Altered MANF Expression in Pancreatic Acinar and Ductal Cells in Chronic Alcoholic Pancreatitis: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, V.K.; Yadav, D.; Garg, P.K. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA 2019, 322, 2422–2434. [Google Scholar] [CrossRef]
- Coté, G.A.; Yadav, D.; Slivka, A.; Hawes, R.H.; Anderson, M.A.; Burton, F.R.; Brand, R.E.; Banks, P.A.; Lewis, M.D.; Disario, J.A. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 266–273. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Hijona, E.; Bañales, J.M.; Cosme, A.; Bujanda, L. Alcohol consumption on pancreatic diseases. World J. Gastroenterol. 2013, 19, 638. [Google Scholar]
- Conwell, D.L.; Banks, P.A.; Sandhu, B.S.; Sherman, S.; Al-Kaade, S.; Gardner, T.B.; Anderson, M.A.; Wilcox, C.M.; Lewis, M.D.; Muniraj, T. Validation of demographics, etiology, and risk factors for chronic pancreatitis in the USA: A report of the North American Pancreas Study (NAPS) Group. Dig. Dis. Sci. 2017, 62, 2133–2140. [Google Scholar] [CrossRef]
- Dufour, M.C.; Adamson, M.D. The epidemiology of alcohol-induced pancreatitis. Pancreas 2003, 27, 286–290. [Google Scholar] [CrossRef]
- Vonlaufen, A.; Wilson, J.S.; Pirola, R.C.; Apte, M.V. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res. Health 2007, 30, 48. [Google Scholar] [PubMed]
- Singhvi, A.; Yadav, D. Myths and realities about alcohol and smoking in chronic pancreatitis. Curr. Opin. Gastroenterol. 2018, 34, 355. [Google Scholar] [CrossRef] [PubMed]
- Pandol, S.J.; Periskic, S.; Gukovsky, I.; Zaninovic, V.; Jung, Y.; Zong, Y.; Solomon, T.E.; Gukovskaya, A.S.; Tsukamoto, H. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology 1999, 117, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Gukovsky, I.; Lugea, A.; Shahsahebi, M.; Cheng, J.H.; Hong, P.P.; Jung, Y.J.; Deng, Q.-G.; French, B.A.; Lungo, W.; French, S.W. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am. J. Physiol. -Gastrointest. Liver Physiol. 2008, 294, G68–G79. [Google Scholar] [CrossRef]
- Lugea, A.; Tischler, D.; Nguyen, J.; Gong, J.; Gukovsky, I.; French, S.W.; Gorelick, F.S.; Pandol, S.J. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 2011, 140, 987–997.e8. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, W.; Luo, J. Targeting Endoplasmic Reticulum Stress as an Effective Treatment for Alcoholic Pancreatitis. Biomedicines 2022, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M. Endoplasmic reticulum stress responses. Cell. Mol. Life Sci. 2008, 65, 862–894. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, M.; Danilova, T.; Palm, E.; Lindholm, P.; Võikar, V.; Hakonen, E.; Ustinov, J.; Andressoo, J.-O.; Harvey, B.K.; Otonkoski, T. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep. 2014, 7, 366–375. [Google Scholar]
- Danilova, T.; Belevich, I.; Li, H.; Palm, E.; Jokitalo, E.; Otonkoski, T.; Lindahl, M. MANF is required for the postnatal expansion and maintenance of pancreatic β-cell mass in mice. Diabetes 2019, 68, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.S.; Raibekas, A.; Pevsner, J.; Vigo, N.; Anafi, M.; Moore, M.K.; Peaire, A.E.; Shridhar, V.; Smith, D.I.; Kelly, J. MANF: A new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J. Mol. Neurosci. 2003, 20, 173–187. [Google Scholar] [CrossRef]
- Yang, S.; Li, S.; Li, X.-J. MANF: A new player in the control of energy homeostasis, and beyond. Front. Physiol. 2018, 9, 1725. [Google Scholar] [CrossRef]
- Wen, W.; Li, H.; Luo, J. Potential Role of MANF, an ER Stress Responsive Neurotrophic Factor, in Protecting Against Alcohol Neurotoxicity. Mol. Neurobiol. 2022, 59, 2992–3015. [Google Scholar] [CrossRef]
- Gaisano, H.Y.; Gorelick, F.S. New insights into the mechanisms of pancreatitis. Gastroenterology 2009, 136, 2040–2044. [Google Scholar] [CrossRef]
- Gukovskaya, A.S.; Gorelick, F.S.; Groblewski, G.E.; Mareninova, O.A.; Lugea, A.; Antonucci, L.; Waldron, R.T.; Habtezion, A.; Karin, M.; Pandol, S.J. Recent insights into the pathogenic mechanism of pancreatitis: Role of acinar cell organelle disorders. Pancreas 2019, 48, 459. [Google Scholar] [CrossRef]
- Pandol, S.J.; Gorelick, F.S.; Gerloff, A.; Lugea, A. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig. Dis. 2010, 28, 776–782. [Google Scholar]
- Glembotski, C.C. Functions for the cardiomyokine, MANF, in cardioprotection, hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2011, 51, 512–517. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Y.; Luo, H.; Zhang, F.; Peng, D.; Jing, L.; Wu, Y.; Xia, X.; Song, Y.; Li, W. MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways. Exp. Neurol. 2018, 308, 59–71. [Google Scholar] [CrossRef]
- Xu, S.; Di, Z.; He, Y.; Wang, R.; Ma, Y.; Sun, R.; Li, J.; Wang, T.; Shen, Y.; Fang, S. Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Aβ toxicity via attenuating Aβ-induced endoplasmic reticulum stress. J. Neuroinflammation 2019, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, W.; Li, H.; Clementino, M.; Xu, H.; Xu, M.; Ma, M.; Frank, J.; Luo, J. MANF is neuroprotective against ethanol-induced neurodegeneration through ameliorating ER stress. Neurobiol. Dis. 2021, 148, 105216. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, H.; Wen, W.; Wang, Y.; Xu, H.; Xu, M.; Frank, J.A.; Wei, W.; Luo, J. MANF protects pancreatic acinar cells against alcohol-induced endoplasmic reticulum stress and cellular injury. J. Hepato-Biliary-Pancreat. Sci. 2021, 28, 883–892. [Google Scholar] [CrossRef]
- Sharer, N.; Schwarz, M.; Malone, G.; Howarth, A.; Painter, J.; Super, M.; Braganza, J. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N. Engl. J. Med. 1998, 339, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.B.; Mizuno, N.; Yatabe, Y.; Yoshikawa, T.; Ishiguro, H.; Yamamoto, A.; Azuma, S.; Naruse, S.; Yamao, K.; Muallem, S. Corticosteroids correct aberrant CFTR localization in the duct and regenerate acinar cells in autoimmune pancreatitis. Gastroenterology 2010, 138, 1988–1996.e1983. [Google Scholar] [CrossRef]
- Maléth, J.; Balázs, A.; Pallagi, P.; Balla, Z.; Kui, B.; Katona, M.; Judák, L.; Németh, I.; Kemény, L.V.; Rakonczay, Z., Jr. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology 2015, 148, 427–439.e416. [Google Scholar] [CrossRef]
- Muniraj, T.; Aslanian, H.R.; Farrell, J.; Jamidar, P.A. Chronic pancreatitis, a comprehensive review and update. Part I: Epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis. Mon. 2014, 60, 530–550. [Google Scholar] [CrossRef]
- Pham, A.; Forsmark, C. Chronic pancreatitis: Review and update of etiology, risk factors, and management. F1000Research 2018, 7, Rev-607. [Google Scholar] [CrossRef]
- Sah, R.P.; Garg, S.K.; Dixit, A.K.; Dudeja, V.; Dawra, R.K.; Saluja, A.K. Endoplasmic reticulum stress is chronically activated in chronic pancreatitis. J. Biol. Chem. 2014, 289, 27551–27561. [Google Scholar] [CrossRef] [PubMed]
- Le Maréchal, C.; Bretagne, J.-F.; Raguénès, O.; Quéré, I.; Chen, J.-M.; Ferec, C. Identification of a novel pancreatitis-associated missense mutation, R116C, in the human cationic trypsinogen gene (PRSS1). Mol. Genet. Metab. 2001, 74, 342–344. [Google Scholar] [CrossRef]
- Tautermann, G.; Ruebsamen, H.; Beck, M.; Dertinger, S.; Drexel, H.; Lohse, P. R116C mutation of cationic trypsinogen in a Turkish family with recurrent pancreatitis illustrates genetic microheterogeneity of hereditary pancreatitis. Digestion 2001, 64, 226–232. [Google Scholar] [CrossRef]
- Teich, N.; Bauer, N.; Mössner, J.; Keim, V. Mutational screening of patients with nonalcoholic chronic pancreatitis: Identification of further trypsinogen variants. Am. J. Gastroenterol. 2002, 97, 341–346. [Google Scholar] [CrossRef]
- Pho-Iam, T.; Thongnoppakhun, W.; Yenchitsomanus, P.-T.; Limwongse, C. A Thai family with hereditary pancreatitis and increased cancer risk due to a mutation in PRSS1 gene. World J. Gastroenterol. 2005, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Kereszturi, É.; Szmola, R.; Kukor, Z.; Simon, P.; Ulrich Weiss, F.; Lerch, M.M.; Sahin-Tóth, M. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: A novel disease mechanism. Hum. Mutat. 2009, 30, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Alahari, S.; Mehmood, R.; Johnson, C.L.; Pin, C.L. The absence of MIST1 leads to increased ethanol sensitivity and decreased activity of the unfolded protein response in mouse pancreatic acinar cells. PloS One 2011, 6, e28863. [Google Scholar] [CrossRef]
- Whitcomb, D.C.; LaRusch, J.; Krasinskas, A.M.; Klei, L.; Smith, J.P.; Brand, R.E.; Neoptolemos, J.P.; Lerch, M.M.; Tector, M.; Sandhu, B.S. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat. Genet. 2012, 44, 1349–1354. [Google Scholar] [CrossRef]
- Ke, Z.; Wang, X.; Liu, Y.; Fan, Z.; Chen, G.; Xu, M.; Bower, K.A.; Frank, J.A.; Li, M.; Fang, S. Ethanol induces endoplasmic reticulum stress in the developing brain. Alcohol Clin. Exp. Res. 2011, 35, 1574–1583. [Google Scholar] [CrossRef]
- Galli, E.; Härkönen, T.; Sainio, M.T.; Ustav, M.; Toots, U.; Urtti, A.; Yliperttula, M.; Lindahl, M.; Knip, M.; Saarma, M. Increased circulating concentrations of mesencephalic astrocyte-derived neurotrophic factor in children with type 1 diabetes. Sci. Rep. 2016, 6, 29058. [Google Scholar] [CrossRef]
- Montaser, H.; Patel, K.A.; Balboa, D.; Ibrahim, H.; Lithovius, V.; Näätänen, A.; Chandra, V.; Demir, K.; Acar, S.; Ben-Omran, T. Loss of MANF causes childhood-onset syndromic diabetes due to increased endoplasmic reticulum stress. Diabetes 2021, 70, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Monroe, K.; Lugea, A.; Yadav, D.; Pandol, S. Uniting epidemiology and experimental disease models for alcohol-related pancreatic disease. Alcohol Res. Curr. Rev. 2017, 38, 173. [Google Scholar]




| Accession Type Accession Reason | # | Pathologic Diagnosis | Age (Average, (Range)) | Sex |
|---|---|---|---|---|
| Total | 87 | 58 (11–82) | 42M/45F | |
| Surgical Pathology | 77 | Primary Histologic Diagnosis | 58 (11–82) | 36M/41F |
| Lesion Removal | 72 | 30 ductal adenocarcinoma 15 intraductal papillary mucinous neoplasm 7 neuroendocrine neoplasm 5 pancreatic pseudocyst 4 solid pseudopapillary tumor 3 pancreatic intraepithelial neoplasia 2 ampullary adenocarcinoma 2 mucinous cystic neoplasm 1 benign non-neoplastic cyst 1 benign common bile duct stenosis 1 lymphoepithelial cyst 1 metastatic cancer | 60 (11–82) | 33M/39F |
| Recurrent Pancreatitis | 3 | 1 obstructive choleliths 1 cystic fibrosis 1 alcoholic pancreatitis | 43 (23–60) | 2M/1F |
| Trauma | 1 | 1 splenic hematoma | 53 (53) | 1M |
| Unsure | 1 | 1 unsure | 47 (47) | 1F |
| Autopsy | 10 | Cause of Death 6 complications of chronic alcoholism 2 pyelonephritis 1 acute pancreatitis (non-alcoholic) 1 bronchopneumonia | 43 (25–59) | 6M/4F |
| Tissue Demonstrated (by case) | # | Accession Reason | Age (Average, (Range)) | Sex |
|---|---|---|---|---|
| CAP only | 15 | 9 autopsy 3 lesion removal 1 recurrent pancreatitis 1 trauma 1 unsure | 48 (28–68) | 10M/5F |
| CNP only | 45 | 43 lesion removal 1 autopsy 1 recurrent pancreatitis | 62 (17–82) | 22M/23F |
| NPT only | 14 | 14 lesion removal | 52 (19–75) | 5M/9F |
| Both CNP and NPT | 13 | 12 lesion removal 1 recurrent pancreatitis | 58 (11–80) | 5M/8F |
| Tissue Demonstrated (total) | # | Accession Reason | Age (Average, (Range)) | Sex |
| CANP | 73 | 58 lesion removal 10 autopsy 3 recurrent pancreatitis 1 trauma 1 unsure | 59 (11–82) | 37M/36F |
| CAP | 15 | 9 autopsy 3 lesion removal 1 recurrent pancreatitis 1 trauma 1 unsure | 48 (28–68) | 10M/5F |
| CNP | 58 | 55 lesion removal 2 recurrent pancreatitis 1 autopsy | 61 (11–82) | 27M/31F |
| NPT | 27 | 26 lesion removal 1 recurrent | 55 (11–80) | 10M/17F |
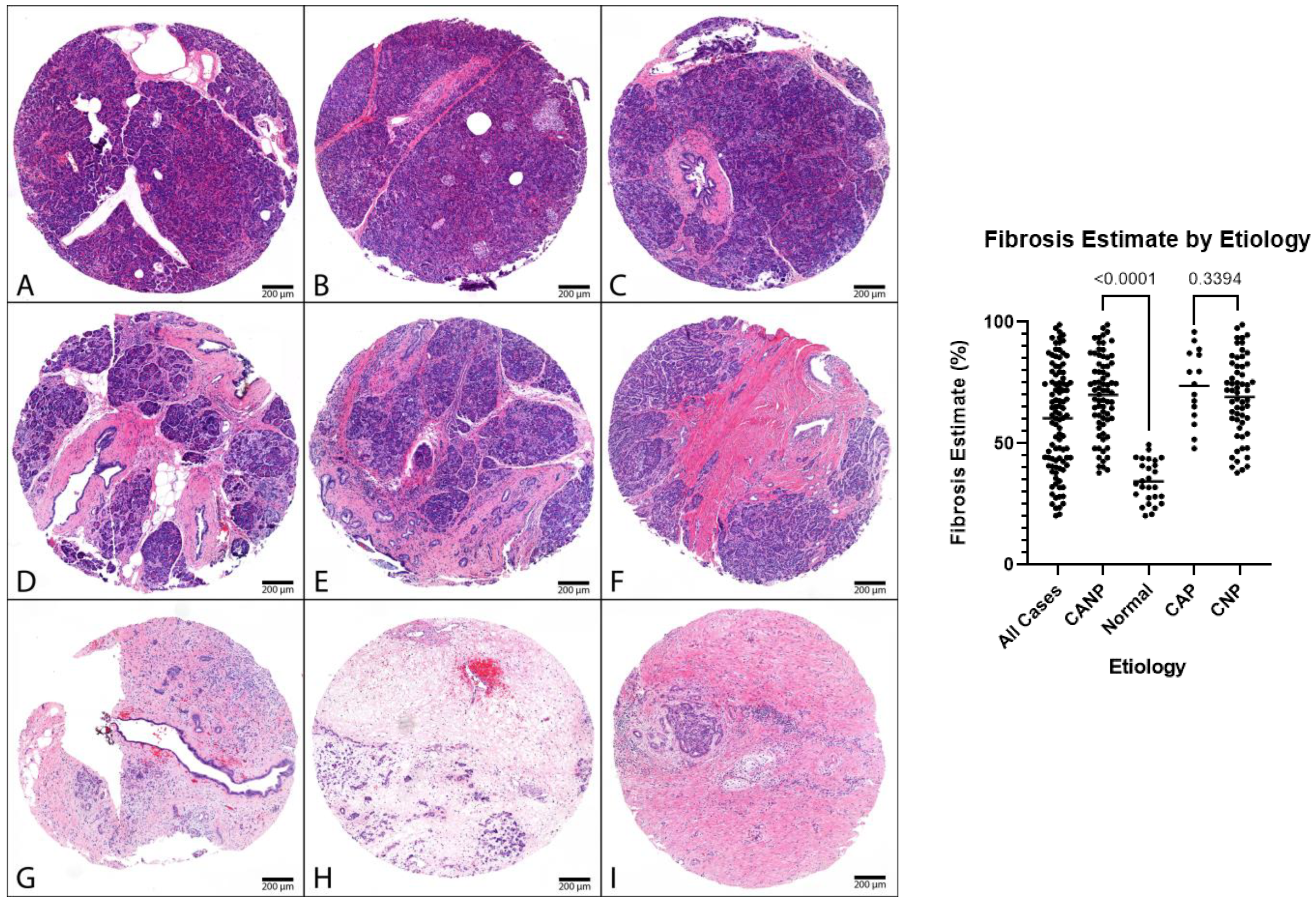
| Fibrosis Estimate | ||||||
|---|---|---|---|---|---|---|
| N | Mean (%) | Range (%) | p-Value | |||
| Both | Male | Female | ||||
| All Cases | 100 | 60 | (20–99) | |||
| Male | 47 | 59 | (23–99) | |||
| Female | 53 | 70 | (20–95) | |||
| CANP | 73 | 70 | (38–99) | |||
| Male | 37 | 70 | (38–99) | |||
| Female | 36 | 71 | (44–95) | |||
| <0.05 | <0.05 | <0.05 | ||||
| Normal | 27 | 34 | (20–50) | |||
| Male | 10 | 36 | (23–48) | |||
| Female | 17 | 34 | (20–50) | |||
| CAP | 15 | 74 | (48–96) | |||
| Male | 10 | 76 | (48–96) | |||
| Female | 5 | 70 | (52–87) | |||
| 0.34 | 0.24 | 0.86 | ||||
| CNP | 58 | 69 | (38–99) | |||
| Male | 27 | 67 | (38–99) | |||
| Female | 31 | 71 | (44–95) | |||
| Duct OD | ||||||
| N | Mean | Range | p-value | |||
| Both | Male | Female | ||||
| All Cases | 98 | 0.17 | (0.01–0.65) | |||
| Male | 45 | 0.17 | (0.01–0.52) | |||
| Female | 53 | 0.16 | (0.01–0.65) | |||
| CANP | 72 | 0.19 | (0.01–0.65) | |||
| Male | 36 | 0.19 | (0.02–0.52) | |||
| Female | 36 | 0.19 | (0.01–0.65) | |||
| <0.05 | <0.05 | 0.07 | ||||
| Normal | 26 | 0.1 | (0.01–0.28) | |||
| Male | 9 | 0.09 | (0.01–0.25) | |||
| Female | 17 | 0.11 | (0.01–0.28) | |||
| Duct OD | ||||||
| N | Mean | Range | p-value | |||
| Both | Male | Female | ||||
| CAP | 15 | 0.27 | (0.08–0.65) | |||
| Male | 10 | 0.25 | (0.10–0.52) | |||
| Female | 5 | 0.32 | (0.08–0.65) | |||
| <0.05 | <0.05 | 0.24 | ||||
| CNP | 57 | 0.17 | (0.01–0.51) | |||
| Male | 26 | 0.17 | (0.02–0.43) | |||
| Female | 31 | 0.17 | (0.01–0.51) | |||
| Acini OD | ||||||
| N | Mean | Range | p-value | |||
| Both | Male | Female | ||||
| All Cases | 92 | 1 | (0.54–1.50) | |||
| Male | 41 | 1.01 | (0.64–1.50) | |||
| Female | 51 | 1 | (0.54–1.44) | |||
| CANP | 65 | 1 | (0.54–1.50) | |||
| Male | 31 | 0.99 | (0.64–1.50) | |||
| Female | 34 | 1.01 | (0.54–1.43) | |||
| 0.96 | 0.46 | 0.51 | ||||
| Normal | 27 | 1 | (0.61–1.44) | |||
| Male | 10 | 1.04 | (0.75–1.23) | |||
| Female | 17 | 0.98 | (0.61–1.44) | |||
| CAP | 12 | 0.81 | (0.54–1.18) | |||
| Male | 7 | 0.83 | (0.64–1.18) | |||
| Female | 5 | 0.8 | (0.54–1.10) | |||
| <0.05 | <0.05 | <0.05 | ||||
| CNP | 53 | 1.05 | (0.62–1.50) | |||
| Male | 24 | 1.04 | (0.68–1.50) | |||
| Female | 29 | 1.05 | (0.62–1.43) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldwell, N.J.; Li, H.; Bellizzi, A.M.; Luo, J. Altered MANF Expression in Pancreatic Acinar and Ductal Cells in Chronic Alcoholic Pancreatitis: A Cross-Sectional Study. Biomedicines 2023, 11, 434. https://doi.org/10.3390/biomedicines11020434
Caldwell NJ, Li H, Bellizzi AM, Luo J. Altered MANF Expression in Pancreatic Acinar and Ductal Cells in Chronic Alcoholic Pancreatitis: A Cross-Sectional Study. Biomedicines. 2023; 11(2):434. https://doi.org/10.3390/biomedicines11020434
Chicago/Turabian StyleCaldwell, Nicholas J., Hui Li, Andrew M. Bellizzi, and Jia Luo. 2023. "Altered MANF Expression in Pancreatic Acinar and Ductal Cells in Chronic Alcoholic Pancreatitis: A Cross-Sectional Study" Biomedicines 11, no. 2: 434. https://doi.org/10.3390/biomedicines11020434
APA StyleCaldwell, N. J., Li, H., Bellizzi, A. M., & Luo, J. (2023). Altered MANF Expression in Pancreatic Acinar and Ductal Cells in Chronic Alcoholic Pancreatitis: A Cross-Sectional Study. Biomedicines, 11(2), 434. https://doi.org/10.3390/biomedicines11020434







