Ponciri Fructus Immatarus Sensitizes the Apoptotic Effect of Hyperthermia Treatment in AGS Gastric Cancer Cells through ROS-Dependent HSP Suppression
Abstract
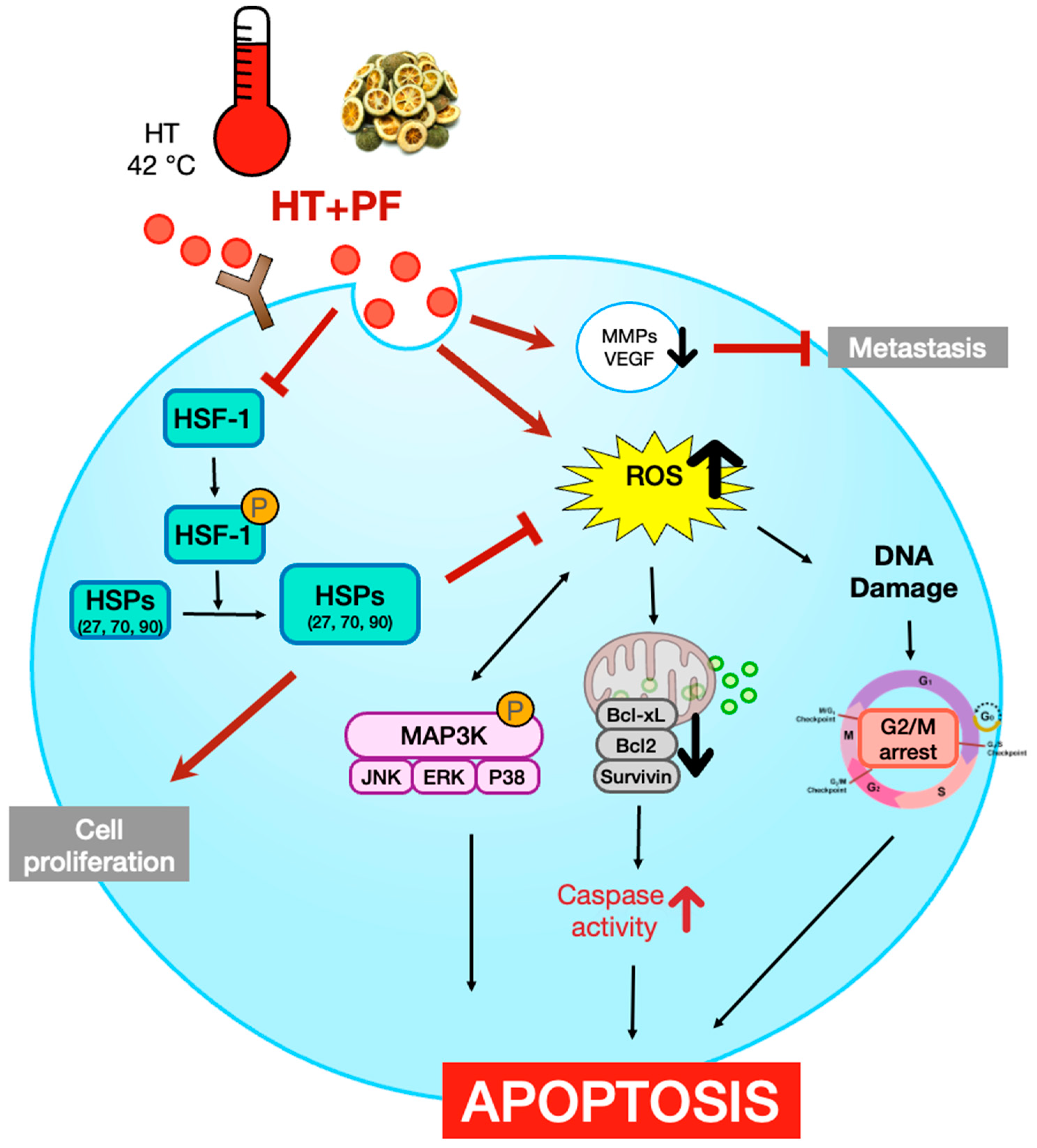
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Liquid Chromatography (LC)-Mass Spectrometry (MS) Analysis
2.3. Cell Culture
2.4. Hyperthermia Treatment
2.5. MTT Assay
2.6. Trypan Blue Assay
2.7. Morphology Assay
2.8. Wound Healing Assay
2.9. Colony Formation Assay
2.10. Western Blot Analysis
2.11. Annexin V Apoptosis Assay
2.12. Cell Cycle Analysis
2.13. ROS Analysis
2.14. Statistical Analysis
3. Results
3.1. UPLC-ESI-QTOF-MS/MS Analysis for Identification of Chemical Components in PF
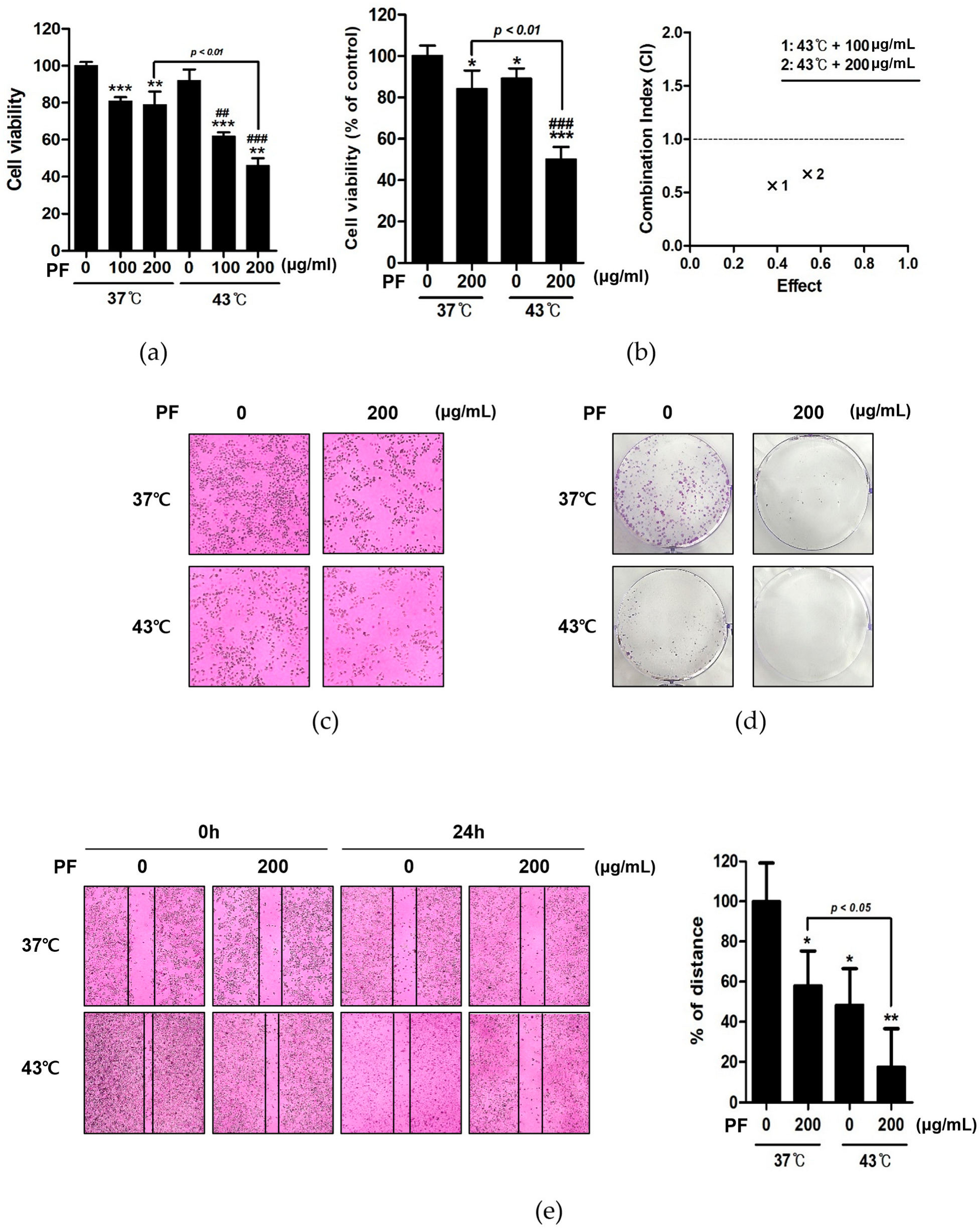
3.2. Co-Treatment with PF and 43 °C Hyperthermia Synergistically Inhibits AGS Cell Proliferation
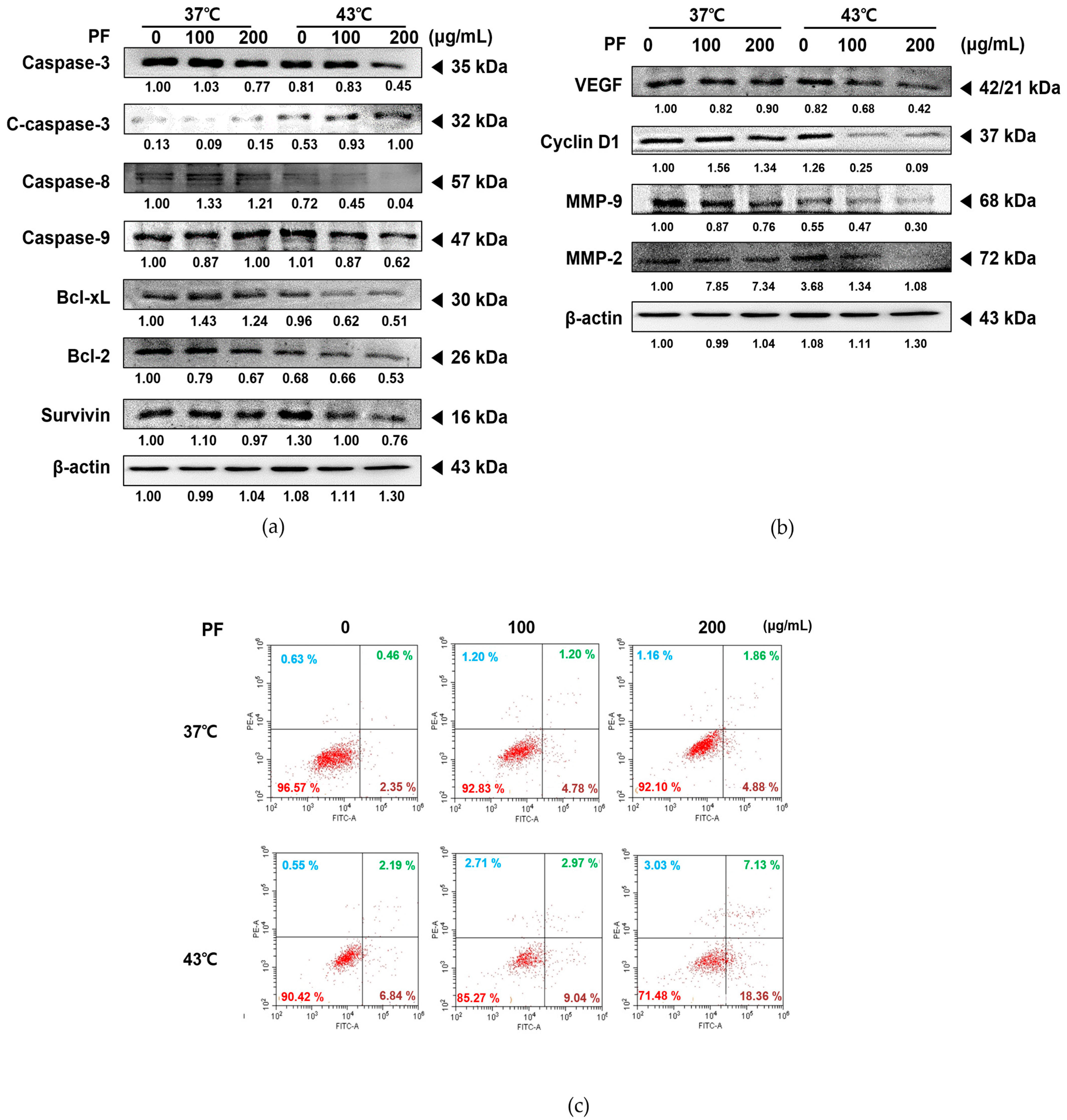
3.3. Co-Treatment with PF and 43 °C Hyperthermia Synergistically Induces Apoptotic Cell Death in AGS Cells
3.4. Co-Treatment with PF and 43 °C Hyperthermia Induces Cell Cycle Arrest in AGS Cells
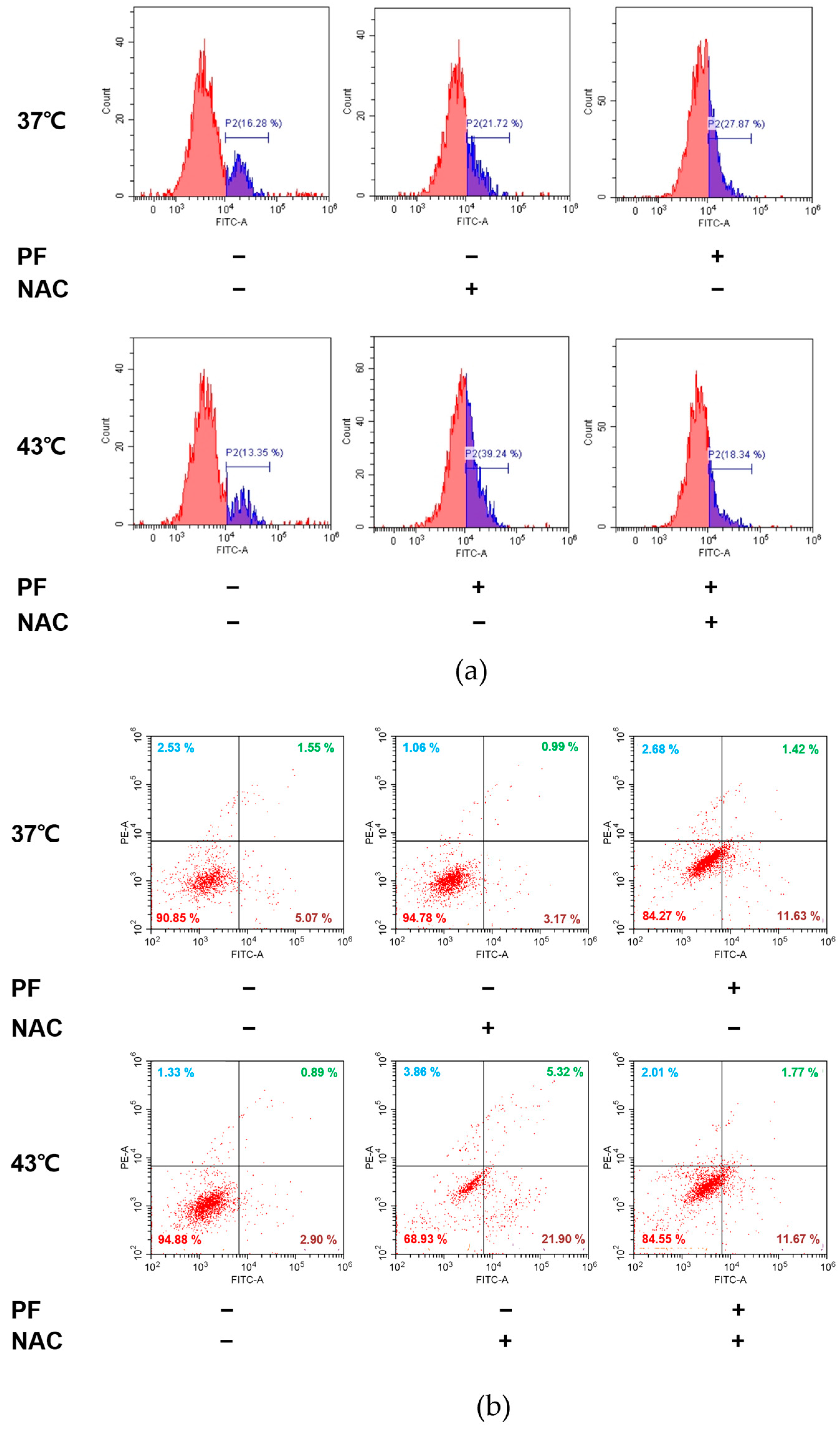
3.5. Co-Treatment with PF and 43 °C Hyperthermia Synergistically Increases ROS Generation and Subsequent Apoptosis in AGS Cells
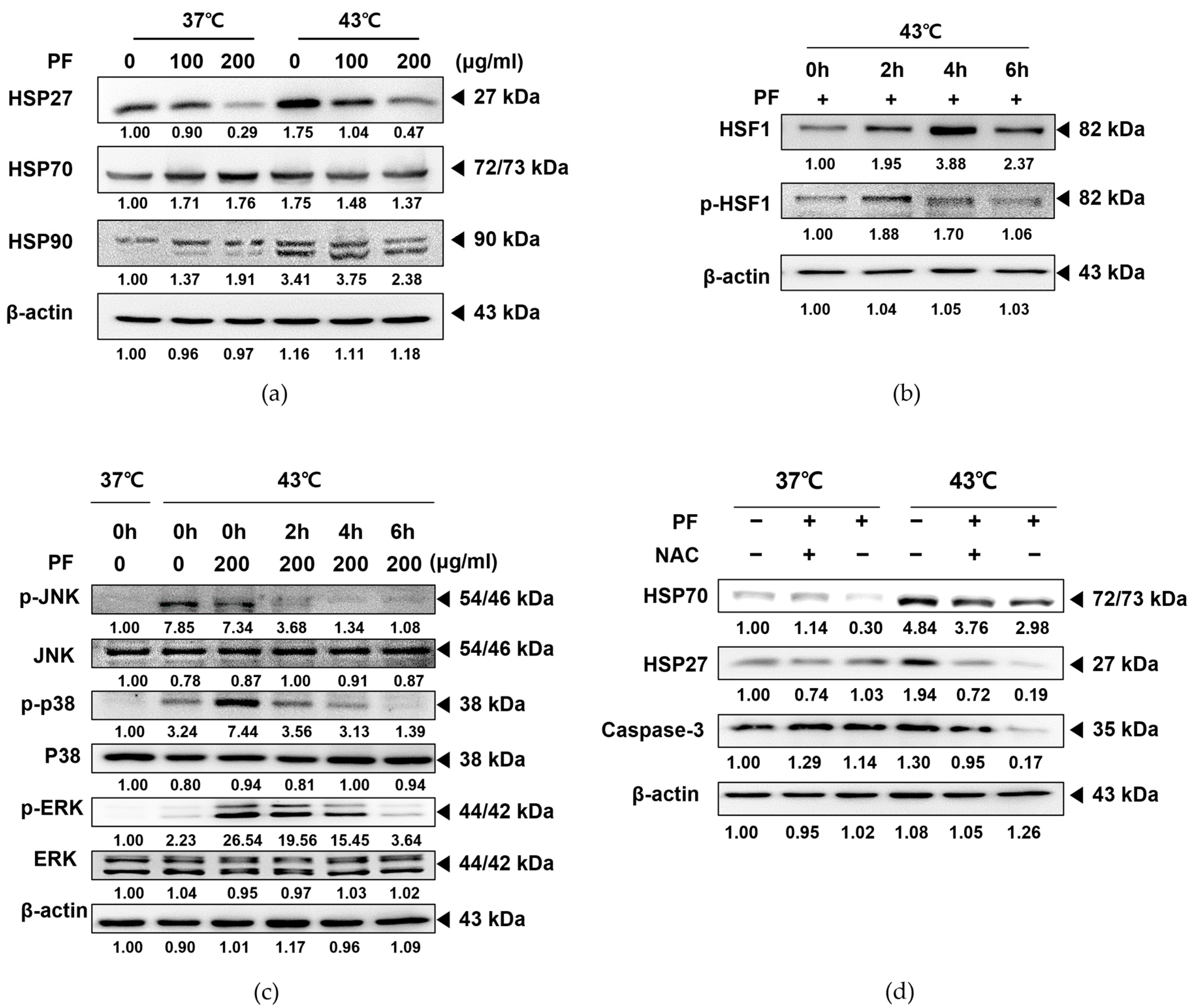
3.6. Co-Treatment with PF and 43 °C Hyperthermia Synergistically HSP via ROS Generation in AGS Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.; Lin, J.; Wang, B.; Chen, C.; Huang, Z.; Tian, Y.; Yuan, Y.; Bu, J. A novel necroptosis-related gene index for predicting prognosis and a cold tumor immune microenvironment in stomach adenocarcinoma. Front. Immunol. 2022, 13, 968165. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Goetze, T.O.; Al-Batran, S.E. Perspectives on the management of oligometastatic disease in esophago-gastric cancer. Cancers 2022, 14, 5200. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Takashima, A.; Yamada, Y.; Nakajima, T.E.; Kato, K.; Hamaguchi, T.; Shimada, Y. Standard first-line chemotherapy for metastatic gastric cancer in Japan has met the global standard: Evidence from recent phase III trials. Gastrointest. Cancer Res. 2009, 3, 239–244. [Google Scholar]
- Cheng, J.; Cai, M.; Shuai, X.; Gao, J.; Wang, G.; Tao, K. First-line systemic therapy for advanced gastric cancer: A systematic review and network meta-analysis. Ther. Adv. Med. Oncol. 2019, 11, 1758835919877726. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Li, J.; Zhang, J.; Liu, D. Regulatory effect of traditional Chinese medicines on signaling pathways of process from chronic atrophic gastritis to gastric cancer. Chin. Herb. Med. 2022, 14, 5–19. [Google Scholar] [CrossRef]
- Yi, J.M.; Kim, M.S.; Koo, H.N.; Song, B.K.; Yoo, Y.H.; Kim, H.M. Poncirus trifoliata fruit induces apoptosis in human promyelocytic leukemia cells. Clin. Chim. Acta 2004, 340, 179–185. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Mandadi, K.K.; Poulose, S.M.; Jadegoud, Y.; Nagana Gowda, G.A.; Patil, B.S. Inhibition of colon cancer cell growth and antioxidant activity of bioactive compounds from Poncirus trifoliata (L.) Raf. Bioorg. Med. Chem. 2007, 15, 4923–4932. [Google Scholar] [CrossRef]
- Hong, J.; Min, H.Y.; Xu, G.H.; Lee, J.G.; Lee, S.H.; Kim, Y.S.; Kang, S.S.; Lee, S.K. Growth inhibition and G1 cell cycle arrest mediated by 25-methoxyhispidol A, a novel triterpenoid, isolated from the fruit of Poncirus trifoliata in human hepatocellular carcinoma cells. Planta Med. 2008, 74, 151–155. [Google Scholar] [CrossRef]
- Munakarmi, S.; Chand, L.; Shin, H.B.; Hussein, U.K.; Yun, B.S.; Park, H.R.; Jeong, Y.J. Anticancer effects of Poncirus fructus on hepatocellular carcinoma through regulation of apoptosis, migration, and invasion. Oncol. Rep. 2020, 44, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Y.; Park, B.S.; Lee, G.S.; Jeong, S.H.; Kim, H.; Ryu, M.H. Autophagic cell death by poncirus trifoliata rafin: A traditional oriental medicine, in human oral cancer HSC-4 cells. Evid. Based Complement. Alternat. Med. 2015, 2015, 394263. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Y.; Ryu, M.H.; Son, Y.; Lee, G.; Jeong, S.H.; Kim, H. Poncirus trifoliata Rafin. induces the apoptosis of triple-negative breast cancer cells via activation of the c-Jun NH(2)-terminal kinase and extracellular signal-regulated kinase pathways. Pharmacogn. Mag. 2015, 11, S237–S243. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Choi, I.H.; Han, M.J.; Yi, H.K.; Yun, B.S.; Park, H.R.; Kim, M. In vitro mitochondrial apoptosis of melanoma cells via immature Poncirus trifoliata fruit extract. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5380–5392. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, E.K.; Shim, W.S. Phytotherapeutic effects of the fruits of Poncirus trifoliata (L.) Raf. on cancer, inflammation, and digestive dysfunction. Phytother. Res. 2018, 32, 616–624. [Google Scholar] [CrossRef]
- Ahmed, K.; Tabuchi, Y.; Kondo, T. Hyperthermia: An effective strategy to induce apoptosis in cancer cells. Apoptosis 2015, 20, 1411–1419. [Google Scholar] [CrossRef]
- Vertrees, R.A.; Das, G.C.; Popov, V.L.; Coscio, A.M.; Goodwin, T.J.; Logrono, R.; Zwischenberger, J.B.; Boor, P.J. Synergistic interaction of hyperthermia and Gemcitabine in lung cancer. Cancer Biol. Ther. 2005, 4, 1144–1153. [Google Scholar] [CrossRef]
- Ohguri, T.; Imada, H.; Narisada, H.; Yahara, K.; Morioka, T.; Nakano, K.; Miyaguni, Y.; Korogi, Y. Systemic chemotherapy using paclitaxel and carboplatin plus regional hyperthermia and hyperbaric oxygen treatment for non-small cell lung cancer with multiple pulmonary metastases: Preliminary results. Int. J. Hyperth. 2009, 25, 160–167. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.; Choi, B.H.; Park, M.T.; Lee, J.; Jeong, S.Y.; Choi, E.K.; Lim, B.U.; Kim, C.; Park, H.J. Hyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cells. Int. J. Hyperth. 2011, 27, 698–707. [Google Scholar] [CrossRef]
- Dou, Y.N.; Dunne, M.; Huang, H.; McKee, T.; Chang, M.C.; Jaffray, D.A.; Allen, C. Thermosensitive liposomal cisplatin in combination with local hyperthermia results in tumor growth delay and changes in tumor microenvironment in xenograft models of lung carcinoma. J. Drug Target 2016, 24, 865–877. [Google Scholar] [CrossRef]
- Deng, H.; Li, B.; Qin, X. The short- and long-term survival of hyperthermic intraperitoneal chemotherapy (HIPEC) in the advanced gastric cancer with/without peritoneal carcinomatosis: A systematic review and meta-analysis of randomized controlled trials. Updates Surg. 2022, 74, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Johnston, F.M. Current role for cytoreduction and HIPEC for gastric cancer with peritoneal disease. J. Surg. Oncol. 2022, 125, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Brenkman, H.J.F.; Päeva, M.; van Hillegersberg, R.; Ruurda, J.P.; Haj Mohammad, N. Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer—A Systematic Review. J. Clin. Med. 2019, 8, 685. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Baek, S.H. Combination therapy with cinnamaldehyde and hyperthermia induces apoptosis of A549 non-small cell lung carcinoma cells via regulation of reactive oxygen species and mitogen-activated protein kinase family. Int. J. Mol. Sci. 2020, 21, 6229. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.R.; Park, J.; Kim, J.E.; Ahn, K.S.; Kim, Y.W.; Jeong, M.; Kim, H.J.; Park, S.H.; Baek, S.H. Cinnamaldehyde and hyperthermia co-treatment synergistically induces ROS-mediated apoptosis in ACHN renal cell carcinoma cells. Biomedicines 2020, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, Y.; Yang, Q.; Yu, G.; Ma, F.; Dong, J. Abamectin causes cardiac dysfunction in carp via inhibiting redox equilibrium and resulting in immune inflammatory response and programmed cell death. Environ. Sci. Pollut. Res. Int. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Figueiredo, C.; Camargo, M.C.; Leite, M.; Fuentes-Pananá, E.M.; Rabkin, C.S.; Machado, J.C. Pathogenesis of gastric cancer: Genetics and molecular classification. Curr. Top. Microbiol. Immunol. 2017, 400, 277–304. [Google Scholar] [CrossRef]
- Anwar, S.; Malik, J.A.; Ahmed, S.; Kameshwar, V.A.; Alanazi, J.; Alamri, A.; Ahemad, N. Can natural products targeting EMT serve as the future anticancer therapeutics? Molecules 2022, 27, 7668. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Teppo, H.R.; Soini, Y.; Karihtala, P. Reactive oxygen species-mediated mechanisms of action of targeted cancer therapy. Oxid. Med. Cell. Longev. 2017, 2017, 1485283. [Google Scholar] [CrossRef]
- Zhitkovich, A. N-Acetylcysteine: Antioxidant, aldehyde scavenger, and more. Chem. Res. Toxicol. 2019, 32, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Vahid, S.; Thaper, D.; Gibson, K.F.; Bishop, J.L.; Zoubeidi, A. Molecular chaperone Hsp27 regulates the Hippo tumor suppressor pathway in cancer. Sci. Rep. 2016, 6, 31842. [Google Scholar] [CrossRef] [PubMed]
- Seigneuric, R.; Mjahed, H.; Gobbo, J.; Joly, A.L.; Berthenet, K.; Shirley, S.; Garrido, C. Heat shock proteins as danger signals for cancer detection. Front. Oncol. 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Guo, Y.; Guettouche, T.; Smith, D.F.; Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 1998, 94, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.L.; Gökmen-Polar, Y. HSF1 as a cancer biomarker and therapeutic target. Curr. Cancer Drug Targets 2019, 19, 515–524. [Google Scholar] [CrossRef]
- Mivechi, N.F.; Koong, A.C.; Giaccia, A.J.; Hahn, G.M. Analysis of HSF-1 phosphorylation in A549 cells treated with a variety of stresses. Int. J. Hyperth. 1994, 10, 371–379. [Google Scholar] [CrossRef]
- Park, J.; Liu, A.Y. JNK phosphorylates the HSF1 transcriptional activation domain: Role of JNK in the regulation of the heat shock response. J. Cell. Biochem. 2001, 82, 326–338. [Google Scholar] [CrossRef]
- Wang, X.; Grammatikakis, N.; Siganou, A.; Stevenson, M.A.; Calderwood, S.K. Interactions between extracellular signal-regulated protein kinase 1, 14-3-3epsilon, and heat shock factor 1 during stress. J. Biol. Chem. 2004, 279, 49460–49469. [Google Scholar] [CrossRef]
- Dayalan Naidu, S.; Sutherland, C.; Zhang, Y.; Risco, A.; de la Vega, L.; Caunt, C.J.; Hastie, C.J.; Lamont, D.J.; Torrente, L.; Chowdhry, S.; et al. Heat shock factor 1 is a substrate for p38 mitogen-activated protein kinases. Mol. Cell. Biol. 2016, 36, 2403–2417. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.K.; Joo, M.C. Effects and safety of aqueous extract of poncirus fructus in spinal cord injury with neurogenic bowel. Evid. Based Complement. Alternat. Med. 2016, 2016, 7154616. [Google Scholar] [CrossRef] [PubMed]
- Shal, B.; Khan, A.; Naveed, M.; Khan, U.N.; Ul-Haq, I.; AlSharari, S.D.; Kim, Y.S.; Khan, S. Effect of 25-methoxy hispidol A isolated from Poncirus trifoliate against bacteria-induced anxiety and depression by targeting neuroinflammation, oxidative stress and apoptosis in mice. Biomed. Pharmacother. 2019, 111, 209–223. [Google Scholar] [CrossRef]
- Yu, D.J.; Jun, J.H.; Kim, T.J.; Suh, D.K.; Youn, D.H.; Kim, T.W. The relaxing effect of Poncirus fructus and its flavonoid content on porcine coronary artery. Lab. Anim. Res. 2015, 31, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Coburn, N.; Cosby, R.; Klein, L.; Knight, G.; Malthaner, R.; Mamazza, J.; Mercer, C.D.; Ringash, J. Staging and surgical approaches in gastric cancer: A systematic review. Cancer Treat. Rev. 2018, 63, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.S.; Boere, I.A.; van Beekhuizen, H.J.; Franckena, M.; Nout, R.; Kruip, M.; Kulawska, M.D.; van Doorn, H.C. Acute and long-term toxicity in patients undergoing induction chemotherapy followed by thermoradiotherapy for advanced cervical cancer. Int. J. Hyperth. 2022, 39, 1440–1448. [Google Scholar] [CrossRef]
- Xia, J.; Wang, L.; Shen, T.; Li, P.; Zhu, P.; Xie, S.; Chen, Z.; Zhou, F.; Zhang, J.; Ling, J.; et al. Integrated manganese (III)-doped nanosystem for optimizing photothermal ablation: Amplifying hyperthermia-induced STING pathway and enhancing antitumor immunity. Acta Biomater. 2022, 155, 601–617. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, X.; Sun, C.; Wang, S.; Ding, S.; Wu, M.; Zhang, J.; Wang, B.; Xue, L.; Yang, L.; et al. Hyperthermic intraperitoneal chemotherapy for patients with gastric cancer based on laboratory tests is safe: A single Chinese center analysis. BMC Surg. 2022, 22, 342. [Google Scholar] [CrossRef]
- van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
- Hatashita, M.; Taniguchi, M.; Baba, K.; Koshiba, K.; Sato, T.; Jujo, Y.; Suzuki, R.; Hayashi, S. Sinodielide A exerts thermosensitizing effects and induces apoptosis and G2/M cell cycle arrest in DU145 human prostate cancer cells via the Ras/Raf/MAPK and PI3K/Akt signaling pathways. Int. J. Mol. Med. 2014, 33, 406–414. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, W.T.; Hsieh, C.H.; Kuo, Y.Y.; Chao, C.Y. Thermal cycling-hyperthermia in combination with polyphenols, epigallocatechin gallate and chlorogenic acid, exerts synergistic anticancer effect against human pancreatic cancer PANC-1 cells. PLoS ONE 2019, 14, e0217676. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Lim, B. Targeting apoptosis in cancer. Curr. Oncol. Rep. 2022, 24, 273–284. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Yao, Z.; Yuan, T.; Wang, H.; Yao, S.; Zhao, Y.; Liu, Y.; Jin, S.; Chu, J.; Xu, Y.; Zhou, W.; et al. MMP-2 together with MMP-9 overexpression correlated with lymph node metastasis and poor prognosis in early gastric carcinoma. Tumour Biol. 2017, 39, 1010428317700411. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Fusté, N.P.; Fernández-Hernández, R.; Cemeli, T.; Mirantes, C.; Pedraza, N.; Rafel, M.; Torres-Rosell, J.; Colomina, N.; Ferrezuelo, F.; Dolcet, X.; et al. Cytoplasmic cyclin D1 regulates cell invasion and metastasis through the phosphorylation of paxillin. Nat. Commun. 2016, 7, 11581. [Google Scholar] [CrossRef]
- Tustumi, F.; Agareno, G.A.; Galletti, R.P.; da Silva, R.B.R.; Quintas, J.G.; Sesconetto, L.A.; Szor, D.J.; Wolosker, N. The role of the heat-shock proteins in esophagogastric cancer. Cells 2022, 11, 2664. [Google Scholar] [CrossRef]
- Ge, H.; He, X.; Guo, L.; Yang, X. Clinicopathological significance of HSP27 in gastric cancer: A meta-analysis. OncoTargets Ther. 2017, 10, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, D.; Kong, X.; Dong, M. Clinicopathological significance of heat shock protein (HSP) 27 expression in gastric cancer: A updated meta-analysis. Evid. Based Complement. Alternat. Med. 2020, 2020, 7018562. [Google Scholar] [CrossRef]
- Wang, X.; Xie, L.; Zhu, L. Clinicopathological significance of HSP70 expression in gastric cancer: A systematic review and meta-analysis. BMC Gastroenterol. 2021, 21, 437. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, S.; Zhabasng, X.; Wu, Y.; Tang, H. High expression of heat shock protein 90 is associated with tumor aggressiveness and poor prognosis in patients with advanced gastric cancer. PLoS ONE 2013, 8, e62876. [Google Scholar] [CrossRef]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat shock proteins and cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef]
- Li, Z.N.; Luo, Y. HSP90 inhibitors and cancer: Prospects for use in targeted therapies (Review). Oncol. Rep. 2023, 49, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.G.; Piao, J.L.; Kondo, T.; Ogawa, R.; Tsuneyama, K.; Zhao, Q.L.; Feril, L.B., Jr.; Inadera, H. Molecular mechanisms of hyperthermia-induced apoptosis enhanced by docosahexaenoic acid: Implication for cancer therapy. Chem. Biol. Interact. 2014, 215, 46–53. [Google Scholar] [CrossRef]
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle 2006, 5, 2592–2601. [Google Scholar] [CrossRef]
- Lianos, G.D.; Alexiou, G.A.; Mangano, A.; Mangano, A.; Rausei, S.; Boni, L.; Dionigi, G.; Roukos, D.H. The role of heat shock proteins in cancer. Cancer Lett. 2015, 360, 114–118. [Google Scholar] [CrossRef]
- Qian, Q.; Chen, W.; Cao, Y.; Cao, Q.; Cui, Y.; Li, Y.; Wu, J. Targeting reactive oxygen species in cancer via chinese herbal medicine. Oxid. Med. Cell. Longev. 2019, 2019, 9240426. [Google Scholar] [CrossRef]


| No. | Name | Formula | Mass (Da) | Expected | Adduct | Found at |
|---|---|---|---|---|---|---|
| RT (min) | Mass (Da) | |||||
| J1 | Citric acid | C6H8O7 | 192.027 | 1.05 | [M−H] − | 191.01958 |
| J2 | Guanosine | C11H15N5O4S | 283.09167 | 1.31 | [M+H] + | 284.09883 |
| [M−H] − | 282.08411 | |||||
| J3 | D-Pantothenic acid | C9H17NO5 | 219.11067 | 2.65 | [M−H] − | 218.10336 |
| J4 | 1-beta-D-glucopyranosyl-L-tryptophan | C17H22N2O7 | 366.1427 | 3.53 | [M−H] − | 365.13525 |
| J5 | Tryptophan | C11H12N2O2 | 204.08988 | 3.71 | [M+H] + | 205.09698 |
| [M−H] − | 203.08254 | |||||
| J6 | Quercetin 3-glucosyl-(1->3)-rhamnosyl-(1->6)-galactoside | C33H40O21 | 772.20621 | 5.32 | [M+H] + | 773.2132 |
| [M−H] − | 771.19788 | |||||
| J7 | Umbelliferone | C9H6O3 | 162.03169 | 6.86 | [M+H] + | 163.03866 |
| [M−H] − | 161.02466 | |||||
| J8 | Rutin | C27H30O16 | 610.15339 | 7.20 | [M+H] + | 611.16014 |
| [M−H] − | 609.14549 | |||||
| J9 | Isoquercitrin | C21H20O12 | 464.09548 | 7.48 | [M−H] − | 463.08739 |
| J10 | Kaempferol 7-neohesperidoside | C27H30O15 | 594.15847 | 7.59 | [M+H] + | 595.16579 |
| [M-H] − | 593.15067 | |||||
| J11 | Narirutin | C27H32O14 | 580.17921 | 7.99 | [M+H] + | 581.18598 |
| [M−H] − | 579.17138 | |||||
| J12 | Prunin (Naringenin-7-O-glucoside) | C21H22O10 | 434.1213 | 8.00 | [M+H] + | 435.12884 |
| J13 | Naringenin | C15H12O5 | 272.06847 | 8.32 | [M+H] + | 273.0757 |
| J14 | Naringin | C27H32O14 | 580.17921 | 8.32 | [M+H] + | 581.18569 |
| [M−H] − | 579.17147 | |||||
| J15 | Hesperidin | C28H34O15 | 610.18977 | 8.60 | [M+H] + | 611.19526 |
| [M−H] − | 609.182 | |||||
| J16 | Heralenol | C16H16O6 | 304.09469 | 9.84 | [M+H] + | 305.10176 |
| J17 | Neoponcirin | C28H34O14 | 594.19486 | 10.55 | [M+H] + | 595.20162 |
| [M−H] − | 595.20162 | |||||
| J18 | Heralenol_1 | C16H16O6 | 304.09469 | 10.58 | [M+H] + | 305.10191 |
| J19 | Heptametoxiflavone | C22H24O9 | 432.14203 | 10.59 | [M+H] + | 433.14889 |
| J20 | Isosakuranetin | C16H14O5 | 286.08412 | 10.89 | [M+H] + | 287.09136 |
| J21 | Poncirin | C28H34O14 | 594.19486 | 10.90 | [M+H] + | 595.20158 |
| [M−H] − | 593.18695 | |||||
| J22 | Heptametoxiflavone_1 | C22H24O9 | 432.14203 | 10.90 | [M+H] + | 433.1487 |
| J23 | Isosakuranin | C22H24O10 | 448.13695 | 11.43 | [M−H] − | 447.12857 |
| J24 | Bergapten | C12H8O4 | 216.04226 | 13.33 | [M+H] + | 217.04951 |
| J25 | Isopimpinellin | C13H10O5 | 246.05282 | 13.5 | [M+H] + | 247.06025 |
| J26 | Oxyimperatorin (Heraclenin) | C16H14O5 | 286.08412 | 14.23 | [M+H] + | 287.09145 |
| J27 | Oxyimperatorin (Heraclenin)_1 | C16H14O5 | 286.08412 | 15.44 | [M+H] + | 287.09152 |
| J28 | Phellopterin | C17H16O5 | 300.09977 | 19.35 | [M+H] + | 301.10746 |
| J29 | Auraptene | C19H22O3 | 298.15689 | 24.83 | [M+H] + | 299.16419 |
| [M−H] − | 297.14938 | |||||
| P25 | Limonene or α-Pinene | C10H16 | 136.1252 | 24.83 | [M+H] + | 137.13244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, C.R.; Kim, H.I.; Kim, J.-E.; Ha, I.J.; Ahn, K.S.; Park, J.; Kim, Y.W.; Baek, S.H. Ponciri Fructus Immatarus Sensitizes the Apoptotic Effect of Hyperthermia Treatment in AGS Gastric Cancer Cells through ROS-Dependent HSP Suppression. Biomedicines 2023, 11, 405. https://doi.org/10.3390/biomedicines11020405
Ahn CR, Kim HI, Kim J-E, Ha IJ, Ahn KS, Park J, Kim YW, Baek SH. Ponciri Fructus Immatarus Sensitizes the Apoptotic Effect of Hyperthermia Treatment in AGS Gastric Cancer Cells through ROS-Dependent HSP Suppression. Biomedicines. 2023; 11(2):405. https://doi.org/10.3390/biomedicines11020405
Chicago/Turabian StyleAhn, Chae Ryeong, Hyo In Kim, Jai-Eun Kim, In Jin Ha, Kwang Seok Ahn, Jinbong Park, Young Woo Kim, and Seung Ho Baek. 2023. "Ponciri Fructus Immatarus Sensitizes the Apoptotic Effect of Hyperthermia Treatment in AGS Gastric Cancer Cells through ROS-Dependent HSP Suppression" Biomedicines 11, no. 2: 405. https://doi.org/10.3390/biomedicines11020405
APA StyleAhn, C. R., Kim, H. I., Kim, J.-E., Ha, I. J., Ahn, K. S., Park, J., Kim, Y. W., & Baek, S. H. (2023). Ponciri Fructus Immatarus Sensitizes the Apoptotic Effect of Hyperthermia Treatment in AGS Gastric Cancer Cells through ROS-Dependent HSP Suppression. Biomedicines, 11(2), 405. https://doi.org/10.3390/biomedicines11020405









