Improved Anti-Biofilm Effect against the Oral Cariogenic Streptococcus mutans by Combined Triclosan/CBD Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacteria Strain and Cultivation
2.3. Planktonic Growth
2.4. Biofilm Formation
2.5. MTT Metabolic Assay
2.6. CV Staining
2.7. High-Resolution Scanning Electron Microscopy (HR-SEM) Imaging
2.8. Scanning Disk Confocal Microscopy (SDCM) Imaging
2.9. Flow Cytometry
2.10. Biocompatibility Assay on Vero Epithelial Cells
2.11. Statistical Analyses
3. Results
3.1. The Anti-Bacterial and Anti-Biofilm Activities of CBD and Triclosan Alone or in Combination
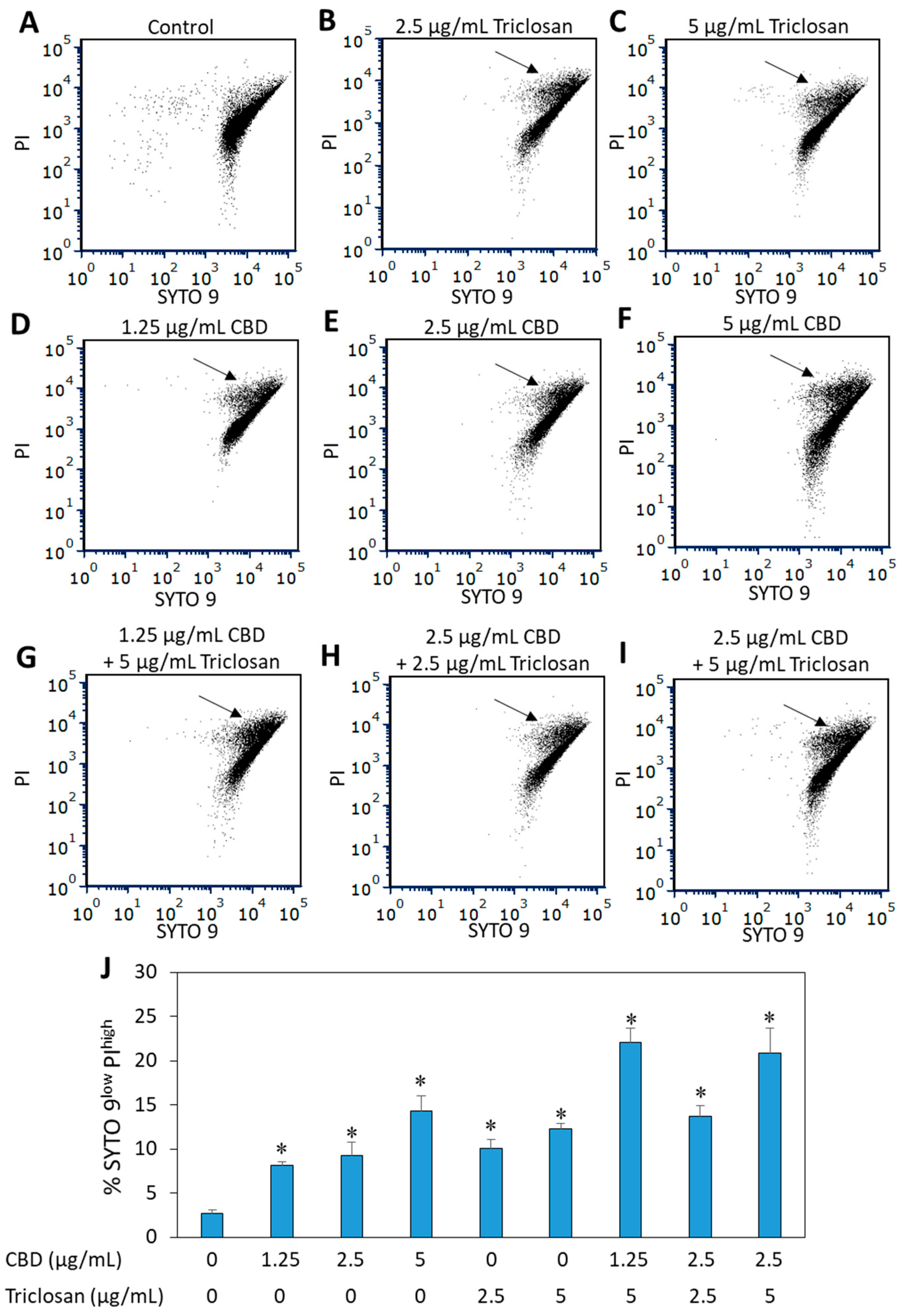
3.2. The Effect of Combined CBD/Triclosan Treatment on Membrane Permeability
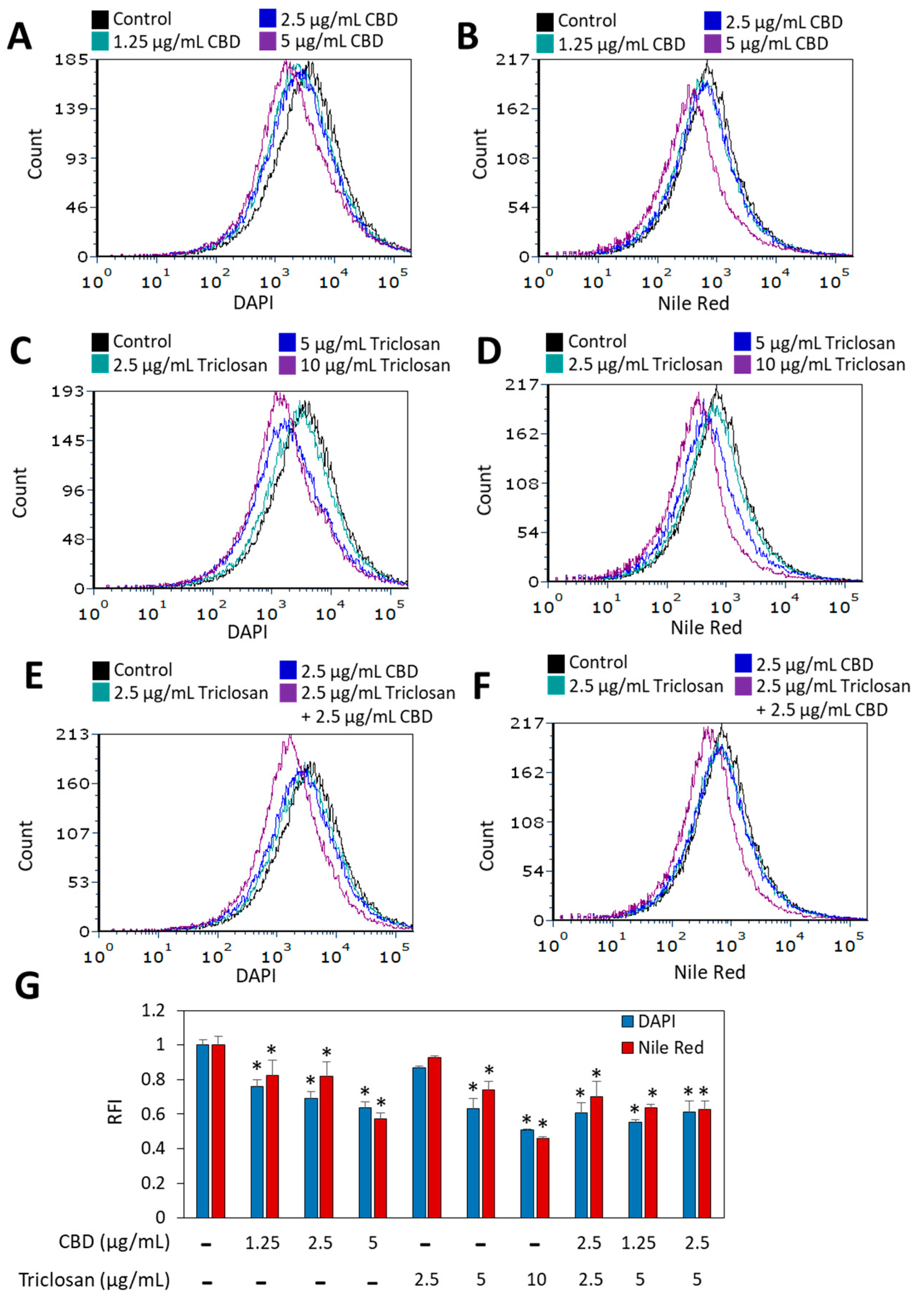
3.3. The Effect of Combined CBD/Triclosan Treatment on Nile Red Membrane Staining
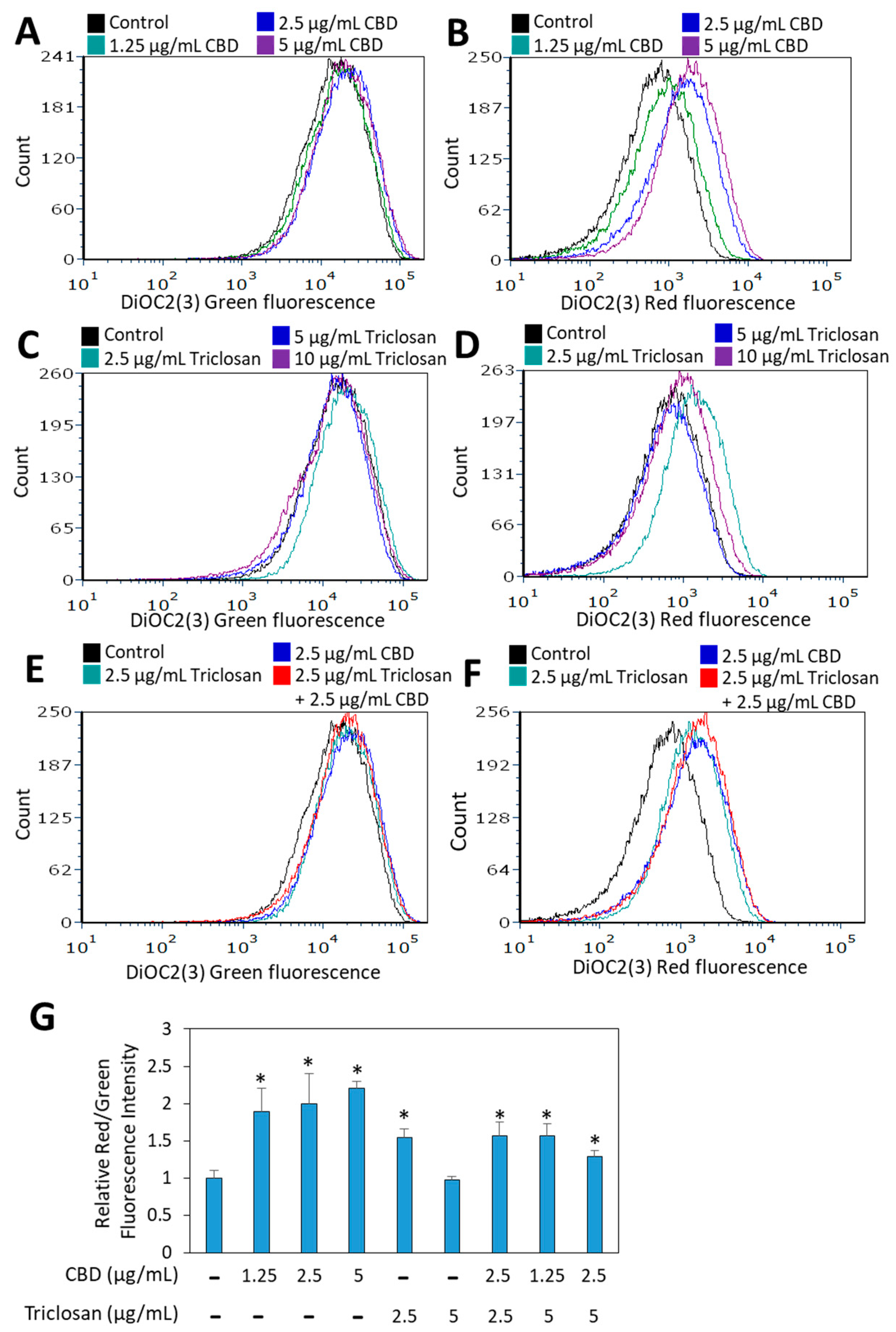
3.4. Both CBD and Triclosan Induces Membrane Hyperpolarization
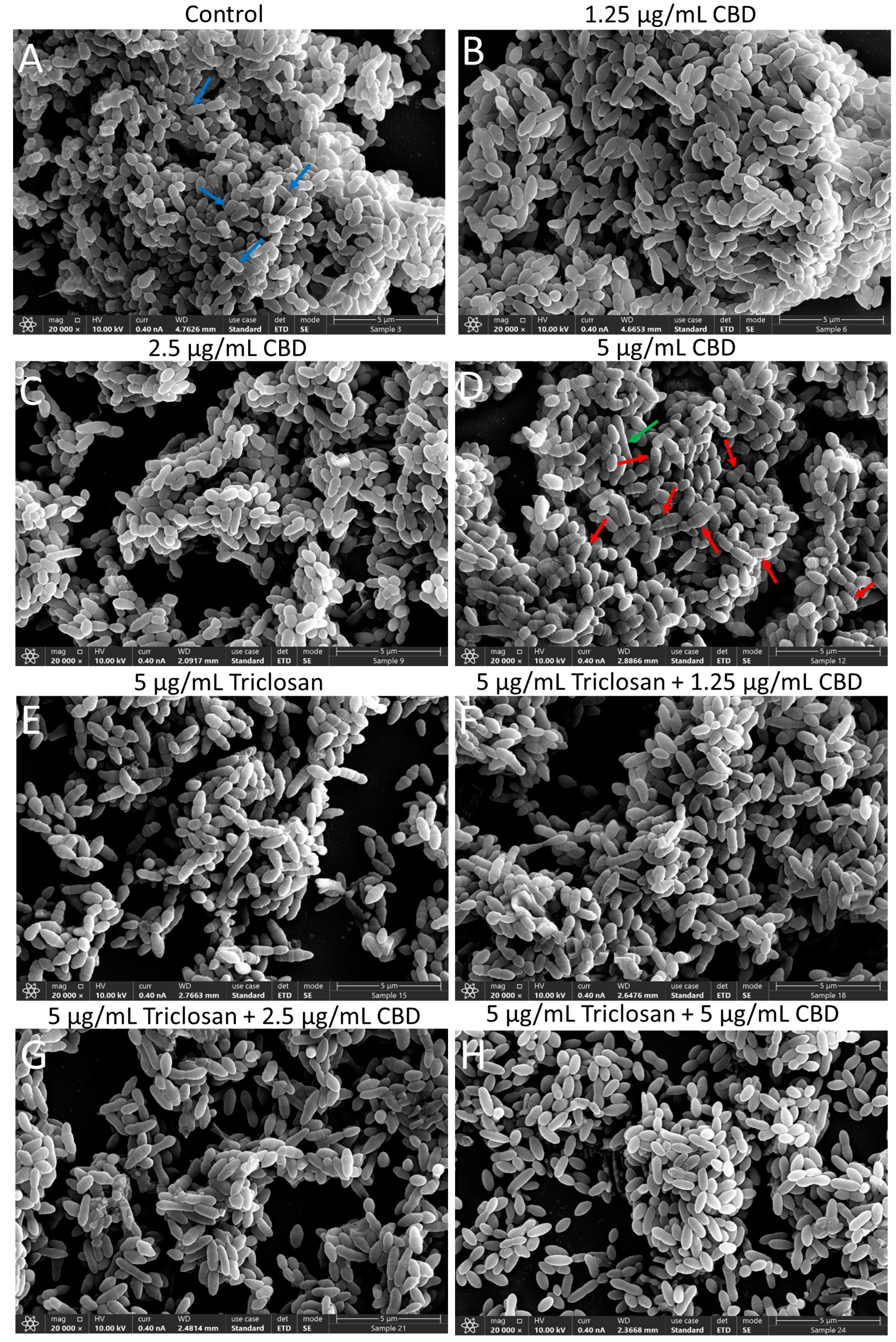
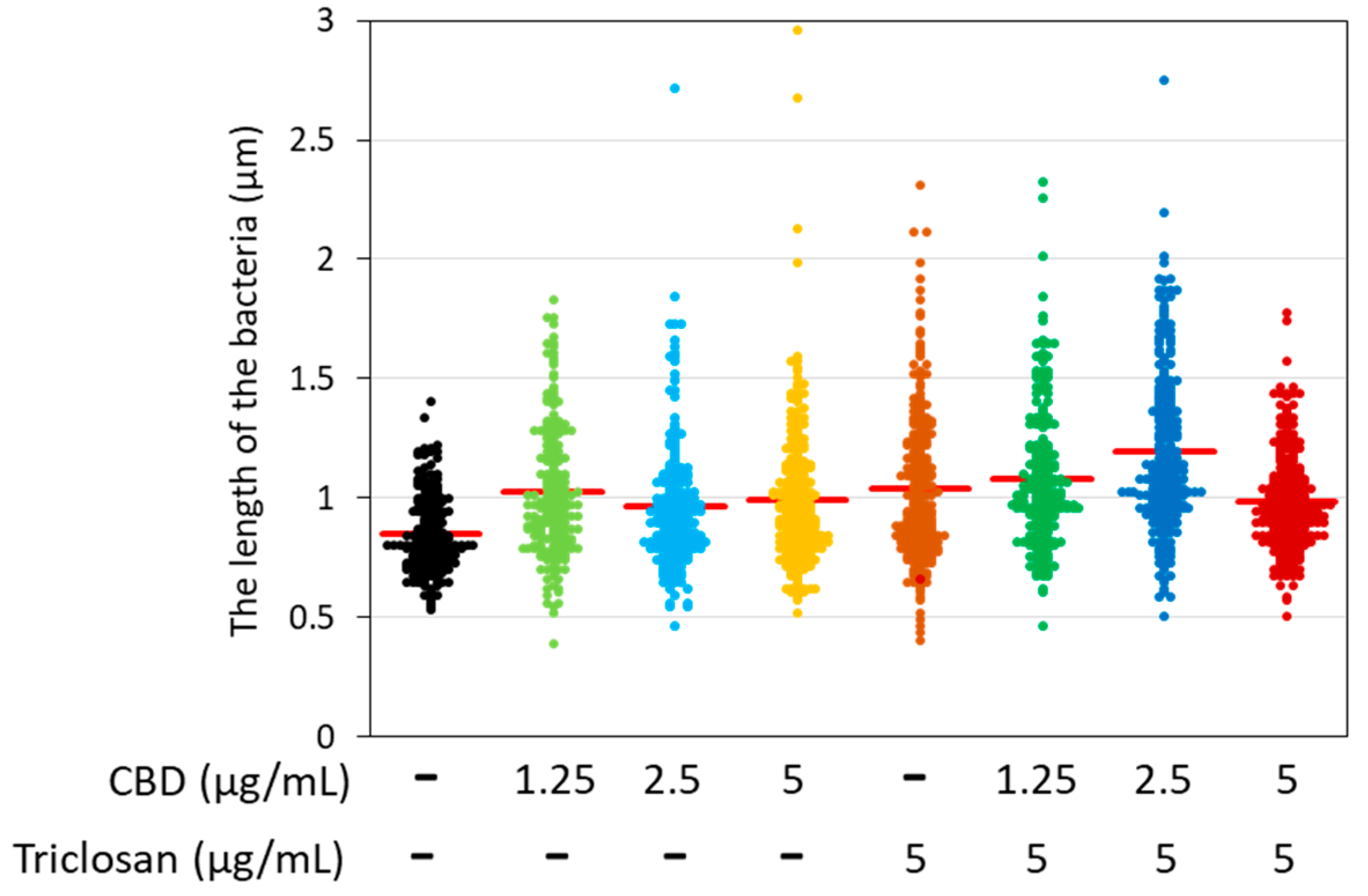
3.5. Morphological Alterations of CBD- and/or Triclosan-Treated S. mutans as Revealed by HR-SEM
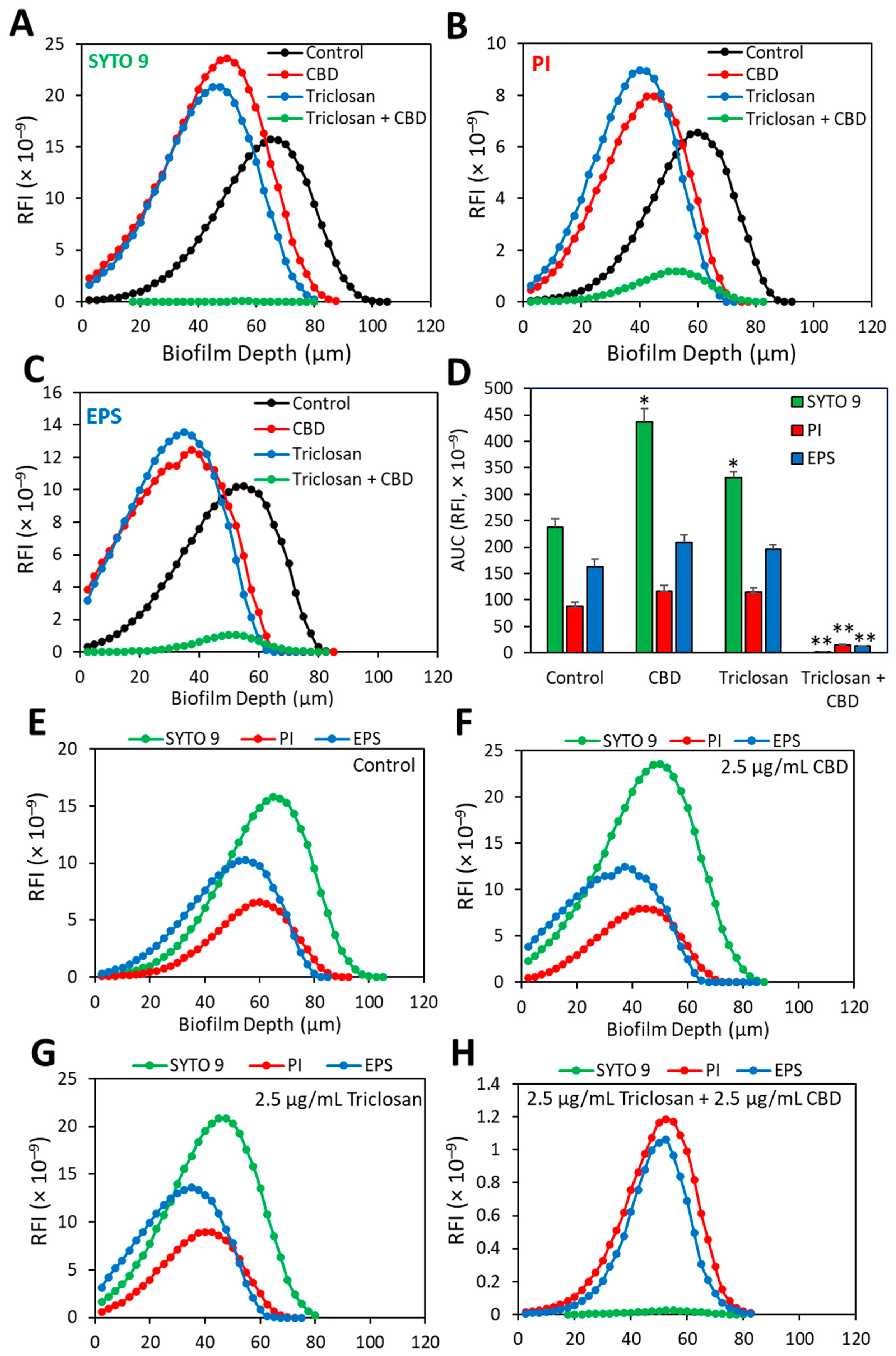
3.6. HR-SEM Images of CBD- and/or Triclosan-Treated S. mutans Biofilms
3.7. Dead/Live Staining of CBD- and/or Triclosan-Treated S. mutans Biofilms
3.8. Cytotoxicity Assay on Vero Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 7-1. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.M.; Dietrich, I.; Klein, C.; von Ohle, C. Dynamic production of soluble extracellular polysaccharides by Streptococcus mutans. Int. J. Dent. 2011, 2011, 435830. [Google Scholar] [CrossRef] [PubMed]
- Scharnow, A.M.; Solinski, A.E.; Wuest, W.M. Targeting S. mutans biofilms: A perspective on preventing dental caries. Medchemcomm 2019, 10, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Varghese, J.; Ramenzoni, L.L.; Shenoy, P.; Nayak, U.Y.; Nayak, N.; Attin, T.; Schmidlin, P.R. In vitro evaluation of substantivity, staining potential, and biofilm reduction of guava leaf extract mouth rinse in combination with its anti-inflammatory effect on human gingival epithelial keratinocytes. Materials 2019, 12, 3903. [Google Scholar] [CrossRef] [PubMed]
- Zayed, S.M.; Aboulwafa, M.M.; Hashem, A.M.; Saleh, S.E. Biofilm formation by Streptococcus mutans and its inhibition by green tea extracts. AMB Express 2021, 11, 73. [Google Scholar] [CrossRef]
- Farkash, Y.; Feldman, M.; Ginsburg, I.; Steinberg, D.; Shalish, M. Polyphenols inhibit Candida albicans and Streptococcus mutans biofilm formation. Dent. J. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Song, Q.; Wang, F.; Sun, L.; Liu, L.; Yang, X.; Yi, J.; Bao, Y.; Ma, H.; et al. Anti-biofilm activities from Bergenia crassifolia leaves against Streptococcus mutans. Front. Microbiol. 2017, 8, 1738. [Google Scholar] [CrossRef]
- Schneider-Rayman, M.; Steinberg, D.; Sionov, R.V.; Friedman, M.; Shalish, M. Effect of epigallocatechin gallate on dental biofilm of Streptococcus mutans: An in vitro study. BMC Oral Health 2021, 21, 447. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed]
- Barak, T.; Sharon, E.; Steinberg, D.; Feldman, M.; Sionov, R.V.; Shalish, M. Anti-bacterial effect of cannabidiol against the cariogenic Streptococcus mutans bacterium: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 15878. [Google Scholar] [CrossRef]
- Stahl, V.; Vasudevan, K. Comparison of efficacy of cannabinoids versus commercial oral care products in reducing bacterial content from dental plaque: A preliminary observation. Cureus 2020, 12, e6809. [Google Scholar] [CrossRef]
- Vasudevan, K.; Stahl, V. Cannabinoids infused mouthwash products are as effective as chlorhexidine on inhibition of total-culturable bacterial content in dental plaque samples. J. Cannabis Res. 2020, 2, 20. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Anti-microbial activity of phytocannabinoids and endocannabinoids in the light of their physiological and pathophysiological roles. Biomedicines 2022, 10, 631. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Hussein, M.; Allobawi, R.; Levou, I.; Blaskovich, M.A.T.; Rao, G.G.; Li, J.; Velkov, T. Mechanisms underlying synergistic killing of Polymyxin B in combination with cannabidiol against Acinetobacter baumannii: A metabolomic study. Pharmaceutics 2022, 14, 786. [Google Scholar] [CrossRef] [PubMed]
- Cortes, E.; Mora, J.; Márquez, E. Modelling the anti-Methicillin-Resistant Staphylococcus aureus (MRSA) activity of cannabinoids: A QSAR and docking study. Crystals 2020, 10, 692. [Google Scholar] [CrossRef]
- Banerjee, S.; Sionov, R.V.; Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Anandamide alters the membrane properties, halts the cell division and prevents drug efflux in multidrug resistant Staphylococcus aureus. Sci. Rep. 2021, 11, 8690. [Google Scholar] [CrossRef]
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Potential combinations of endocannabinoid/endocannabinoid-like compounds and antibiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2020, 15, e0231583. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Banerjee, S.; Bogomolov, S.; Smoum, R.; Mechoulam, R.; Steinberg, D. Targeting the Achilles’ Heel of Multidrug-Resistant Staphylococcus aureus by the endocannabinoid anandamide. Int. J. Mol. Sci. 2022, 23, 7798. [Google Scholar] [CrossRef] [PubMed]
- Abichabki, N.; Zacharias, L.V.; Moreira, N.C.; Bellissimo-Rodrigues, F.; Moreira, F.L.; Benzi, J.R.L.; Ogasawara, T.M.C.; Ferreira, J.C.; Ribeiro, C.M.; Pavan, F.R.; et al. Potential cannabidiol (CBD) repurposing as antibacterial and promising therapy of CBD plus polymyxin B (PB) against PB-resistant gram-negative bacilli. Sci. Rep. 2022, 12, 6454. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, C.S.; Højrup, P.; Klitgaard, J.K. Cannabidiol is an effective helper compound in combination with bacitracin to kill Gram-positive bacteria. Sci. Rep. 2020, 10, 4112. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z. Avidekel Cannabis extracts and cannabidiol are as efficient as Copaxone in suppressing EAE in SJL/J mice. Inflammopharmacology 2019, 27, 167–173. [Google Scholar] [CrossRef]
- Weiss, L.; Zeira, M.; Reich, S.; Slavin, S.; Raz, I.; Mechoulam, R.; Gallily, R. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology 2008, 54, 244–249. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Aziz, A.I.; Nguyen, L.C.; Oumeslakht, L.; Bensussan, A.; Ben Mkaddem, S. Cannabinoids as immune system modulators: Cannabidiol potential therapeutic approaches and limitations. Cannabis Cannabinoid Res. 2022. [Google Scholar] [CrossRef]
- Feldman, M.; Gati, I.; Sionov, R.V.; Sahar-Helft, S.; Friedman, M.; Steinberg, D. Potential combinatory effect of cannabidiol and triclosan incorporated into sustained release delivery system against oral candidiasis. Pharmaceutics 2022, 14, 1624. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-bacterial properties of cannabigerol toward Streptococcus mutans. Front. Microbiol. 2021, 12, 656471. [Google Scholar] [CrossRef]
- Hall, M.J.; Middleton, R.F.; Westmacott, D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother. 1983, 11, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-biofilm activity of cannabigerol against Streptococcus mutans. Microorganisms 2021, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; Park, M.; Chon, J.; Khan, S. Methods to grow and measure in vitro static biofilms. Encycl. Infect. Immun. 2022, 4, 408–429. [Google Scholar]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 2019, 9, 6483. [Google Scholar] [CrossRef] [PubMed]
- Khruengsai, S.; Sripahco, T.; Rujanapun, N.; Charoensup, R.; Pripdeevech, P. Chemical composition and biological activity of Peucedanum dhana A. Ham essential oil. Sci. Rep. 2021, 11, 19079. [Google Scholar] [CrossRef] [PubMed]
- Lobos, O.; Padilla, A.; Padilla, C. In vitro antimicrobial effect of bacteriocin PsVP-10 in combination with chlorhexidine and triclosan against Streptococcus mutans and Streptococcus sobrinus strains. Arch. Oral. Biol. 2009, 54, 230–234. [Google Scholar] [CrossRef]
- Bedran, T.B.; Grignon, L.; Spolidorio, D.P.; Grenier, D. Subinhibitory concentrations of triclosan promote Streptococcus mutans biofilm formation and adherence to oral epithelial cells. PLoS ONE 2014, 9, e89059. [Google Scholar] [CrossRef]
- Dong, L.; Tong, Z.; Linghu, D.; Lin, Y.; Tao, R.; Liu, J.; Tian, Y.; Ni, L. Effects of sub-minimum inhibitory concentrations of antimicrobial agents on Streptococcus mutans biofilm formation. Int. J. Antimicrob. Agents 2012, 39, 390–395. [Google Scholar] [CrossRef]
- Sugimoto, A.; Maeda, A.; Itto, K.; Arimoto, H. Deciphering the mode of action of cell wall-inhibiting antibiotics using metabolic labeling of growing peptidoglycan in Streptococcus pyogenes. Sci. Rep. 2017, 7, 1129. [Google Scholar] [CrossRef]
- Whittle, E.E.; Legood, S.W.; Alav, I.; Dulyayangkul, P.; Overton, T.W.; Blair, J.M.A. Flow cytometric analysis of efflux by dye accumulation. Front. Microbiol. 2019, 10, 2319. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ogawa, W.; Nishioka, T.; Tsuchiya, T.; Kuroda, T. Functionally cloned pdrM from Streptococcus pneumoniae encodes a Na(+) coupled multidrug efflux pump. PLoS ONE 2013, 8, e59525. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Luo, W.; Xu, L.; Yang, B.; Zhao, W.; Cang, H. Progress of antimicrobial discovery against the major cariogenic pathogen Streptococcus mutans. Curr. Issues Mol. Biol. 2019, 32, 601–644. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.R.; Ren, Z.; Hwang, G.; Liu, Y.; Combs, A.; Söderström, B.; Lara Vasquez, P.; Khosravi, Y.; Brady, L.J.; Koo, H.; et al. Streptococcus mutans yidC1 and yidC2 impact cell envelope biogenesis, the biofilm matrix, and biofilm biophysical properties. J. Bacteriol. 2019, 201, e00396-18. [Google Scholar] [CrossRef]
- ISO. 10993–5: 2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Watanabe, E.; Nascimento, A.P.; Guerreiro-Tanomaru, J.M.; Razaboni, A.M.; de Andrade, D.; Tanomaru-Filho, M. Antiseptic mouthwashes: In vitro antibacterial activity. Acta Odontológica Latinoam. 2015, 28, 180–184. [Google Scholar]
- Tafazoli, A.; Tafazoli Moghadam, E. Camellia Sinensis mouthwashes in oral care: A systematic review. J. Dent. 2020, 21, 249–262. [Google Scholar] [CrossRef]
- Kamarehei, F.; Mehdiabadi, M.; Naderi, F. Antibacterial effects of natural compounds on biofilm formation of Streptococcus mutans. Clin. Exp. Dent. Res. 2022, 8, 1426–1433. [Google Scholar] [CrossRef]
- Zeng, Y.; Nikitkova, A.; Abdelsalam, H.; Li, J.; Xiao, J. Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch. Oral. Biol. 2019, 98, 9–16. [Google Scholar] [CrossRef]
- Li, X.; Yin, L.; Ramage, G.; Li, B.; Tao, Y.; Zhi, Q.; Lin, H.; Zhou, Y. Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans. Microbiologyopen 2019, 8, e937. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Kolishetti, N.; Arias, A.Y.; Vashist, A.; Nair, M. Cannabidiol for neurodegenerative disorders: A comprehensive review. Front. Pharmacol. 2022, 13, 989717. [Google Scholar] [CrossRef] [PubMed]
- Gildea, L.; Ayariga, J.A.; Xu, J.; Villafane, R.; Robertson, B.K.; Samuel-Foo, M.; Ajayi, O.S. Cannabis sativa CBD extract exhibits synergy with broad-spectrum antibiotics against Salmonella enterica subsp. Enterica serovar Typhimurium. Microorganisms 2022, 10, 2360. [Google Scholar] [CrossRef]
- Aqawi, M.; Gallily, R.; Sionov, R.V.; Zaks, B.; Friedman, M.; Steinberg, D. Cannabigerol prevents quorum sensing and biofilm formation of Vibrio harveyi. Front. Microbiol. 2020, 11, 858. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the holy triangle of quorum sensing, biofilm formation, and antibiotic resistance in pathogenic bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Feldman, M.; Sionov, R.V.; Mechoulam, R.; Steinberg, D. Anti-biofilm activity of cannabidiol against Candida albicans. Microorganisms 2021, 9, 441. [Google Scholar] [CrossRef]
- Patel, M. Oral cavity and Candida albicans: Colonisation to the development of infection. Pathogens 2022, 11, 335. [Google Scholar] [CrossRef]
- Sztajer, H.; Szafranski, S.P.; Tomasch, J.; Reck, M.; Nimtz, M.; Rohde, M.; Wagner-Döbler, I. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 2014, 8, 2256–2271. [Google Scholar] [CrossRef]
- Benarroch, J.M.; Asally, M. The microbiologist’s guide to membrane potential dynamics. Trends Microbiol. 2020, 28, 304–314. [Google Scholar] [CrossRef]
- Phan, T.-N.; Marquis, R.E. Triclosan inhibition of membrane enzymes and glycolysis of Streptococcus mutans in suspensions and biofilms. Can. J. Microbiol. 2006, 52, 977–983. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avraham, M.; Steinberg, D.; Barak, T.; Shalish, M.; Feldman, M.; Sionov, R.V. Improved Anti-Biofilm Effect against the Oral Cariogenic Streptococcus mutans by Combined Triclosan/CBD Treatment. Biomedicines 2023, 11, 521. https://doi.org/10.3390/biomedicines11020521
Avraham M, Steinberg D, Barak T, Shalish M, Feldman M, Sionov RV. Improved Anti-Biofilm Effect against the Oral Cariogenic Streptococcus mutans by Combined Triclosan/CBD Treatment. Biomedicines. 2023; 11(2):521. https://doi.org/10.3390/biomedicines11020521
Chicago/Turabian StyleAvraham, Maayan, Doron Steinberg, Tamar Barak, Miriam Shalish, Mark Feldman, and Ronit Vogt Sionov. 2023. "Improved Anti-Biofilm Effect against the Oral Cariogenic Streptococcus mutans by Combined Triclosan/CBD Treatment" Biomedicines 11, no. 2: 521. https://doi.org/10.3390/biomedicines11020521
APA StyleAvraham, M., Steinberg, D., Barak, T., Shalish, M., Feldman, M., & Sionov, R. V. (2023). Improved Anti-Biofilm Effect against the Oral Cariogenic Streptococcus mutans by Combined Triclosan/CBD Treatment. Biomedicines, 11(2), 521. https://doi.org/10.3390/biomedicines11020521





