Transforming Cross-Linked Cyclic Dimers of KR-12 into Stable and Potent Antimicrobial Drug Leads
Abstract
1. Introduction
2. Results
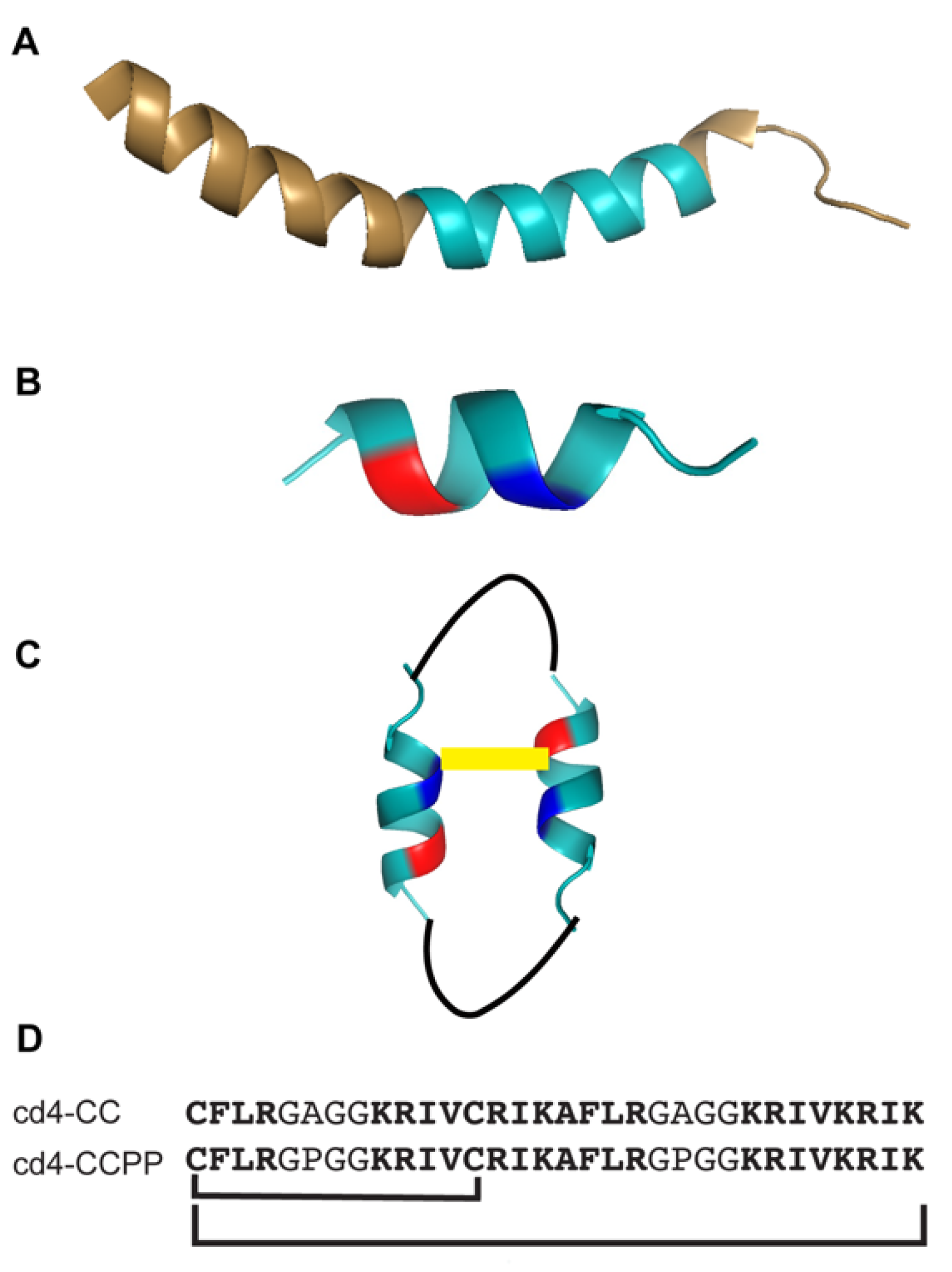
2.1. Design, Synthesis, and Amino Acid Composition of Cross-linked Cyclic Dimer
2.2. Cross-linked Cyclic Dimer Adopts an Alpha-helical Conformation in Membrane Mimetic Environments
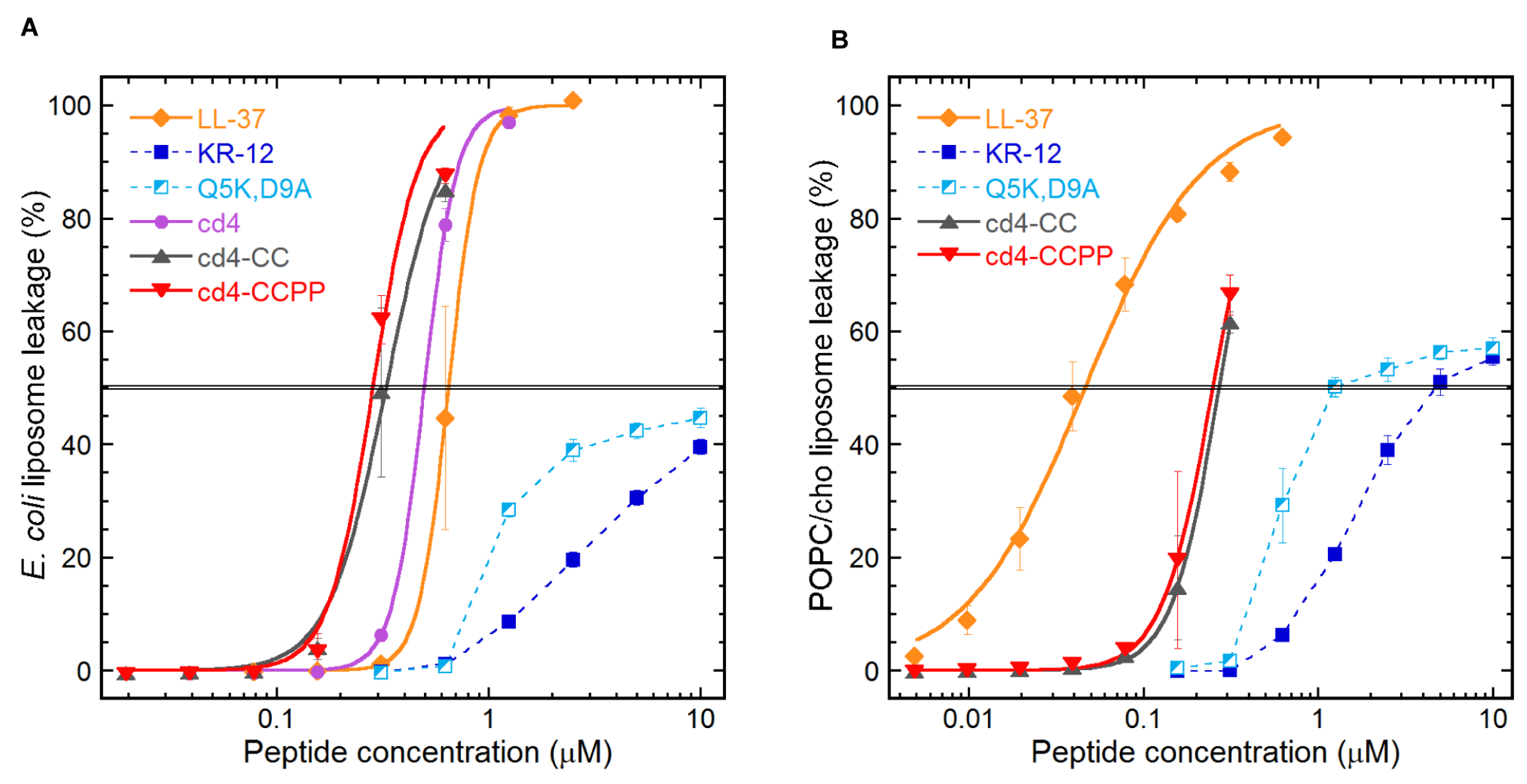
2.3. Quantifying the Membrane-disrupting Mechanism of Cross-linked Cyclic Dimers
2.4. Cross-Linked Cyclic Dimers Are More Potent Than LL-37 in a Two-step Microdilution Assay
2.5. Influence of Physiological Salt Concentrations on the Antimicrobial Activity of Cross-linked Cyclic Dimers
2.6. Cross-linked Cyclic Dimers Retained Antimicrobial Activity in the Presence of Human Serum and Rich Growth Media
2.7. Bacteria Are More Susceptible to Cross-linked Cyclic Dimers in Ionic Environment
2.8. Cross-linked Cyclic Dimers Are Active against LL-37-resistant Strains
2.9. Cross-linked Cyclic Dimers Are Less Hemolytic
2.10. Protease Resistance of Cross-linked Cyclic Dimers
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Nuclear Magnetic Resonance (NMR) Experiments
4.3. Circular Dichroism Spectrum Analysis
4.4. E. coli Liposome Leakage Assay
4.5. Bacterial and Fungal Strains were Used
4.6. Antimicrobial Peptide Susceptibility Testing
4.7. Resistance of Peptides to Salts and Human Serum
4.8. MIC in Mueller–Hinton Broth (MHB) and Ionic Environment
4.9. MIC against Resistant Strains
5. Resistance to Proteolytic Digestion
5.1. Stability Human Serum
5.2. Stability in Commercial Proteases
5.3. Hemolysis Assay
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | Antimicrobial Peptide |
| CD | Circular Dichroism |
| CFU | Colony Forming Unit |
| FMOC | Fluorenylmethyloxycarbonyl |
| HPLC | High-Performance Liquid Chromatography |
| MHB | Mueller–Hinton Broth |
| MIC | Minimum Inhibitory Concentration |
| MS | Mass Spectrometry |
| NMR | Nuclear Magnetic Spectroscopy |
| OD | Optical Density |
| PBS | Phosphate Buffer Saline |
| RP-HPLC | Reverse Phase High-Performance Liquid Chromatography |
| SPPS | Solid-Phase Peptide Synthesis |
| TSB | Tryptic Soy Broth |
References
- Sørensen, O.E.; Gram, L.; Johnsen, A.H.; Andersson, E.; Bangsbøll, S.; Tjabringa, G.S.; Hiemstra, P.S.; Malm, J.; Egesten, A.; Borregaard, N. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: A novel mechanism of generating antimicrobial peptides in vagina. J. Biol. Chem. 2003, 278, 28540–28546. [Google Scholar] [CrossRef] [PubMed]
- Dürr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37--A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta 2016, 1858, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Giacometti, A.; Ghiselli, R.; Bergnach, C.; Orlando, F.; Silvestri, C.; Mocchegiani, F.; Licci, A.; Skerlavaj, B.; Rocchi, M.; et al. LL-37 protects rats against lethal sepsis caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2006, 50, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Nell, M.J.; Tjabringa, G.S.; Wafelman, A.R.; Verrijk, R.; Hiemstra, P.S.; Drijfhout, J.W.; Grote, J.J. Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides 2006, 27, 649–660. [Google Scholar]
- Bowdish, D.M.; Davidson, D.J.; Speert, D.P.; Hancock, R.E.W. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J. Immunol. 2004, 172, 3758–3765. [Google Scholar]
- Grönberg, A.; Mahlapuu, M.; Stahle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: A randomized, placebo-controlled clinical trial. Wound Repair Regen. 2014, 22, 613–621. [Google Scholar] [CrossRef]
- Ciornei, C.D.; Sigurdardottir, T.; Schmidtchen, A.; Bodelsson, M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob. Agents Chemother. 2005, 49, 2845–2850. [Google Scholar] [CrossRef]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: Relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999, 341, 501–513. [Google Scholar]
- Schmidtchen, A.; Frick, I.M.; Andersson, E.; Tapper, H.; Björck, L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 2002, 46, 157–168. [Google Scholar]
- Sieprawska-Lupa, M.; Mydel, P.; Krawczyk, K.; Wojcik, K.; Puklo, M.; Lupa, B.; Suder, P.; Silberring, J.; Reed, M.; Pohl, J.; et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob. Agents Chemother. 2004, 48, 4673–4679. [Google Scholar] [PubMed]
- Abou Alaiwa, M.H.; Reznikov, L.R.; Gansemer, N.D.; Sheets, K.A.; Horswill, A.R.; Stoltz, D.A.; Zabner, J.; Welsh, M.J. pH modulates the activity and synergism of the airway surface liquid antimicrobials beta-defensin-3 and LL-37. Pro. Natl. Acad. Sci. USA 2014, 111, 18703–18708. [Google Scholar]
- Pletzer, D.; Mansour, S.C.; Hancock, R.E.W. Synergy between conventional antibiotics and anti-biofilm peptides in a murine, sub-cutaneous abscess model caused by recalcitrant ESKAPE pathogens. PLoS Pathog. 2018, 14, e1007084. [Google Scholar] [CrossRef] [PubMed]
- Pollini, S.; Brunetti, J.; Sennati, S.; Rossolini, G.M.; Bracci, L.; Pini, A.; Falciani, C. Synergistic activity profile of an antimicrobial peptide against multidrug-resistant and extensively drug-resistant strains of Gram-negative bacterial pathogens. J. Pept. Sci. 2017, 4, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Alanine and Lysine Scans of the LL-37-Derived Peptide Fragment KR-12 Reveal Key Residues for Antimicrobial Activity. Chembiochem 2018, 19, 931–939. [Google Scholar]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Backbone cyclization and dimerization of LL-37-derived peptides enhance antimicrobial activity and proteolytic stability. Front. Microbiol. 2020, 11, 168. [Google Scholar]
- White, J.K.; Muhammad, T.; Alsheim, E.; Mohanty, S.; Blasi-Romero, A.; Gunasekera, S.; Strömstedt, A.A.; Ferraz, N.; Göransson, U.; Brauner, A. A stable cyclized antimicrobial peptide derived from LL-37 with host immunomodulatory effects and activity against uropathogens. Cell Mol. Life Sci. 2022, 79, 411. [Google Scholar] [CrossRef]
- Strömstedt, A.A.; Park, S.; Burman, R.; Göransson, U. Bactericidal activity of cyclotides where phosphatidylethanolamine-lipid selectivity determines antimicrobial spectra. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1986–2000. [Google Scholar] [CrossRef]
- Wang, Y.; Agerberth, B.; Löthgren, A.; Almstedt, A.; Johansson, J. Apolipoprotein A-I Binds and Inhibits the Human Antibacterial/Cytotoxic Peptide LL-37. J. Biol. Chem. 1998, 273, 33115–33118. [Google Scholar] [CrossRef]
- Dorschner, R.A.; Lopez-Garcia, B.; Peschel, A.; Kraus, D.; Morikawa, K.; Nizet, V.; Gallo, R.L. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J. 2006, 1, 35–42. [Google Scholar]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [PubMed]
- Dijksteel, G.S.; Ulrich, M.; Middelkoop, E.; Boekema, B.K.H.L. Lessons learned from clinical trials using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [PubMed]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [PubMed]
- Domadia, P.N.; Bhunia, A.; Ramamoorthy, A.; Bhattacharjya, S. Structure, interactions, and antibacterial activities of MSI-594 derived mutant peptide MSI-594F5A in lipopolysaccharide micelles: Role of the helical hairpin conformation in outer-membrane permeabilization. J. Am. Chem. Soc. 2010, 132, 18417–18428. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Lin, Y.M.; Wang, C.F.; Liao, Y.D. Outer membrane lipoprotein Lpp is Gram-negative bacterial cell surface receptor for cationic antimicrobial peptides. J. Biol. Chem. 2012, 287, 418–428. [Google Scholar] [CrossRef]
- Jacob, B.; Park, I.S.; Bang, J.K.; Shin, S.Y. Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity. J. Pept. Sci. 2013, 19, 700–707. [Google Scholar]
- Verjans, E.T.; Zels, S.; Luyten, W.; Landuyt, B.; Schoofs, L. Molecular mechanisms of LL-37-induced receptor activation: An overview. Peptides 2016, 85, 16–26. [Google Scholar] [CrossRef]
- Ge, Y.; MacDonald, D.L.; Holroyd, K.J.; Thornsberry, C.; Wexler, H.; Zasloff, M. In Vitro antibacterial properties of pexiganan, an analog of Magainin. Antimicrob. Agents Chemother. 1999, 43, 782–788. [Google Scholar]
- Park, I.Y.; Cho, J.H.; Kim, K.S.; Kim, Y.-B.; Kim, M.S.; Kim, S.C. Helix Stability Confers Salt Resistance upon Helical Antimicrobial Peptides. J. Biol. Chem. 2004, 279, 13896–13901. [Google Scholar] [CrossRef]
- Yu, H.Y.; Tu, C.H.; Yip, B.S.; Chen, H.L.; Cheng, H.T.; Huang, K.C.; Lo, H.J.; Cheng, J.W. Easy strategy to increase salt resistance of antimicrobial peptides. Antimicrob. Agents Chemother. 2011, 55, 4918–4921. [Google Scholar] [CrossRef]
- Mohanram, H.; Bhattacharjya, S. Salt-resistant short antimicrobial peptides. Biopolymers 2016, 106, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Lu, Y.A.; Yang, J.L. Design of salt-insensitive glycine-rich antimicrobial peptides with cyclic tricystine structures. Biochemistry 2000, 39, 7159–7169. [Google Scholar] [PubMed]
- Turner, J.; Cho, Y.; Dinh, N.N.; Waring, A.J.; Lehrer, R.I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 1998, 42, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Nijnik, A.; Hancock, R.E.W. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 2009, 16, 41–47. [Google Scholar] [CrossRef]
- Lofton, H.; Pränting, M.; Thulin, E.; Andersson, D.I. Mechanisms and Fitness Costs of Resistance to Antimicrobial Peptides LL-37, CNY100HL and Wheat Germ Histones. PLoS ONE 2013, 8, e68875. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Malik, S.Z. Interaction of Cyclotides and Bacteria: A Study of the Cyclotide Action and the Bacterial Reaction. Ph.D. Dissertation, Uppsala University, Uppsala, Sweden, 2017. ISBN 978-91-554-9870-2. [Google Scholar]
- Strömstedt, A.A.; Kristiansen, P.K.; Gunasekera, S.; Grob, N.; Skjeldal, L. Göransson, U. Selective membrane disruption by the cyclotide kalata B7: Complex ions and essential functional groups in the phosphatidylethanolamine binding pocket. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1317–1327. [Google Scholar] [CrossRef] [PubMed]



| Strain/Peptides | Control 1 | NaCl | NH4Cl | KCl | CaCl2 | MgCl2 | FeCl3 | 25% Serum | MHB 2 | IE 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | ||||||||||
| LL-37 | 0.625 | 5 | 1.25 | 1.25 | >10 | 5 | 2.5 | >40 | 20 | 10 |
| KR2-12 | 2.5 | >10 | 10 | 10 | >10 | >10 | 10 | >40 | 80 | >20 |
| KR-12 (Q5K,D9A) | 1.25 | >10 | 5 | 2.5 | >10 | >10 | 2.5 | >40 | 80 | 10 |
| cd4-CC | 0.625 | 2.5 | 2.5 | 1.25 | 2.5 | 1.25 | 2.5 | 5 | 5 | 1.25 |
| cd4-CCPP | 0.312 | 2.5 | 1.25 | 0.625 | 1.25 | 1.25 | 1.25 | 2.5 | 5 | 0.625 |
| P. aeruginosa | ||||||||||
| LL-37 | 1.25 | 5 | 1.25 | 1.25 | >10 | 5 | 2.5 | >40 | 20 | 10 |
| KR-12 | 10 | >10 | >10 | 10 | >10 | >10 | 10 | >40 | 80 | >20 |
| KR-12 (Q5K,D9A) | 1.25 | >10 | 5 | 2.5 | >10 | >10 | 5 | >40 | 80 | 10 |
| cd4-CC | 0.625 | 2.5 | 1.25 | 1.25 | 2.5 | 2.5 | 1.25 | 5 | 5 | 1.25 |
| cd4-CCPP | 0.625 | 1.25 | 1.25 | 1.25 | 2.5 | 1.25 | 0.625 | 2.5 | 5 | 0.625 |
| S. aureus | ||||||||||
| LL-37 | 1.25 | >10 | 2.5 | 1.25 | 2.5 | 5 | 2.5 | >40 | >80 | 10 |
| KR-12 | 10 | >10 | 10 | >10 | >10 | >10 | 10 | >40 | >80 | >20 |
| KR-12 (Q5K,D9A) | 1.25 | >10 | 2.5 | >10 | >10 | 5 | 2.5 | >40 | >80 | 10 |
| cd4-CC | 0.625 | 2.5 | 2.5 | 0.625 | 1.25 | 1.25 | 1.25 | 20 | >40 | 1.25 |
| cd4-CCPP | 0.625 | 2.5 | 1.25 | 0.625 | 0.625 | 0. 625 | 0.625 | 10 | >40 | 0.625 |
| C. albicans | ||||||||||
| LL-37 | 2.5 | >10 | 2,5 | 2,5 | >10 | >10 | 2.5 | np 4 | np | np |
| KR-12 | 10 | >10 | 10 | >10 | >10 | >10 | 10 | np | np | np |
| KR-12 (Q5K,D9A) | 1.25 | >10 | 10 | 10 | >10 | >10 | 5 | np | np | np |
| cd4-CC | 0.625 | 5 | 1.25 | 1.25 | 2.5 | 2.5 | 1.25 | np | np | np |
| cd4-CCPP | 0.625 | 5 | 0.625 | 0.625 | 2.5 | 1.25 | 0.625 | np | np | np |
| Peptides | Bacterial Species and Strains | |||||
|---|---|---|---|---|---|---|
| S. typhimurium | E. coli | |||||
| WT 1 | waaY | phoP | WT 1 | DA54114 | DA57107 | |
| LL-37 | 2.5 | 5 | 10 | 1 | 1 | 2.25 |
| KR-12 | 20 | 20 | 20 | 1 | 1 | 1 |
| KR-12 (Q5K,D9A) | 10 | 10 | 10 | 1 | 1 | 1 |
| cd4-CCPP | 2.5 | 1.25 | 1.25 | 0.37 | 0.25 | 0.25 |
| cd4-CC | 1.25 | 2.5 | 1.25 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, T.; Strömstedt, A.A.; Gunasekera, S.; Göransson, U. Transforming Cross-Linked Cyclic Dimers of KR-12 into Stable and Potent Antimicrobial Drug Leads. Biomedicines 2023, 11, 504. https://doi.org/10.3390/biomedicines11020504
Muhammad T, Strömstedt AA, Gunasekera S, Göransson U. Transforming Cross-Linked Cyclic Dimers of KR-12 into Stable and Potent Antimicrobial Drug Leads. Biomedicines. 2023; 11(2):504. https://doi.org/10.3390/biomedicines11020504
Chicago/Turabian StyleMuhammad, Taj, Adam A. Strömstedt, Sunithi Gunasekera, and Ulf Göransson. 2023. "Transforming Cross-Linked Cyclic Dimers of KR-12 into Stable and Potent Antimicrobial Drug Leads" Biomedicines 11, no. 2: 504. https://doi.org/10.3390/biomedicines11020504
APA StyleMuhammad, T., Strömstedt, A. A., Gunasekera, S., & Göransson, U. (2023). Transforming Cross-Linked Cyclic Dimers of KR-12 into Stable and Potent Antimicrobial Drug Leads. Biomedicines, 11(2), 504. https://doi.org/10.3390/biomedicines11020504






