Graves’ Eye Disease: Clinical and Radiological Diagnosis
Abstract
1. Introduction
2. Graves’ Eye Disease
3. GED Grading Systems
4. Clinical Diagnosis
5. Radiological Diagnosis
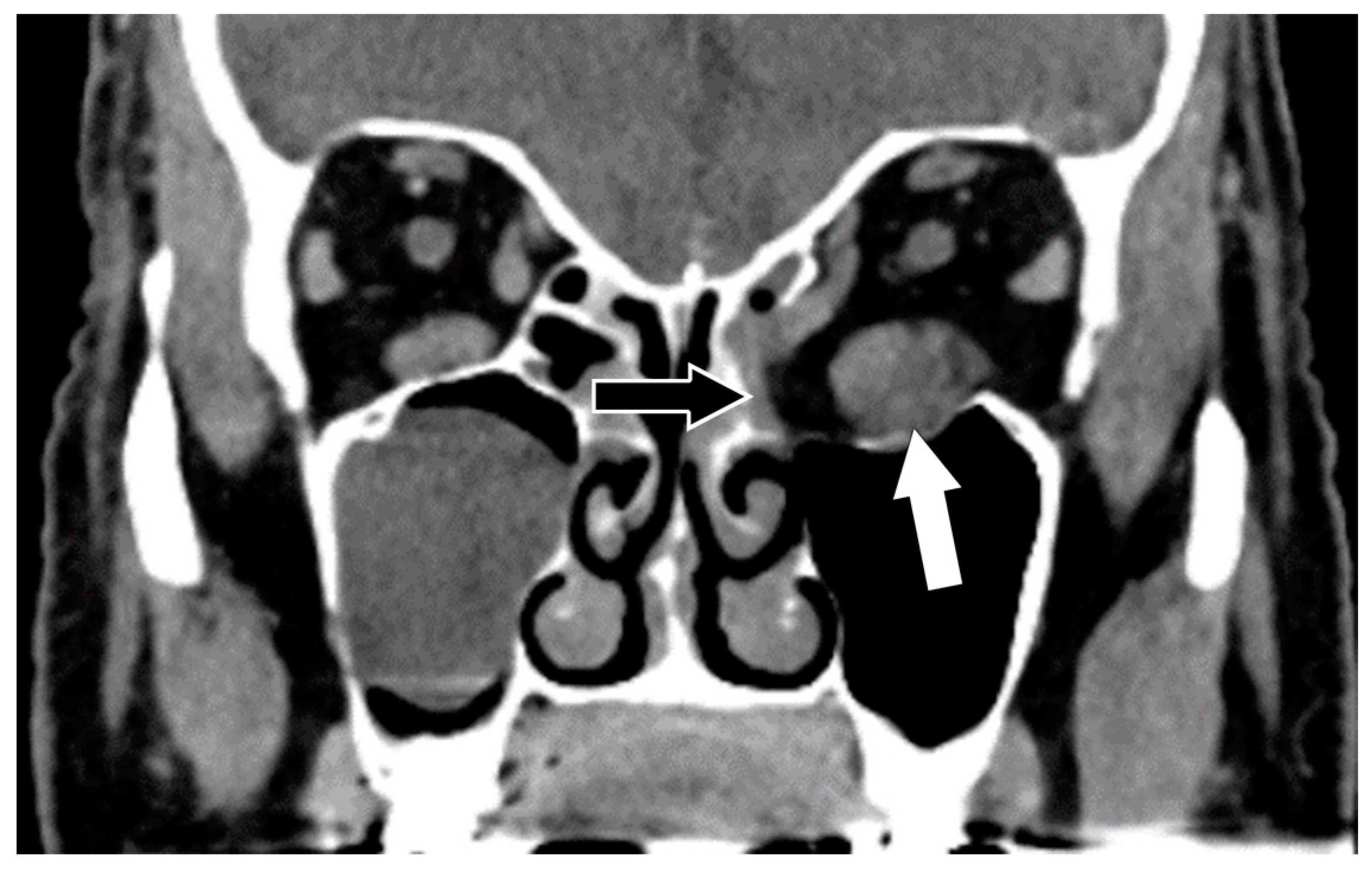
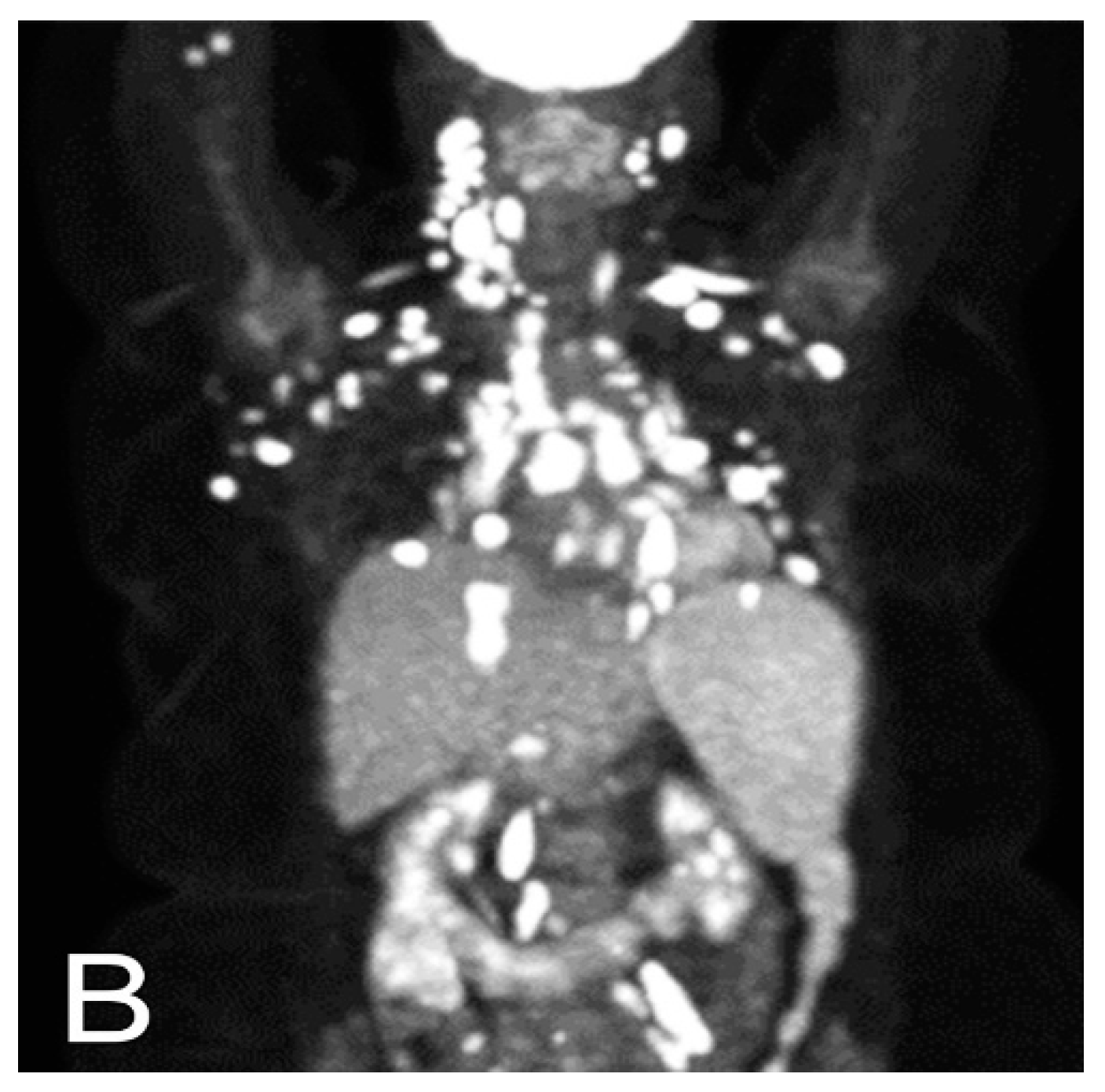
5.1. Computed Tomography (CT)
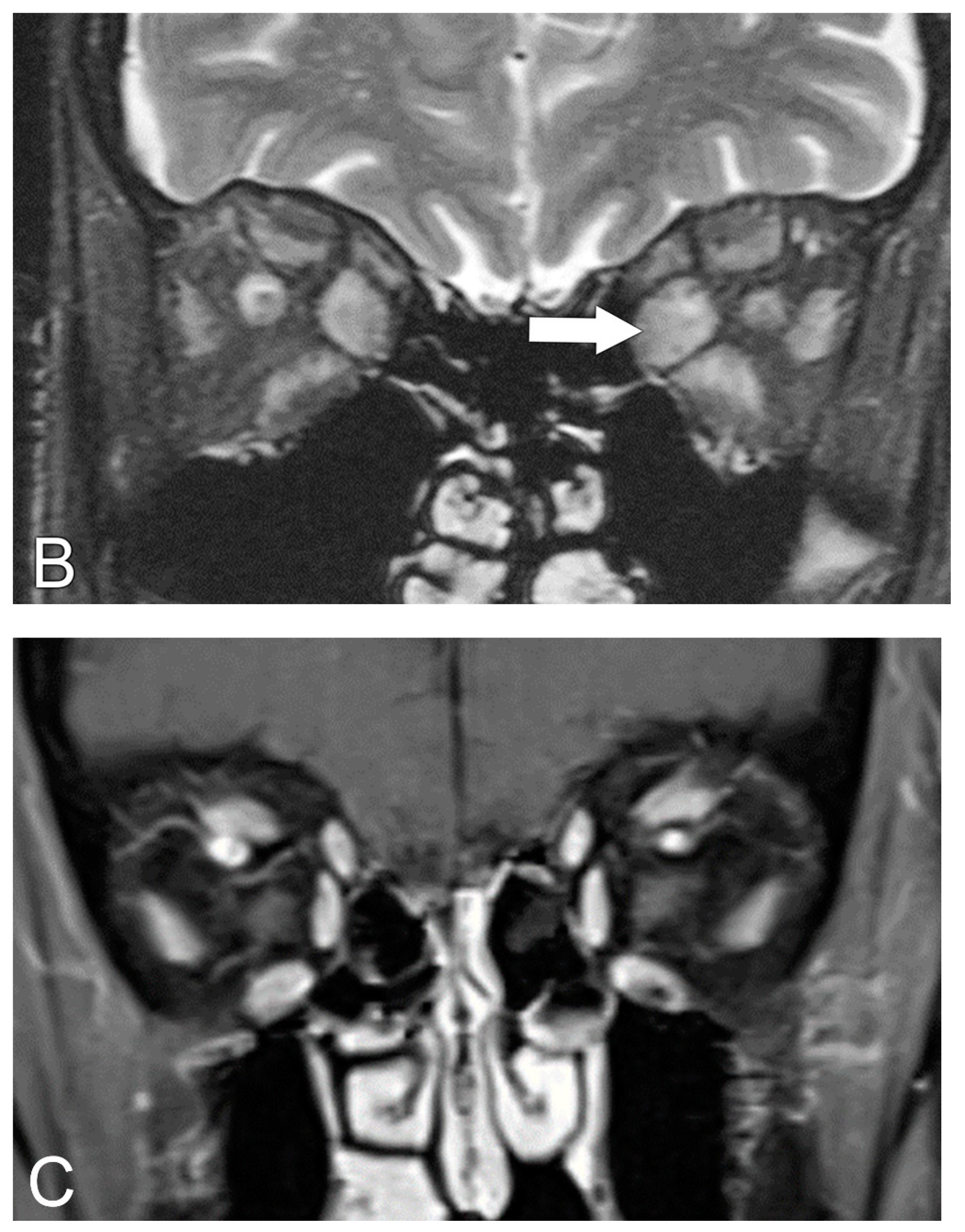
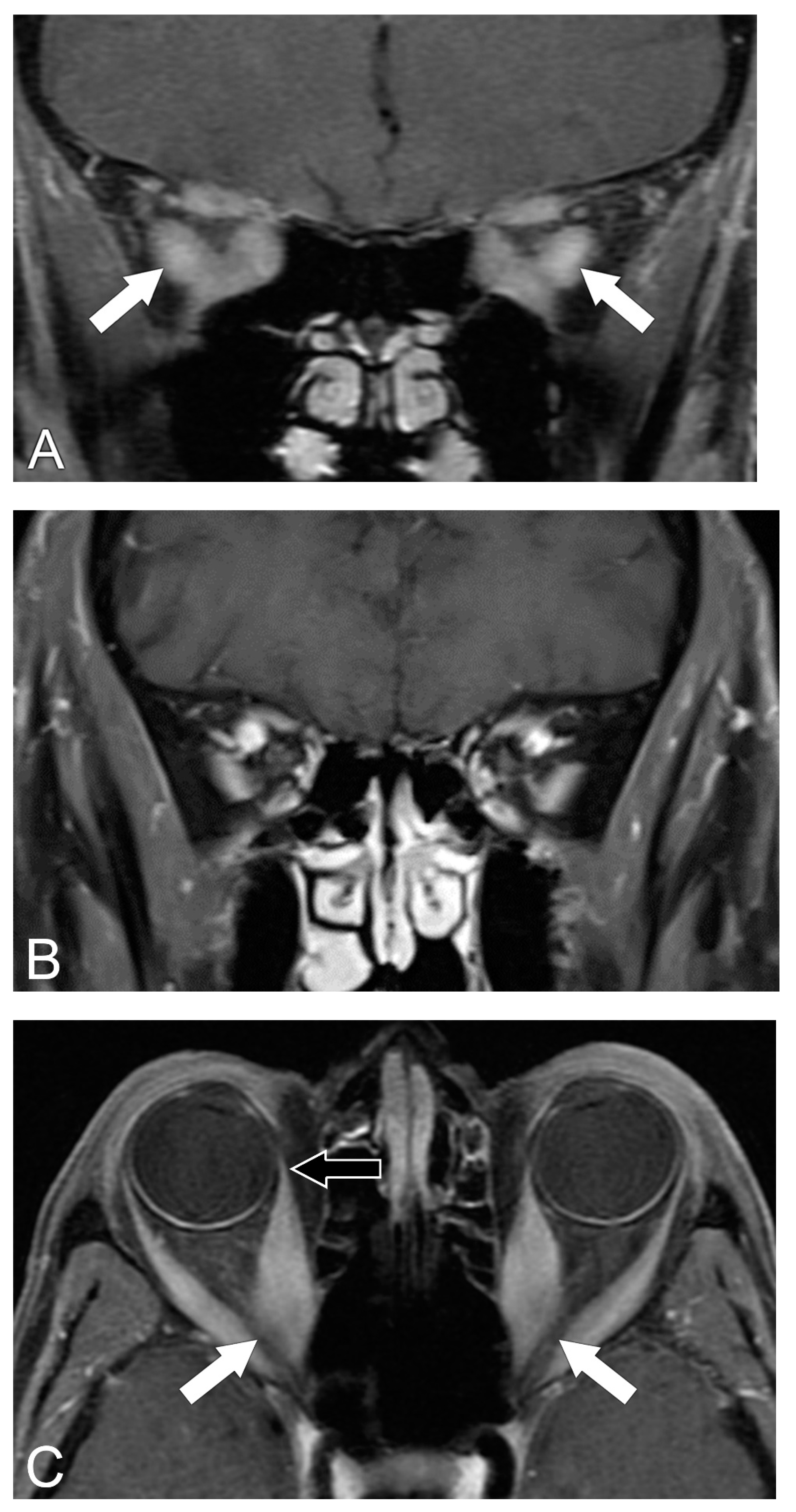
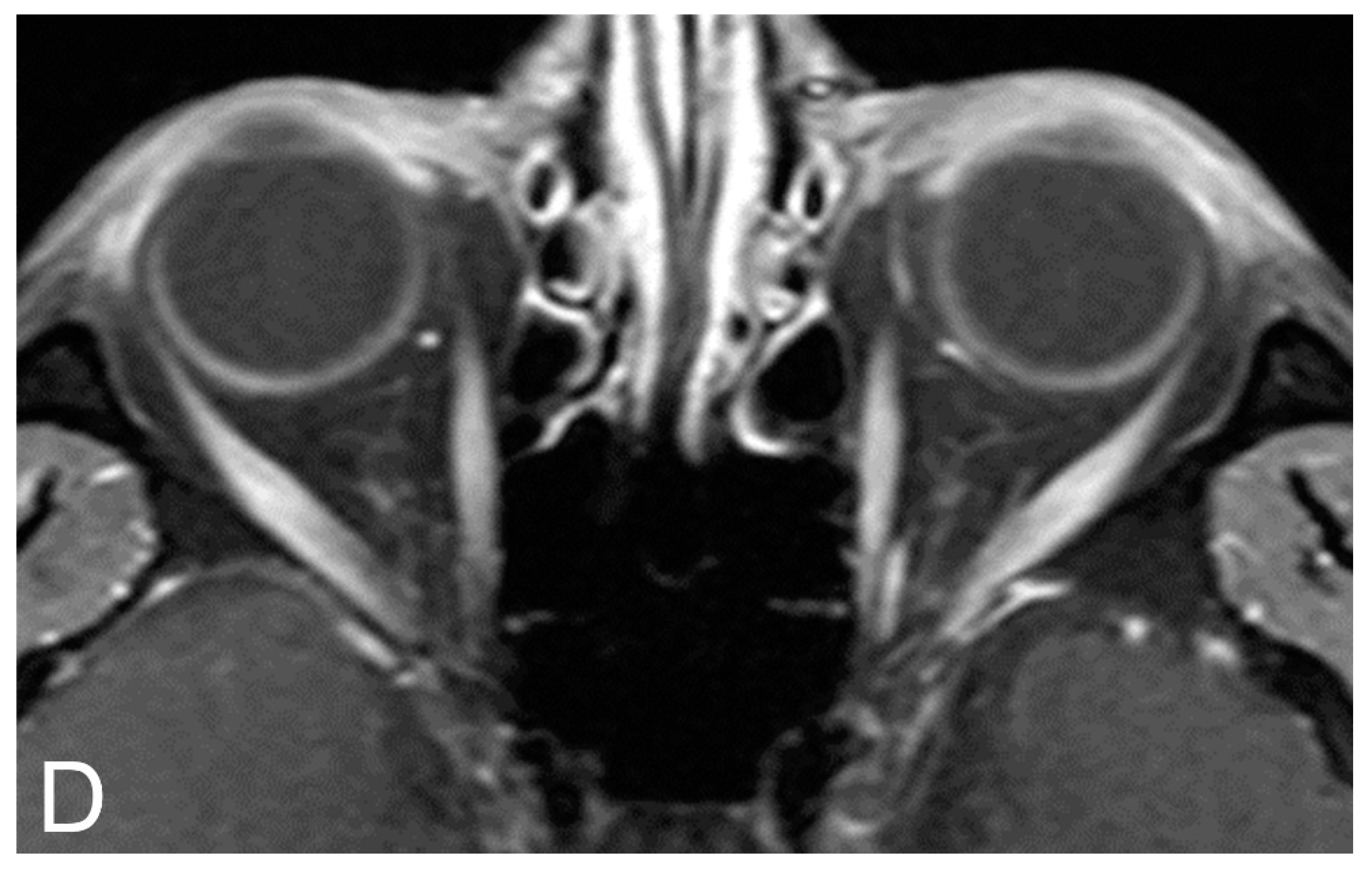
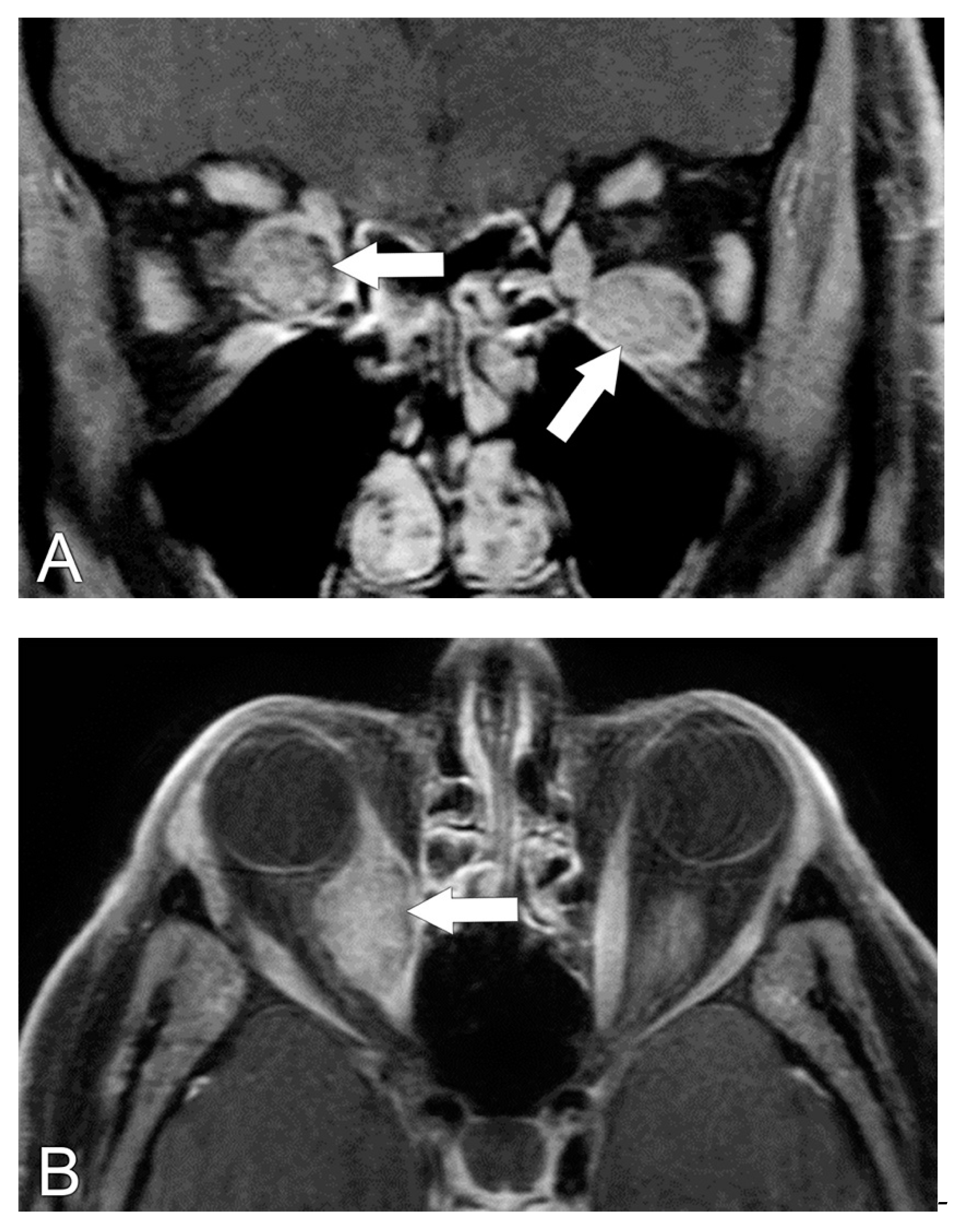
5.2. Magnetic Resonance Imaging (MRI)
5.3. MRI Correlation with Disease Severity
5.4. Diagnosis of Compressive Optic Neuropathy (CON)
6. Radiological Differential Diagnosis
7. Treatment
Author Contributions
Funding
Conflicts of Interest
References
- Ferrari, S.M.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S.R.; Patrizio, A.; Mazzi, V.; Antonelli, A.; Fallahi, P. Chemokines in hyperthyroidism. J. Clin. Transl. Endocrinol. 2019, 16, 100196. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, S.M.; Rapoport, B. Breaking Tolerance to Thyroid Antigens: Changing Concepts in Thyroid Autoimmunity. Endocr. Rev. 2013, 35, 59–105. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Ragusa, F.; Elia, G.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Giusti, C.; Gonnella, D.; Cristaudo, A.; et al. Graves’ disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101387. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hegedus, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef]
- Boelaert, K.; Torlinska, B.; Holder, R.L.; Franklyn, J.A. Older Subjects with Hyperthyroidism Present with a Paucity of Symptoms and Signs: A Large Cross-Sectional Study. J. Clin. Endocrinol. Metab. 2010, 95, 2715–2726. [Google Scholar] [CrossRef]
- Devereaux, D.; Tewelde, S.Z. Hyperthyroidism and Thyrotoxicosis. Emerg. Med. Clin. N. Am. 2014, 32, 277–292. [Google Scholar] [CrossRef]
- Schwartz, K.M.; Fatourechi, V.; Ahmed, D.D.F.; Pond, G.R. Dermopathy of Graves’ Disease (Pretibial Myxedema): Long-Term Outcome. J. Clin. Endocrinol. Metab. 2002, 87, 438–446. [Google Scholar] [CrossRef]
- Fatourechi, V.; Ahmed, D.D.F.; Schwartz, K.M. Thyroid Acropachy: Report of 40 Patients Treated at a Single Institution in a 26-Year Period. J. Clin. Endocrinol. Metab. 2002, 87, 5435–5441. [Google Scholar] [CrossRef]
- Perini, N.; Santos, R.B.; Romaldini, J.H.; Villagelin, D. Thyroid acropachy: A rare manifestation of graves’ disease in joints. AACE Clin. Case Rep. 2019, 5, e369–e371. [Google Scholar] [CrossRef]
- Lindgren, A.L.; Sidhu, S.; Welsh, K.M. Periorbital myxedema treated with intralesional hyaluronidase. Am. J. Ophthalmol. Case Rep. 2020, 19, 100751. [Google Scholar] [CrossRef]
- Wei, Y.; Kang, X.L.; Monte, M.A.D. Enlargement of the superior rectus and superior oblique muscles causes intorsion in Graves’ eye disease. Br. J. Ophthalmol. 2016, 100, 1280–1284. [Google Scholar] [CrossRef]
- Cockerham, K.P.; Kennerdell, J.S. Does radiotherapy have a role in the management of thyroid orbitopathy? View 1. Br. J. Ophthalmol. 2002, 86, 102–104. [Google Scholar]
- Glatt, H.J. Optic nerve dysfunction in thyroid eye disease: A clinician’s perspective. Radiology 1996, 200, 26–27. [Google Scholar] [CrossRef]
- Eckstein, A.; Losch, C.; Glowacka, D.; Schott, M.; Mann, K.; Esser, J.; Morgenthaler, N.G. Euthyroid and primarily hypothyroid patients develop milder and significantly more asymmetrical Graves ophthalmopathy. Br. J. Ophthalmol. 2009, 93, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Kendall-Taylor, P.; Perros, P. Clinical Presentation of Thyroid Associated Orbitopathy. Thyroid 1998, 8, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Noth, D.; Gebauer, M.; Müller, B.; Bürgi, U.; Diem, P. Graves’ ophthalmopathy: Natural history and treatment outcomes. Swiss Med. Wkly. 2001, 131, 603–609. [Google Scholar] [PubMed]
- Wiersinga, W.M.; Smit, T.; Van Der Gaag, R.; Koornneef, L. Temporal relationship between onset of Graves’ ophthalmopathy and onset of thyroidal Graves’ disease. J. Endocrinol. Investig. 1988, 11, 615–619. [Google Scholar] [CrossRef]
- Dolman, P.J. Grading Severity and Activity in Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2018, 34, S34–S40. [Google Scholar] [CrossRef]
- McKeag, D.; Lane, C.; Lazarus, J.H.; Baldeschi, L.; Boboridis, K.; Dickinson, A.J.; Hullo, A.I.; Kahaly, G.; Krassas, G.; Marcocci, C.; et al. Clinical features of dysthyroid optic neuropathy: A European Group on Graves’ Orbitopathy (EUGOGO) survey. Br. J. Ophthalmol. 2006, 91, 455–458. [Google Scholar] [CrossRef]
- Dolman, P.J. Evaluating Graves’ orbitopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 229–248. [Google Scholar] [CrossRef]
- Kendler, D.L.; Lippa, J.; Rootman, J. The initial clinical characteristics of Graves’ orbitopathy vary with age and sex. Arch. Ophthalmol. 1993, 111, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Regensburg, N.I.; Wiersinga, W.M.; Berendschot, T.T.; Saeed, P.; Mourits, M.P. Effect of smoking on orbital fat and muscle volume in Graves’ orbitopathy. Thyroid 2011, 21, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Frueh, B.R.; Musch, D.C.; Garber, F.W. Lid retraction and levator aponeurosis defects in Graves’ eye disease. Ophthalmic Surg. 1986, 17, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Dolman, P.J. Levator muscle enlargement in thyroid eye disease-related upper eyelid retraction. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 35–39. [Google Scholar] [CrossRef]
- Crisp, M.; Starkey, K.J.; Lane, C.; Ham, J.; Ludgate, M. Adipogenesis in thyroid eye disease. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3249–3255. [Google Scholar]
- Feldon, S.E.; Weiner, J.M. Clinical significance of extraocular muscle volumes in Graves’ ophthalmopathy: A quantitative computed tomography study. Arch. Ophthalmol. 1982, 100, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Villadolid, M.C.; Yokoyama, N.; Izumi, M.; Nishikawa, T.; Kimura, H.; Ashizawa, K.; Kiriyama, T.; Uetani, M.; Nagataki, S. Untreated Graves’ disease patients without clinical ophthalmopathy demonstrate a high frequency of extraocular muscle (EOM) enlargement by magnetic resonance. J. Clin. Endocrinol. Metab. 1995, 80, 2830–2833. [Google Scholar] [CrossRef] [PubMed]
- Starks, V.S.; Reinshagen, K.L.; Lee, N.G.; Freitag, S.K. Visual field and orbital computed tomography correlation in dysthyroid optic neuropathy due to thyroid eye disease. Orbit 2019, 39, 77–83. [Google Scholar] [CrossRef]
- Bartley, G.B. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans. Am. Ophthalmol. Soc. 1994, 92, 477–588. [Google Scholar]
- Neigel, J.M.; Rootman, J.; Belkin, R.I.; Nugent, R.A.; Drance, S.M.; Beattie, C.W.; Spinelli, J.A. Dysthyroid optic neuropathy: The crowded orbital apex syndrome. Ophthalmology 1988, 95, 1515–1521. [Google Scholar] [CrossRef]
- Trobe, J.D.; Glaser, J.S.; Laflamme, P. Dysthyroid optic neuropathy. Clinical profile and rationale for management. Arch. Ophthalmol. 1978, 96, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Mourits, M.P.; Prummel, M.F.; Wiersinga, W.M.; Koornneef, L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin. Endocrinol. 1997, 47, 9–14. [Google Scholar] [CrossRef]
- Boboridis, K.; Perros, P. General management plan. In Graves’ Orbitopathy; Karger: Basel, Switzerland, 2010; pp. 88–95. [Google Scholar]
- Werner, S.C. Classification of the eye changes of Graves’ disease. Am. J. Ophthalmol. 1969, 68, 646–648. [Google Scholar] [CrossRef]
- Barrio-Barrio, J.; Sabater, A.L.; Bonet-Farriol, E.; Velázquez-Villoria, A.; Galofré, J.C. Graves’ ophthalmopathy: VISA versus EUGOGO classification, assessment, and management. J. Ophthalmol. 2015, 2015, 249125. [Google Scholar] [CrossRef]
- Bahn, R.S.; Kazim, M. Thyroid eye disease. In Diseases and Disorders of the Orbit and Ocular Adnexa; Fay, A., Dolman, P.J., Eds.; Elsevier: Amsterdam, the Netherlands, 2017; pp. 219–234. [Google Scholar]
- Polito, E.; Leccisotti, A. MRI in Graves orbitopathy: Recognition of enlarged muscles and prediction of steroid response. Ophthalmologica 1995, 209, 182–186. [Google Scholar] [CrossRef]
- Regensburg, N.I.; Wiersinga, W.M.; Berendschot, T.T.J.M.; Saeed, P.; Mourits, M.P. Densities of Orbital Fat and Extraocular Muscles in Graves Orbitopathy Patients and Controls. Ophthalmic Plast. Reconstr. Surg. 2011, 27, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Rootman, J. Diseases of the orbit: A multidisciplinary approach. Arch. Ophthalmol. 1988, 64, 143–240. [Google Scholar]
- Miller, M.T.; Mafee, M.F. Computed tomography scanning in the evaluation of ocular motility disorders. Radiol. Clin. North Am. 1987, 25, 733–752. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.Y.Q.; Leong, Y.-Y.; Lang, S.S.; Htoon, Z.M.; Young, S.M.; Sundar, G. Radiologic parameters of orbital bone remodeling in thyroid eye disease. Investig. Opthalmol. Vis. Sci. 2017, 58, 2527. [Google Scholar] [CrossRef]
- Cohen, L.M.; Liou, V.D.; Cunnane, M.E.; Yoon, M.K. Radiographic analysis of fatty infiltration of the extraocular muscles in thyroid eye disease. Orbit 2020, 41, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kvetny, J.; Puhakka, K.B.; Røhl, L. Magnetic resonance imaging determination of extraocular eye muscle volume in patients with thyroid-associated ophthalmopathy and proptosis. Acta Ophthalmol. Scand. 2006, 84, 419–423. [Google Scholar] [CrossRef]
- Tortora, F.; Cirillo, M.; Ferrara, M.; Belfiore, M.P.; Carella, C.; Caranci, F.; Cirillo, S. Disease activity in Graves’ ophthalmopathy: Diagnosis with orbital MR imaging and correlation with clinical score. Neuroradiol. J. 2013, 26, 555–564. [Google Scholar] [CrossRef]
- Higashiyama, T.; Iwasa, M.; Ohji, M. Quantitative analysis of inflammation in orbital fat of thyroid-associated ophthalmopathy using MRI signal intensity. Sci. Rep. 2017, 7, 16874. [Google Scholar] [CrossRef]
- Ollitrault, A.; Charbonneau, F.; Herdan, M.-L.; Bergès, O.; Zuber, K.; Giovansili, L.; Launay, P.; Savatovsky, J.; Lecler, A. Dixon-T2WI magnetic resonance imaging at 3 tesla outperforms conventional imaging for thyroid eye disease. Eur. Radiol. 2021, 31, 5198–5205. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, H.; Chen, W.; Chen, H.-H.; Chen, L.; Xu, X.-Q.; Wu, F.-Y. Morphological and microstructural brain changes in thyroid-associated ophthalmopathy: A combined voxel-based morphometry and diffusion tensor imaging study. J. Endocrinol. Investig. 2020, 43, 1591–1598. [Google Scholar] [CrossRef]
- Barrett, L.; Glatt, H.J.; Burde, R.M.; Gado, M.H. Optic nerve dysfunction in thyroid eye disease: CT. Radiology 1988, 167, 503–507. [Google Scholar] [CrossRef]
- Birchall, D.; Goodall, K.L.; Noble, J.L.; Jackson, A. Graves ophthalmopathy: Intracranial fat prolapse on CT images as an indicator of optic nerve compression. Radiology 1996, 200, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Feldon, S.E.; Lee, C.P.; Muramatsu, S.K. Quantitative computed tomography of Graves’ ophthalmopathy. Extraocular muscle and orbital fat in development of optic neuropathy. Arch. Ophthalmol. 1985, 103, 213–215. [Google Scholar] [CrossRef]
- Goncalves, A.C.P.; Silva, L.; Gebrim, E.; Monteiro, M. Quantification of Orbital apex crowding for screening of dysthyroid optic neuropathy using multidetector CT. Am. J. Neuroradiol. 2012, 33, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.A.; Belkin, R.I.; Neigel, J.M.; Rootman, J.; Robertson, W.D.; Spinelli, J.; Graeb, D.A. Graves orbitopathy: Correlation of CT and clinical findings. Radiology 1990, 177, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Feldon, S.E.; Muramatsu, S.; Weiner, J.M. Clinical classification of Graves’ ophthalmopathy. Identification of risk factors for optic neuropathy. Arch. Ophthalmol. 1984, 102, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Gorman, C.A. The Measurement of Change in Graves’ Ophthalmopathy. Thyroid 1998, 8, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, K. Intraocular pressure and proptosis in 95 patients with graves ophthalmopathy. Am. J. Ophthalmol. 1997, 124, 570–572. [Google Scholar] [CrossRef]
- Peyster, R.; Ginsberg, F.; Silber, J.; Adler, L. Exophthalmos caused by excessive fat: CT volumetric analysis and differential diagnosis. Am. J. Roentgenol. 1986, 146, 459–464. [Google Scholar] [CrossRef]
- Dodds, N.I.; Atcha, A.W.; Birchall, D.; Jackson, A. Use of high-resolution MRI of the optic nerve in Graves’ ophthalmopathy. Br. J. Radiol. 2009, 82, 541–544. [Google Scholar] [CrossRef]
- Chen, G.; Cheuk, W.; Chan, J.K.-C. IgG4-related sclerosing disease: A critical appraisal of an evolving clinicopathologic entity. Adv. Anat. Pathol. 2010, 39, 303–332. [Google Scholar]
- Stone, J.H.; Zen, Y.; Deshpande, V. IgG4-related disease. N. Engl. J. Med. 2012, 366, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Tiegs-Heiden, C.; Eckel, L.; Hunt, C.; Diehn, F.; Schwartz, K.; Kallmes, D.; Salomão, D.; Witzig, T.; Garrity, J. Immunoglobulin G4–related disease of the orbit: Imaging features in 27 patients. Am. J. Neuroradiol. 2014, 35, 1393–1397. [Google Scholar] [CrossRef]
- Rothfus, W.E.; Curtin, H.D. Extraocular muscle enlargement: A CT review. Radiology 1984, 151, 677–681. [Google Scholar] [CrossRef]
- Yuen, S.J.; Rubin, P.A. Idiopathic orbital inflammation: Distribution, clinical features, and treatment outcome. Arch. Ophthalmol. 2003, 121, 491–499. [Google Scholar] [CrossRef]
- Martinez, R. Erdheim-Chester disease: MR of intraaxial and extraaxial brain stem lesions. AJNR Am. J. Neuroradiol. 1995, 16, 1787–1790. [Google Scholar]
- Veyssier-Belot, C.; Cacoub, P.; Caparros-Lefebvre, D.; Wechsler, J.; Brun, B.; Remy, M.; Wallaert, B.; Petit, H.; Grimaldi, A.; Wechsler, B.; et al. Erdheim-Chester disease clinical and radiologic characteristics of 59 cases. Medicine 1996, 75, 157–169. [Google Scholar] [CrossRef]
- Albayram, S.; Kizilkilic, O.; Zulfikar, Z.; Işlak, C.; Kocer, N. Spinal dural involvement in Erdheim-Chester disease: MRI findings. Neuroradiology 2002, 44, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Allmendinger, A.M.; Krauthamer, A.V.; Spektor, V.; Aziz, M.S.; Zablow, B. Atypical spine involvement of Erdheim-Chester disease in an elderly male. J. Neurosurg. Spine 2010, 12, 257–260. [Google Scholar] [CrossRef]
- Drier, A.; Haroche, J.; Savatovsky, J.; Godenèche, G.; Dormont, D.; Chiras, J.; Amoura, Z.; Bonneville, F. Cerebral, facial, and orbital involvement in Erdheim-Chester disease: CT and MR imaging findings. Radiology 2010, 255, 586–594. [Google Scholar] [CrossRef]
- Coha, B.; Vucinic, I.; Mahovne, I.; Vukovic-Arar, Z. Extranodal lymphomas of head and neck with emphasis on NK/T-cell lymphoma, nasal type. J. Cranio-Maxillofac. Surg. 2014, 42, 149–152. [Google Scholar] [CrossRef]
- Surov, A.; Behrmann, C.; Holzhausen, H.-J.; Kösling, S. Lymphomas and metastases of the extra-ocular musculature. Neuroradiology 2011, 53, 909–916. [Google Scholar] [CrossRef] [PubMed]
- El-Hadad, C.; Koka, K.; Dong, W.; Do, T.; Haider, M.; Ursua, J.D.; Ning, J.; Debnam, J.M.; Esmaeli, B. Multidisciplinary management of orbital metastasis and survival outcomes. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaibani, A.H.; Carter, K.D.; Nerad, J.A.; Lee, A.G. Prostate carcinoma metastasis to extraocular muscles. Ophthalmic Plast. Reconstr. Surg. 2008, 24, 233–235. [Google Scholar] [CrossRef]
- Lacey, B.; Chang, W.; Rootman, J. Nonthyroid causes of extraocular muscle disease. Surv. Ophthalmol. 1999, 44, 187–213. [Google Scholar] [CrossRef]
- Shafi, F.; Mathewson, P.; Mehta, P.; Ahluwalia, H.S. The enlarged extraocular muscle: To relax, reflect or refer? Eye 2016, 31, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Towbin, R.; Han, B.K.; Kaufman, R.; Burke, M. Postseptal cellulitis: CT in diagnosis and management. Radiology 1986, 158, 735–737. [Google Scholar] [CrossRef]
- Dolman, P.J.; Rath, S. Orbital radiotherapy for thyroid eye disease. Curr. Opin. Ophthalmol. 2012, 23, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Selva-Nayagam, D.; Chen, C.; King, G. Late reactivation of thyroid orbitopathy. Clin. Exp. Ophthalmol. 2004, 32, 46–50. [Google Scholar] [CrossRef] [PubMed]

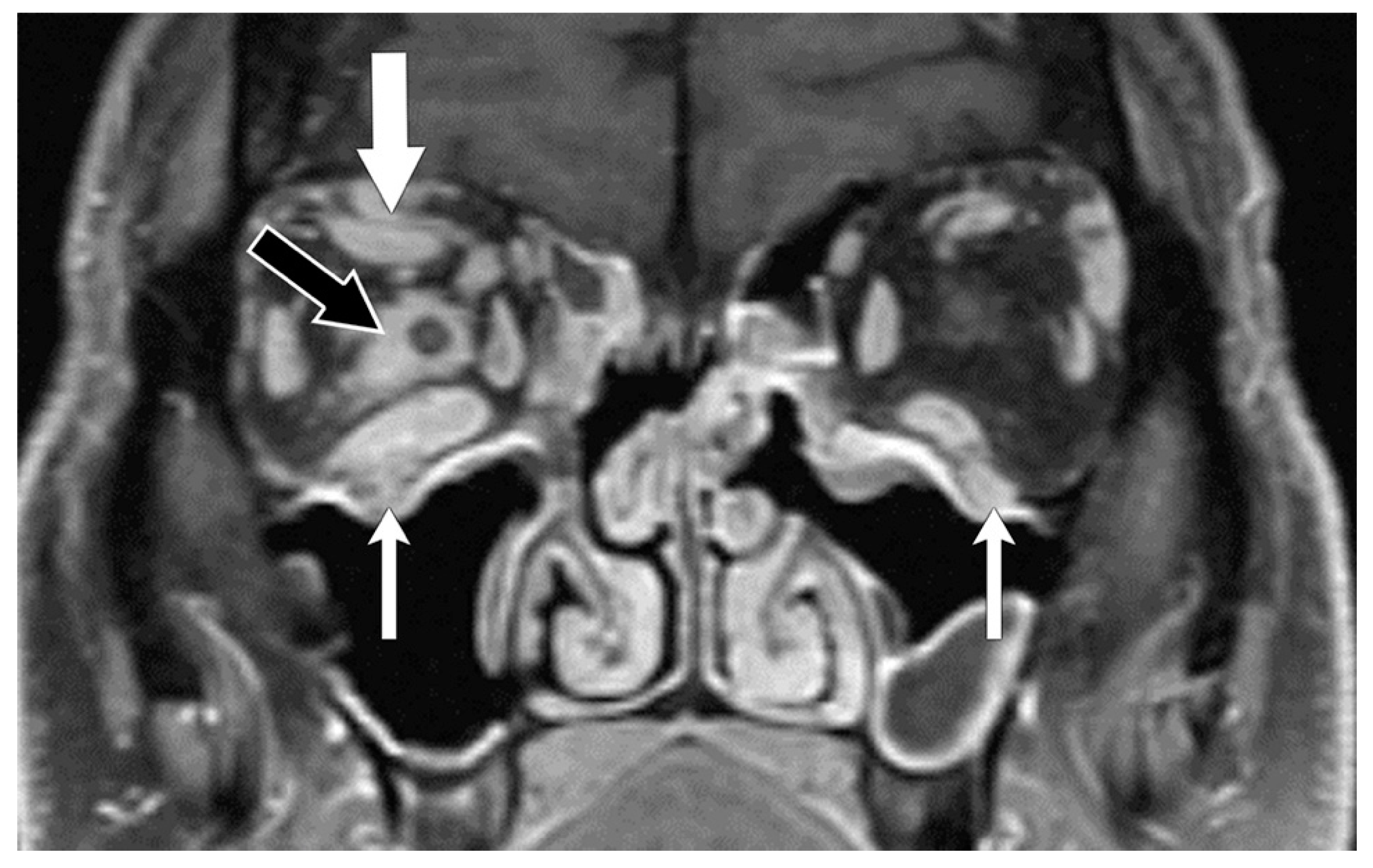
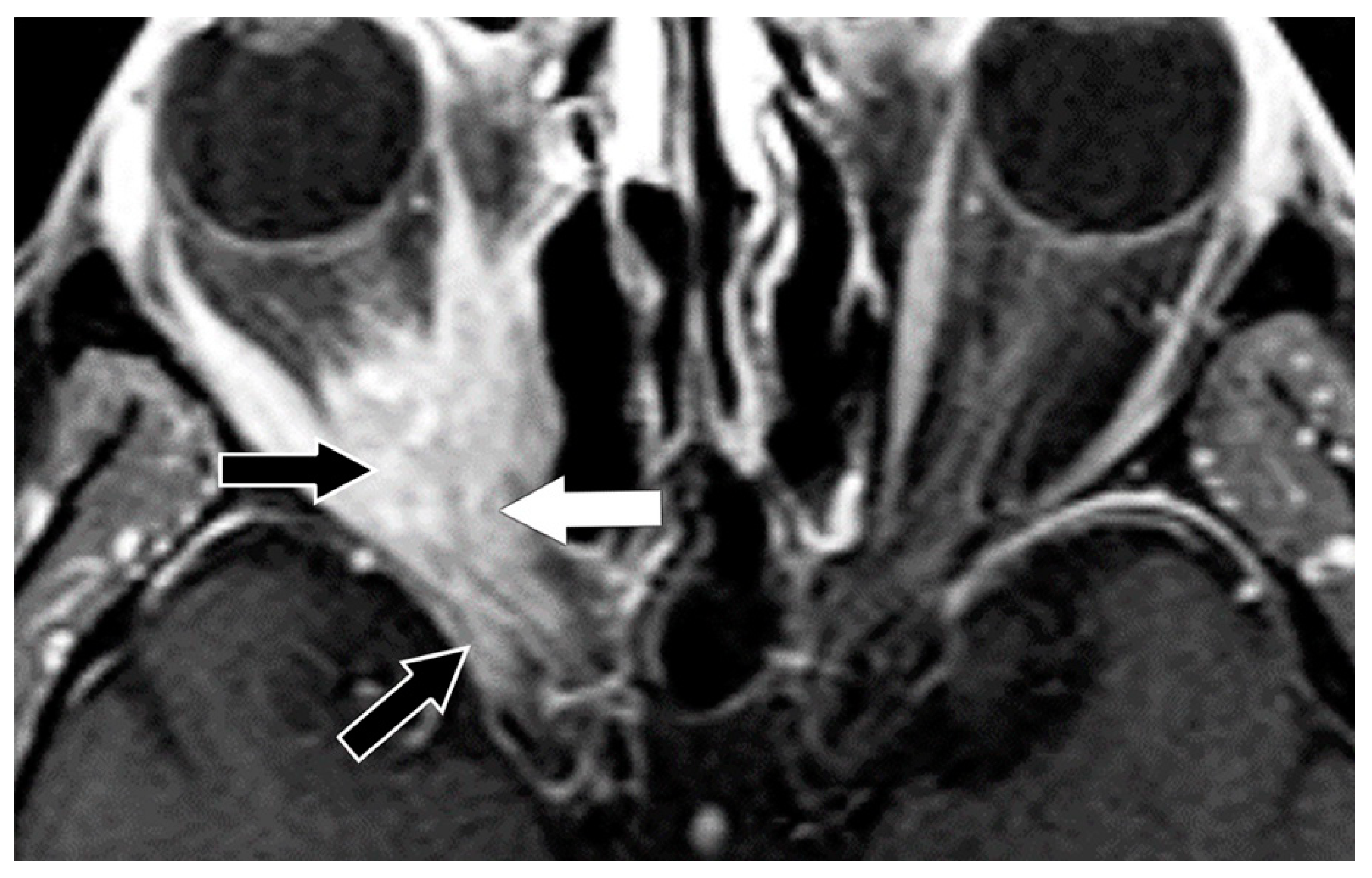
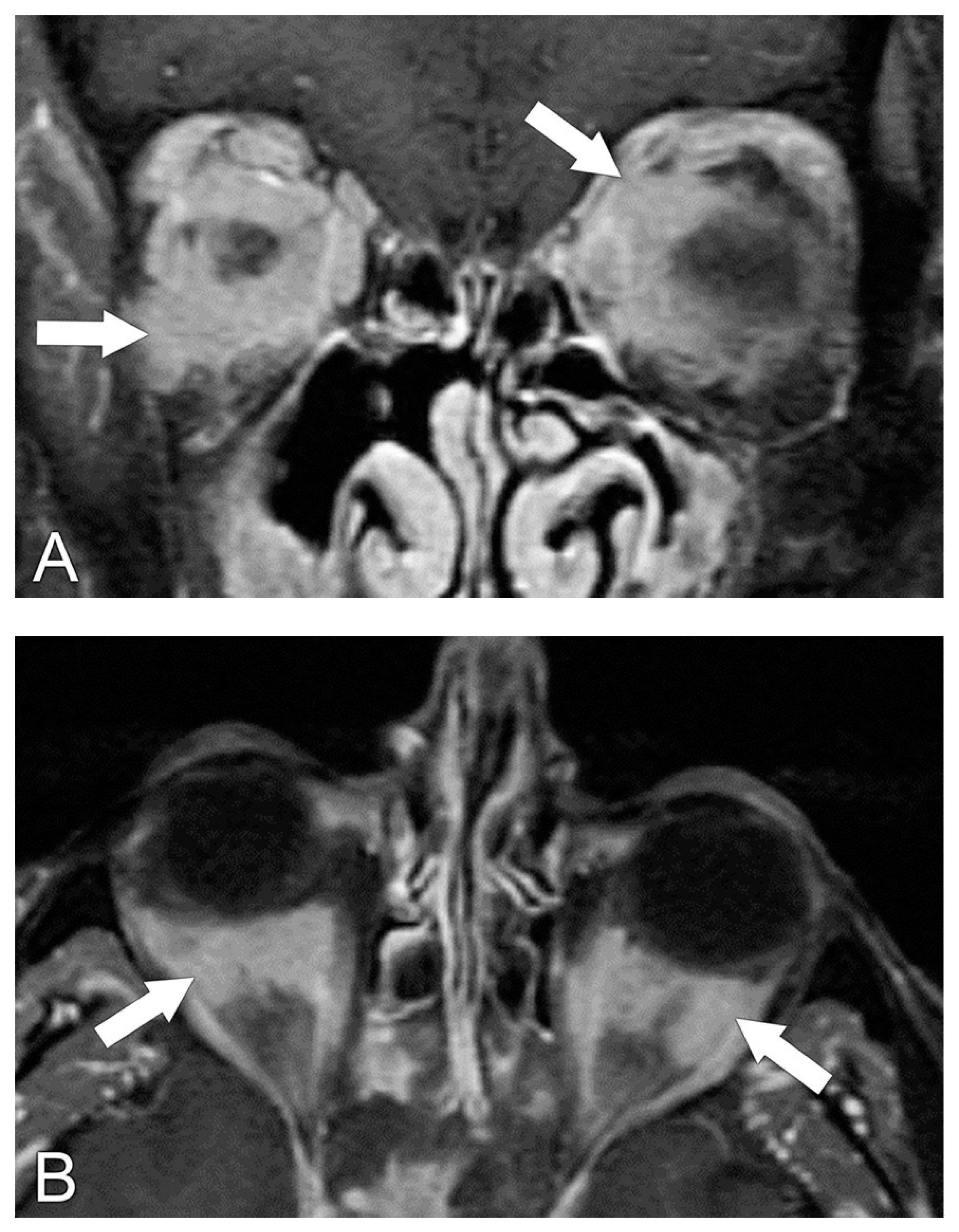
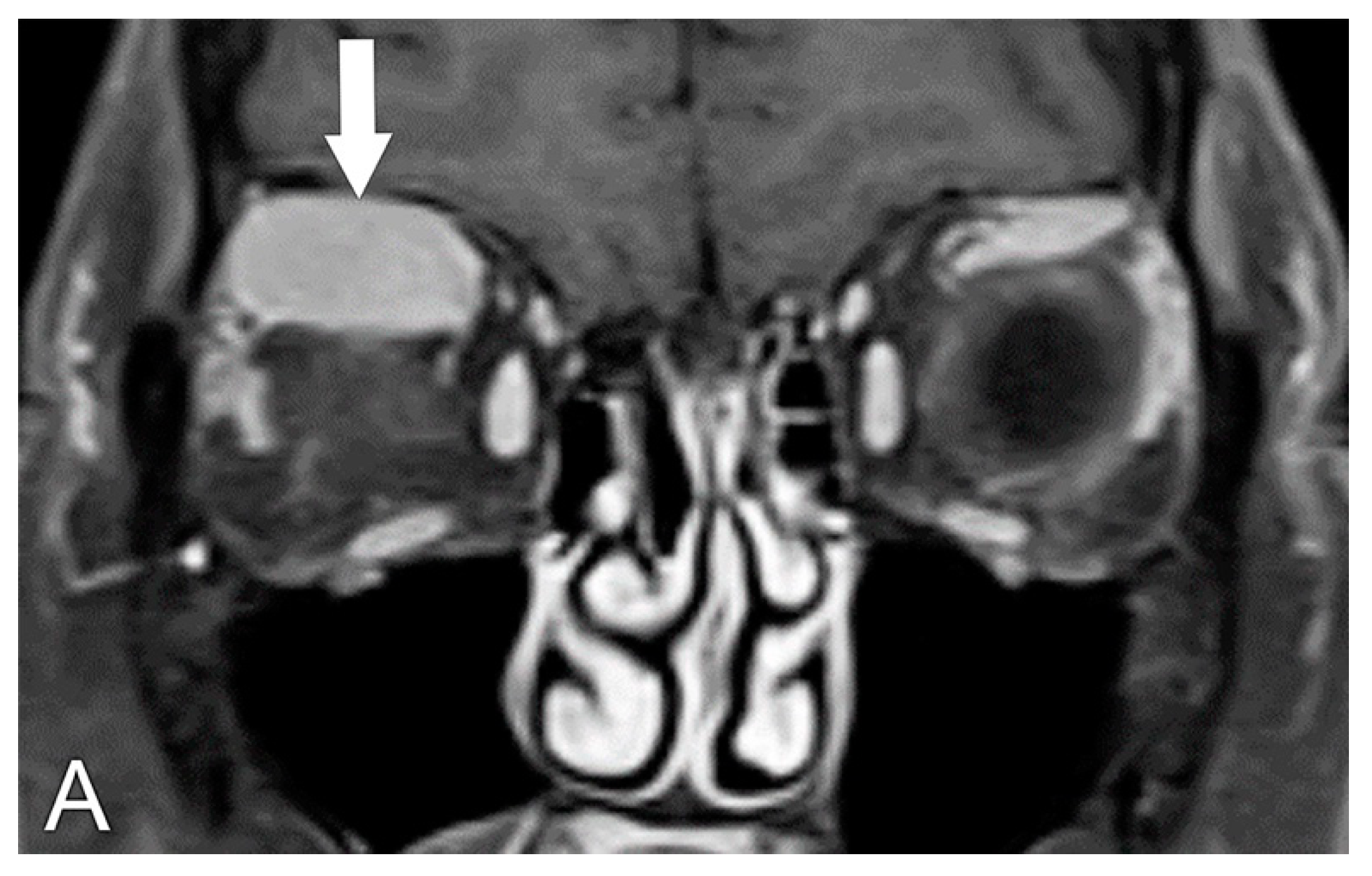
| 1—Orbital pain |
| 2—Pain evoked by gaze |
| 3—Eyelid swelling |
| 4—Eyelid redness |
| 5—Injected conjunctiva |
| 6—Chemosis |
| 7—Inflamed caruncle |
| Classification | Signs and Symptoms (Grade) |
|---|---|
| 0 | Asymptomatic, no physical exam findings |
| I | Physical signs only |
| II | Involvement of soft tissue |
| 0: absent | |
| a: minimal | |
| b: moderate | |
| c: marked | |
| III | Proptosis |
| 0: absent | |
| a: minimal: 3 to 4 mm above normal | |
| b: moderate: 5 to 7 mm above normal | |
| c: marked: 8+ mm above normal | |
| IV | Extraocular muscles |
| 0: absent | |
| a: minimal: gaze limitations at extremes | |
| b: moderate: motion restriction, no fixation | |
| c: marked: globe fixation | |
| V | Cornea |
| 0: absent | |
| a: minimal: stippling | |
| b: moderate: ulceration | |
| c: marked: necrosis, perforation, clouding | |
| VI | Optic nerve compression/vision loss |
| 0: absent | |
| a: minimal: 20/20–20/60 vision; disc pallor | |
| b: moderate: 20/70–20/200 vision; disc pallor | |
| c: marked: <20/200 vision; blindness |
| • Inflamed caruncle | 0: absent | 1: present | |
| • Injected conjunctiva | 0: absent | 1: present | |
| • Eyelid redness | 0: absent | 1: present | |
| • Diurnal variation of symptoms | 0: absent | 1: present | |
| • Chemosis | 0: absent | ||
| 1: conjunctiva behind lid | |||
| 2: conjunctiva beyond lid | |||
| • Painful sensation behind globe | |||
| At rest | 0: absent | 1: present | |
| With gaze | 0: absent | 1: present | |
| • Eyelid edema | 0: absent | ||
| 1: present without festoons (bags under eyes) | |||
| 2: present with festoons | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchings, K.R.; Fritzhand, S.J.; Esmaeli, B.; Koka, K.; Zhao, J.; Ahmed, S.; Debnam, J.M. Graves’ Eye Disease: Clinical and Radiological Diagnosis. Biomedicines 2023, 11, 312. https://doi.org/10.3390/biomedicines11020312
Hutchings KR, Fritzhand SJ, Esmaeli B, Koka K, Zhao J, Ahmed S, Debnam JM. Graves’ Eye Disease: Clinical and Radiological Diagnosis. Biomedicines. 2023; 11(2):312. https://doi.org/10.3390/biomedicines11020312
Chicago/Turabian StyleHutchings, Kasen R., Seth J. Fritzhand, Bita Esmaeli, Kirthi Koka, Jiawei Zhao, Salmaan Ahmed, and James Matthew Debnam. 2023. "Graves’ Eye Disease: Clinical and Radiological Diagnosis" Biomedicines 11, no. 2: 312. https://doi.org/10.3390/biomedicines11020312
APA StyleHutchings, K. R., Fritzhand, S. J., Esmaeli, B., Koka, K., Zhao, J., Ahmed, S., & Debnam, J. M. (2023). Graves’ Eye Disease: Clinical and Radiological Diagnosis. Biomedicines, 11(2), 312. https://doi.org/10.3390/biomedicines11020312




