [Google Scholar] [CrossRef]
- Birtić, S. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef]
- Razboršek, M.I.; Ivanović, M. Stability studies and determination of carnosic acid and its oxidative degradation products by gas chromatography–mass spectrometry. Inter. J. Mass Spectr. 2016, 407, 29–39. [Google Scholar] [CrossRef]
- Buchin, Y.; Sakemi, Y.; Hamashima, R.; Morioka, Y.; Yamanaka, D.; Hakuno, F.; Takahashi, S.-I.; Shindo, K. Structures and biological activities of new carnosic acid- and carnosol-related compounds generated by heat treatment of rosemary. Phytochem. Lett. 2019, 30, 43–48. [Google Scholar] [CrossRef]
- Oh, J.; Yu, T.; Choi, S.J.; Yang, Y.; Baek, H.S.; An, S.A.; Kwon, L.K.; Kim, J.; Rho, H.S.; Shin, S.S.; et al. Syk/Src pathway-targeted inhibition of skin inflammatory responses by carnosic acid. Mediators Inflamm. 2012, 2012, 781375. [Google Scholar] [CrossRef]
- Chae, I.G.; Yu, M.H.; Im, N.K.; Jung, Y.T.; Lee, J.; Chun, K.S.; Lee, I.S. Effect of Rosemarinus officinalis L. on MMP-9, MCP-1 levels, and cell migration in RAW 264.7 and smooth muscle cells. J. Med. Food. 2012, 15, 879–886. [Google Scholar] [CrossRef]
- Mengoni, E.S.; Vichera, G.; Rigano, L.A.; Rodriguez-Puebla, M.L.; Galliano, S.R.; Cafferata, E.E.; Pivetta, O.H.; Moreno, S.; Vojnov, A.A. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia 2011, 82, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Wei, W.H.; Zhang, X.W.; Liu, D.; Zeng, K.W.; Tu, P.F. An Integrated Proteomics and Bioinformatics Approach Reveals the Anti-inflammatory Mechanism of Carnosic Acid. Front. Pharmacol. 2018, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.H.; Liang, Y.C.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis 2002, 23, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Hwang, C.J.; Choi, J.Y.; Park, M.H.; Song, M.J.; Oh, K.W.; Son, D.J.; Lee, S.H.; Han, S.B.; Hong, J.T. Inhibitory Effect of Carnosol on Phthalic Anhydride-Induced Atopic Dermatitis via Inhibition of STAT3. Biomol. Ther. 2017, 25, 535–544. [Google Scholar] [CrossRef]
- Schwager, J.; Richard, N.; Fowler, A.; Seifert, N.; Raederstorff, D. Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes. Molecules 2016, 21, 465. [Google Scholar] [CrossRef]
- Shi, W.; Xu, G.; Zhan, X.; Gao, Y.; Wang, Z.; Fu, S.; Qin, N.; Hou, X.; Ai, Y.; Wang, C.; et al. Carnosol inhibits inflammasome activation by directly targeting HSP90 to treat inflammasome-mediated diseases. Cell Death. Dis. 2020, 11, 252. [Google Scholar] [CrossRef]
- Bauer, J.; Kuehnl, S.; Rollinger, J.M.; Scherer, O.; Northoff, H.; Stuppner, H.; Werz OKoeberle, A. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1. J. Pharmacol. Exp. Ther. 2012, 342, 169–176. [Google Scholar] [CrossRef]
- Maione, F.; Cantone, V.; Pace, S.; Chini, M.G.; Bisio, A.; Romussi, G.; Pieretti, S.; Werz, O.; Koeberle, A.; Mascolo, N.; et al. Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions. Br. J. Pharmacol. 2017, 174, 1497–1508. [Google Scholar] [CrossRef]
- Crozier, R.W.E.; Yousef, M.; Coish, J.M.; Fajardo, V.A.; Tsiani, E.; MacNeil, A.J. Carnosic acid inhibits secretion of allergic inflammatory mediators in IgE-activated mast cells via direct regulation of Syk activation. J. Biol. Chem. 2023, 102867. [Google Scholar] [CrossRef]
- Foresti, R.; Bains, S.K.; Pitchumony, T.S.; de Castro Brás, L.E.; Drago, F.; Dubois-Randé, J.-L.; Bucolo, C.; Motterlini, R. Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol. Res. 2013, 76, 132–148. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hosokawa, Y.; Ozaki, K.; Matsuo, T. Carnosic acid inhibits inflammatory cytokines production in human periodontal ligament cells. Immunopharmacol. Immunotoxicol. 2020, 42, 373–378. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hosokawa, Y.; Ozaki, K.; Matsuo, T. Carnosic Acid Inhibits CXCR3 Ligands Production in IL-27-Stimulated Human Oral Epithelial Cells. Inflammation 2019, 42, 1311–1316. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, X.; Zhou, L.; Liu, Z.; Yuan, J.; Cheng, J.; Zhao, J.; Wu, L.; Li, H.; Qiu, H.; et al. Carnosic acid inhibits inflammation response and joint destruction on osteoclasts, fibroblast-like synoviocytes, and collagen-induced arthritis rats. J. Cell Physiol. 2018, 233, 6291–6303. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Yang, S.-C.; Hsu, Y.-H.; Chen, C.-Y.; Chen, P.-J.; Syu, Y.-T.; Lin, C.-H.; Hwang, T.G.-L. Carnosic acid inhibits reactive oxygen species-dependent neutrophil extracellular trap formation and ameliorates acute respiratory distress syndrome. Life Sci. 2022, 121334. [Google Scholar] [CrossRef]
- Kawamura, T.; Momozane, T.; Sanosaka, M.; Sugimura, K.; Iida, O.; Fuchino, H.; Funaki, S.; Shintani, Y.; Inoue, M.; Minami, M.; et al. Carnosol Is a Potent Lung Protective Agent: Experimental Study on Mice. Transplant. Proceed. 2015, 47, 1657–1661. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; de Souza, I.C.C.; Fürstenau, C.R. Carnosic Acid Induces Anti- Inflammatory Effects in Paraquat-Treated SH-SY5Y Cells Through a Mechanism Involving a Crosstalk Between the Nrf2/HO-1 Axis and NF-κB. Mol. Neurobiol. 2018, 55, 890–897. [Google Scholar] [CrossRef]
- Martin, D.; Rojo, A.I.; Salinas, M.; Diaz, R.; Gallardo, G.; Alam, J.; de Galarreta, C.M.R.; Cuadrado, A. Regulation of Heme Oxygenase-1 Expression through the Phosphatidylinositol 3-Kinase/Akt Pathway and the Nrf2 Transcription Factor in Response to the Antioxidant Phytochemical Carnosol. J. Biol. Chem. 2004, 279, 8919–8929. [Google Scholar] [CrossRef]
- Wu, C.-R.; Tsai, C.-W.; Chang, S.-W.; Lin, C.-Y.; Huang, L.C.; Tsai, C.-W. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: Involvement of antioxidative enzymes induction. Chemico-Biol. Inter. 2015, 225, 40–46. [Google Scholar] [CrossRef]
- Hou, C.-W.; Lin, Y.-T.; Chen, Y.-L.; Wang, Y.H.; Chou, J.L.; Ping, L.Y.; Jeng, K.C. Neuroprotective effects of carnosic acid on neuronal cells under ischemic and hypoxic stress. Nutr. Neurosci. 2012, 15, 257–263. [Google Scholar] [CrossRef]
- Meng, P.; Yoshida, H.; Matsumiya, T.; Imaizumi, T.; Tanji, K.; Xing, F.; Hayakari, R.; Dempoya, J.; Tatsut, T.; Aizawa-Yashiro, T.; et al. Carnosic acid suppresses the production of amyloid-β 1-42 by inducing the metalloprotease gene TACE/ADAM17 in SH-SY5Y human neuroblastoma cells. Neurosci. Res. 2013, 75, 94–102. [Google Scholar] [CrossRef]
- Yoshida, H.; Meng, P.; Matsumiya, T.; Tanji, K.; Hayakari, R.; Xing, F.; Wang, L.; Tsuruga, K.; Tanaka, H.; Mimura, J.; et al. Carnosic acid suppresses the production of amyloid-β 1-42 and 1-43 by inducing an α-secretase TACE/ADAM17 in U373MG human astrocytoma cells. Neurosci. Res. 2014, 79, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Liu, K.L.; Lin, Y.R.; Kuo, W.C. The mechanisms of carnosic acid attenuates tumor necrosis factor-α-mediated inflammation and insulin resistance in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2014, 58, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Dewanjee, S.; Joardar, S.; Chakraborty, P.; Bhattacharya, H.; Bhanja, S.; Bhattacharyya, C.; Bhowmik, M.; Bhowmick, S.; Saha, A.; et al. Carnosic acid attenuates doxorubicin-induced cardiotoxicity by decreasing oxidative stress and its concomitant pathological consequences. Food Chem. Toxicol. 2022, 166, 113205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Yang, J.J.; Zhang, H.S. Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomed. Pharmacother. 2019, 109, 71–83. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Momeni-Moghaddam, M.A.; Chini, M.G.; Saviano, A.; Maione, F.; Bifulco, G.; Rahmanian-Devin, P.; Jebalbarezy, A.; Askari, V.R. Carnosol Attenuates LPS- Induced Inflammation of Cardiomyoblasts by Inhibiting NF-κB: A Mechanistic in Vitroand in SilicoStudy. Evid. Based Complement. Alternat. Med. 2022, 2022, 7969422. [Google Scholar] [CrossRef]
- Gao, L.; Shan, W.; Zeng, W.; Hu, Y.; Wang, G.; Tian, X.; Zhang, N.; Shi, X.; Zhao, Y.; Ding, C.; et al. Carnosic acid alleviates chronic alcoholic liver injury by regulating the SIRT1/ChREBP and SIRT1/p66shc pathways in rats. Mol. Nutr. Food Res. 2016, 60, 1902–1911. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.J.; Chen, G.Y.; Wang, W.W.; Xie, Z.T.; Tang, G.F.; Wei, S.D. Carnosic acid nanoparticles suppress liver ischemia/reperfusion injury by inhibition of ROS, Caspases and NF-κB signaling pathway in mice. Biomed. Pharmacother. 2016, 82, 237–246. [Google Scholar] [CrossRef]
- Park, M.Y.; Mun, S.T. Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS- stimulated 3T3-L1 adipocytes. Nutr. Res. Pract. 2014, 8, 516–620. [Google Scholar] [CrossRef]
- D’Agata, V.; D’Amico, A.G.; Maugeri, G.; Bucolo, C.; Rossi, S.; Giunta, S. Carnosol attenuates high glucose damage in human retinal endothelial cells through regulation of ERK/Nrf2/HO-1 pathway. J. Asian Nat. Prod. Res. 2022; in press. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Hou, N.; Li, J.; Liu, M.; Peng, S.; Zhang, Y.; Luo, Y.; Zhao, B.; Wang, S.; et al. Carnosol as a Nrf2 Activator Improves Endothelial Barrier Function Through Antioxidative Mechanisms. Int. J. Mol. Sci. 2019, 20, 880. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, G.; Peng, K.; Gao, Y.; Wang, J.; Gao, C.; He, C.; Lu, F. Carnosol Maintains Intestinal Barrier Function and Mucosal Immune Homeostasis in DSS-Induced Colitis. Front. Nutr. 2022, 9, 894307. [Google Scholar] [CrossRef]
- Chrastina, M.; Poništ, S.; Tóth, J.; Czigle, S.; Pašková, Ľ.; Vyletelová, V.; Švík, K.; Bauerová, K. Combination Therapy of Carnosic Acid and Methotrexate Effectively Suppressed the Inflammatory Markers and Oxidative Stress in Experimental Arthritis. Molecules 2022, 27, 7115. [Google Scholar] [CrossRef]
- Xia, G.; Wang, X.; Sun, H.; Qin, Y.; Fu, M. Carnosic acid (CA) attenuates collagen- induced arthritis in db/db mice via inflammation suppression by regulating ROS- dependent p38 pathway. Free Radic. Biol. Med. 2017, 108, 418–432. [Google Scholar] [CrossRef]
- Li, L.; Pan, Z.; Ning, D.; Fu, Y. Rosmanol and Carnosol Synergistically Alleviate Rheumatoid Arthritis through Inhibiting TLR4/NF-κB/MAPK Pathway. Molecules 2022, 27, 78. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Sun, H.; Cao, K. Carnosic acid protects against lipopolysaccharide-induced acute lung injury in mice. Exp. Ther. Med. 2019, 18, 3707–3714. [Google Scholar] [CrossRef]
- Kalantar, H.; Sadeghi, E.; Abolnezhadian, F.; Goudarzi, M.; Hemmati, A.A.; Basir, Z.; Kalantar, M. Carnosol attenuates bleomycin-induced lung damage via suppressing fibrosis, oxidative stress and inflammation in rats. Life Sci. 2021, 287, 120059. [Google Scholar] [CrossRef]
- Lee, J.E.; Im, D.S. Suppressive Effect of Carnosol on Ovalbumin-Induced Allergic Asthma. Biomol. Ther. 2021, 29, 58–63. [Google Scholar] [CrossRef]
- da Rosa, J.S.; Facchin, B.M.; Bastos, J.; Siqueira, M.A.; Micke, G.A.; Dalmarco, E.M.; Pizzolatti, M.G.; Fröde, T.S. Systemic administration of Rosmarinus officinalis attenuates the inflammatory response induced by carrageenan in the mouse model of pleurisy. Planta Med. 2013, 79, 1605–1614. [Google Scholar] [CrossRef]
- Yeo, I.J.; Park, J.H.; Jang, J.S.; Lee, D.Y.; Park, J.E.; Choi, Y.E.; Joo, J.H.; Song, J.K.; Jeon, H.O.; Hong, J.T. Inhibitory effect of Carnosol on UVB-induced inflammation via inhibition of STAT3. Arch. Pharm. Res. 2019, 42, 274–283. [Google Scholar] [CrossRef]
- AlKahtane, A.A.; Ghanem, E.; Bungau, S.G.; Alarifi, S.; Ali, D.; AlBasher, G.; Alkahtani, S.; Aleya, L.; Abdel-Daim, M.M. Carnosic acid alleviates chlorpyrifos-induced oxidative stress and inflammation in mice cerebral and ocular tissues. Environ. Sci. Pollut. Res. Int. 2020, 27, 11663–11670. [Google Scholar] [CrossRef]
- Maynard, M.E.; Underwood, E.L.; Redell, J.B.; Zhao, J.; Kobori, N.; Hood, K.N.; Moore, A.N.; Dash, P.K. Carnosic Acid Improves Outcome after Repetitive Mild Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Xie, Y.X.; Zhang, J.W.; Qiu, X.H.; Cheng, A.B.; Tian, L.; Ma, B.Y.; Hou, Y.B. Carnosol protects against spinal cord injury through Nrf-2 upregulation. J. Recept. Signal Transduct. Res. 2016, 36, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Singh, I.N.; Wang, J.A.; Hall, E.D. Nrf2–ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp. Neurol. 2015, 264, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Fan, L.; Peng, Y.; He, X.; Chen, H.; Duan, H.; Yang, F.; Lin, D.; Lin, Z.; Li, H.; et al. Carnosic Acid Mitigates Early Brain Injury After Subarachnoid Hemorrhage: Possible Involvement of the SIRT1/p66shc Signaling Pathway. Front. Neurosci. 2019, 13, 26. [Google Scholar] [CrossRef]
- Yi-Bin, W.; Xiang, L.; Bing, Y.; Qi, Z.; Fei-Tong, J.; Minghong, W.; Xiangxiang, Z.; Le, K.; Yan, L.; Ping, S.; et al. Inhibition of the CEBPβ-NFκB interaction by nanocarrier-packaged Carnosic acid ameliorates glia-mediated neuroinflammation and improves cognitive function in an Alzheimer’s disease model. Cell Death Dis. 2022, 13, 318. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Han, J.J.; Zhang, F.; Liu, S.; Zhu, L.; Wang, Z.Z.; Zhang, G.X.; Zhang, Y. Carnosol Modulates Th17 Cell Differentiation and Microglial Switch in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2018, 9, 1807. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A.; Farkhondeh, T. Evaluation of Antidiabetic Activity of Carnosol (Phenolic Diterpene in Rosemary) in Streptozotocin-Induced Diabetic Rats. Cardiovasc. Hematol. Disord. Drug Targets 2017, 17, 11–17. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Zhao, D.; Du, B.; Wang, M. Protective effect of rosmarinic acid and carnosic acid against streptozotocin-induced oxidation, glycation, inflammation and microbiota imbalance in diabetic rats. Food Funct. 2018, 9, 851–860. [Google Scholar] [CrossRef]
- Xie, Z.; Zhong, L.; Wu, Y.; Wan, X.; Yang, H.; Xu, X.; Li, P. Carnosic acid improves diabetic nephropathy by activating Nrf2/ARE and inhibition of NF-κB pathway. Phytomedicine 2018, 47, 161–173. [Google Scholar] [CrossRef]
- Hu, M.; Li, T.; Bo, Z.; Xiang, F. The protective role of carnosic acid in ischemic/reperfusion injury through regulation of autophagy under T2DM. Exp. Biol. Med. 2019, 244, 602–611. [Google Scholar] [CrossRef]
- Song, H.M.; Li, X.; Liu, Y.Y.; Lu, W.P.; Cui, Z.H.; Zhou, L.; Yao, D.; Zhang, H.M. Carnosic acid protects mice from high-fat diet-induced NAFLD by regulating MARCKS. Int. J. Mol. Med. 2018, 42, 193–207. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, Z.; Wang, Y.; Xiao, H.; Wu, W.; Xiao, C.; Liu, X. Carnosic acid attenuates lipopolysaccharide-induced liver injury in rats via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2013, 53, 1–9. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hong, H.L.; Kim, G.M.; Leem, J.; Kwon, H.H. Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Molecules 2021, 26, 7589. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Zheng, Y.; Zhang, N. Carnosol protects against renal ischemia-reperfusion injury in rats. Exp. Anim. 2018, 67, 545–553. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Hu, M.; Li, Y.H.; Cao, X.H. Carnosic acid alleviates brain injury through NF-κB-regulated inflammation and Caspase-3-associated apoptosis in high fat-induced mouse models. Mol. Med. Rep. 2019, 20, 495–504. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chuang, L.T.; Lien, T.J.; Liing, Y.R.; Chen, W.Y.; Tsai, P.J. Rosmarinus officinalis extract suppresses Propionibacterium acnes-induced inflammatory responses. J. Med. Food. 2013, 16, 324–333. [Google Scholar] [CrossRef]
- Yousef, M.; Crozier, R.W.E.; Hicks, N.J.; Watson, C.J.F.; Boyd, T.; Tsiani, E.; MacNeil, A.J. Attenuation of allergen-mediated mast cell activation by rosemary extract (Rosmarinus officinalis L.). J. Leukoc. Biol. 2020, 107, 843–857. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; Yang, Y.; Ding, Y.; Sun, X. Carnosol suppresses microglia cell inflammation and apoptosis through PI3K/AKT/mTOR signaling pathway. Immunopharmacol. Immunotoxicol. 2022, 44, 656–662. [Google Scholar] [CrossRef]
- Pungsrinont, T.; Kallenbach, J.; Baniahmad, A. Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 11088. [Google Scholar] [CrossRef]
- Inoue, K.; Takano, H.; Shiga, A.; Fujita, Y.; Makino, H.; Yanagisawa, R.; Ichinose, T.; Kato, Y.; Yamada, T.; Yoshikawa, T. Effects of volatile constituents of a rosemary extract on allergic airway inflammation related to house dust mite allergen in mice. Int. J. Mol. Med. 2005, 16, 315–319. [Google Scholar] [CrossRef]
- Rodrigues, M.R.; Kanazawa, L.K.; das Neves, T.L.; da Silva, C.F.; Horst, H.; Pizzolatti, M.G.; Santos, A.R.; Baggio, C.H.; Werner, M.F. Antinociceptive and anti-inflammatory potential of extract and isolated compounds from the leaves of Salvia officinalis in mice. J. Ethnopharmacol. 2012, 139, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Ali-Beig, H.; Farahbakhsh, S.; Mojabi, N.; Rastegar-Moghadam, B.; Arbabian, S.; Kazemi, M.; Tekieh, E.; Golmanesh, L.; Ranjbaran, M.; et al. Hydroalcoholic extract of Rosemary (Rosmarinus officinalis L.) and its constituent carnosol inhibit formalin-induced pain and inflammation in mice. Pak. J. Biol. Sci. 2013, 16, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bakirel, T.; Bakirel, U.; Keleş, O.U.; Ulgen, S.G.; Yardibi, H. In Vivo Assessment of Antidiabetic and Antioxidant Activities of Rosemary (Rosmarinus officinalis) in Alloxan-Diabetic Rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Eidi, M.; Eidi, A.; Zamanizadeh, H. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 310–313. [Google Scholar] [CrossRef]
- Lima, C.F.; Azevedo, M.F.; Araujo, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Metformin-like effect of Salvia officinalis (common sage): Is it useful in diabetes prevention? Br. J. Nutr. 2006, 96, 326–333. [Google Scholar] [CrossRef]
- Emam, M. Comparative Evaluation of Antidiabetic Activity of Rosmarinus officinalis L. and Chamomile Recutita in Streptozotocin Induced Diabetic Rats. Agric. Biol. J. N. Am. 2012, 3, 247–252. [Google Scholar] [CrossRef]
- Ramadan, K.S.; Khalil, O.A.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Hypoglycemic and Hepatoprotective Activity of Rosmarinus officinalis Extract in Diabetic Rats. J. Physiol. Biochem. 2013, 69, 779–783. [Google Scholar] [CrossRef]
- Hasei, S.; Yamamotoya, T.; Nakatsu, Y.; Ohata, Y.; Itoga, S.; Nonaka, Y.; Matsunaga, Y.; Sakoda, H.; Fujishiro, M.; Kushiyama, A.; et al. Carnosic Acid and Carnosol Activate AMPK, Suppress Expressions of Gluconeogenic and Lipogenic Genes, and Inhibit Proliferation of HepG2 Cells. Int. J. Mol. Sci. 2021, 22, 4040. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Tsiani, E. Attenuation of Free Fatty Acid-Induced Muscle Insulin Resistance by Rosemary Extract. Nutrients 2018, 10, 1623. [Google Scholar] [CrossRef]
- Naimi, M.; Tsakiridis, T.; Stamatatos, T.C.; Alexandropoulos, D.I.; Tsiani, E. Increased Skeletal Muscle Glucose Uptake by Rosemary Extract through AMPK Activation. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 407–413. [Google Scholar] [CrossRef]
- Naimi, M.; Vlavcheski, F.; Murphy, B.; Hudlicky, T.; Tsiani, E. Carnosic Acid as a Component of Rosemary Extract Stimulates Skeletal Muscle Cell Glucose Uptake via AMPK Activation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Afonso, M.S.; de O Silva, A.M.; Carvalho, E.B.; Rivelli, D.P.; Barros, S.B.; Rogero, M.M.; Lottenberg, A.M.; Torres, R.P.; Mancini-Filho, J. Phenolic Compounds from Rosemary (Rosmarinus officinalis L.) Attenuate Oxidative Stress and Reduce Blood Cholesterol Concentrations in Diet-Induced Hypercholesterolemic Rats. Nutr. Metab. 2013, 10, 19. [Google Scholar] [CrossRef]
- Christensen, K.B.; Jørgensen, M.; Kotowska, D.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Activation of the nuclear receptor PPARγ by metabolites isolated from sage (Salvia officinalis L.). J. Ethnopharmacol. 2010, 132, 127–133. [Google Scholar] [CrossRef]
- Park, M.-Y.; Mun, S.T. Dietary Carnosic Acid Suppresses Hepatic Steatosis Formation via Regulation of Hepatic Fatty Acid Metabolism in High-Fat Diet-Fed Mice. Nutr. Res. Pract. 2013, 7, 294–301. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Selma, M.V.; Larrosa, M.; Obiol, M.; García-Villalba, R.; González-Barrio, R.; Issaly, N.; Flanagan, J.; Roller, M.; Tomás-Barberán, F.A.; et al. A rosemary extract rich in carnosic acid selectively modulates caecum microbiota and inhibits β-glucosidase activity, altering fiber and short chain fatty acids fecal excretion in lean and obese female rats. PLoS ONE 2014, 9, e94687. [Google Scholar] [CrossRef]
- Zhao, Y.; Sedighi, R.; Wang, P.; Chen, H.; Zhu, Y.; Sang, S. Carnosic Acid as a Major Bioactive Component in Rosemary Extract Ameliorates High-Fat-Diet-Induced Obesity and Metabolic Syndrome in Mice. J. Agric. Food Chem. 2015, 63, 4843–4852. [Google Scholar] [CrossRef]
- Rau, O.; Wurglics, M.; Paulke, A.; Zitzkowski, J.; Meindl, N.; Bock, A.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006, 72, 881–887. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Li, L.; Wang, W.; Tan, H.Y.; Qu, Y.; Wang, D. The involvement of gut microbiota in the anti-tumor effect of carnosic acid via IL-17 suppression in colorectal cancer. Chem. Biol. Interact. 2022, 365, 110080. [Google Scholar] [CrossRef]
- He, X.; Zhang, M.; Li, S.T.; Li, X.; Huang, Q.; Zhang, K.; Zheng, X.; Xu, X.T.; Zhao, D.G.; Ma, Y.Y. Alteration of gut microbiota in high-fat diet-induced obese mice using carnosic acid from rosemary. Food Sci. Nutr. 2022, 10, 2325–2332. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Bahjat, F.R.; Pine, P.R.; Reitsma, A.; Cassafer, G.; Baluom, M.; Grillo, S.; Chang, B.; Zhao, F.F.; Payan, D.G.; Grossbard, E.B.; et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum. 2008, 58, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.K.; Sriskantharajah, S.; Hessel, E.M.; Okkenhaug, K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr. Opin. Pharmacol. 2015, 23, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S.; Kim, H.G.; Kim, J.H.; Yang, W.S.; Kim, E.; Jeong, D.; Park, J.G.; Aziz, N.; Kim, S.; Parameswaran, N.; et al. Syk-MyD88 Axis Is a Critical Determinant of Inflammatory-Response in Activated Macrophages. Front. Immunol. 2021, 12, 767366. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, R.; Cao, Y.; Guo, M.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Yang, Z.; Zhang, N. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2014, 20, 54–58. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.F.; Niu, X.L. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhan, L.; Liao, H.; Chen, L.; Lv, X. Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27 expression via the TLR4/NF-κB signaling pathway. Plant. Med. 2013, 79, 102–109. [Google Scholar] [CrossRef]
- Youn, H.S.; Lee, J.Y.; Saitoh, S.I.; Miyake, K.; Kang, K.W.; Choi, Y.J.; Hwang, D.H. Suppression of MyD88- and TRIF-dependent signaling pathways of Toll-like receptor by (−)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochem. Pharmacol. 2006, 72, 850–859. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell Immunol. 2016, 310, 131–140. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. In Vitro 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Jakus, P.B.; Kalman, N.; Antus, C.; Radnai, B.; Tucsek, Z.; Gallyas, F., Jr.; Sumegi, B.; Veres, B. TRAF6 is functional in inhibition of TLR4-mediated NF-κB activation by resveratrol. J. Nutr. Biochem. 2013, 24, 819–823. [Google Scholar] [CrossRef]
- Machado, T.R.; Machado, T.R.; Pascutti, P.J. The p38 MAPK Inhibitors and Their Role in Inflammatory Diseases. Chem. Eur. 2021, 6, 5729–5742. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell. Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef]
- Westenberger, G.; Sellers, J.; Fernando, S.; Junkins, S.; Han, S.M.; Min, K.; Lawan, A. Function of Mitogen-Activated Protein Kinases in Hepatic Inflammation. J. Cell Signal. 2021, 2, 172–180. [Google Scholar]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef]
- Du, L. Doxorubicin-induced Toxicity Through the p38 MAPK Protein Kinase Pathway. Highlights Sci. Eng. Technol. 2022, 19, 9–15. [Google Scholar] [CrossRef]
- Guo, R.M.; Xu, W.M.; Lin, J.C.; Mo, L.Q.; Hua, X.X.; Chen, P.X.; Wu, K.; Zheng, D.D.; Feng, J.Q. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol. Med. Rep. 2013, 8, 603–608. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Jiang, S.; Qiu, Y.; Wang, Z.; Ji, Y.; Zhang, X.; Yan, X.; Zhan, Z. Carnosic Acid Induces Antiproliferation and Anti-Metastatic Property of Esophageal Cancer Cells via MAPK Signaling Pathways. J. Oncol. 2021, 2021, 4451533. [Google Scholar] [CrossRef]
- Wang, X.; Gupta, P.; Jramne, Y.; Danilenko, M.; Liu, D.; Studzinski, G.P. Carnosic acid increases sorafenib-induced inhibition of ERK1/2 and STAT3 signaling which contributes to reduced cell proliferation and survival of hepatocellular carcinoma cells. Oncotarget 2020, 11, 3129–3143. [Google Scholar] [CrossRef]
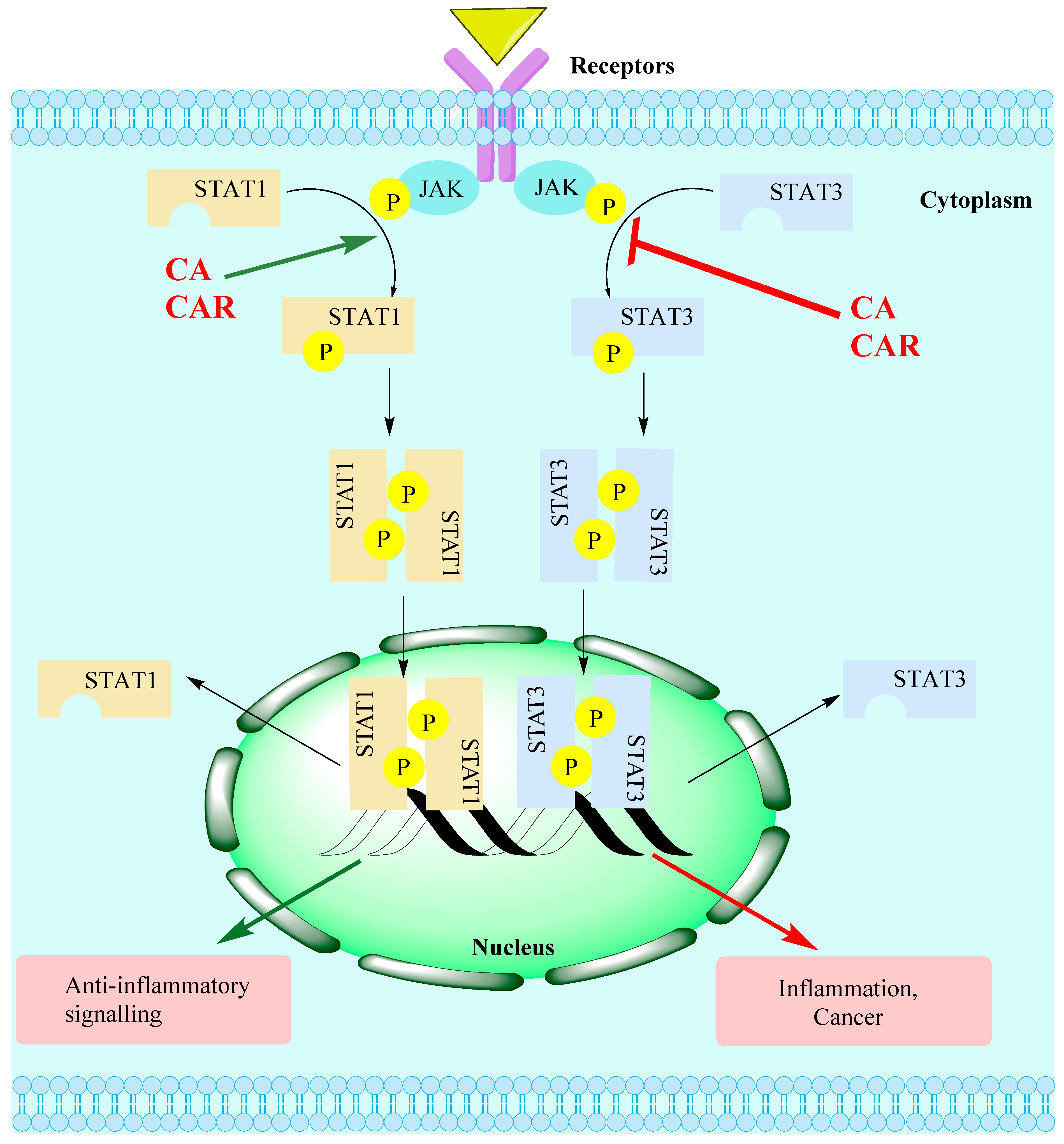
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.-Q.; Sethi, G.; Goh, B.-C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Kasembeli, M.M.; Bharadwaj, U.; Robinson, P.; Tweardy, D.J. Contribution of STAT3 to Inflammatory and Fibrotic Diseases and Prospects for its Targeting for Treatment. Int. J. Mol. Sci. 2018, 19, 2299. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, L.; Chen, X.; Lu, Q.; Yang, Y.; Liu, J.; Ma, X. SIRT1 counteracted the activation of STAT3 and NF-κB to repress the gastric cancer growth. Int. J. Clin. Exp. Med. 2014, 7, 5050–5058. [Google Scholar] [PubMed]
- Li, L.; Sun, Q.; Li, Y.; Yang, Y.; Yang, Y.; Chang, T.; Man, M.; Zheng, L. Overexpression of SIRT1 Induced by Resveratrol and Inhibitor of miR-204 Suppresses Activation and Proliferation of Microglia. J. Mol. Neurosci. 2015, 56, 858–867. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Zhang, Q.; Lu, Y.; Liu, J.; Li, W.; Lv, S.; Zhou, M.; Zhang, X.; Hang, C. Resveratrol Attenuates Early Brain Injury after Experimental Subarachnoid Hemorrhage via Inhibition of NLRP3 Inflammasome Activation. Front. Neurosci. 2017, 11, 611. [Google Scholar] [CrossRef]
- Zou, P.; Liu, X.; Li, G.; Wang, Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol. Med. Rep. 2018, 17, 3212–3217. [Google Scholar] [CrossRef]
- He, Q.; Li, Z.; Wang, Y.; Hou, Y.; Li, L.; Zhao, J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int. Immunopharmacol. 2017, 50, 208–215. [Google Scholar] [CrossRef]
- Sadeghi, A.; Seyyed Ebrahimi, S.S.; Golestani, A.; Meshkani, R. Resveratrol ameliorates palmitate-induced inflammation in skeletal muscle cells by attenuating oxidative stress and JNK/NF-κB pathway in a SIRT1-independent mechanism. J. Cel. Biochem. 2017, 118, 2654–2663. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Q.; Wang, M.; Liang, M.; Yang, X.; Xu, X.; Zou, H.; Qiu, J. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS ONE 2011, 6, e27081. [Google Scholar] [CrossRef]
- Li, D.; Liu, N.; Zhao, H.H.; Zhang, X.; Kawano, H.; Liu, L.; Zhao, L.; Li, H.P. Interactions between Sirt1 and MAPKs regulate astrocyte activation induced by brain injury in vitro and in vivo. J. Neuroinflamm. 2017, 14, 67. [Google Scholar] [CrossRef]
- Yang, H.; Gu, Z.T.; Li, L.; Maegele, M.; Zhou, B.Y.; Li, F.; Zhao, M.; Zhao, K.S. SIRT1 plays a neuroprotective role in traumatic brain injury in rats via inhibiting the p38 MAPK pathway. Acta Pharmacol. Sin. 2017, 38, 168–181. [Google Scholar] [CrossRef]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol. Nutr. Food Res. 2015, 59, 1905–1917. [Google Scholar] [CrossRef]
- Hu, T.; Shi, J.J.; Fang, J.; Wang, Q.; Chen, Y.B.; Zhang, S.J. Quercetin ameliorates diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice. Aging 2020, 12, 7015–7029. [Google Scholar] [CrossRef]
- Chen, D.L.; Yang, K.Y. Berberine Alleviates Oxidative Stress in Islets of Diabetic Mice by Inhibiting miR-106b Expression and Up-Regulating SIRT1. J. Cell Biochem. 2017, 118, 4349–4357. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Zhang, L.; Wu, Z.X.; Shan, T.T.; Xiong, C. Berberine Ameliorates Doxorubicin-Induced Cardiotoxicity via a SIRT1/p66Shc-Mediated Pathway. Oxid. Med. Cell Longev. 2019, 2019, 2150394. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, W.; Lin, Y.; Yi, W.; Liang, Z.; Yan, J.; Wang, N.; Deng, C.; Zhang, S.; Li, Y.; et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic. Biol. Med. 2013, 65, 667–679. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Ye, B.; Wang, Q.; Xie, X.; Shen, H. Protective effect of curcumin on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway. BMC Complement. Altern. 2016, 16, 299. [Google Scholar] [CrossRef]
- Habtemariam, S. The Molecular Pharmacology of Phloretin: Anti-Inflammatory Mechanisms of Action. Biomedicines 2023, 11, 143. [Google Scholar] [CrossRef]
- Habtemariam, S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxid. Med. Cell Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Cao, X.; Chen, X.; Zou, T.; You, J. Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction. Antioxidants 2022, 11, 2379. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, M.A.; Rucker, L.G.; Russo, H.M.; Dubyak, G.R. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J. Immunol. 2015, 194, 3937–3952. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.; Bartok, E.; Rieger, A.; Franchi, L.; Nuñez, G.; Hornung, V. Cutting edge: Reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011, 187, 613–617. [Google Scholar] [CrossRef]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef]
- Kong, R.; Sun, L.; Li, H.; Wang, D. The role of NLRP3 inflammasome in the pathogenesis of rheumatic disease. Autoimmunity 2022, 55, 1–7. [Google Scholar] [CrossRef]
- Lu, X.; Tan, Q.; Ma, J.; Zhang, J.; Yu, P. Emerging Role of LncRNA Regulation for NLRP3 Inflammasome in Diabetes Complications. Front. Cell. Dev. Biol. 2022, 9, 792401. [Google Scholar] [CrossRef]
- Vafaei, S.; Taheri, H.; Hajimomeni, Y.; Fakhre Yaseri, A.; Abolhasani Zadeh, F. The role of NLRP3 inflammasome in colorectal cancer: Potential therapeutic target. Clin. Transl. Oncol. 2022, 24, 1881–1889. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, Y.; Wu, S.; Chen, Q.; Wang, L. The Role of NLRP3 Inflammasome in Alzheimer’s Disease and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 845185. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar]
- Lee, H.E.; Yang, G.; Kim, N.D.; Jeong, S.; Jung, Y.; Choi, J.Y.; Park, H.H.; Lee, J.Y. Targeting ASC in NLRP3 inflammasome by caffeic acid phenethyl ester: A novel strategy to treat acute gout. Sci. Rep. 2016, 6, 38622. [Google Scholar] [CrossRef]
- Kong, F.; Ye, B.; Cao, J.; Cai, X.; Lin, L.; Huang, S.; Huang, W.; Huang, Z. Curcumin represses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB and P2X7R signaling in PMA-induced macrophages. Front. Pharmacol. 2016, 7, 369. [Google Scholar] [CrossRef]
- Chang, Y.P.; Ka, S.M.; Hsu, W.H.; Chen, A.; Chao, L.K.; Lin, C.C.; Hsieh, C.C.; Chen, M.C.; Chiu, H.W.; Ho, C.L.; et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell Physiol. 2015, 230, 1567–1579. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Eltutan, B.I.; Isci, K.B.; Genc, S. Resveratrol Inhibits NLRP3 Inflammasome-Induced Pyroptosis and miR-155 Expression in Microglia Through Sirt1/AMPK Pathway. Neurotox. Res. 2021, 39, 1812–1829. [Google Scholar] [CrossRef]
- Xue, Y.; Du, M.; Zhu, M.J. Quercetin suppresses NLRP3 inflammasome activation in epithelial cells triggered by Escherichia coli O157:H7. Free Radic. Biol. Med. 2017, 108, 760–769. [Google Scholar] [CrossRef]
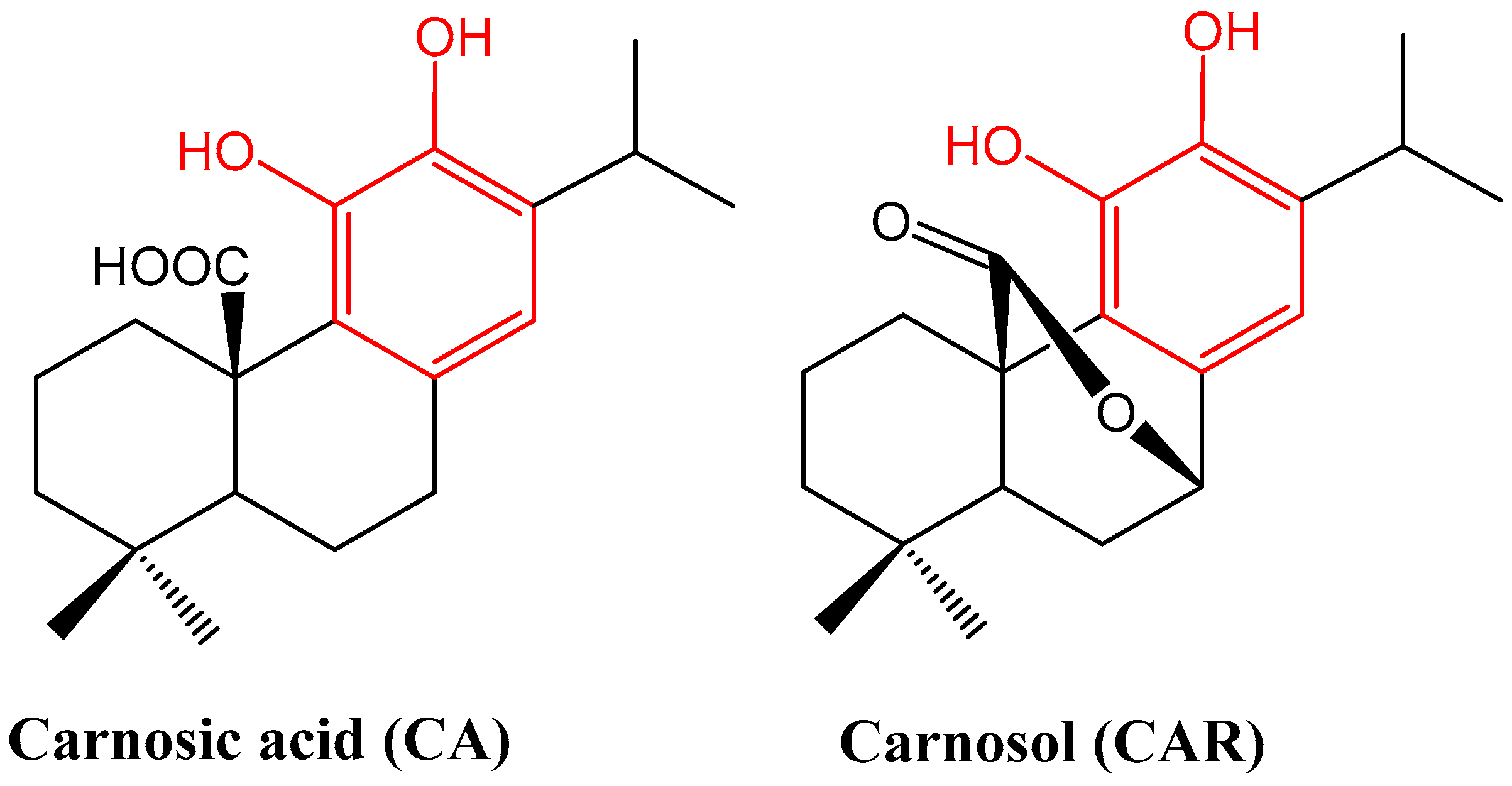


| Experimental Model | Compound (Dose) | Main Finding | Reference |
|---|---|---|---|
| RAW 264.7 cells activated by Gram-positive bacteria-derived peptidoglycan, pam3CSK or LPS | CA (5–20 μg/mL) | Inhibits the release of NO, TNF-α and PGE₂; inhibits NF-κB activation and the phosphorylation of Syk/Src, PI3K, Akt, IκBα, IKK and IκBα. | Oh et al. [19] |
| RAW 264.7 cells activated by LPS | CA and CAR (10 μM) | Suppresses MMP-9 and MCP-1 release. | Chae et al. [20] |
| RAW 264.7 cells activated by LPS | CA and CAR (12.5–50 μg/mL) | Suppresses NO production. | Mengoni et al. [21] |
| RAW 264.7 cells activated by LPS | CA (2.5–20 μM) | Inhibits NO, TNF-α and COX-2 expression; suppresses the transcription of inflammatory genes (Nos2, Tnfα, Cox2 and Mcp1); inhibits IKKβ/IκB-α/NF-κB, MAPKs (ERK, JNK and p38) and FoxO1/3 signalling pathways. | Wang et al. [22] |
| RAW 264.7 cells activated by LPS | CAR (IC50 9.4 μM) | Inhibits NO production and iNOS expression (mRNA and protein); inhibits NF-κB translocation and DNA binding activity; inhibits IKK activity and degradation of IκBα; inhibits MAPK (p38 and p44/42) activation. | Lo et al. [23] |
| RAW 264.7 cells activated by LPS | CAR (1, 2 and 5 μM) | Inhibits NO and expression of iNOS and COX-2; inhibits STAT3 phosphorylation and DNA binding activity. | Lee et al. [24] |
| RAW 264.7 cells activated by LPS | CA and CAR (5–15 μM) | Inhibits NO and PGE₂, cytokine (IL-1α and IL-6) and chemokine (CCL5/RANTES, CXCL10/IP-10) production, along with gene expression of iNOS; suppresses nuclear translocation of NF-κBp65. | Schwager et al. [25] |
| Primary mouse bone-marrow-derived macrophages (BMDMs) simulated by LPS | CAR (2.5–40 µM) | Inhibits NLRP3 inflammasome activation and HSP90; inhibits pro-inflammatory cytokine (pro-IL-1β, TNF-α and IL-6) expression. | Shi et al. [26] |
| Human whole-blood simulated by LPS | CA and CAR (IC50 1.9–3.5 μg/mL) | Inhibits the activity of microsomal PGE2 synthase (mPGES)-1. | Bauer et al. [27] Maione et al. [28] |
| Mouse bone-marrow-derived mast cells stimulated by anti-TNP IgE | CA (15 and 50 μM) | Inhibits ROS generation, Ca2+ mobilisation and degranulation; suppresses protein and gene expression of pro-inflammatory cytokines (IL-6, IL-13 and TNF) and chemokines (CCL2, CCL3 and CCL9); reduces phosphorylation of IKK and IκBα, Syk (Tyr352 and 525/526), TAK1 (Ser412) and Akt; decreases the level of NFKB2 mRNA and genes (c-jun, Egr1 and Egr2). | Crozier et al. [29] |
| BV2 mouse microglial cells stimulated by LPS and INF-γ | CA and CAR (5 μM) | Inhibits NO and TNF-α, and PGE2 production; induces HO-1 expression. | Foresti et al. [30] |
| IL-1β- or TNF-α-stimulated human periodontal ligament cells | CA (3.125–50 µM) | Suppresses the release of IL-6 and chemokines’ (CXCL10, CCL2 and CCL20) production; inhibits JNK, NF-κB and STAT3. | Hosokawa et al., 2020 [31] |
| Human oral epithelial cell line (TR146 cells) stimulated by IL-27 | CA (3.125–50 µM) | Suppresses chemokine (CXCL9, CXCL10 and CXCL11) production; inhibits the phosphorylation of STAT1, STAT3 and Akt. | Hosokawa et al., 2019 [32] |
| Bone marrow cells and osteoblasts stimulated by M-CSF | CA (10 or 20 μM) | Inhibits ROS production while augmenting SOD and GPx activity; inhibits the RANKL-mediated activation of NF-κB and MAPKs (JNK and p38) and expression of cytokines (TNF-α, IL-1β and IL-18) and COX2. | Liu et al. [33] |
| Chondrosarcoma cell line SW1353 and primary human chondrocytes stimulated by IL-1β | CA, and CAR (5–15 µM) | Inhibits catabolic genes such as MMP-13 and ADAMTS-4 and nuclear translocation of NF-κBp65. | Schwager et al. [25] |
| Human neutrophils stimulated by fMLF, MMK1 or PMA | CA (1–10 μM) | Suppresses the expression of integrin adhesion molecules (CD11b) and adhesion of neutrophils to endothelial (bEND 3) cells; inhibits the phosphorylation of MAPKs (ERK, JNK and p38). | Tsai et al. [34] |
| Human lung NCI-H1975 cells for H2O2-induced cell death; excised-lung organ culture ischemia model | CAR (3 μM) | Cytoprotection via upregulation of HO-1. | Kawamura et al. [35] |
| Keratinocyte HaCaT cells stimulated with SLS and RA | CA (5–20 μg/mL) | Suppresses the production of IL-6, IL-8 and MCP-1. | Oh et al. [19] |
| SH-SY5Y cells exposed to paraquat | CA (1 μM) | Inhibits NF-κB transcription and IL-1β, TNF-α and COX-2 expression; effect mediated via activation of the Nrf2 and HO-1 signalling pathway. | de Oliveira et al. [36] |
| PC12 cells subjected to serum starvation | CAR (10 µM) | Cytoprotective effect via activation of the HO-1 and Nrf2 pathway. | Martin et al. [37] |
| 6-OHDA-induced neuronal (SH-SY5Y) cell death | CA (1 µM) | Cytoprotective effect through inhibition of the MAPK pathway; inhibition of phosphorylation of JNK and p38. | Wu et al. [38] |
| PC12 cells; hypoxia-induced neuronal cell injury model | CA (1 μM) | Improves cell viability; suppresses ROS generation and lipid peroxidation; PGE2 (also COX-2 activation) NO and pro-inflammatory cytokines (IL-1 and IL-6) production; and ERK, JNK and p38 MAPK activation. | Hou et al. [39] |
| SH-SY5Y | CA (30 µM) | Inhibits Aβ (1-40 and 1-42) production by activating α-secretase, TACE. | Meng et al. [40] |
| U373MG human astrocytoma cells | CA (50 μM) | Inhibits Aβ peptides (1-40, 1-42 and 1-43) by increasing mRNA expression of α-secretase (TACE). | Yoshida et al. [41] |
| 3T3-L1 adipocytes stimulated by TNF-α | CA (1–20 µM) | Inhibits mRNA expression of inflammatory genes (IL-6 and MCP-1), the activation of ERK and JNK, the phosphorylation of IκB and IKK, the nuclear translocation of p65 and the DNA-binding activity of NF-κB and AP-1. | Tsai et al. [42] |
| Rat cardiomyocytes (H9C2 cells); DOX-induced cardiotoxicity | CA (2.4–10 µM) | Suppresses production of ROS and NO and activation or phosphorylation of p38 and JNK; inhibits NF–κB (p65) activation; upregulates Nrf2 and HO-1 levels. | Manna et al. [43] |
| H9C2 cells; DOX-induced cardiotoxicity | CA (5–20 μM) | Suppresses the level of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β and IL-18) and COX-2; inhibits NF-κB activation. | Zhang et al. [44] |
| H9C2 cells stimulated by LPS | CAR (5–20 μM) | Inhibits NF-κB activation and cytokine (TNF-α, IL-1β, IL-6) and COX-2 (as well as PGE2) expression; possible direct interaction with IKKβ (in silico study). | Baradaran Rahimi et al. [45] |
| HepG2 cells exposed to ethanol (100 mM) | CA (10 µM) | Inhibits oxidative stress, inflammation and cell death; effect mediated by activation of SIRT1 (see also in vivo effect). | Gao et al. [46] |
| LPS-treated hepatic stellate cells from mice | CA nanoparticles | Deactivates phosphorylated IKKα, IκBα and NF-κB; decreases TNF-α, IL-1β and IL-18 expression; suppresses ROS production while increasing SOD1, SOD2, HO-1 and Nrf-2 levels. | Li et al. [47] |
| 3T3-L1 adipocytes stimulated by LPS | CA (up to 20 µM) | Suppresses TNF-α, IL-6 and MCP-1 mRNA levels; downregulates NF-κB and ERK. | Park and Mun, [48] |
| Human retinal endothelial cells challenged by high glucose | CAR (2.5–20 µM) | Upregulates the expression and activity of Nrf2, HO-1 and ERK1/2; suppresses ROS production and apoptosis. | D’Agata et al. [49] |
| Human lung microvascular endothelial cells (HMVEC-L) challenged by t-BHP | CAR (10 µM) | Increases the expression of Nrf2 and HO-1 while it also interrupts the Nrf2-Keap1 protein−protein interaction; inhibits cell death. | Li et al. [50] |
| HCT-116 cells challenged by thapsigargin | CAR (10 µM) | Ameliorates the induced endoplasmic reticulum stress; suppresses the expression of pro-inflammatory mediators (TNF-α, IL-6, IFN-γ, CXCL10). | Xu et al. [51] |
| Experimental Model | Compound (Dose) | Main Finding | Reference |
|---|---|---|---|
| LPS-induced septic shock in mice | CAR (20 or 40 mg/kg, i.p.) | Prevents NLRP3 inflammasome activation; downregulates the serum levels of IL-1β and TNF-α. | Shi et al. [26] |
| Methionine- and choline-deficient (MCD) diet-fed mouse NASH model | CAR (20 or 40 mg/kg, i.p.) | Suppresses liver injury, fibrosis, NLRP3 inflammasome activation, IL-1β, TNF-α and profibrotic marker alpha-smooth muscle actin (α-SMA). | Shi et al. [26] |
| Adjuvant arthritis model in rats | Methotrexate (0.3 mg/kg) in combination with CA (100 mg/kg, p.o.) | Suppresses hind paw swelling, the levels of IL-17A, MMP-9 and MCP-1 in plasma, and GGT activity in the joint; increases mRNA expression levels of HO-1 and CAT; suppresses IL-1β level in the liver. | Chrastina et al. [52] |
| Collagen-induced arthritis-db/db mice model of rheumatoid arthritis | CA (30 and 60 mg/kg, i.p.) | Improves bone loss coupled with antidiabetic effects (e.g., OGTT and ITT). | Xia et al. [53] |
| Type II collagen-induced arthritis model in mice | CAR (40 mg/kg, p.o.) or rosmanol (40 mg/kg/d, p.o.) | Alleviates swelling, redness and synovitis; decreases the arthritis index score and the serum level of pro-inflammatory cytokines (IL-6, MCP-1 and TNF-α); blocks NF-κB and MAPK (JNK and p38 MAPK) pathways; better result in drug combination with rosmanol. | Li et al. [54] |
| ARDS in mice induced by LPS | CA (5 or 10 mg/kg, i.v.) | Improves inflammatory status (histology); reduces MPO activities, neutrophil infiltration and lipid peroxidation. | Tsai et al. [34] |
| LPS-induced acute lung injury (ALI) experimental model in mice | CA (10, 20 and 40 mg/kg, i.p.) | In addition to histological improvement, reduces the production (mRNA and protein) of IL-1β, IL-6, TNF-α, TLR4 and NF-κB expression and NF-κB phosphorylation in lung tissues. | Li et al. [55] |
| Bleomycin-induced lung damage in rats | CAR (10, 20 and 40 mg/kg, p.o.) | Reduces oxidative markers (MDA, NO, protein carbonyl), proinflammatory cytokines (TNF-α and IL-6 levels) and MPO activity in the lungs; increases GSH content and activities of CAT, GPx and SOD; reduces lung fibrosis. | Kalantar et al. [56] |
| Ovalbumin-induced allergic asthma in mice | CAR (5 mg/kg, i.p.) | Reduces eosinophils in the bronchoalveolar lavage fluids, and pro-inflammatory cytokines’ production (IL-4 and IL-13) in the bronchoalveolar lavage fluids and the lungs. | Lee and Im [57] |
| PMA-induced ear inflammation in mice | CA and CAR-EC50 values for reduction of oedema of 10.20 μg/cm2 and 10.70 μg/cm2, respectively | Reduces oedema, ulceration, leucocyte infiltration and expression levels of IL-1β, TNF-α and COX-2. | Mengoni et al. [21] |
| Carrageenan-induced oedema model in mice | CAR (1–10 mg/ kg, i.p.) | Reduces oedema; decreases MPO, NO and IL-17A; increases the level of anti-inflammatory cytokine, IL-10. | da Rosa et al. [58] |
| Atopic dermatitis in mice induced by 5% phthalic anhydride | CAR (0.05 µg/cm2) | Inhibits the expression of iNOS and COX-2 in skin tissue; inhibits STAT3 in skin tissue; reduces the serum levels of TNF-α, IL-1β and IgE. | Lee et al., 2017 [24] |
| UVB-induced skin inflammation in mice | CAR (0.05 µg/cm2) | Reduces erythema, epidermal thickness and serum levels of IgE and IL-1β; suppresses iNOS and COX-2; decreases activation of STAT3 and JAK. | Yeo et al. [59] |
| Carrageenan-induced oedema model in mice | CA (30 or 100 µg per paw) | Reduces oedema and levels of microsomal prostaglandin E synthase-1 (mPGES-1) and 5-LO-derived products. | Maione et al. [28] |
| 6-OHDA model of PD in rats | CA (20 mg/kg, p.o.) | Improves behavioural changes along with LPO, GSH and SOD. | Wu et al. [38] |
| Chlorpyrifos-induced neuronal damage in mice | CA (30 and 60 mg/kg p.o.) | Suppresses the serum level of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in cerebral and ocular tissues, reverses the decrease in AChE and antioxidant markers (GSH, GPx, SOD and CAT) and reduces pro-oxidant (MDA and NO) markers. | AlKahtane et al. [60] |
| Mild TBI in mice | CA (1 mg/kg, i.p.) | Improves motor and cognitive dysfunction, activates Nrf2 and suppresses NF-κB. | Maynard et al. [61] |
| Spinal cord injury in rats | CAR (5 mg/kg, i.p.) | Activates Nrf2; reduces ROS generation, LPO content, protein carbonyl and sulfhydryl levels; increases antioxidant status (SOD, CAT GPx, GSH, GSH-S-transferase); inhibits NF-κB and COX-2 expression; reverses the reduction in phosphor-Akt. | Wang et al. [62] |
| Traumatic brain injury in mice | CA (0.3, 1.0 or 3.0 mg/kg, i.p.) | Activates the Nrf2–ARE pathways; improves mitochondrial respiratory dysfunction, lipid peroxidation and protein nitration in brain tissues. | Miller et al. [63] |
| Subarachnoid haemorrhage brain injury model in rats | CA (3 mg/kg, i.p.) | Increases SIRT1, MnSOD and Bcl-2 in addition to improving brain oedema and neuronal structure and function. | Teng et al. [64] |
| APP/PS1 mouse model of AD | CA (10 or 30 mg/kg, p.o) | Reduces Aβ deposition, cognitive decline and levels of pro-inflammatory cytokine (IL-1β, TNFα and IL-6) production; inhibits Aβ secretion and interaction between CEBPβ and NFκB p65. | Yi-Bin et al. [65] |
| Experimental autoimmune encephalomyelitis in mice | CAR (50 mg/kg, i.p.) | Reduces demyelination and inhibits Th17 cell differentiation and STAT3 phosphorylation; blocks translocation of NF-κB; switches macrophage/microglia to non-inflammatory phenotype. | Li et al. [66] |
| STZ-induced diabetic rats | CAR (1, 5, 10 mg/kg/day, i.p. for 4 weeks) | Suppresses serum levels of glucose, IL-6, TNF-α, MDA, TG, TC, LDL-C, GST, SOD, CAT and HDL-C in a dose-dependent manner. | Samarghandian et al. [67] |
| STZ-induced diabetes in rats | CA (30 mg/kg) | Reduces glucose level in diabetic rats; reduces MDA and glycated end products, tissue damage and inflammation score; reverses change in the gut microbiota population. | Ou et al. [68] |
| STZ-induced diabetic mice db/db mice | CA (15 or 30 mg/kg, i.g.) | Nephroprotective effect coupled with activation of Nrf2 and inhibition of NF-κB. | Xie et al. [69] |
| Ischaemia/reperfusion model in diabetic mice | CA (50 mg/kg, p.o.) | Suppresses ROS and pro-inflammatory cytokine (IL-6 and TNF-α) production. | Hu et al. [70] |
| DOX-induced cardiotoxicity in rats | CA (10 mg/kg, p.o.) | Decreases the levels of ROS, NO, phospho-p38, phospho-JNK1 proteins and NF–κB (p65); reverses the downregulation of Nrf2 in the nucleus and HO-1 in the cardiomyocytes. | Manna et al. [43] |
| DOX-induced cardiotoxicity mice | CA or Carvedilol (5 mg/kg, p.o.) | Ameliorates cardiac injury and suppresses the levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β and IL-18) and COX-2, and NF-κB; reverses the reduced antioxidant level (GSH) or activity (SOD, CAT and NQO-1) and the increased oxidative stress (MDA level); increases Nrf2 in heart tissue; drug combination offers better result. | Zhang et al. [44] |
| Chronic alcoholic liver injury model in rats | (15 or 30 mg/kg, i.g.) | Activates SIRT1 and increases MnSOD; suppresses NF-κB and serum level of TNF-α. | Gao et al. [46] |
| Ischemia/reperfusion model of liver damage in rats | CA (10 and 20 mg/kg, i.p.) | Normalises the levels of SOD, CAT and GSH and GPx) and the NF-κB signalling pathway of pro-inflammatory cytokine (TNF-α and IL-1β) expression. | Li et al. [47] |
| HFD-induced NAFLD model in mice | CA (15 mg/kg, p.o.) | Improves glucose and insulin tolerance; suppresses the serum and hepatic levels of IL-1β, IL-18, TNF-α, IL-2, IL-4, IL-6, IL-12 and IFN-γ; reverses the low-level MARCKS under diabetes; ameliorates the diabetes-associated activation of PI3K/Akt, NLRP3/NF-κB and SREBP-1c signalling pathway. | Song et al. [71] |
| LPS-induced liver injury in rats | CA (30 or 60 mg/kg, p.o.) | Ameliorates liver damage (histology and biochemical markers) and suppresses inflammatory cells’ infiltration and the serum level of pro-inflammatory cytokines (TNF-α and IL-6); increases antioxidant levels (SOD, GSH and GPx) in serum and liver. | Xiang et al. [72] |
| LPS-induced liver injury in mice | CA (40 mg/kg, i.p.) | Inhibits the expression of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α and MCP-1, mRNA and protein), NOX4 (mRNA and protein) immune cell (neutrophil) infiltration and NF-κB activation; increases GSH, CAT and MnSOD. | Kim et al. [73] |
| Renal ischemia-reperfusion injury in rats | CAR (3 mg/kg, i.v.) | Inhibits apoptotic tubular cell death and activation of the p38 pathway. | Zheng et al., 2018 [74] |
| NASH model in mice | CAR (20 or 40 mg/kg, i.p.) | Suppresses NLRP3 inflammasome activity via direct effect on heat-shock protein 90 (HSP90). | Shi et al. [26] |
| HFD-induced mouse obesity and metabolic syndrome model | CA (10 or 20 mg/kg, p.o.) | Downregulates the levels of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in serum and brain tissues, and the NF-κB signalling pathway. | Liu et al. [75] |
| Dextran sulphate sodium (DSS) experimental model of colitis mice | CAR (50 mg/kg i.p.) | Reduces inflammatory cell infiltration and pro-inflammatory cytokine (TNF-α, IL-1β, IL-6 and IFN-γ) expression. | Xu et al. [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habtemariam, S. Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines 2023, 11, 545. https://doi.org/10.3390/biomedicines11020545
Habtemariam S. Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines. 2023; 11(2):545. https://doi.org/10.3390/biomedicines11020545
Chicago/Turabian StyleHabtemariam, Solomon. 2023. "Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol" Biomedicines 11, no. 2: 545. https://doi.org/10.3390/biomedicines11020545
APA StyleHabtemariam, S. (2023). Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines, 11(2), 545. https://doi.org/10.3390/biomedicines11020545






