Abstract
Nanomedicine, a promising area of medicine, employs nanosized tools for the diagnosis, prevention, and treatment of disease. Particularly, liposomes, lipid-based nanovesicles, are currently one of the most successful nanosystems, with extensive applications in the clinic and an increasing pipeline of products in preclinical and clinical development. These versatile nanotechnological tools are biocompatible and biodegradable, and can load a variety of molecules and, ultimately, improve the therapeutic performance of drugs while minimizing undesired side effects. In this review, we provide a brief description on liposomes’ composition and classification and mainly focus on their clinical use in various areas, including disease management (e.g., cancer, fungal and bacterial infections, ocular pathologies), analgesia, vaccination, diagnostics, and immunosuppression in organ transplantation. Herein are described examples of current liposomal products already in the clinic, as well as the most recent clinical trials involving liposomes as effective and safe nanomedicine tools.
1. Introduction
The constant evolution of science is prompted by knowledge exchange between different areas. In the biology field, it was in the late 1600s that Robert Hooke carried out the first observations of the unit of life, the cell, with only a thirty-times magnification microscope. In parallel, Anton van Leeuwenhoek developed and improved the microscopy field, constructing a microscope capable of up to 300 times magnification that allowed the observation of different types of mammalian cells, tissues and bacteria [1]. In 1931, Ernst Ruska and Max Knoll, two German scientists, achieved a major breakthrough in microscopy technology by creating the first transmission electron microscope [2].
1.1. A Brief History of Liposomes
Since the observation of cells under a microscope, scientists have tried to understand how lipids and biological membranes behave [3]. In 1890, Lord Raleigh studied the interfacial tension between a triglyceride (castor oil) and water [4]. Later, in 1925, Gorter and Grendel demonstrated that the cell membrane was constituted by phospholipid molecules, the “lipid bilayers” [5]. Following these discoveries, Singer and Nicolson suggested the “Fluid Mosaic Model”, in 1972, still accepted today [6]. The introduction of electron microscopy allowed the visualization of biological membranes in greater detail. These appeared as two “opaque” bands divided by “a less opaque interspace” [7], an observation that was interpreted as two opposed phospholipid monolayers [8]. It was around 1962 that Alec Bangham, using a friend’s electron microscope, observed that, in aqueous negative stain, the phospholipid lecithin or its mixture with cholesterol spontaneously formed closed structures, with concentric lamellae [9]. This apparently simple discovery was a revolutionary step in the course of lipid research [3]. At that time, the systems visualized by Bangham were designated as “multilamellar smectic mesophases” and, later on, Gerald Weissmann proposed the name “liposomes” [10]. In the 70 s, Bangham postulated that “something like liposomes must have been available to house the first forms of cellular life” that, together with other studies, remarkably impacted evolutionary history [10].
1.2. Liposome Properties and Composition
Liposomes are defined as small synthetic vesicles composed of one or more lipid bilayers separated by aqueous compartments (Figure 1a) [3]. Liposomes are mainly composed of phospholipids, a group of amphiphile molecules that includes two main categories: glycerophospholipids and sphingomyelins [3,11]. Examples of glycerophospholipids are phosphatidyl choline (PC), phosphatidyl ethanolamine (PE), and phosphatidyl glycerol (PG) [12,13]. An important characteristic of lipids that affects the bilayer properties, including its fluidity, is the phase transition temperature (Tc). This parameter is defined as the temperature at which the physical state of the lipid changes from an ordered and rigid gel-state to a disordered and more fluid liquid-crystalline phase [14]. Tc highly depends on the length and saturation degree of nonpolar chains, with longer and more saturated chains corresponding to higher Tc [14]. Examples of phospholipids with different chain length and saturation degree are distearoyl phosphatidyl choline (DSPC; Tc ≈ +55 °C), dipalmitoyl phosphatidyl choline (DPPC; Tc ≈ +41 °C), dimyristoyl phosphatidyl choline (DMPC; Tc ≈ +24 °C), and dioleoyl phosphatidyl choline (DOPC; Tc ≈ −17 °C) (Figure 1b) [13,14].
These lipid vesicles are extremely versatile, as they can load a variety of molecules, protecting them from premature degradation, changing the pharmacokinetics and improving the biodistribution profile, and ultimately enhancing the therapeutic effect of incorporated drugs [15,16]. Different factors can directly influence the properties of developed liposomal formulations, such as lipid composition, surface charge, bilayer fluidity, size, and preparation method [11,13]. For instance, surface modification with polyethylene glycol (PEG) covalently linked to distearoyl phosphatidyl ethanolamine (DSPE-PEG; Figure 1) is able to decrease the opsonization by plasmatic proteins, avoiding premature detection and uptake by the mononuclear phagocytic system [17,18,19]. Consequently, in vivo, this increases the half-life of liposomes in the bloodstream and their ability to extravasate to affected sites [17,18,19].
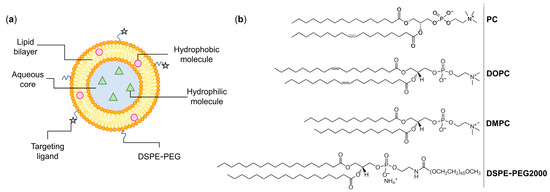
Figure 1.
(a) Schematic representation of a liposome. These lipid-based nanosystems mimic biological membranes and are composed of one (unilamellar) or more (multilamellar) concentric lipid bilayers separated by aqueous compartments. Liposomes are able to accommodate both hydrophilic and hydrophobic molecules and their surface may be coated with specific ligands that recognize receptors overexpressed at tumor cells. (b) Chemical structures of commonly used phospholipids for the preparation of liposomes. PC: phosphatidyl choline; DOPC: dioleoyl phosphatidyl choline; DMPC: dimyristoyl phosphatidyl choline; DSPE-PEG: poly(ethylene glycol) 2000 covalently linked to distearoyl phosphatidyl ethanolamine. Images adapted from [20].
1.3. Classification and Main Applications of Liposomes
As depicted in Figure 2, liposomes are usually classified into three big groups based on size and number of bilayers: unilamellar vesicles (ULVs; one lipid bilayer); oligolamellar vesicles (OLVs; 2–5 lipid bilayers); and multilamellar vesicles (MLVs; more than five lipid bilayers, MLVs). In terms of diameter, ULVs are categorized into small unilamellar vesicles (SUVs; 20–100 nm), large unilamellar vesicles (LUVs; 100–1000 nm), and giant unilamellar vesicles (GUVs; >1000 nm) [11,21,22,23]. In some cases, concentric phospholipid spheres are produced within larger liposomes, forming multivesicular vesicles (MVV) [21,24,25,26].
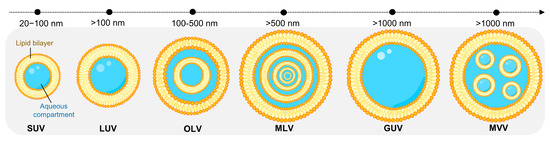
Figure 2.
Visual representation of the different classes of liposomes based on size and lamellarity. SUV: small unilamellar vesicle; LUV: large unilamellar vesicle; OLV: oligolamellar vesicle; MLV: multilamellar vesicle; GUV: giant unilamellar vesicle; MVV: multivesicular vesicle.
Liposomes can also be further subdivided according to their composition and application (Table 1) [22,26,27].

Table 1.
Classification of liposomes based on their composition and properties [22,26,27].
The first research studies using liposomes began in the the 1960s, being applied as models of biological membranes [3]. In 1971, Gregory Gregoriadis introduced liposomes as delivery systems for enzymes (lysozyme) [28,29] and, since then, this lipid-based nanosystem has been used to entrap L-asparaginase [30,31], catalase [32], superoxide dismutase [33], among other enzymes [34]. Besides functioning as models of biological membranes, liposomes are versatile and ideal nanotechnological tools for various purposes, as detailed in Table 2.

Table 2.
Description of some applications of liposomes [27,35].
As previously mentioned, liposomes are extremely useful as delivery systems, with countless examples of high and low molecular weight molecules (e.g., enzymes, proteins, metal-based complexes, antibiotics) that can be loaded in this lipid-based nanoplatform [30,31,33,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. As part of the drug development pipeline, these liposomal formulations must first undergo extensive characterization and evaluation in both in vitro and in vivo models. In Table 3 are presented some examples of preclinical reports with different liposomal formulations, highlighting the advantages of using this nanosystem for therapeutic applications.

Table 3.
Selected preclinical studies of liposomes loaded with high and low molecular weight molecules.
2. Liposomes as Nanomedicine Tools
Over the years, liposomes have been employed as tools to maximize the therapeutic index of a panoply of molecules, including anticancer drugs, antibiotics, genetic material and antifungals [16,19,27,56,57,58]. Nowadays, the success of liposomes is evidenced by several approved products (Table 4; Figure 3) or undergoing clinical trials [59,60,61].

Table 4.
Examples of liposomal formulations approved for clinical use [11,21,23,27,61,62].
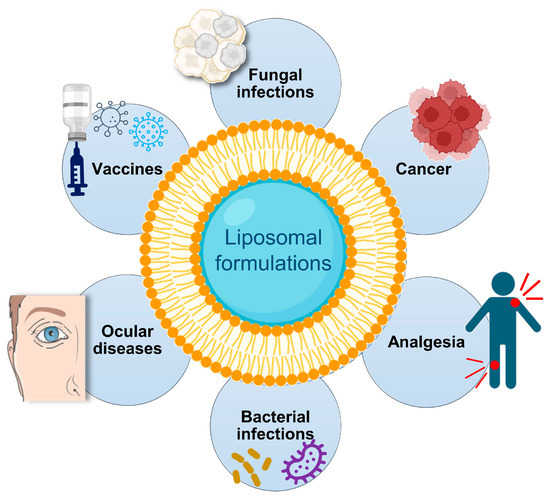
Figure 3.
Clinical applications of liposomes.
2.1. Liposomes for the Treatment of Fungal Infections
AmBisome® was the first approved liposomal formulation to be used in the clinic against fungal infections, including aspergillosis, mucormycosis, invasive candidiasis, and cryptococcal meningitis [63]. Initially, the drug amphotericin B was developed for the treatment of local mycotic infections, being subsequently approved as a systemic antifungal agent [64]. Due to associated nephrotoxicity and infusion-related reactions, the liposomal formulation AmBisome® was designed. This retained the antifungal activity of the drug, while significantly reducing toxicity [63]. In 2022, results from a phase III clinical trial in patients with HIV-associated cryptococcal meningitis demonstrated that a single dose of AmBisome® (together with flucytosine and fluconazole) was equivalent to the current standard of care, with less toxicity [65].
2.2. Liposomes for Cancer Management
In cancer treatment, one of the most well-known examples is Doxil®/Caelyx®, the commercial name for liposomal doxorubicin. This drug, when intravenously administered in the free form, presents high cardiotoxicity. However, following its encapsulation in liposomes, a drastic toxicity reduction was achieved, while the antitumor efficacy was maintained [66], reinforcing the advantages of using this delivery nanosystem. The loading of daunorubicin into liposomes (DaunoXome®) also proved to be very advantageous, increasing tumor drug delivery by approximately 10-fold compared to the free drug and promoting a sustained release in vivo [67].
Another example of an antineoplastic liposomal product is Vyxeos®, which was approved for the treatment of acute myeloid leukemia [68]. This nanoformulation contains two cytotoxic drugs, cytarabine and daunorubicin (5:1). As each one of these drugs displays distinct mechanisms of action, the simultaneous delivery of both drugs in a liposomal formulation resulted in a synergistic effect, increasing treatment efficacy with a lower dosing [68,69].
Continuous advances in nanomedicine are witnessed every day, with novel liposomes being developed and entering clinical trials. Liposomes with ligands attached to their surface (immunoliposomes) are currently under investigation to selectively recognize receptors overexpressed at tumor cells and to promote cellular internalization [70]. This, in turn, results in increased therapeutic efficacy and reduced unwanted side effects. For instance, the endothelial growth factor receptor (EGFR) is known to promote tumorigenesis and it is recognized as a biomarker of drug resistance [71]. Anti-EGFR immunoliposomes loading doxorubicin are in phase II clinical trials for patients with advanced triple-negative breast cancer (NCT02833766) [72]. Also, constant improvements of existing nanomedicines are being made. For example, a novel liposomal formulation of doxorubicin (Talidox) was developed and is currently under a phase I clinical trial (NCT03387917) to assess safety, maximal tolerated dose, pharmacokinetics, and preliminary efficacy. This new liposomal product is expected to improve the benefit/risk profile when compared to established doxorubicin liposomes, namely Doxil®/Caelyx® [72]. Topotecan, a hydrophilic anticancer drug derived from camptothecin, was encapsulated in dihydrosphingomyelin-based liposomes (FF-10850). Currently, liposomal topotecan is undergoing phase I clinical trials (NCT04047251) for the treatment of advanced solid tumors [72,73]. Cancer resistance to drug therapy is a challenge that greatly affects clinical outcomes. In the case of platinum-resistant small cell lung cancer, Onivyde® (liposomal irinotecan) is being tested in a phase 3 clinical trial, showing promising antitumor activity and safety [74].
2.3. Liposomes for the Delivery of Antibacterial Drugs
Liposomes also function as tools to enhance the therapeutic performance of antibiotics, being advantageous against antimicrobial resistance [56]. Arikayce® is a liposomal formulation of amikacin, an antibiotic that belongs to the aminoglycoside class [75,76]. Due to limited safety data, the Food and Drug Administration (FDA) approved this liposomal product only for adult patients with nontuberculous mycobacterial lung disease, in a combination treatment regimen. Inhalation of Arikayce® through a nebulizer improves lung drug delivery compared to intravenously administered free amikacin, effectively clearing pulmonary infections caused by Mycobacterium avium complex [75,76]. A clinical trial (NCT04163601) with liposomal amikacin for inhalation is being conducted to assess the therapeutic efficacy against infections caused by Mycobacterium abscessus, which are difficult to treat and are commonly found in patients with cystic fibrosis [72].
2.4. Liposomes for Ophthalmologic Applications
In ocular diseases, Visudyne® is used for the therapeutic management of age-related macular degeneration by photodynamic therapy [77]. The treatment starts with an intravenous infusion of Visudyne®, followed by nonthermal light activation of the photosensitive drug, verteporfin [77]. Moreover, for the relief of dry eye symptoms, the liposomal products Lacrisek® (Fidia Farmaceutici S.p.A., Abano Terme, Italy) and Optrex™ ActiMist™ (Reckitt Bensicker, Slough, UK) are currently commercialized [78]. In the prevention of macular edema after femtosecond laser-assisted cataract surgery, liposomes loading triamcinolone acetonide were as effective as a combination therapy, with better visual outcomes [79]. Furthermore, in a phase I/II clinical trial (NCT02006147), a liposome ophthalmic formulation of dexamethasone sodium phosphate (TLC399) effectively improved macular edema secondary to retinal vein occlusion [72,80].
2.5. Liposomes in Analgesia
Another useful application of liposomes is in pain management. Opioid analgesia is a major part of post-operative pain control. However, the excessive prescription of opioids brings troubling health consequences for the patients. DepoDur™ (Pacira Pharmaceuticals, Inc., San Diego, CA, USA) is a liposomal formulation of morphine sulfate for a single-dose administration into the lumbar epidural space, providing up to 48 h of analgesia [81,82]. Compared to standard epidural morphine, this nanoformulation decreased the need for supplemental analgesics and ameliorated post-Cesarean delivery pain, with improved mobility of patients [81,82]. In addition, liposomal bupivacaine (Exparel®) is a long-acting analgesia formulation that effectively decreased post-surgery pain and reduced opioid needs [83]. Furthermore, the analgesic efficacy of a single dose of liposomal bupivacaine is being assessed in a phase IV study (NCT03737604) in renal transplant recipients [72].
2.6. Liposomes in Vaccination
In the field of immunization, the introduction of mRNA vaccines was a breakthrough since these elicit a potent and long-lasting immunity. The application of lipid-based nanosystems for mRNA vaccination constitutes an effective strategy and, with the 2019 outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), this nanotechnology further proved its importance in the response against this global health crisis [84]. Moreover, the composition of liposomes can be modeled to exert an immunostimulant effect (e.g., containing monophosphoryl lipid A), being useful as antigen carriers and adjuvants for vaccines [85]. For instance, a vaccine to prevent and reduce human immunodeficiency virus (HIV) spread has been designed (ACTHIVE-001). This vaccine consists in a native-like HIV-1 envelope adjuvanted with MPLA liposomes and is currently in phase I clinical trial (NCT03961438) to evaluate the safety and immunogenicity in healthy adults [72].
2.7. Liposomes for the Delivery of Immunosuppressive Drugs
Organ transplantation is considered one of the major advances of modern medicine and it is often the only chance for patient survival. Organ rejection still represents a challenge, and constant refinement of immunosuppression protocols is required [86] since treatment duration and intensity is associated with increased risk of developing malignancies [87]. Therefore, it is urgent to find tools that provide a more effective and safer use of immunosuppressant drugs. An example is the application of liposomes for the delivery for cyclosporin A. To prevent bronchiolitis obliterans syndrome after allogenic hematopoietic stem cell transplantation or after lung transplant, aerosolized liposomal cyclosporine A was developed (L-CsA) [88,89]. Phase II (NCT04107675) and phase III (NCT03657342, NCT03656926, NCT04039347) studies are being conducted to assess the tolerability and safety, as well as to evaluate the pharmacokinetics and therapeutic efficacy [72].
2.8. Liposomes for Diagnostic Applications
In Alzheimer’s disease, important diagnostic information can be obtained by different imaging modalities, such as magnetic resonance imaging (MRI) and positive emission tomography (PET), which allow the early detection of changes in the brain. One of the hallmarks of this irreversible neurodegenerative disease that impacts cognition and function is the progressive accumulation of extracellular amyloid beta plaques [90]. For contrast-enabled MRI of amyloid plaques, a novel liposomal platform loading the contrast agent gadolinium (ADx-001; single intravenous infusion) is under phase I clinical trial. This study will evaluate the safety and provide the proof-of-concept in patients with suspected Alzheimer’s disease (NCT05453539) [72].
Overall, liposomes have brought significant advances in medicine, with a positive outcome in terms of efficacy and safety. As previously mentioned, one of the benefits of liposomes is the ability to change the biodistribution profile of loaded drugs, depending on the lipid composition, leading to a higher concentration at target sites and minimizing exposure of healthy tissues in comparison with the unloaded drug. Nevertheless, this modified biodistribution may cause some unexpected effects [26]. This is the case of doxorubicin encapsulated in pegylated liposomes. Although its encapsulation in liposomes increases blood circulation and reduces the cardiotoxicity associated to this chemotherapeutic agent, a skin toxic effect has been described, named as hand-foot syndrome [26]. This effect is mainly due to the accumulation at hands and feet of the polymer PEG included in the lipid composition. Fortunately, this occurrence is frequently mild and patients are not usually required to withdraw treatment [26,91].
Another issue associated with liposome administration is the complement activation-related pseudoallergy (CARPA) [92]. This hypersensitivity reaction is sometimes observed upon first exposure to phospholipids included in liposome formulation. However, with subsequent administrations, the symptoms usually decrease or resolve [92,93]. In problematic clinical circumstances, CARPA can be managed by decreasing the infusion rate and by pre-treating patients with steroids and antihistamines to decrease its severity [92,93]. In preclinical studies, for the particular case of Doxil®, premedication with unloaded PEGylated liposomes (placebo liposomes) is being evaluated as a strategy to mitigate CARPA. These in vivo studies demonstrated that placebo liposomes induced tachyphylaxis, resulting in a substantial decrease or almost complete remission of the symptoms [94].
In summary, scientific knowledge is continuously evolving, providing solutions for current and emerging challenges in the area of nanomedicine. Preclinical research and clinical evaluation are crucial to further improve the efficacy and safety of liposomal formulations, ensuring their successful approval for the benefit of patients. All the limitations that may be observed for the new nanomedicines have to be evaluated case by case. Nevertheless, for each clinical condition, a rigorous balance between efficacy and safety have to be carefully considered.
3. Conclusions
Nanotechnology is, undoubtedly, vital to tackle complex medical situations. Liposomes, in particular, have revolutionized the pharmaceutical industry and medicine, providing innovative solutions for disease management and improving patients’ quality of life. Over the years, several liposomal products have successfully reached the market worldwide, with many being researched at the preclinical stage or undergoing clinical trials. These biocompatible lipid-based nanosystems improve the solubility and stability of drugs, prolong half-lives, and promote drug accumulation at target sites (e.g., tumor, infection, inflammation), enhancing treatment efficacy while minimizing unwanted side effects. From a regulatory point of view, the diversity of liposomal products requires appropriate guidelines to ensure their quality, effectiveness and safety, with each phase of research, development and production being tightly controlled. For instance, minor changes in the lipid composition might result in significant variations in the pharmacodynamics and pharmacokinetics of the loaded drug, having repercussions in the therapeutic performance. Globally, lipid-based nanosystems continue to provide innovative solutions for clinical challenges, advancing the treatment and diagnosis of human pathologies.
Author Contributions
Writing—original draft preparation, H.L. and J.O.P.; writing—review and editing, J.O.P. and M.M.G.; supervision and funding acquisition, M.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Fundação para a Ciência e Tecnologia (FCT), projects UIDB/04138/2020 and UIDP/04138/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ribatti, D. An historical note on the cell theory. Exp. Cell Res. 2018, 364, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L. Electron microscope inventors share Nobel Physics Prize. Science 1986, 234, 821–822. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V. Liposomes Came First: The Early History of Liposomology. In Liposomes. Methods in Molecular Biology; D’Souza, G.G.M., Ed.; Humana Press: New York, NY, USA, 2017; pp. 1–15. ISBN 978-1-4939-6591-5. [Google Scholar]
- Fisher, L.; Mitchell, E.; Parker, N. Interfacial tensions of commercial vegetable oils with water. J. Food Sci. 1985, 50, 1201–1202. [Google Scholar] [CrossRef]
- Gorter, E.; Grendel, F. On bimolecular layers of lipoids on the chromocytes of the blood. J. Exp. Med. 1925, 41, 439–443. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Sjöstrand, F.S.; Andersson-Cedergren, E.; Dewey, M.M. The ultrastructure of the intercalated discs of frog, mouse and Guinea pig cardiac muscle. J. Ultrastruct. Res. 1958, 1, 271–287. [Google Scholar] [CrossRef]
- Robertson, J.D. The molecular structure and contact relationships of cell membranes. Prog. Biophys. Mol. Biol. 1960, 10, 343–418. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660-IN10. [Google Scholar] [CrossRef]
- Deamer, D.W. From “Banghasomes” to liposomes: A memoir of Alec Bangham, 1921–2010. FASEB J. 2010, 24, 1308–1310. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Perrie, Y. Liposomes. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 1–8. ISBN 9780470015902. [Google Scholar]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Koynova, R.; Tenchov, B. Phase Transitions and Phase Behavior of Lipids. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1841–1854. ISBN 978-3-642-16712-6. [Google Scholar]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Perche, F.; Torchilin, V.P. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J. Drug Deliv. 2013, 2013, 1–32. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef]
- Allen, T.M.; Hansen, C.B.; de Menezes, D.E.L. Pharmacokinetics of long-circulating liposomes. Adv. Drug Deliv. Rev. 1995, 16, 267–284. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Avanti Polar Lipids. Available online: https://avantilipids.com/ (accessed on 8 November 2022).
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Sheoran, R.; Khokra, S.L.; Chawla, V.; Dureja, H. Recent patents, formulation techniques, classification and characterization of liposomes. Rec. Pat. Nanotechnol. 2019, 13, 17–27. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Milcovich, G.; Lettieri, S.; Antunes, F.E.; Medronho, B.; Fonseca, A.C.; Coelho, J.F.J.; Marizza, P.; Perrone, F.; Farra, R.; Dapas, B.; et al. Recent advances in smart biotechnology: Hydrogels and nanocarriers for tailored bioactive molecules depot. Adv. Colloid Interface Sci. 2017, 249, 163–180. [Google Scholar] [CrossRef]
- Storm, G.; Crommelin, D.J. Liposomes: Quo Vadis? Pharm. Sci. Technol. Today 1998, 1, 19–31. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Sessa, G.; Weissmann, G. Incorporation of lysozyme into liposomes. J. Biol. Chem. 1970, 245, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G.; Ryman, B.E. Liposomes as carriers of enzymes or drugs: A new approach to the treatment of storage diseases. Biochem. J. 1971, 124, 58P. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Perez-Soler, R.; Cruz, M.E.M. Biological characterization of L-asparaginase liposomal formulations. Cancer Chemother. Pharmacol. 1996, 38, 373–377. [Google Scholar] [CrossRef]
- Do, T.T.; Do, T.P.; Nguyen, T.N.; Nguyen, T.C.; Vu, T.T.P.; Nguyen, T.G.A. Nanoliposomal L-asparaginase and its antitumor activities in Lewis lung carcinoma tumor-induced BALB/c mice. Adv. Mater. Sci. Eng. 2019, 2019, 3534807. [Google Scholar] [CrossRef]
- Hei, Y.; Teng, B.; Zeng, Z.; Zhang, S.; Li, Q.; Pan, J.; Luo, Z.; Xiong, C.; Wei, S. Multifunctional immunoliposomes combining catalase and PD-L1 antibodies overcome tumor hypoxia and enhance immunotherapeutic effects against melanoma. Int. J. Nanomed. 2020, 15, 1677–1691. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Boerman, O.C.; Laverman, P.; Corvo, M.L.; Storm, G.; Cruz, M.E.M. Enzymosomes with surface-exposed superoxide dismutase: In vivo behaviour and therapeutic activity in a model of adjuvant arthritis. J. Control. Release 2007, 117, 186–195. [Google Scholar] [CrossRef]
- Cruz, M.E.M.; Corvo, M.L.; Martins, M.B.; Simões, S.; Gaspar, M.M. Liposomes as tools to improve therapeutic enzyme performance. Pharmaceutics 2022, 14, 531. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 302–315. [Google Scholar] [CrossRef]
- Simões, S.; Filipe, A.; Faneca, H.; Mano, M.; Penacho, N.; Düzgünes, N.; de Lima, M.P. Cationic liposomes for gene delivery. Expert Opin. Drug Deliv. 2005, 2, 237–254. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, C.; Zhang, X.; Ning, P.; Wang, Z.; Tian, J.; Chen, X. Liposome-based probes for molecular imaging: From basic research to the bedside. Nanoscale 2019, 11, 5822–5838. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; You, D.G.; Oh, B.H.; Bui, V.D.; An, J.Y.; Um, W.; Park, J.H. Diselenide-bearing liposomes for intracellular delivery of a vitamin C derivative in cancer cells. Macromol. Res. 2021, 29, 327–330. [Google Scholar] [CrossRef]
- Rip, J.; Chen, L.; Hartman, R.; van den Heuvel, A.; Reijerkerk, A.; van Kregten, J.; van der Boom, B.; Appeldoorn, C.; de Boer, M.; Maussang, D.; et al. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood–brain barrier in rats. J. Drug Target. 2014, 22, 460–467. [Google Scholar] [CrossRef]
- Soni, V.; Chandel, S.; Jain, P.; Asati, S. Role of Liposomal Drug-Delivery System in Cosmetics. In Nanobiomaterials in Galenic Formulations and Cosmetics; Grumezescu, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 93–120. ISBN 978-0-323-42868-2. [Google Scholar]
- Corvo, M.L.; Martins, M.B.; Francisco, A.P.; Morais, J.G.; Cruz, M.E.M. Liposomal formulations of Cu,Zn-superoxide dismutase: Physico-chemical characterization and activity assessment in an inflammation model. J. Control. Release 1997, 43, 1–8. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Calado, S.; Pereira, J.; Ferronha, H.; Correia, I.; Castro, H.; Tomás, A.M.; Cruz, M.E.M. Targeted delivery of paromomycin in murine infectious diseases through association to nano lipid systems. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1851–1860. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Neves, S.; Portaels, F.; Pedrosa, J.; Silva, M.T.; Cruz, M.E.M. Therapeutic efficacy of liposomal rifabutin in a Mycobacterium avium model of infection. Antimicrob. Agents Chemother. 2000, 44, 2424–2430. [Google Scholar] [CrossRef]
- Marcelino, P.; Marinho, H.S.; Campos, M.C.; Neves, A.R.; Real, C.; Fontes, F.S.; Carvalho, A.; Feio, G.; Martins, M.B.F.; Corvo, M.L. Therapeutic activity of superoxide dismutase-containing enzymosomes on rat liver ischaemia-reperfusion injury followed by magnetic resonance microscopy. Eur. J. Pharm. Sci. 2017, 109, 464–471. [Google Scholar] [CrossRef]
- Kim, I.-S.; Choi, H.-G.; Choi, H.-S.; Kim, B.-K.; Kim, C.-K. Prolonged systemic delivery of streptokinase using liposome. Arch. Pharm. Res. 1998, 21, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, T.; Zhang, X.; Rao, H.; Jin, Z.; Pan, X.; Li, J.; Mo, Y.; Cai, Y.; Wu, J. CRGD urokinase liposomes for thrombolysis in rat model of acute pulmonary microthromboembolism. Drug Des. Devel. Ther. 2022, 16, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, M.M.; Cruz, A.; Penha, A.F.; Reymão, J.; Sousa, A.C.; Eleutério, C.V.; Domingues, S.A.; Fraga, A.G.; Filho, A.L.; Cruz, M.E.M.; et al. Rifabutin encapsulated in liposomes exhibits increased therapeutic activity in a model of disseminated tuberculosis. Int. J. Antimicrob. Agents 2008, 31, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Mandal, M.; Pinho, J.O.; Catalão, M.J.; Almeida, A.J.; Azevedo-Pereira, J.M.; Gaspar, M.M.; Anes, E. Liposomal Delivery of Saquinavir to Macrophages Overcomes Cathepsin Blockade by Mycobacterium tuberculosis and Helps Control the Phagosomal Replicative Niches. Int. J. Mol. Sci. 2023, 24, 1142. [Google Scholar] [CrossRef]
- Jorge, J.C.S.; Perez-Soler, R.; Morais, J.G.; Cruz, M.E.M. Liposomal palmitoyl-L-asparaginase: Characterization and biological activity. Cancer Chemother. Pharmacol. 1994, 34, 230–234. [Google Scholar] [CrossRef]
- Pinho, J.O.; Matias, M.; Marques, V.; Eleutério, C.; Fernandes, C.; Gano, L.; Amaral, J.D.; Mendes, E.; Perry, M.J.; Moreira, J.N.; et al. Preclinical validation of a new hybrid molecule loaded in liposomes for melanoma management. Biomed. Pharmacother. 2023, 157, 114021. [Google Scholar] [CrossRef]
- Pinho, J.O.; Amaral, J.D.; Castro, R.E.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine 2019, 14, 835–850. [Google Scholar] [CrossRef]
- Matos, C.P.; Albino, M.; Lopes, J.; Viana, A.S.; Côrte-Real, L.; Mendes, F.; Pessoa, J.C.; Tomaz, A.I.; Reis, C.P.; Gaspar, M.M.; et al. New iron(III) anti-cancer aminobisphenolate/phenanthroline complexes: Enhancing their therapeutic potential using nanoliposomes. Int. J. Pharm. 2022, 623, 121925. [Google Scholar] [CrossRef]
- Pinho, J.O.; da Silva, I.V.; Amaral, J.D.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Therapeutic potential of a copper complex loaded in pH-sensitive long circulating liposomes for colon cancer management. Int. J. Pharm. 2021, 599, 120463. [Google Scholar] [CrossRef]
- Ribeiro, N.; Albino, M.; Ferreira, A.; Escrevente, C.; Barral, D.; Pessoa, J.; Reis, C.; Gaspar, M.; Correia, I. Liposomal formulations of a new zinc(II) complex exhibiting high therapeutic potential in a murine colon cancer model. Int. J. Mol. Sci. 2022, 23, 6728. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as antibiotic delivery systems: A promising nanotechnological strategy against antimicrobial resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- James, N.D.; Coker, R.J.; Tomlinson, D.; Harris, J.R.W.; Gompels, M.; Pinching, A.J.; Stewart, J.S.W. Liposomal doxorubicin (Doxil): An effective new treatment for Kaposi’s sarcoma in AIDS. Clin. Oncol. 1994, 6, 294–296. [Google Scholar] [CrossRef]
- Ferreira, M.; Pinto, S.N.; Aires-da-Silva, F.; Bettencourt, A.; Aguiar, S.I.; Gaspar, M.M. Liposomes as a nanoplatform to improve the delivery of antibiotics into Staphylococcus aureus biofilms. Pharmaceutics 2021, 13, 321. [Google Scholar] [CrossRef]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Pinho, J.O.; Matias, M.; Gaspar, M.M. Emergent nanotechnological strategies for systemic chemotherapy against melanoma. Nanomaterials 2019, 9, 1455. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles–From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal amphotericin B (AmBisome®): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An overview of the main antifungal agent used to treat invasive fungal infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Jarvis, J.N.; Lawrence, D.S.; Meya, D.B.; Kagimu, E.; Kasibante, J.; Mpoza, E.; Rutakingirwa, M.K.; Ssebambulidde, K.; Tugume, L.; Rhein, J.; et al. Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N. Engl. J. Med. 2022, 386, 1109–1120. [Google Scholar] [CrossRef]
- Rafiyath, S.M.; Rasul, M.; Lee, B.; Wei, G.; Lamba, G.; Liu, D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: A meta-analysis. Exp. Hematol. Oncol. 2012, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D.; et al. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.O.; Pottier, A.; Schiffelers, R.; Ceccaldi, A.; Schmid, R. Delivering the power of nanomedicine to patients today. J. Control. Release 2020, 326, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, L.; Saunders, D.N.; Ranson, M.; Thurecht, K.J.; Storm, G.; Vine, K.L. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J. Control. Release 2018, 277, 1–13. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine Clinical Trials. Available online: https://clinicaltrials.gov/ (accessed on 29 November 2022).
- Matulonis, U.A.; Janku, F.; Moser, J.C.; Fu, S.; Wages, D.S.; Wheeler, C.A.; Mori, M.; Shimoyama, S.; Yamada, N.; Subach, R.A.; et al. A first-in-human phase 1 dose escalation study of FF-10850 (liposomal topotecan) in patients with advanced solid tumors. J. Clin. Oncol. 2022, 40, 3101. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Spigel, D.R.; Chen, Y.; Jove, M.; Juan-Vidal, O.; Rich, P.; Hayes, T.; Calderón, V.G.; Caro, R.B.; Navarro, A.; et al. RESILIENT part 1: A phase 2 dose-exploration and dose-expansion study of second-line liposomal irinotecan in adults with small cell lung cancer. Cancer 2022, 128, 1801–1811. [Google Scholar] [CrossRef]
- Khan, O.; Chaudary, N. The use of amikacin liposome inhalation suspension (Arikayce) in the treatment of refractory nontuberculous mycobacterial lung disease in adults. Drug Des. Devel. Ther. 2020, 14, 2287–2294. [Google Scholar] [CrossRef]
- Leong, E.W.X.; Ge, R. Lipid nanoparticles as delivery vehicles for inhaled therapeutics. Biomedicines 2022, 10, 2179. [Google Scholar] [CrossRef]
- Bressler, N.M.; Bressler, S.B. Photodynamic therapy with verteporfin (Visudyne): Impact on ophthalmology and visual sciences. Investig. Ophthalmol. Vis. Sci. 2000, 41, 624–628. [Google Scholar]
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538. [Google Scholar] [CrossRef]
- Gonzalez-De la Rosa, A.; Navarro-Partida, J.; Altamirano-Vallejo, J.C.; Jauregui-Garcia, G.D.; Acosta-Gonzalez, R.; Ibanez-Hernandez, M.A.; Mora-Gonzalez, G.F.; Armendáriz-Borunda, J.; Santos, A. Novel triamcinolone acetonide-loaded liposomal topical formulation improves contrast sensitivity outcome after femtosecond laser-assisted cataract surgery. J. Ocul. Pharmacol. Ther. 2019, 35, 512–521. [Google Scholar] [CrossRef]
- Bonilla, L.; Espina, M.; Severino, P.; Cano, A.; Ettcheto, M.; Camins, A.; García, M.L.; Souto, E.B.; Sánchez-López, E. Lipid nanoparticles for the posterior eye segment. Pharmaceutics 2021, 14, 90. [Google Scholar] [CrossRef]
- Alam, M.; Hartrick, C.T. Extended-release epidural morphine (DepoDurTM): An old drug with a new profile. Pain Pract. 2005, 5, 349–353. [Google Scholar] [CrossRef]
- Carvalho, B.; Roland, L.M.; Chu, L.F.; Campitelli, V.A.; Riley, E.T. Single-dose, extended-release epidural morphine (DepoDurTM) compared to conventional epidural morphine for post-cesarean pain. Anesth. Analg. 2007, 105, 176–183. [Google Scholar] [CrossRef]
- Hellums, R.N.; Adams, M.D.; Purdy, N.C.; Lindemann, T.L. Impact of liposomal bupivacaine on post-operative pain and opioid usage in thyroidectomy. Ann. Otol. Rhinol. Laryngol. 2023, 132, 77–81. [Google Scholar] [CrossRef]
- Lopez-Cantu, D.O.; Wang, X.; Carrasco-Magallanes, H.; Afewerki, S.; Zhang, X.; Bonventre, J.V.; Ruiz-Esparza, G.U. From bench to the clinic: The path to translation of nanotechnology-enabled mRNA SARS-CoV-2 vaccines. Nano-Micro Lett. 2022, 14, 41. [Google Scholar] [CrossRef]
- Rao, M.; Peachman, K.K.; Alving, C.R. Liposome Formulations as Adjuvants for Vaccines. In Nanoparticles for Rational Vaccine Design. Current Topics in Microbiology and Immunology, 433; Gill, H.S., Compans, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–28. ISBN 978-3-030-85067-8. [Google Scholar]
- Bezinover, D.; Saner, F. Organ transplantation in the modern era. BMC Anesthesiol. 2019, 19, 32. [Google Scholar] [CrossRef]
- Cangemi, M.; Montico, B.; Faè, D.A.; Steffan, A.; Dolcetti, R. Dissecting the multiplicity of immune effects of immunosuppressive drugs to better predict the risk of de novo malignancies in solid organ transplant patients. Front. Oncol. 2019, 9, 160. [Google Scholar] [CrossRef]
- Behr, J.; Zimmermann, G.; Baumgartner, R.; Leuchte, H.; Neurohr, C.; Brand, P.; Herpich, C.; Sommerer, K.; Seitz, J.; Menges, G.; et al. Lung deposition of a liposomal cyclosporine A inhalation solution in patients after lung transplantation. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Neurohr, C.; Kneidinger, N.; Ghiani, A.; Monforte, V.; Knoop, C.; Jaksch, P.; Parmar, J.; Ussetti, P.; Sole, A.; Müller-Quernheim, J.; et al. A randomized controlled trial of liposomal cyclosporine A for inhalation in the prevention of bronchiolitis obliterans syndrome following lung transplantation. Am. J. Transplant. 2022, 22, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of early Alzheimer’s disease: Clinical practice in 2021. J. Prev. Alzheimer’s Dis. 2021, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef]
- Szebeni, J.; Storm, G. Complement activation as a bioequivalence issue relevant to the development of generic liposomes and other nanoparticulate drugs. Biochem. Biophys. Res. Commun. 2015, 468, 490–497. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef]
- Szebeni, J.; Bedőcs, P.; Urbanics, R.; Bünger, R.; Rosivall, L.; Tóth, M.; Barenholz, Y. Prevention of infusion reactions to PEGylated liposomal doxorubicin via tachyphylaxis induction by placebo vesicles: A porcine model. J. Control. Release 2012, 160, 382–387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).