Colorectal Cancer (CRC): Investigating the Expression of the Suppressor of Fused (SuFu) Gene and Its Relationship with Several Inflammatory Blood-Based Biomarkers
Abstract
:1. Introduction
2. Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Inclusion and Exclusion Criteria
2.4. RNA Isolation, and cDNA Synthesis and Real-Time PCR of SuFu
2.5. Protein Expression and Localization of SuFu via Immunohistochemistry (IHC)
2.6. Protocol for IHC
2.7. Evaluation of IHC
2.8. Follow-Up
2.9. Ethics
2.10. Statistical Analysis
3. Results
3.1. Patient Characteristics
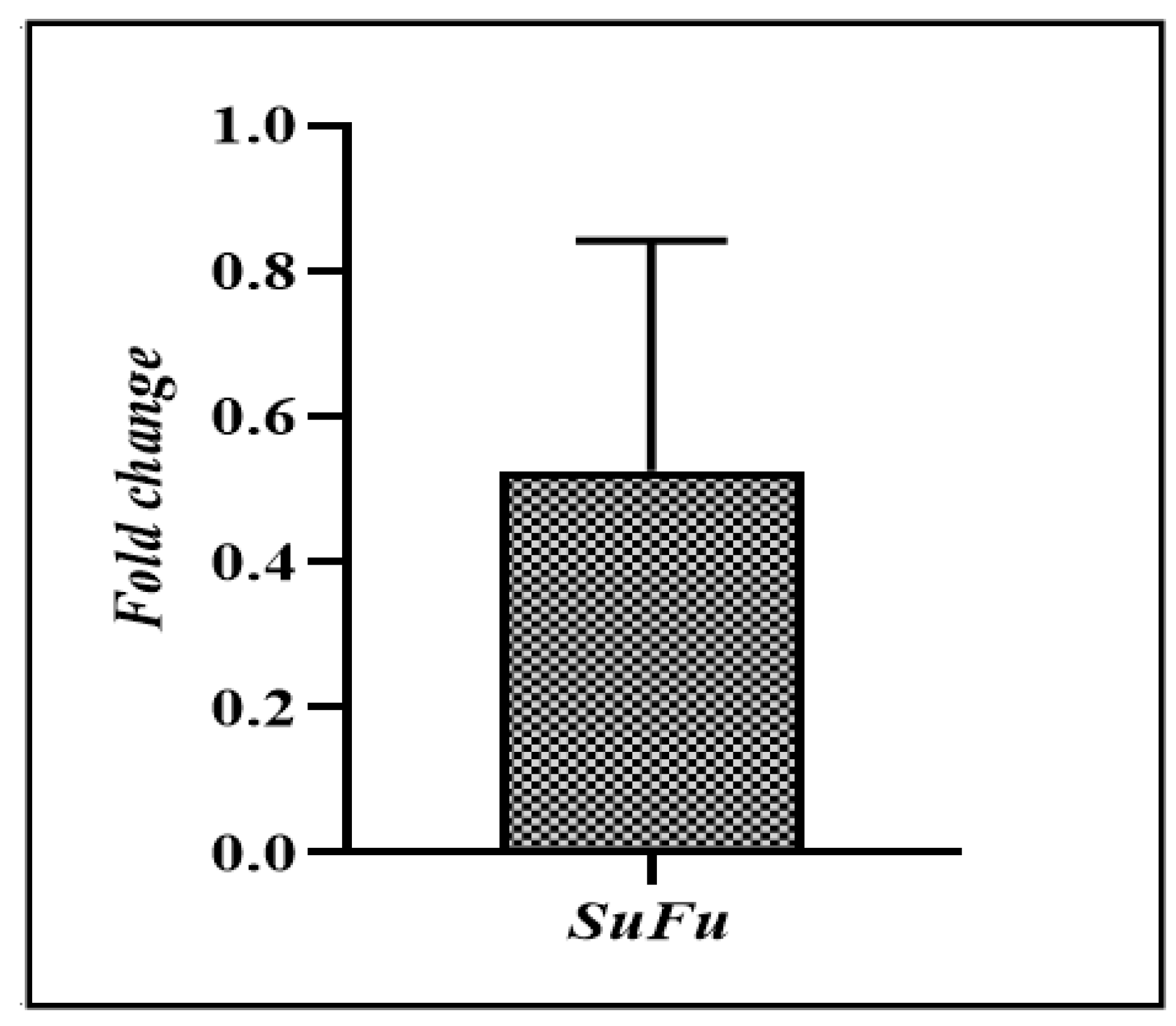
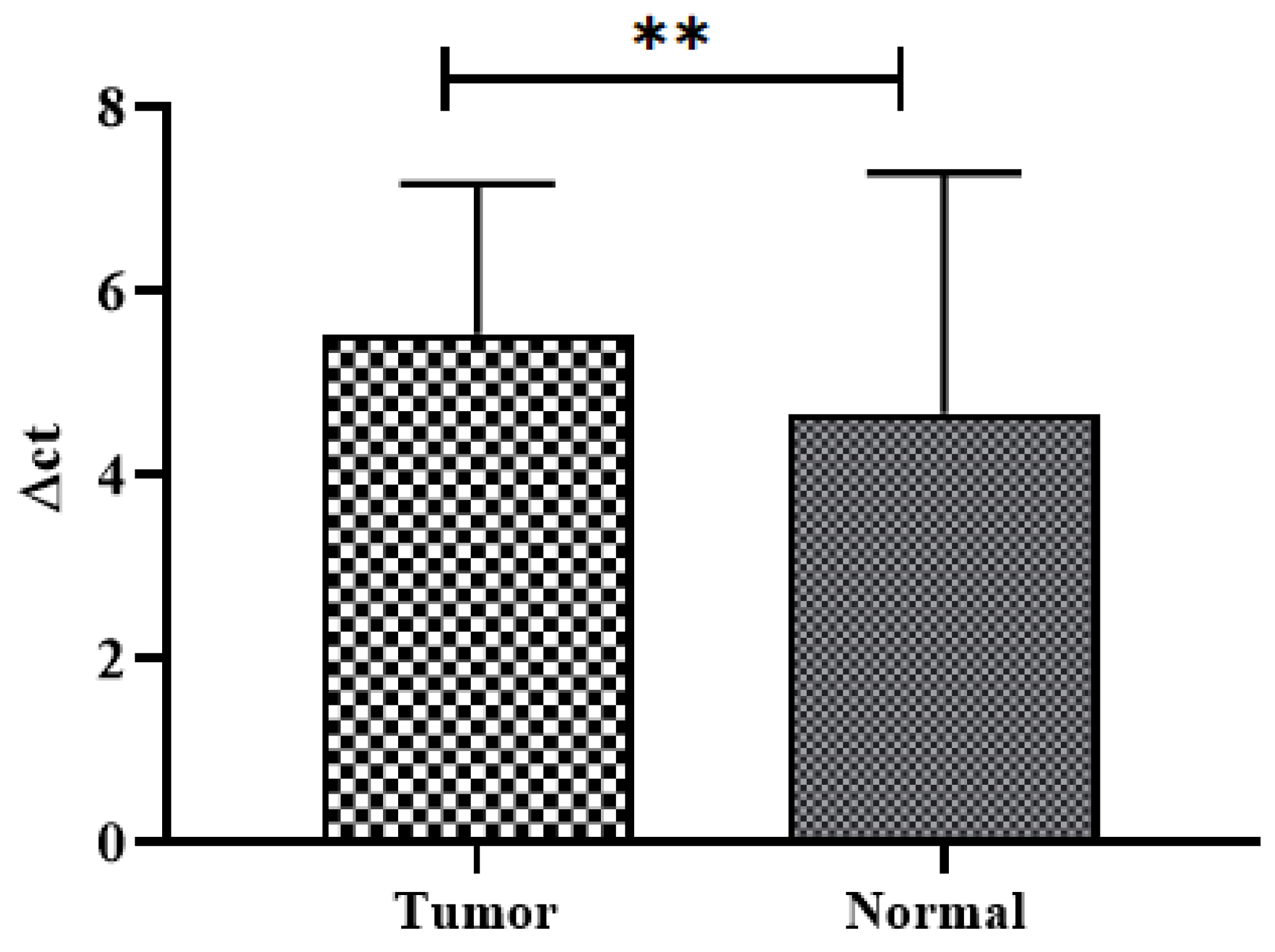
3.2. SuFu mRNA Expression in CRC
3.3. Comparison of SuFu mRNA Expression with Various Clinicopathological and Laboratory Parameters
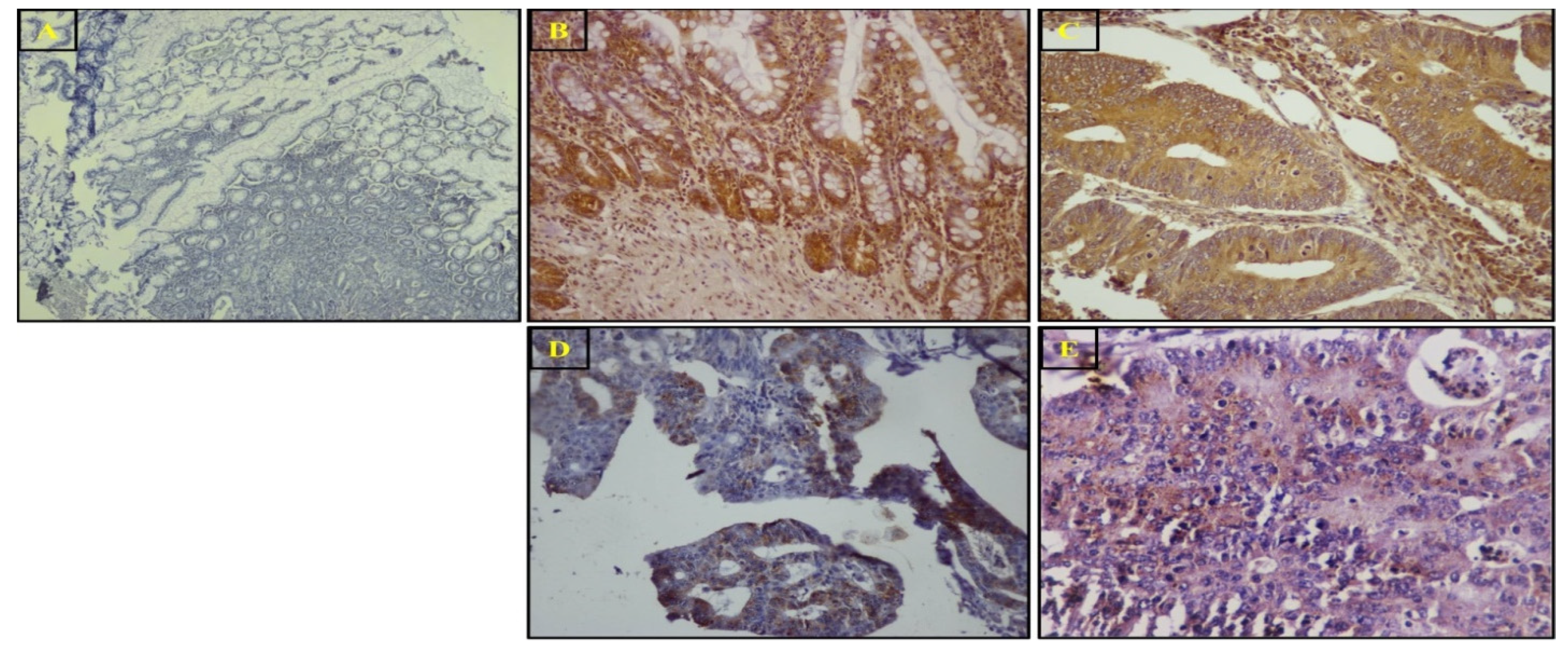
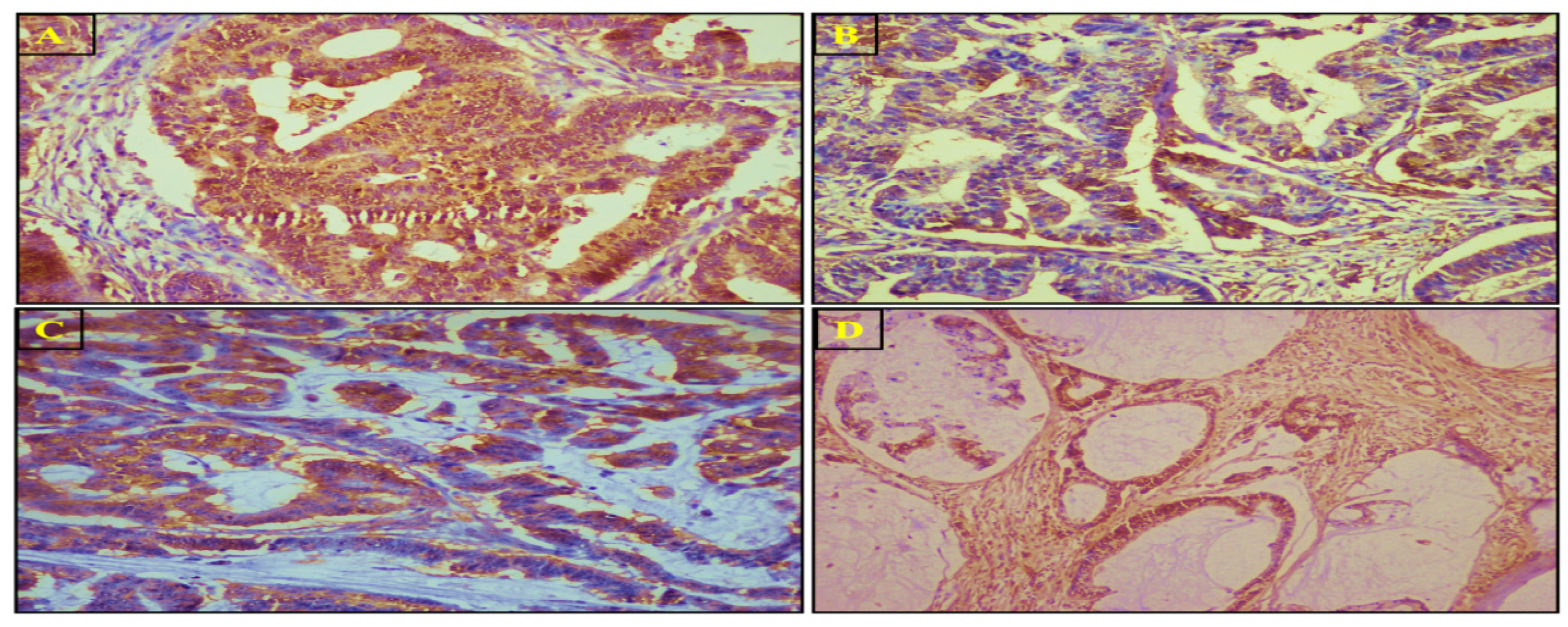
3.4. SuFu Protein Expression and Localization via IHC
3.5. Comparison of Laboratory Parameters between CRC Group and Healthy Controls
3.6. Correlation of Laboratory Parameters with Different Clinicopathological Parameters in Patients with CRC
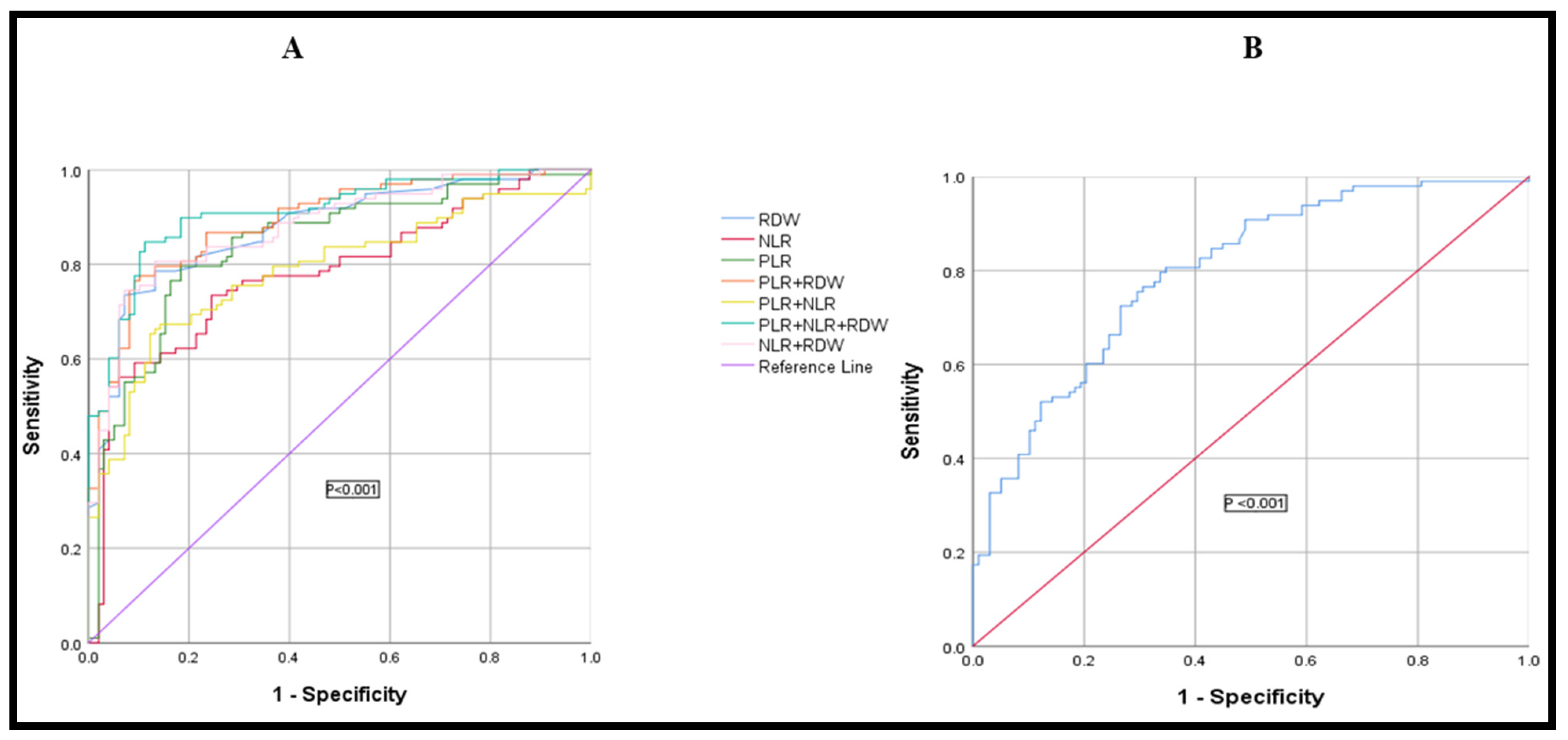
3.7. Diagnostic Efficacy of RDW, PLR, NLR, and HPR, Used Alone or in Combination, in Differentiating Colon Cancer from the Normal Healthy Control Group
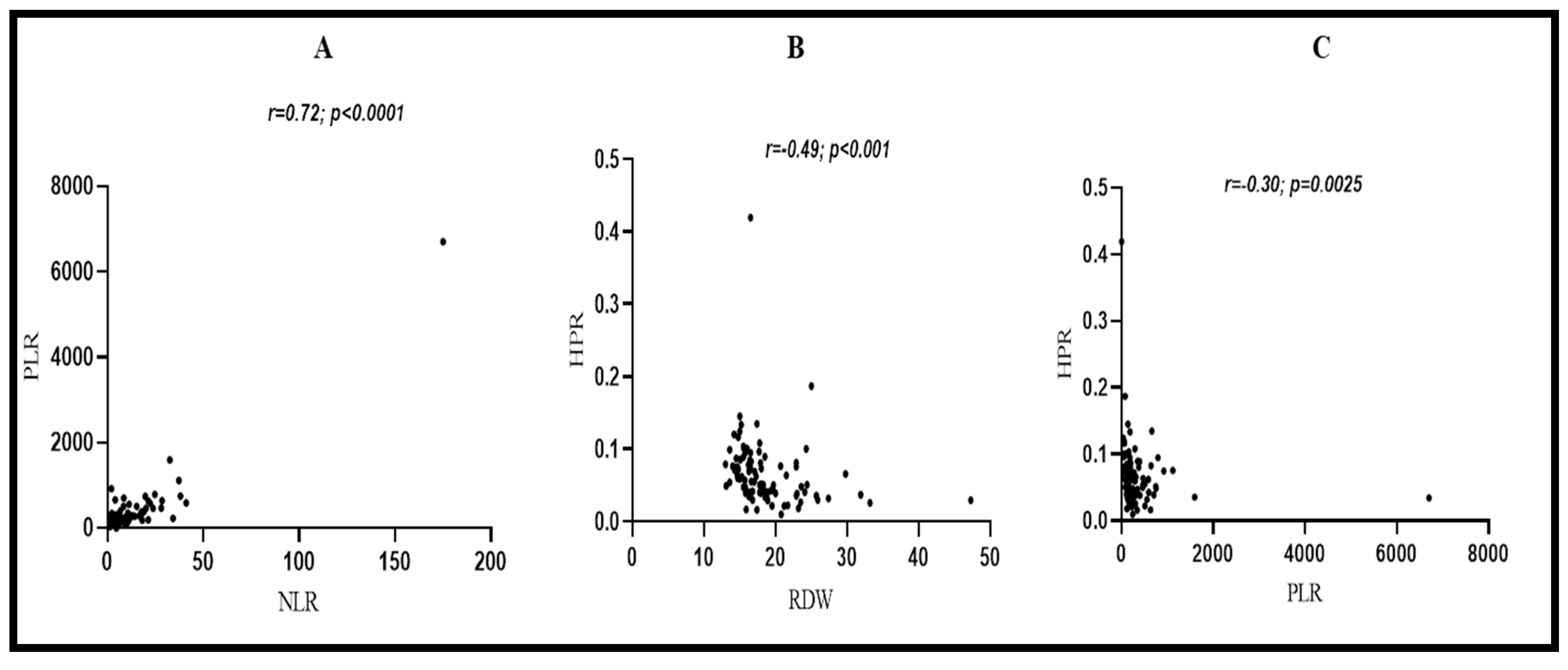
3.8. Correlations between Laboratory Parameters in CRC Patients
3.9. HPR Value’s Association with Cancer Stage and Tumor Invasion Depth
3.10. Prognostic Analysis of Various Clinicopathological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Peterson, C.Y.; Sriram, D.; Mahipal, A. Early-stage colon cancer: Current treatment standards, evolving paradigms, and future directions. World J. Gastrointest. Oncol. 2020, 12, 808–832. [Google Scholar] [CrossRef] [PubMed]
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Mosconi, S.; Mandalà, M.; Cervantes, A.; Arnold, D. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24 (Suppl 6), vi64–vi72. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Molecular Biologic and Epidemiologic Insights for Preventability of Colorectal Cancer. Gynecol. Oncol. 2022, 114, 645–650. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Toma, M.; Beluşică, L.; Stavarachi, M.; Apostol, P.; Spandole, S.; Radu, I.; Cimponeriu, D. Rating the environmental and genetic risk factors for colorectal cancer. J. Med. Life 2012, 5, 152–159. [Google Scholar] [PubMed]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon. Rectal. Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef]
- Hong, K.D.; Lee, Y.; Kim, B.-H.; Lee, S.I.; Moon, H.Y. Expression of GLI1 Correlates with Expression of Lymphangiogenesis Proteins, Vascular Endothelial Growth Factor C and Vascular Endothelial Growth Factor Receptor 3, in Colorectal Cancer. Am. Surg. 2013, 79, 198–204. [Google Scholar] [CrossRef]
- Douard, R.; Moutereau, S.; Pernet, P.; Chimingqi, M.; Allory, Y.; Manivet, P.; Conti, M.; Vaubourdolle, M.; Cugnenc, P.-H.; Loric, S. Sonic Hedgehog–dependent proliferation in a series of patients with colorectal cancer. Surgery 2006, 139, 665–670. [Google Scholar] [CrossRef]
- Bian, Y.H.; Huang, S.H.; Yang, L.; Ma, X.L.; Xie, J.W.; Zhang, H.W. Sonic hedgehog-Gli1 pathway in colorectal adenocarcinomas. World J. Gastroenterol. 2007, 13, 1659–1665. [Google Scholar] [CrossRef] [Green Version]
- Yue, S.; Chen, Y.; Cheng, S.Y. Hedgehog signaling promotes the degradation of tumor suppressor Sufu through the ubiquitin–proteasome pathway. Oncogene 2008, 28, 492–499. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Tang, J.; Luo, S. Molecular mechanisms of suppressor of fused in regulating the hedgehog signalling pathway (Review). Oncol. Lett. 2018, 15, 6077–6086. [Google Scholar] [CrossRef]
- Taylor, M.D.; Liu, L.; Raffel, C.; Hui, C.C.; Mainprize, T.G.; Zhang, X.; Agatep, R.; Chiappa, S.; Gao, L.; Lowrance, A.; et al. Mutations in SUFU predispose to medulloblastoma. Nat. Genet. 2002, 31, 306–310. [Google Scholar] [CrossRef]
- Sheng, T.; Li, C.; Zhang, X.; Chi, S.; He, N.; Chen, K.; McCormick, F.; Gatalica, Z.; Xie, J. Activation of the hedgehog pathway in advanced prostate cancer. Mol. Cancer 2004, 3, 29. [Google Scholar] [CrossRef]
- Reifenberger, J.; Wolter, M.; Knobbe, C.B.; Köhler, B.; Schönicke, A.; Scharwächter, C.; Kumar, K.; Blaschke, B.; Ruzicka, T.; Reifenberger, G. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br. J. Dermatol. 2005, 152, 43–51. [Google Scholar] [CrossRef]
- Yan, R.; Peng, X.; Yuan, X.; Huang, D.; Chen, J.; Lu, Q.; Lv, N.; Luo, S. Suppression of growth and migration by blocking the hedgehog signaling pathway in gastric cancer cells. Cell. Oncol. 2013, 36, 421–435. [Google Scholar] [CrossRef]
- Li, Z.J.; Nieuwenhuis, E.; Nien, W.; Zhang, X.; Zhang, J.; Puviindran, V.; Wainwright, B.; Kim, P.C.W.; Hui, C.-C. Kif7 regulates Gli2 through Sufu-dependent and -independent functions during skin development and tumorigenesis. Development 2012, 139, 4152–4161. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhu, X.; Liu, W.; Ruan, T.; Tao, K. Hedgehog signaling pathway in colorectal cancer: Function, mechanism, and therapy. Onco. Targets Ther. 2017, 10, 3249–3259. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Reports on progress in physics. Phys. Soc. (Great Br.) 2014, 77, 076602. [Google Scholar] [CrossRef]
- Mouasni, S.; Tourneur, L. FADD at the Crossroads between Cancer and Inflammation. Trends Immunol. 2018, 39, 1036–1053. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, Z.; Kang, Y.; Lin, Z.; Lu, P.; Lin, Q.; Cai, Z.; Cao, Y.; Zhu, X. Clinical Usefulness and Prognostic Value of Red Cell Distribution Width in Colorectal Cancer. BioMed Res. Int. 2018, 2018, 9858943. [Google Scholar] [CrossRef]
- Ay, S.; Eryilmaz, M.A.; Aksoy, N.; Okus, A.; Unlu, Y.; Sevinc, B. Is Early Detection of Colon Cancer Possible with Red Blood Cell Distribution Width? Asian Pac. J. Cancer Prev. Asian Pac. Organ. Cancer Prev. 2015, 16, 753–756. [Google Scholar] [CrossRef]
- Hu, Z.; Tan, S.; Chen, S.; Qin, S.; Chen, H.; Qin, S.; Huang, Z.; Zhou, F.; Qin, X. Diagnostic value of hematological parameters platelet to lymphocyte ratio and hemoglobin to platelet ratio in patients with colon cancer. Clin. Chim. Acta 2019, 501, 48–52. [Google Scholar] [CrossRef]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef]
- Garcea, G.; Ladwa, N.; Neal, C.P.; Metcalfe, M.S.; Dennison, A.R.; Berry, D.P. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J. Surg. 2011, 35, 868–872. [Google Scholar] [CrossRef]
- Brown, D.J.F.; Milroy, R.; Preston, T.; McMillan, D. The relationship between an inflammation-based prognostic score (Glasgow Prognostic Score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J. Clin. Pathol. 2007, 60, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.; McMillan, D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Futur. Oncol. 2010, 6, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Q.; Hu, T.; Gu, C.; Bi, L.; Wang, Z. Elevated red blood cell distribution width contributes to poor prognosis in patients undergoing resection for nonmetastatic rectal cancer. Medicine 2018, 97, e9641. [Google Scholar] [CrossRef]
- Yang, D.; Quan, W.; Wu, J.; Ji, X.; Dai, Y.; Xiao, W.; Chew, H.; Sun, Z.; Li, D. The value of red blood cell distribution width in diagnosis of patients with colorectal cancer. Clin. Chim. Acta 2018, 479, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Compton, C.C.; Greene, F.L. The Staging of Colorectal Cancer: 2004 and Beyond. CA A Cancer J. Clin. 2004, 54, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Sobin, L.H. Fleming IDJCIIJotACS. TNM classification of malignant tumors. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1997, 80, 1803–1804. [Google Scholar]
- Dukes, C. Histological Grading of Rectal Cancer: (Section of Pathology). Proc. R Soc. Med. 1937, 30, 371–376. [Google Scholar] [PubMed]
- Grinnell, R.S. The Grading and Prognosis of Carcinoma of the Colon and Rectum. Ann. Surg. 1939, 109, 500–533. [Google Scholar] [CrossRef]
- Stewart, F.W.; Spies, J.W. Biopsy Histology in the Grading of Rectal Carcinoma. Am. J. Pathol. 1929, 5, 109–116.9. [Google Scholar] [PubMed]
- Sobin, L.H.; Wittekind, C.H. TNM Classification of Malignant Tumours; Wiley-Blackwell: New York, NY, USA, 2009. [Google Scholar]
- Ueno, H.; Kajiwara, Y.; Shimazaki, H.; Shinto, E.; Hashiguchi, Y.; Nakanishi, K.; Maekawa, K.; Katsurada, Y.; Nakamura, T.; Mochizuki, H.; et al. New criteria for histologic grading of colorectal cancer. Am. J. Surg. Pathol. 2012, 36, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S. Socioeconomic status scales: Revised Kuppuswamy, BG Prasad, and Udai Pareekh’s scale updated for 2021. J. Fam. Med. Prim. Care 2021, 10, 3964–3967. [Google Scholar] [CrossRef]
- Chapman, G.; Maclean, H. “Junk food” and “healthy food”: Meanings of food in adolescent women’s culture. J. Nutr. Educ. 1993, 25, 108–113. [Google Scholar] [CrossRef]
- Sappayatosok, K.; Maneerat, Y.; Swasdison, S.; Viriyavejakul, P.; Dhanuthai, K.; Zwang, J.; Chaisri, U. Expression of pro-inflammatory protein, iNOS, VEGF and COX-2 in oral squamous cell carcinoma (OSCC), relationship with angiogenesis and their clinico-pathological correlation. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, E319–E324. [Google Scholar]
- Wang, Z.C.; Gao, J.; Zi, S.M.; Yang, M.; Du, P.; Cui, L. Aberrant expression of sonic hedgehog pathway in colon cancer and melanosis coli. J. Dig. Dis. 2013, 14, 417–424. [Google Scholar] [CrossRef]
- Islami, F.; Kamangar, F.; Nasrollahzadeh, D.; Aghcheli, K.; Sotoudeh, M.; Abedi-Ardekani, B.; Merat, S.; Nasseri-Moghaddam, S.; Semnani, S.; Sepehr, A.; et al. Socio-economic status and oesophageal cancer: Results from a population-based case–control study in a high-risk area. Leuk. Res. 2009, 38, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, F.; Zanetti, R.; Costa, G. Cancer risk and social inequalities in Italy. J. Epidemiol. Community Health 1994, 48, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiq, R.; Shah, I.A.; Bhat, G.A.; Lone, M.M.; Islami, F.; Boffetta, P.; Dar, N.A. Secondhand Smoking and the Risk of Esophageal Squamous Cell Carcinoma in a High Incidence Region, Kashmir, India. Medicine 2016, 95, e2340. [Google Scholar] [CrossRef]
- Dar, N.A.; Shah, I.A.; Bhat, G.A.; Makhdoomi, M.A.; Iqbal, B.; Rafiq, R.; Nisar, I.; Bhat, A.B.; Nabi, S.; Masood, A.; et al. Socioeconomic status and esophageal squamous cell carcinoma risk in Kashmir, India. Cancer Sci. 2013, 104, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Tostar, U.; Malm, C.J.; Kindblom, L.-G.; Toftgård, R.; Undèn, A.B.; Meis-Kindblom, J.M. Deregulation of the hedgehog signalling pathway: A possible role for thePTCH andSUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J. Pathol. 2005, 208, 17–25. [Google Scholar] [CrossRef]
- Svärd, J.; Heby-Henricson, K.; Persson-Lek, M.; Rozell, B.; Lauth, M.; Bergström, A.; Ericson, J.; Toftgård, R.; Teglund, S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev. Cell 2006, 10, 187–197. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Du, W.; Chen, L.; Wang, G.; Cui, Y.; Liu, Y.; Dou, Z.; Wang, H.; Zhang, P.; et al. Suppressor of fused (Sufu) represses Gli1 transcription and nuclear accumulation, inhibits glioma cell proliferation, invasion and vasculogenic mimicry, improving glioma chemo-sensitivity and prognosis. Oncotarget 2014, 5, 11681–11694. [Google Scholar] [CrossRef]
- Shi, C.; Xie, M.; Li, L.; Li, K.; Hu, B.-L. The association and diagnostic value of red blood cell distribution width in colorectal cancer. Medicine 2019, 98, e15560. [Google Scholar] [CrossRef]
- Mo, C.; Hu, Z.; Qin, S.; Chen, H.; Huang, L.; Li, S.; Cao, Z. Diagnostic value of platelet-lymphocyte ratio and hemoglobin-platelet ratio in patients with rectal cancer. J. Clin. Lab. Anal. 2020, 34, e23153. [Google Scholar] [CrossRef]
- Irawan, C.; Rachman, A.; Rahman, P.; Mansjoer, A. Role of Pretreatment Hemoglobin-to-Platelet Ratio in Predicting Survival Outcome of Locally Advanced Nasopharyngeal Carcinoma Patients. J. Cancer Epidemiol. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhen, Y.; Xie, W.; Wang, Y.; Chen, F.; Qin, C.; Yang, H.; Du, Z.; Shen, Z.; Zhang, B.; et al. Preoperative hemoglobin-platelet ratio can significantly predict progression and mortality outcomes in patients with T1G3 bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget 2018, 9, 18627–18636. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.X.; Yang, L.; He, B.S.; Pan, Y.Q.; Ying, H.Q.; Sun, H.L.; Lin, K.; Hu, X.X.; Xu, T.; Wang, S.K. Combination of preoperative NLR, PLR and CEA could increase the diagnostic efficacy for I-III stage CRC. J. Clin. Lab. Anal. 2017, 31, e22075. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Number (n) & Percentage (%) |
|---|---|
| Age | |
| <50 | 29(29.6) |
| ≥50 | 69(70.4) |
| Gender | |
| Male | 57(58.2) |
| Female | 41(41.8) |
| Dwelling | |
| Rural | 69(70.4) |
| Urban | 29(29.6) |
| Social Class | |
| Low | 40(40.8) |
| Middle & High | 58(59.2) |
| Education | |
| lower | 65(65.3) |
| higher | 33(33.7) |
| Blood Group | |
| A | 23(23.5) |
| B | 33(33.7) |
| AB | 18(18.3) |
| O | 24(24.5) |
| A+B | 56(57.14) |
| AB+O | 42(42.90) |
| BMI | |
| <24.9 | 59(60.2) |
| 25–29.9 | 31(31.6) |
| ≥30 | 8(8.2) |
| <25 | 59(60.2) |
| ≥25 | 39(39.8) |
| Family History | |
| Yes | 24(24.5) |
| No | 74(75.5) |
| Smoking Status | |
| Yes | 38(38.8) |
| No | 60(61.2) |
| Lifestyle | |
| Active | 68(69.4) |
| Sedentary | 30(30.6) |
| Comorbid Status | |
| Present | 40(40.8) |
| Absent | 58(59.2) |
| HTN | 24(24.5) |
| HTN+T2D | 16(16.3) |
| Absent | 58(59.2) |
| Salt Intake | |
| Yes | 82(83.7) |
| No | 16(16.3) |
| Red Meat Consumption | |
| Yes | 74(75.5) |
| No | 24(24.5) |
| Sundried Vegetables | |
| Yes | 76(77.6) |
| No | 22(22.4) |
| Source of Drinking Water | 56(57.2) |
| Tap Water (R) | 26(26.5) |
| Tap Water (L) | 16(16.3) |
| Others | |
| Pickles | |
| Yes | 73(74.5) |
| No | 25(25.5) |
| Pesticide Exposure | |
| Yes | 31(31.6) |
| No | 67(68.4) |
| Junk Food Consumption | |
| Yes | 16(16.3) |
| No | 82(83.7) |
| Frying | |
| Shallow | 57(58.2) |
| Deep | 41(41.8) |
| Histological type | |
| Adenocarcinoma | 88(89.8) |
| Mucinous | 10(10.2) |
| Site of Tumor | |
| Colon(C) | 56(57.1) |
| Rectum(R) | 29(29.6) |
| Rectosigmoid(RS) | 13(13.3) |
| RC | 24(24.5) |
| TC | 8(8.1) |
| LC | 24(24.5) |
| RS | 13(13.3) |
| R | 29(29.6) |
| Colon | 69(70.4) |
| Rectum | 29(29.6) |
| Tumor configuration | |
| Ulcerated | 27(27.6) |
| Ulceroinfilitrative | 71(72.4) |
| Tumor size (cm) | |
| 1–3 | 38(38.8) |
| ≥3 | 60(61.2) |
| Tumor Differentiation | |
| Well | 15(15.3) |
| Moderate | 69(70.4) |
| Poor | 14(14.3) |
| Tumor Invasion Depth | |
| T1 | 3(3.1) |
| T2 | 22(22.5) |
| T3 | 61(62.2) |
| T4 | 12(12.2) |
| T1 + T2 | 25(25.5) |
| T3 + T4 | 73(74.5) |
| TNM Staging | |
| I | 17(17.3) |
| II | 39(39.8) |
| III | 38(38.8) |
| IV | 4(4.1) |
| I + II | 56(57.1) |
| III + IV | 42(42.9) |
| Tumor Grade | |
| 1 | 15(15.3) |
| 2 | 69(70.4) |
| 3 | 14(14.3) |
| Node Status | |
| Absent | 57(58.2) |
| Present | 41(41.8) |
| Necrosis | |
| Present | 29(29.6) |
| Absent | 69(70.4) |
| LVI | |
| Present | 66(67.3) |
| Absent | 32(32.7) |
| PNI | |
| Present | 14(14.3) |
| Absent | 84(85.7) |
| Distant Metastasis | |
| Present | 3(3.1) |
| Absent | 95(96.9) |
| TALNR | |
| Present | 62(63.3) |
| Absent | 36(36.7) |
| Poor | 12(12.3) |
| Mild-moderate | 39(39.8) |
| High | 11(11.22) |
| Necrosis | |
| Yes | 29(29.6) |
| No | 69(70.4) |
| Recurrence | |
| Yes | 26(26.5) |
| No | 72(73.5) |
| Vital Status | |
| Alive | 88(89.8) |
| Dead | 10(10.2) |
| Characteristics | Low Expression n (%) | Same as Normal n (%) | OR (95%CI) | Chi2 | p-Value |
|---|---|---|---|---|---|
| Age | |||||
| ≤50 | 22(75.8) | 7(24.2) | 1.47(0.54–3.95) | 0.44 | 0.581 |
| >50 | 47(68.1) | 22(31.9) | |||
| Gender | |||||
| Male | 35(61.4) | 22(38.6) | 0.32 (0.124–0.866) | 5.32 | 0.021 |
| Female | 34(83) | 7(17) | |||
| Dwelling | |||||
| Rural | 48(69.5) | 21(30.4) | 0.87(0.33–2.27) | 0.80 | 0.781 |
| Urban | 21(72.4) | 8(27.6) | |||
| Social Class | |||||
| Low | 29(72.5) | 11(27.5) | 1.18(0.48–2.88) | 1.42 | 0.712 |
| Middle & High | 40(70) | 18(30) | |||
| Education | |||||
| lower | 52(80) | 13(20) | 3.7(1.52–9.31) | 8.52 | 0.004 |
| higher | 17(51.5) | 16(48.5) | |||
| Blood Group | |||||
| A+B | 42(75) | 14(25) | 1.6(0.69–3.99) | 1.32 | 0.250 |
| AB+O | 27(64.3) | 15(35.7) | |||
| BMI | |||||
| <25 | 43(72.9) | 16(27.1) | 1.3(0.55–3.23) | 0.42 | 0.509 |
| ≥25 | 26(66.7) | 13(33.3) | |||
| Family History | |||||
| Yes | 16(66.7) | 8(33.3) | 1.2(0.47—3.38) | 2.14 | 0.64 |
| No | 53(71.6) | 21(28.4) | |||
| Comorbid Status | |||||
| Present | 27(67.5) | 13(32.5) | 1.2(0.52–3.03) | 0.271 | 0.621 |
| Absent | 42(72.4) | 16(27.6) | |||
| Smoking Status | |||||
| Yes | 20(52.6) | 18(47.4) | 1.1(0.91–9.98) | 9.41 | 0.058 |
| No | 49(81.7) | 11(18.3) | |||
| Passive smoking | |||||
| Yes | 58(82.8) | 12(17.2) | 0.13(0.051–0.351) | 18.2 | 0.001 |
| No | 11(39.3) | 17(60.7) | |||
| Lifestyle | |||||
| Active | 51(75) | 17(25) | 0.50(0.20–1.2) | 2.24 | 0.134 |
| Sedentary | 18(60) | 12(40) | |||
| Source of Drinking Water | |||||
| Tap (River+Lake) | 55(67.1) | 27(32.9) | 0.29(0.062–1.37) | 2.6 | 0.102 |
| Other | 14(87.5) | 2(12.5) | |||
| Frying | |||||
| Shallow | 38(66.7) | 19(33.3) | 0.64(0.26–1.58) | 0.913 | 0.339 |
| Deep | 31(75.6) | 10(24.4) | |||
| Pesticide Exposure | |||||
| Yes | 19(61.3) | 12(38.7) | 1.8(0.749–4.60) | 1.80 | 0.179 |
| No | 50(74.6) | 17(25.4) | |||
| Junk Food Consumption | |||||
| Yes | 10(62.5) | 6(37.5) | 1.5(0.502–4.72) | 0.57 | 0.449 |
| No | 59(72) | 23(28) | |||
| Histological type | |||||
| Adenocarcinoma | 62(70.4) | 26(29.6) | 0.97(0.23–4.08) | 0.01 | 0.976 |
| Mucinous | 7(70) | 3(30) | |||
| Site of Tumour | |||||
| Colon | 38(67.8) | 18(32.2) | 0.3(0.07–1.91) | 1.46 | 0.481 |
| Recto sigmoid | 11(84.6) | 2(15.4) | |||
| Rectum | 20(69) | 9(31) | |||
| Tumor configuration | |||||
| Ulcerated | 19(70) | 8(30) | 0.98(0.37–2.63) | 0.088 | 0.996 |
| Ulceroinfilitrative | 50(70.4) | 21(29.6) | |||
| Tumour Differentiation | |||||
| Well | 12(80) | 3(20) | 2.1(0.5–8.3) | 3.12 | 0.209 |
| Moderate | 45(65.2) | 24(34.8) | |||
| Poor | 12(85.7) | 2(14.3) | |||
| Tumor size | |||||
| <3cm | 28(66.7) | 14(33.3) | 0.73(0.30–17) | 4.94 | 0.482 |
| ≥3cm | 41(73.2) | 15(26.8) | |||
| Tumor Invasion Depth | |||||
| T1 + T2 | 14(56) | 11(44) | 0.41(0.16–1.08) | 3.34 | 0.067 |
| T3 + T4 | 55(75.3) | 18(24.7) | |||
| TNM Staging | |||||
| I | 7(41.2) | 10(58.8) | 2(0.08–0.90) - 0.39(0.15–0.91) | 10.01 3.92 | 0.018 0.048 |
| II | 28(71.8) | 11(28.2) | |||
| III | 30(79) | 8(21) | |||
| IV | 4(100) | 0(0) | |||
| I + II | 35(62.5) | 21(37.5) | |||
| III + IV | 34(81) | 8(19) | |||
| Necrosis | |||||
| Present | 22(76) | 7(24) | 0.68(0.25–1.82) | 0.58 | 0.443 |
| Absent | 47(68) | 22(32) | |||
| Node status | |||||
| Present | 34(83) | 7(17) | 0.32(0.12–0.86) | 5.32 | 0.021 |
| Absent | 35(61) | 22(39) | |||
| LVI | |||||
| Present | 48(72.7) | 18(27.3) | 0.71(0.28–1.77) | 0.52 | 0.470 |
| Absent | 21(65.5) | 11(34.5) | |||
| PNI | |||||
| Present | 10(71.4) | 4(28.6) | 0.94(0.27–3.29) | 0.08 | 0.928 |
| Absent | 59(70.2) | 25(29.8) | |||
| Distant Metastasis | |||||
| Present | 3(100) | 0(0) | – | 1.30 | 0.254 |
| Absent | 66(69.4) | 29(30.6) | |||
| TALNR | |||||
| Present | 45(72.5) | 17(27.5) | 0.75(0.31–1.83) | 0.38 | 0.536 |
| Absent | 24(52.1) | 22(47.9) | |||
| Recurrence | |||||
| Yes | 23(88.5) | 3(11.5) | 0.25(0.03–0.82) | 5.53 | 0.019 |
| No | 46(63.9) | 26(36.1) | |||
| Vital Status | |||||
| Alive | 64(72.7) | 24(27.3) | 0.21(0.04–0.98) | 4.52 | 0.371 |
| Dead | 5(50) | 5(50) |
| Characteristics | Low Expression (IRS ≤ 4) | Same Expression (IRS > 4) | OR (95%CI) | Chi2 | p-Value |
|---|---|---|---|---|---|
| Site of Tumour | |||||
| Colon | 35(65.5) | 21(37.5) | 0.91(0.2–2.6) 1.1(0.4–2.9) | 0.94 | 0.925 |
| Recto sigmoid | 8(61.5) | 5(38.5) | |||
| Rectum | 19(65.5) | 10(34.5) | |||
| Tumor configuration | |||||
| Ulcerated | 16(59.3%) | 11(40%) | 0.7(0.3–1.9) | 0.257 | 0.612 |
| Ulceroinfilitrative | 46(64.8%) | 25(35%) | |||
| Tumour Differentiation (Grade) | |||||
| Well(I) | 5 (33.3%) | 10(66.7%) | 0.2(0.04–0.8) 0.2(0.08–0.9) | 4.64 4.92 | 0.042 0.021 |
| Moderate(II) | 27(39.4%) | 42(60.7%) | |||
| Poor(III) | 10(71.4%) | 4(28.6%) | |||
| Tumor size | |||||
| <3 cm | 15(78.9%) | 4(21.1%) | 2.5(0.7–8.4) | 2.49 | 0.114 |
| ≥3 cm | 47(59.5%) | 32(40%) | |||
| Tumor Invasion Depth | |||||
| T1 + T2 | 21(84%) | 4(16%) | 4(1.2–13.13) | 6.2 | 0.013 |
| T3 + T4 | 41(56.2%) | 32(43.8%) | |||
| TNM Staging | |||||
| I + II | 27(48.2%) | 29(51.8%) | 3.1(1.5–6.3) | 12.7 | 0.003 |
| III + IV | 35(83.3%) | 7(16%) | |||
| Necrosis | |||||
| Absent | 44(71%) | 25(69.4%) | 1.02(0.7–1.3) | 0.025 | 0.873 |
| Present | 18(29%) | 11(30.6%) | |||
| Node status | |||||
| Absent | 28(49.1%) | 29(50.9%) | 2.9(1.4–6.1) | 11.75 | 0.001 |
| Present | 34(82.9%) | 7(17.1%) | |||
| LVI | |||||
| Absent | 16(50%) | 16(50%) | 0.4(0.1–1.03) | 3.59 | 0.058 |
| Present | 46(69.7%) | 20(30.3%) | |||
| LNM | |||||
| Present | 17(85%) | 3(15%) | 2.8(0.9–8.2) | 5.1 | 0.024 |
| Absent | 45(57.7%) | 33(42.3%) | |||
| PNI | |||||
| Present | 14(100%) | 0(0%) | 0.57(0.4–0.6) | 9.4 | 0.002 |
| Absent | 48(57.1%) | 36(42.9%) | |||
| Distant Metastasis | |||||
| Present | 3(100%) | 0(0%) | 0.6(0.5–0.7) | 1.7 | 0.180 |
| Absent | 59(62.1%) | 36(37.9%) | |||
| TALNR | |||||
| Present | 39(62.9%) | 23(37.1%) | 1.04(0.44–2.4) | 0.010 | 0.922 |
| Absent | 23(63.9%) | 13(36.1%) | |||
| Recurrence | |||||
| No | 39(54.2) | 33(45.8) | 3.9(1.3–11) | 9.66 | 0.002 |
| Yes | 23(88.5) | 3(11.5) | |||
| Vital Status | |||||
| Dead | 10 (100%) | 0(0%) | 1.6 (1.4–2.0) | 6.4 | 0.011 |
| Alive | 52(59%) | 36(40%) |
| Protein Expression | Chi2 | p-Value | |||
|---|---|---|---|---|---|
| mRNA expression | Low expression | Same as normal | 20.06 | 0.0001 | |
| Low expression | 53 (82.81%) | 11 (17.19%) | |||
| Same as normal | 13 (38.24%) | 21 (61.76%) | |||
| Parameters | CRC Group | Healthy Controls | p-Value |
|---|---|---|---|
| Mean/Median ± SD/IQR | Mean/Median ± SD/IQR | ||
| Age | 57.51 ± 13.9 | 55.16 ± 16.4 | 0.298 |
| HB (g/L) | 11.25 (10.10–12.30) | 13.10 (11.9–14.6) | 0.001 |
| WBC (* 109/L) | 7.1 (5.60–9.12) | 7.30 (5.13–8.76) | 0.247 |
| Neutrophils (* 109/L) | 4.95 (3.5,7.7) | 4.01 (2.6–5.3) | 0.001 |
| Lymphocytes (* 109/L) | 1.10 (0.57–1.82) | 1.96 (1.33–2.72) | 0.001 |
| PLT (* 109/L) | 186.50 (137.75–258) | 129 (81.67–178.33) | 0.001 |
| PLR | 187.85 (114.00–342.41) | 65.10 (43.81–102.20) | 0.001 |
| NLR | 4.93 (2.10–12.03) | 1.93 (1.48–2.15) | 0.001 |
| HPR | 0.055 (0.039–0.0811) | 0.104 (0.073–0.1553) | 0.001 |
| RDW% | 17.4 (15.57–20.72) | 14.36 (13.48–15.09) | 0.001 |
| RDW | |||
|---|---|---|---|
| Clinicopathological Parameter | N | Median(IQR) | p-Value |
| Tumor site | |||
| Right colon | 24 | 21.25(18.12–24.25) | 0.001 |
| Left colon | 24 | 17.40(15.20–18.50) | |
| Tumor size | |||
| <3 | 38 | 17.20(15.18–20.03) | 0.473 |
| ≥3 | 60 | 17.80(15.80–21.65) | |
| Necrosis | |||
| Absent | 69 | 16.60(15.40–19.65) | 0.025 |
| Present | 29 | 18.50(16.80–22.90) | |
| Tumor configuration | |||
| Ulcerated | 27 | 16.80(15.20–20.02) | 0.510 |
| Ulceroinflitrative | 71 | 17.80(15.80–21.65) | |
| Node status | |||
| Absent | 57 | 16.60(15.15–19.90) | 0.038 |
| Present | 41 | 18.50(16.10–22.90) | |
| LNM | 0.380 | ||
| Absent | 78 | 17.35(15.50–20.73) | |
| Present | 20 | 18.60(16.45–20.90) | |
| Tumor depth | |||
| T1 + T2 | 25 | 16.50(14.70–17.60) | 0.010 |
| T3 + T4 | 73 | 18.0(15.85–21.35) | |
| Stage | |||
| I + II | 56 | 16.70(15.12–19.47) | 0.026 |
| III + IV | 42 | 18.50(15.95–22.92) | |
| PNI | |||
| Absent | 84 | 16.9(15.50–19.65) | 0.041 |
| Present | 14 | 19.8(17.42–23.80) | |
| HPR | |||
| Necrosis | |||
| Absent | 69 | 0.063(0.0401–0.086) | 0.015 |
| Present | 29 | 0.041(0.029–0.068) | |
| Tumor configuration | |||
| Ulcerated | 27 | 0.054(0.04–0.07) | 0.671 |
| Ulceroinflitrative | 71 | 0.051(0.03–0.08) | |
| Tumor depth | |||
| T1 + T2 | 25 | 0.075(0.057–0.112) | 0.001 |
| T3 + T4 | 73 | 0.050(0.035–0.076) | |
| Stage | |||
| I + II | 56 | 0.0661(0.039–0.086) | 0.126 |
| III + IV | 42 | 0.0478(0.038–0.073) | |
| I | 17 | 0.080(0.046–0.120) | 0.008 |
| III | 38 | 0.049(0.0381–0.073) | |
| LNM | |||
| Absent | 78 | 0.05(0.03–0.08) | 0.341 |
| Present | 20 | 0.04(0.03–0.06) | |
| LVI | |||
| Absent | 32 | 0.075(0.049–0.093) | 0.013 |
| Present | 66 | 0.049(0.037–0.074) | |
| Distant Metastasis | |||
| Absent | 95 | 0.05(0.03–0.08) | 0.236 |
| Present | 3 | 0.03(0.03–0.04) | |
| Tumor Size | |||
| <3 cm | 42 | 0.069(0.041–0.091) | 0.015 |
| 3 and above | 43 | 0.051(0.039–0.078) | |
| CEA (ng/mL) | 0.033 | ||
| 0–3 | 37 | 0.049(0.037–0.071) | |
| >3 | 61 | 0.063(0.040–0.089) | |
| PLR | |||
| Necrosis | |||
| Absent | 69 | 150.90(103.40–299.52) | |
| Present | 29 | 258.33(160.00–547.50) | 0.010 |
| LNM | |||
| Absent | 78 | 177(108.4–320.7) | 0.172 |
| Present | 20 | 235(134.7469.3) | |
| Tumor depth | |||
| T1 + T2 | 25 | 214(120.8–455.8) | 0.634 |
| T3 + T4 | 73 | 188.7(116.5–307.1) | |
| Distant Metastasis | |||
| Absent | 95 | 184(122.85–314.00) | |
| Present | 3 | 705(245.12–889.32) | 0.0131 |
| Stage | |||
| I + II | 56 | 177(177–395.4) | 0.851 |
| III + IV | 42 | 207(119–306.3) | |
| NLR | |||
| TALNR | |||
| Not seen | 36 | 2.96(1.881–7.939) | 0.048 |
| Seen | 62 | 6.15(2.247–16.700) | |
| Tumor depth | |||
| T1 + T2 | 25 | 8.8(2.15–17.23) | 0.276 |
| T3 + T4 | 73 | 4.8(2.1–10.54) | |
| Stage | |||
| I + II | 56 | 5.54(2.18–15.46) | 0.456 |
| III + IV | 42 | 5.04(1.95–11.07) | |
| Tumor site | |||
| RC | 24 | 4.5(2.11–14.72) | 0.006 |
| TC | 8 | 15.02(6.85–21.00) | |
| LC | 24 | 3.52(1.86–9.09) | |
| RS | 13 | 5.33(2.05–24.91) | |
| R | 29 | 4.87(1.93,11.05) | |
| LNM | |||
| Absent | 78 | 4.98(2.06–10.96) | 0.177 |
| Present | 20 | 7.54(2.37–19.81) | |
| Tumor configuration | |||
| Ulcerated | 27 | 5.83(2.11–16.80) | 0.694 |
| Ulceroinflitrative | 71 | 5.00(2.00–11.25) | |
| Parameters | J | Cut-Off | Sensitivity (%) | Specificity (%) | +LR | −LR | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| PLR | 0.61 | >105.574 | 79.6 | 81.6 | 4.33 | 0.25 | 0.842 (0.796–0.900) |
| NLR | 0.50 | >3.34 | 59.18 | 90.82 | 6.44 | 0.45 | 0.782 (0.720–0.840) |
| RDW | 0.66 | >15.7 | 73.5 | 92.9 | 10.29 | 0.29 | 0.876 (0.561–0.755) |
| HPR | 0.45 | ≤0.0781 | 72.45 | 73.47 | 2.73 | 0.38 | 0.796 (0.747–0.861) |
| NLR + RDW | 0.67 | >0.4286 | 80.61 | 86.73 | 6.08 | 0.22 | 0.879 (0.825–0.921) |
| PLR + NLR | 0.53 | >0.4761 | 67.3 | 85.7 | 4.71 | 0.38 | 0.787 (0.401–0.612) |
| PLR + RDW | 0.67 | >0.4631 | 77.6 | 89.8 | 7.60 | 0.25 | 0.891 (0.561–0.744) |
| PLR + NLR + RDW | 0.73 | >0.4223 | 84.69 | 88.78 | 7.55 | 0.17 | 0.910 (0.860–0.943) |
| Parameters | N | 3-Year OS | Chi2 | p-Value | 3-Year DFS | Chi2 | p-Value |
|---|---|---|---|---|---|---|---|
| Tumor Site | |||||||
| Colon | 56 | 91.10% | 73.20% | ||||
| Rectosigmoid | 13 | 100% | 3.9 | 0.14 | 61.50% | 0.51 | 0.77 |
| Rectum | 29 | 82.80% | 79.30% | ||||
| Tumor Grade | |||||||
| WD | 15 | 93.30% | 66.70% | 3 | 0.223 | ||
| MD | 69 | 88.40% | 1.88 | 0.39 | 79.70% | ||
| PD | 14 | 92.90% | 50% | ||||
| LVI | |||||||
| Absent | 32 | 96.40% | 0.93 | 0.33 | 84.40% | 1.6 | 0.307 |
| Present | 66 | 86.40% | 68.20% | ||||
| Node Status | |||||||
| Present | 41 | 80.70% | 6.4 | 0.011 | 53.70% | 14.2 | 0.01 |
| Absent | 57 | 96.50% | 87.70% | ||||
| PNI | |||||||
| Present | 14 | 71.70% | 5.7 | 0.017 | 42.90% | 6.5 | 0.011 |
| Absent | 84 | 92.50% | 77.40% | ||||
| LNM | |||||||
| Absent | 78 | 94.90% | 7.8 | 0.005 | 84.60% | 19.3 | 0.001 |
| Present | 20 | 70% | 30% | ||||
| Tumor Depth | |||||||
| T1 + T2 | 25 | 90.90% | 86.30% | ||||
| T3 + T4 | 73 | 66.70% | 0.702 | 0.402 | 51.60% | 14.47 | 0.074 |
| Stage | |||||||
| I + II | 56 | 98.90% | 8.72 | 0.003 | 89.30% | 13.2 | 0.001 |
| III + IV | 42 | 75.70% | 52.40% | ||||
| SuFu Expression | |||||||
| Low | 62 | 83.80% | 5.22 | 0.023 | 62.70% | 8.15 | 0.003 |
| Same as normal | 36 | 100% | 91.70% | ||||
| CA 19.9 | |||||||
| ≤35 | 66 | 89.40% | 0.94 | 0.331 | 78.80% | 8.09 | 0.004 |
| >35 | 32 | 90.60% | 62.50% |
| OS | DFS | |||||
|---|---|---|---|---|---|---|
| Parameters | H.R | 95% CI | p-Value | H.R | 95% CI | p-Value |
| Tumor site | 0.44 | 0.05–3.6 | 0.451 | 1.4 | 0.38–5.5 | 0.573 |
| PLR | 3.1 | 0.63–15.65 | 0.162 | 0.6 | 0.25–1.72 | 0.404 |
| NLR | 0.2 | 0.05–1.24 | 0.090 | 1.5 | 0.61–3.94 | 0.404 |
| RDW | 0.310 | 0.510–1.763 | 0.187 | 1.4 | 0.33–6.53 | 0.601 |
| CA 19.9 | 2.06 | 0.438–9.75 | 0.397 | 3.07 | 1.32–7.11 | 0.009 |
| CEA | 3.5 | 0.71–17.41 | 0.120 | 1.3 | 0.574–3.060 | 0.511 |
| Tumor grade | 2.1 | 0.03–6.3 | 0.052 | 1.3 | 0.34–5.4 | 0.658 |
| LVI | 3.8 | 0.32–45.5 | 0.281 | 0.93 | 0.28–3.06 | 0.911 |
| Node status | 0.8 | 0.21–3.7 | 0.870 | 1.02 | 0.21–4.9 | 0.98 |
| PNI | 1.06 | 0.08–13.59 | 0.936 | 1.8 | 0.64–5.1 | 0.260 |
| Tumor depth | 0.031 | 1.23–2.63 | 0.082 | 1.21 | 1.16–3.65 | 0.095 |
| Stage | 4.23 | 0.67–18.3 | 0.089 | 3.09 | 0.62–15.2 | 0.166 |
| SuFu expression | 2.9 | 0.83–10.45 | 0.093 | 0.34 | 0.10–1.13 | 0.080 |
| Lab Parameters | SuFu Expression | ||||
|---|---|---|---|---|---|
| Low | Same as Normal | Odds Ratio | Chi2 | p-Value | |
| RDW | |||||
| <15 | 10 | 4 | 0.008 | 0.928 | |
| ≥15 | 59 | 25 | 1.01 (0.30–3.69) | ||
| PLR | |||||
| <150 | 24 | 12 | 0.75 (0.31–1.75) | 0.38 | 0.536 |
| ≥150 | 45 | 17 | |||
| HPR | |||||
| <0.07 | 55 | 19 | 2.23 | 0.136 | |
| ≥0.07 | 14 | 10 | 2.06 (0.78–5.42) | ||
| NLR | |||||
| <5 | 33 | 16 | 0.74 (0.31–1.78) | 0.44 | 0.501 |
| ≥5 | 36 | 13 | |||
| CEA (ng/mL) | |||||
| 0–3 | 30 | 7 | 2.4 (0.91–6.41) | 3.25 | 0.071 |
| >3 | 39 | 22 | |||
| CA 19.9 (IU/mL) | |||||
| ≤35 | 44 | 22 | 0.56 (0.21–1.49) | 1.35 | 0.244 |
| >35 | 25 | 7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rather, T.B.; Parveiz, I.; Bhat, G.A.; Rashid, G.; Akhtar, K.; Haque, R.; Ola, M.S.; Ali, M.; Wani, R.A.; Khan, I.Y.; et al. Colorectal Cancer (CRC): Investigating the Expression of the Suppressor of Fused (SuFu) Gene and Its Relationship with Several Inflammatory Blood-Based Biomarkers. Biomedicines 2023, 11, 540. https://doi.org/10.3390/biomedicines11020540
Rather TB, Parveiz I, Bhat GA, Rashid G, Akhtar K, Haque R, Ola MS, Ali M, Wani RA, Khan IY, et al. Colorectal Cancer (CRC): Investigating the Expression of the Suppressor of Fused (SuFu) Gene and Its Relationship with Several Inflammatory Blood-Based Biomarkers. Biomedicines. 2023; 11(2):540. https://doi.org/10.3390/biomedicines11020540
Chicago/Turabian StyleRather, Tahseen Bilal, Ishrat Parveiz, Gulzar A Bhat, Gowhar Rashid, Kulsum Akhtar, Rizwanul Haque, Mohammad Shamsul Ola, Mehboob Ali, Rauf A Wani, Ishrat Younas Khan, and et al. 2023. "Colorectal Cancer (CRC): Investigating the Expression of the Suppressor of Fused (SuFu) Gene and Its Relationship with Several Inflammatory Blood-Based Biomarkers" Biomedicines 11, no. 2: 540. https://doi.org/10.3390/biomedicines11020540
APA StyleRather, T. B., Parveiz, I., Bhat, G. A., Rashid, G., Akhtar, K., Haque, R., Ola, M. S., Ali, M., Wani, R. A., Khan, I. Y., Besina, S., & Mudassar, S. (2023). Colorectal Cancer (CRC): Investigating the Expression of the Suppressor of Fused (SuFu) Gene and Its Relationship with Several Inflammatory Blood-Based Biomarkers. Biomedicines, 11(2), 540. https://doi.org/10.3390/biomedicines11020540







