Mesenchymal Stem/Stromal Cells in Three-Dimensional Cell Culture: Ion Homeostasis and Ouabain-Induced Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Experimental Design
2.2. Spheroid Formation
2.3. Analysis of Cell K+ and Na+ Content and K+ Influx
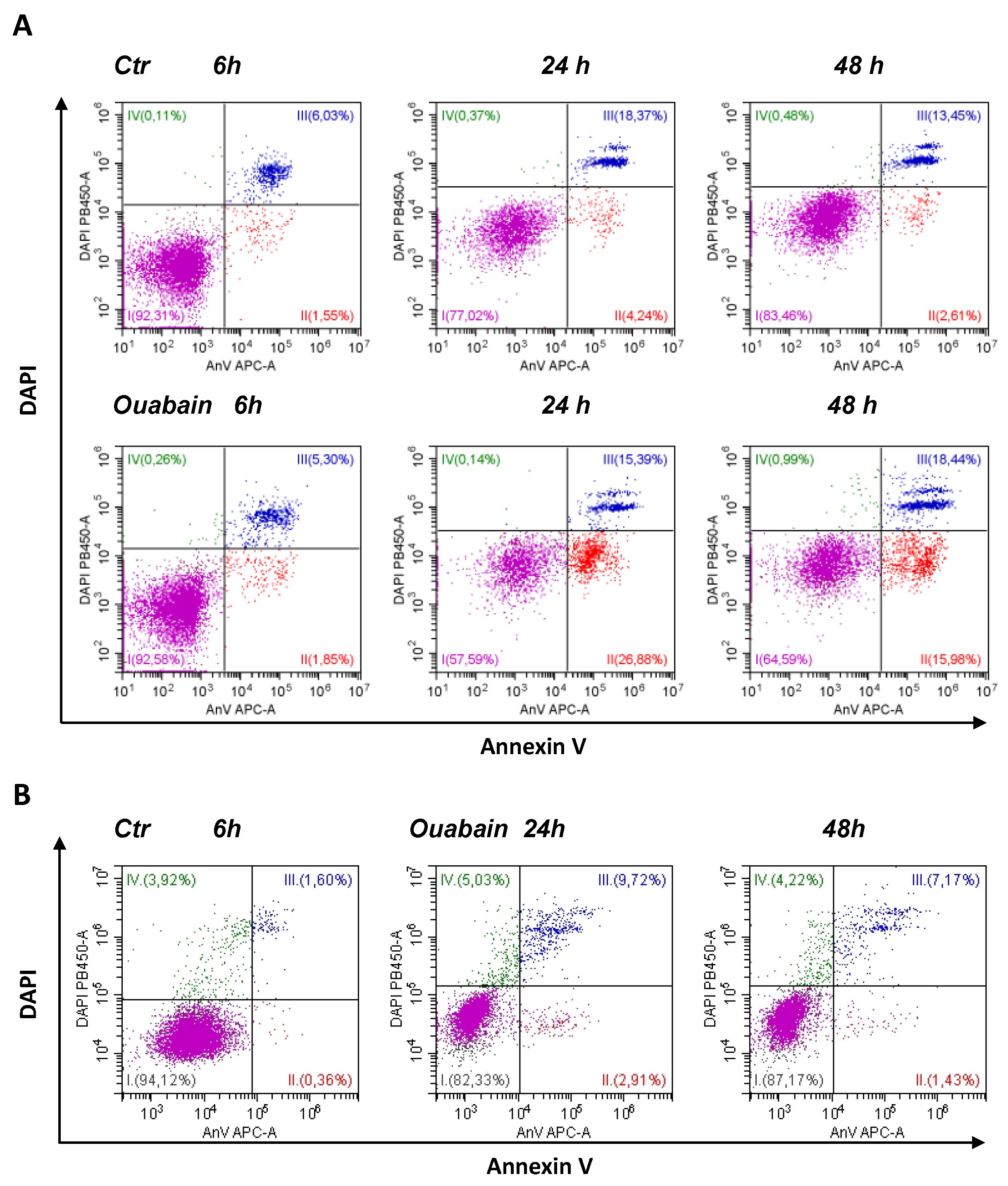
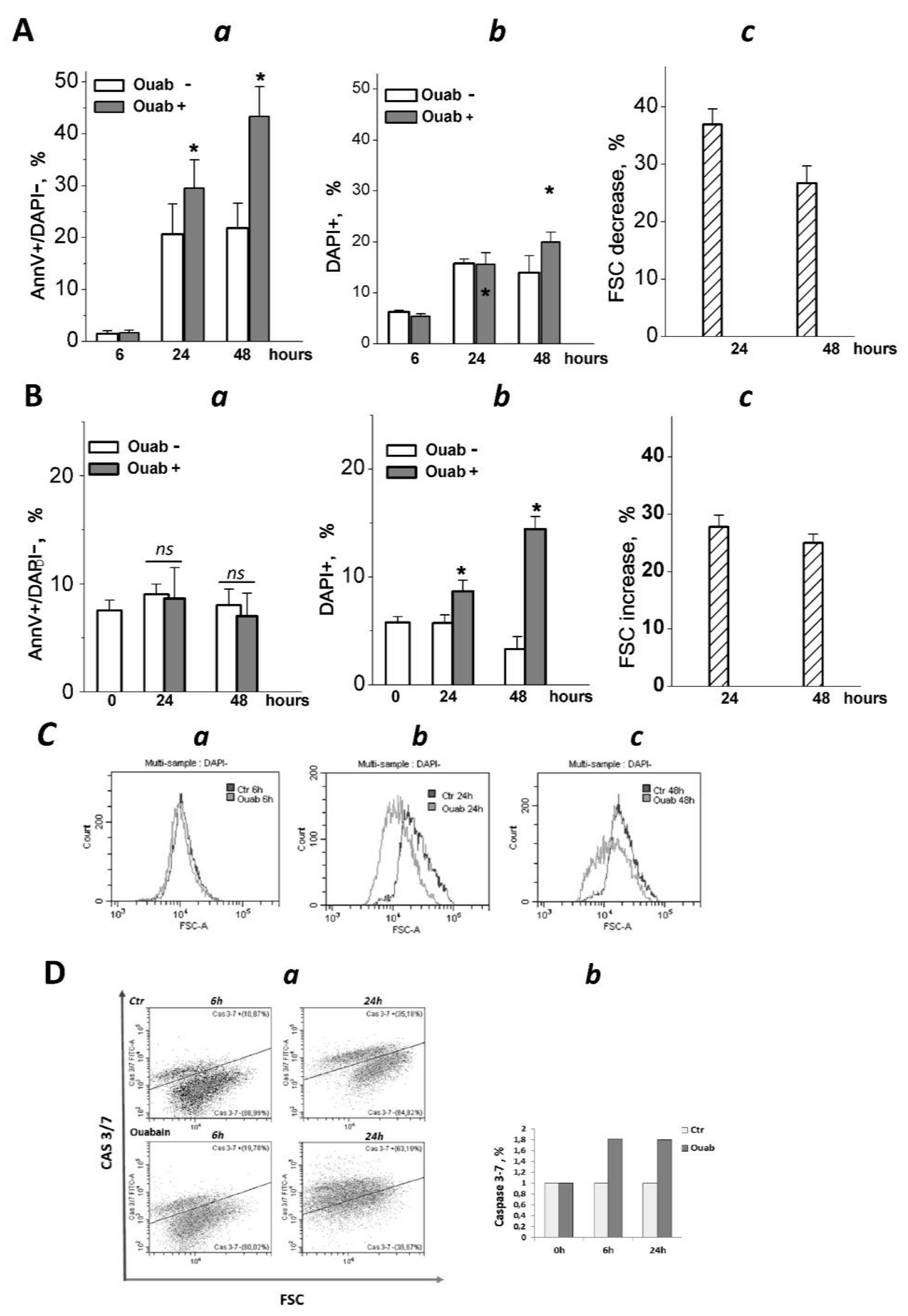
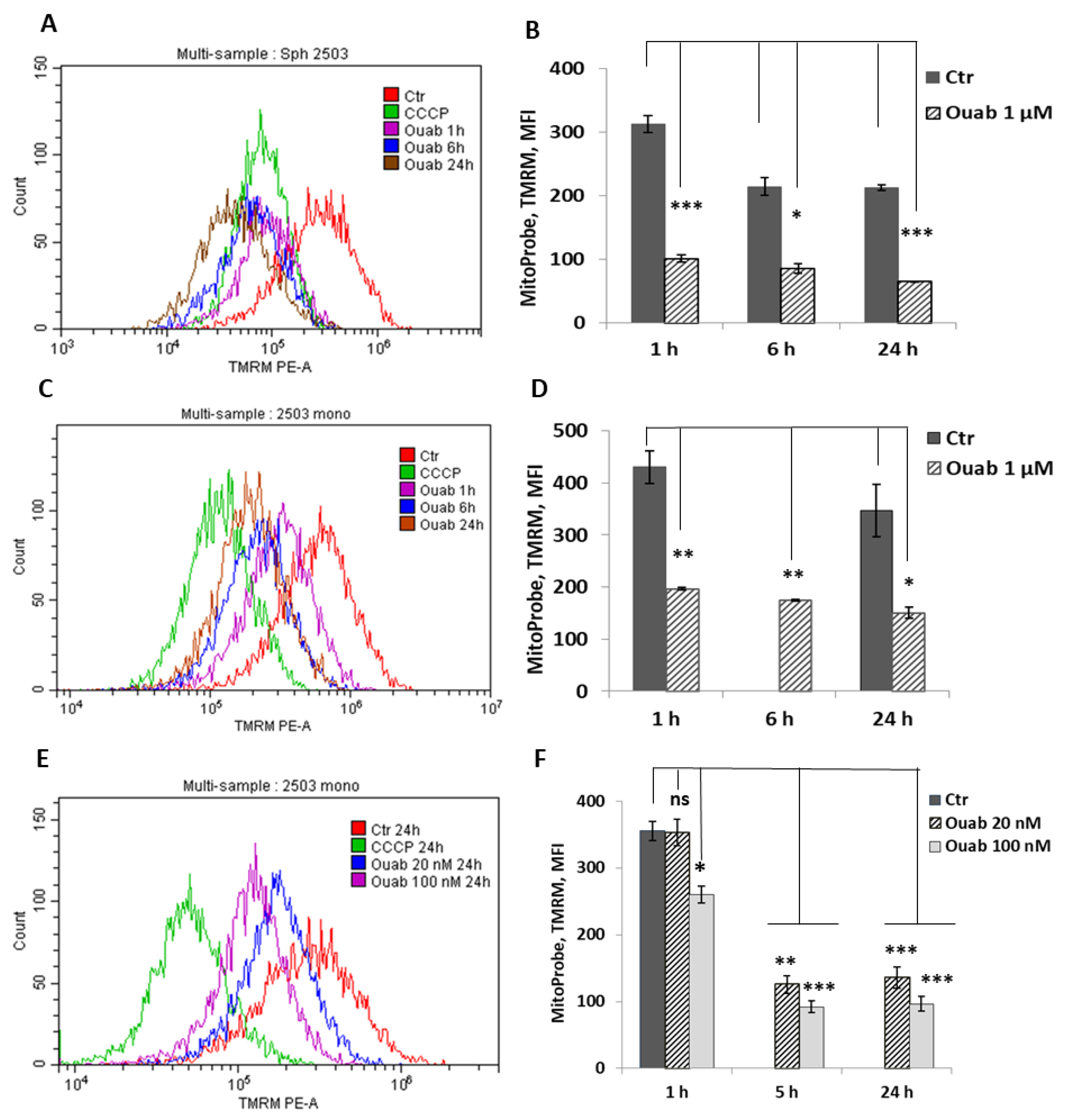
2.4. Flow Cytometry Assay of Apoptosis and Mitochondrial Membrane Potential
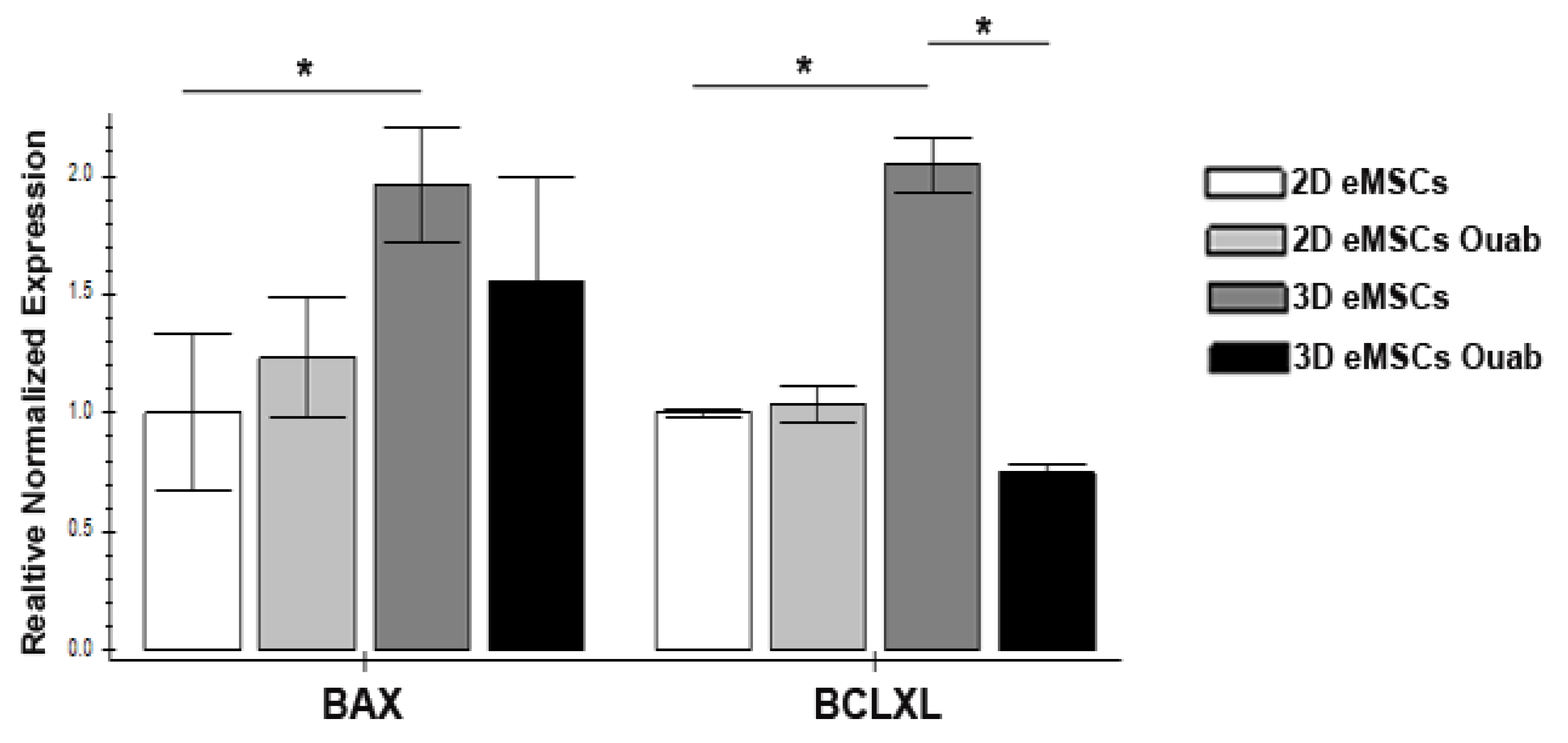
2.5. Quantitative RT-PCR
2.6. Statistical Analysis
3. Results
3.1. Intracellular K+ and Na+ Content and Rb+ (K+) Influxes during eMSCs Transit from 2D Monolayer Culture to 3D Spheroids
3.2. Ouabain Induced Apoptosis in eMSCs Spheroids
3.3. Effect of Ouabain on Cellular K+ Content and Mitochondrial Membrane Potential in 2D and 3D eMSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trounson, A.; McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Parker, A.M.; Katz, A.J. Adipose-derived stem cells for the regeneration of damaged tissues. Expert Opin. Biol. Ther. 2006, 6, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.T.; Badowski, M.; Ahmad, N.; Gaballa, M.A. The potential of cord blood stem cells for use in regenerative medicine. Expert Opin. Biol. Ther. 2007, 7, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Cho, N.H.; Park, Y.K.; Kim, Y.T.; Yang, H.; Kim, S.K. Lifetime expression of stem cell markers in the uterine endometrium. Fertil. Steril. 2004, 81, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E. Identification and characterisation of human endometrial stem/progenitor cels. Aust. New Zealand J. Obstet. Gynaecol. 2006, 46, 250–253. [Google Scholar] [CrossRef]
- Zemelko, V.I.; Grinchuk, T.M.; Domnina, A.P.; Artzibasheva, I.V.; Zenin, V.V.; Kirsanov, A.A.; Bichevaia, N.K.; Korsak, V.S.; Nikolsky, N.N. Multipotent mesenchymal stem cells of desquamated endometrium: Isolation, characterization and use as feeder layer for maintenance of human embryonic stem cell lines. Cell Tissue Biol. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Prockop, D.J. The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Prockop, D.J.; Kota, D.J.; Bazhanov, N.; Regerl, R.L. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J. Cell. Mol. Med. 2010, 14, 2190–2199. [Google Scholar] [CrossRef]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.-H.; Gargett, C.E. Endometrial and menstrual blood mesenchymal stem/stromal cells: Biological properties and clinical fpplication. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Murphy, M.P.; Wang, H.; Patel, A.N.; Kambhampati, S.; Angle, N.; Chan, K.; Marleau, A.M.; Pyszniak, A.; Carrier, E.; Ichim, T.E.; et al. Allogeneic endometrial regenerative cells: An “Off the shelf solution” for critical limb ischemia? J. Transl. Med. 2008, 6, 45. [Google Scholar] [CrossRef]
- Baptista, L.S.; Kronemberger, G.S.; Côrtes, I.; Charelli, L.E.; Matsui, R.A.M.; Palhares, T.N.; Sohier, J.; Rossi, A.M.; Granjeiro, J.M. Adult stem cells spheroids to optimize cell colonization in scaffolds for cartilage and bone tissue engineering. Int. J. Mol. Sci. 2018, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Nimiritsky, P.P.; Eremichev, R.Y.; Alexandrushkina, N.A.; Efimenko, A.Y.; Tkachuk, V.A.; Makarevich, P.I. Unveiling mesenchymal stromal cells’ organizing function in regeneration. Int. J. Mol. Sci. 2019, 20, 823. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Wang, R.; Wang, Y.; Zhang, Y.; Hu, T.; Song, W.; Li, Z.; Huang, S.; Fue, X. Biochemical and structural cues of 3D-printed matrix synergistically direct MSC differentiation for functional sweat gland regeneration. Sci. Adv. 2020, 6, eaaz1094. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-W.; Asano, S.; Hsu, S.-H. Cellular spheroids of mesenchymal stem cells and their perspectives in future healthcare. Appl. Sci. 2019, 9, 627. [Google Scholar] [CrossRef]
- Sant, S.; Johnston, P.A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov. Today Technol. 2017, 23, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Darwin, J.P. Aggregation of human mesenchymal stromal cells (MSCS) into 3d spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012, 347, 701–711. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, Y.S.; Lee, S.H. Long-duration three-dimensional spheroid culture promotes angiogenic activities of adipose-derived mesenchymal stem cells. Biomol. Ther. 2016, 24, 260–267. [Google Scholar] [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef]
- Domnina, A.; Novikova, P.; Obidina, J.; Fridlyanskaya, I.; Alekseenko, L.; Kozhukharova, I.; Lyublinskaya, O.; Zenin, V.; Nikolsky, N. Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium. Stem Cell Res. Ther. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.Y.; Liu, B.H.; Sieber, M.; Hsu, S.H. Substrate-dependent gene regulation of self-assembled human MSC spheroids on chitosan membranes. BMC Genom. 2014, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Korff, T.; Augustin, H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell. Biol. 1998, 143, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.C.; Whitehead, J.; Falahee, P.C.; Zhou, D.; Simon, S.I.; Leach, J.K. Multifactorial experimental design to optimize the anti-inflammatory and proangiogenic potential of mesenchymal stem cell spheroids. Stem Cells 2017, 35, 1493–1504. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef]
- Domnina, A.; Ivanova, J.; Alekseenko, L.; Kozhukharova, I.; Borodkina, A.; Pugovkina, N.; Smirnova, I.; Lyublinskaya, O.; Fridlyanskaya, I.; Nikolsky, N. Three-dimensional compaction switches stress response programs and enhances therapeutic efficacy of endometrial mesenchymal stem/stromal cells. Front. Cell Dev. Biol. 2020, 8, 473. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, C.H.; Hwang, S.M.; Lin, W.W.; Huang, C.H.; Lee, W.Y.; Chang, Y.; Sung, H.-W. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells 2009, 27, 724–732. [Google Scholar] [CrossRef]
- Amos, P.J.; Kapur, S.K.; Stapor, P.C.; Shang, H.; Bekiranov, S.; Khurgel, M.; Rodeheaver, G.T.; Peirce, S.M.; Katz, A.J. Human adipose-derived stromal cells accelerate diabetic wound healing: Impact of cell formulation and delivery. Tissue Eng. Part A 2010, 16, 1595–1606. [Google Scholar] [CrossRef]
- Bhang, S.H.; Lee, S.; Shin, J.Y.; Lee, T.J.; Kim, B.S. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng. Part A 2012, 18, 2138–2147. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Wolint, P.; Wickboldt, N.; Gemayel, G.; Weber, B.; Brokopp, C.E.; Boni, A.; Falk, V.; Bosman, A.; Jaconi, M.E.; et al. Human stem cell-based three-dimensional microtissues for advanced cardiac cell therapies. Biomaterials 2013, 34, 6339–6654. [Google Scholar] [CrossRef]
- Domnina, A.; Alekseenko, L.; Kozhukharova, I.; Lyublinskaya, O.; Shorokhova, M.; Zenin, V.; Fridlyanskaya, I.; Nikolsky, N. Generation of therapeutically potent spheroids from human endometrial mesenchymal stem/stromal cells. J. Pers. Med. 2021, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Herranz, N.; Sun, B.; Wagner, V.; Gallage, S.; Guiho, R.; Wolter, K.; Pombo, J.; Irvine, E.E.; Innes, A.J.; et al. Cardiac glycosides are broad-spectrum senolytics. Nat. Metab. 2019, 1, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Triana-Martinez, F.; Picallos-Rabina, P.; Da Silva-Álvarez, S.; Pietrocola, F.; Llanos, S.; Rodilla, V.; Soprano, T.; Pedroso, P.; Ferreirós, A.; Barradas, M.; et al. Identification and characterization of cardiac glycosides as senolytic compounds. Nat. Commun. 2019, 10, 4731. [Google Scholar] [CrossRef] [PubMed]
- Marakhova, I.I.; Vereninov, A.A.; Toropova, F.V.; Vinogradova, T.A. Na,K-ATPase pump in activated human lymphocytes: On the mechanisms of rapid and long-term increase in K influxes during the initiation of phytohemagglutinin-induced proliferation. Biochim. Biophys. Acta 1998, 1368, 61–72. [Google Scholar] [CrossRef]
- Marakhova, I.; Domnina, A.; Shatrova, A.; Borodkina, A.; Burova, E.; Pugovkina, N.; Zemelko, V.; Nickolsky, N. Proliferation-related changes in K+ content in human mesenchymal stem cells. Sci. Rep. 2019, 9, 346. [Google Scholar] [CrossRef]
- Sudarikova, A.V.; Vasileva, V.Y.; Vassilieva, I.O.; Negulyaev, Y.A.; Morachevskaya, E.A.; Chubinskiy-Nadezhdin, V.I. Extracellular protease trypsin activates amiloride-insensitive sodium channels in human leukemia cells. J. Cell. Biochem. 2019, 120, 461–469. [Google Scholar] [CrossRef]
- Marakhova, I.; Yurinskaya, V.; Aksenov, N.; Zenin, V.; Shatrova, A.; Vereniniv, A. Intracellular K+ and water content in human blood lymphocytes during transition from quiescence to proliferation. Sci. Rep. 2019, 9, 16253. [Google Scholar] [CrossRef]
- Jakobsson, E. Interactions of cell volume, membrane potential, and membrane transport parameters. Am. J. Physiol. Cell Physiol. 1980, 238, C196–C206. [Google Scholar] [CrossRef]
- Vereninov, I.A.; Yurinskaya, V.E.; Model, M.A.; Lang, F.; Vereninov, A.A. Computation of pump-leak flux balance in animal cells. Cell. Physiol. Biochem. 2014, 34, 1812–1823. [Google Scholar] [CrossRef]
- Shatrova, A.; Burova, E.; Pugovkina, N.; Domnina, A.; Nikolsky, N.; Marakhova, I. Monovalent ions and stress-induced senescence in human mesenchymal endometrial stem/stromal cells. Sci. Rep. 2022, 12, 11194. [Google Scholar] [CrossRef]
- Vanden-Berghe, T.; Grootjans, S.; Goossens, V.; Dondelinger, Y.; Krysko, D.; Takahashi, N.; Vandenabeele, P. Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods 2013, 61, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Yurinskaya, V.; Aksenov, N.; Moshkov, A.; Model, M.; Goryachaya, T.; Vereninov, A. A comparative study of U937 cell size changes during apoptosis initiation by flow cytometry, light scattering, water assay and electronic sizing. Apoptosis 2017, 22, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.H.; Liu, K.L.; Shih, Y.L.; Chuang, Y.Y.; Chou, J.; Lu, H.F.; Jair, H.W.; Lee, M.Z.; Au, M.K.; Chung, J.G. Ouabain induces apoptotic cell death through caspase- and mitochndria-dependent pathways in human osteosarcoma U-2 OS cells. Anticancer Res. 2018, 38, 169–178. [Google Scholar] [PubMed]
- Lee, E.J.; Park, S.J.; Kang, S.K.; Kim, G.-H.; Kang, H.-J.; Lee, S.-W.; Jeon, H.B.; Kim, H.-S. Spherical bullet formation via e-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol. Ther. 2012, 20, 1424–1433. [Google Scholar] [CrossRef]
- Mege, R.M.; Ishiyama, N. Integration of cadherin adhesion and cytoskeleton at adherens junctions. Cold Spring Harb. Perspect. Biol. 2017, 9, a028738. [Google Scholar] [CrossRef]
- Powan, P.; Luanpitpong, S.; He, X.; Rojanasaku, Y.; Chanvorachote, P. Detachment-induced E-cadherin expression promotes 3D tumor spheroid formation but inhibits tumor formation and metastasis of lung cancer cells. Am. J. Physiol. Cell. Physiol. 2017, 313, C556–C566. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.; Chen, H.; Li, H.; Wu, Y. 3D culture increases pluripotent gene expression in mesenchymal stem cells through relaxation of cytoskeleton tension. J. Cell. Mol. Med. 2017, 21, 1073–1084. [Google Scholar]
- Colombo, E.; Cattaneo, M.G. Multicellular 3D models to study tumour-stroma interactions. Int. J. Mol. Sci. 2021, 22, 1633. [Google Scholar] [CrossRef]
- Belusa, R.; Aizman, O.; Andersson, R.M.; Aperia, A. Changes in Na+-K+-ATPase activity influence cell attachment to fibronectin. Am. J. Physiol. Cell. Physiol. 2001, 282, C302–C309. [Google Scholar] [CrossRef]
- Vagin, O.; Dada, L.A.; Tokhtaeva, E.; Sachs, G. The Na-K-ATPase α1β1 heterodimer as cell adhesion molecule in epithelia. Am. J. Physiol. Cell. Physiol. 2012, 302, C1271–C1281. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The structure and function of the NA,K-ATPase isoforms in health and disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Rotoli, D.; Cejas, M.; Maeso, M.D.; Pérez-Rodríguez, N.D.; Morales, M.; Ávila, J.; Mobasheri, A.; Martín-Vasallo, P. The Na, K-ATPase β-Subunit Isoforms Expression in Glioblastoma Multiforme: Moonlighting Roles. Int. J. Mol. Sci. 2017, 18, 2369. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.L.; Ramírez-Salinas, G.L.; Martinez-Archundia, M.; Cuellar-Perez, F.; Vilchis-Nestor, C.A.; Cancino-Diaz, J.C.; Shoshani, L. The β2-Subunit (AMOG) of human Na+, K+-ATPase is a homophilic adhesion molecule. Int. J. Mol. Sci. 2022, 23, 7753. [Google Scholar] [CrossRef]
- Vereninov, A.A.; Gusev, E.V.; Kazakova, O.M.; Klimenko, E.M.; Marakhova, I.I.; Osipov, V.V.; Toropova, F.V. Transport and distribution of monovalent cations in human peripheral blood lymphocytes activated by phytohemagglutinin. Tsitologiia 1991, 33, 78–93. [Google Scholar] [PubMed]
- Tosteson, D.C.; Hoffman, J.F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J. Gen. Physiol. 1960, 44, 169–194. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef] [PubMed]
- Yurinskaya, V.; Goryachaya, T.; Guzhova, I.; Moshkov, A.; Rozanov, Y.; Sakuta, G.; Shirokova, A.; Shumilina, E.; Vassilieva, I.; Lang, F.; et al. Potassium and sodium balance in U937 cells during apoptosis with and without cell shrinkage. Cell Physiol. Biochem. 2005, 16, 155–162. [Google Scholar] [CrossRef]
- Figueroa, B.; Xu, F.X.; Hu, R.; Men, S.; Dan Fu, D. Quantitative imaging of intracellular density with ratiometric stimulated Raman scattering microscopy. J. Phys. Chem. B. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Burg, M.B. Macromolecular crowding as a cell volume sensor. Cell. Physiol. Biochem. 2000, 10, 251–256. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Pedersen, S.F. Cell volume homeostatic mechanisms: Effectors and signalling pathways. Acta Physiol. 2011, 202, 465–485. [Google Scholar] [CrossRef]
- Akabayov, B.; Akabayov, S.R.; Lee, S.J.; Wagner, G.; Richardson, C.C. Impact of macromolecular crowding on DNA replication. Nat. Commun. 2013, 4, 1615. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Putzel, G.G.; Szleifer, I.; Szleifer, I. Macromolecular crowding as a regulator of gene transcription. Biophys. J. 2014, 106, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Model, M.A.; Hollembeak, J.T.; Kurokawa, M. Macromolecular crowding: A hidden link between cell volume and everything else. Cell. Physiol. Biochem. 2021, 55, 25–40. [Google Scholar] [PubMed]
- Rosen, K.; Shi, W.; Calabretta, B.; Filmus, J. Cell detachment triggers p38 mitogen-activated protein kinase-dependent overexpression of Fas ligand. A novel mechanism of Anoikis of intestinal epithelial cells. J. Biol. Chem. 2002, 277, 46123–46130. [Google Scholar] [CrossRef]
- Grossmann, J. Molecular mechanisms of “detachment-induced apoptosis-anoikis. Apoptosis 2002, 7, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Zhao, H.; Han, Z.C. Signalling mechanisms of anoikis. Histol Histopathol. 2004, 19, 973–983. [Google Scholar]
- Xie, Z.; Askari, A. Na+/K+-ATPase as a signal transducer. Eur. J. Biochem. 2002, 269, 2434–2439. [Google Scholar] [CrossRef]
- Aperia, A. New Roles for an old Na,K-ATPase emerges as an interesting drug target. J. Intern. Med. 2007, 261, 44–52. [Google Scholar] [CrossRef]
- Orlov, S.N.; Tverskoi, A.M.; Sidorenko, S.V.; Smolyaninova, L.V.; Lopina, O.D.; Dulin, N.O.; Klimanova, E.A. Na,K-ATPase as a target for endogenous cardiotonic steroids: What’s the evidence? Genes Dis. 2020, 8, 259–271. [Google Scholar] [CrossRef]
- Lopina, O.D.; Tverskoi, A.M.; Klimanova, E.A.; Sidorenko, S.V.; Orlov, S.N. Ouabain-induced cell death and survival. Role of α1-Na,K-ATPase-mediated signaling and [Na+]i/[K+]i-dependent gene expression. Front. Physiol. 2020, 11, 1060. [Google Scholar] [CrossRef]
- Lopachev, A.V.; Lagarkova, M.A.; Lebedeva, O.S.; Ezhova, M.A.; Kazanskaya, R.B.; Timoshina, Y.A.; Khutorova, A.V.; Akkuratov, E.E.; Fedorova, T.N.; Gainetdinov, R.R. Ouabain-induced gene expression changes in human iPSC-derived neuron culture expressing dopamine and cAMP-regulated phosphoprotein 32 and GABA receptors. Brain Sci. 2021, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Klimanova, E.A.; Sidorenko, S.V.; Abramicheva, P.A.; Tverskoi, A.M.; Orlov, S.N.; Lopina, O.D. Transcriptomic changes in endothelial cells triggered by Na,K-ATPase inhibition: A search for upstream Na+i/K+i sensitive genes. Int. J. Mol. Sci. 2020, 21, 7992. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.A.; Yang, P.; Pawlus, A.D.; Block, K.I. Cardiac glycosides as novel cancer therapeutic agents. Mol. Interv. 2008, 8, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Prassas, I.; Diamandis, E.P. Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Alekseenko, L.L.; Zemelko, V.I.; Zenin, V.V.; Pugovkina, N.A.; Kozhukharova, I.V.; Kovaleva, Z.V.; Grinchuk, T.M.; Fridlyanskaya, I.I.; Nikolsky, N.N. Heat shock induces apoptosis in human embryonic stem cells but a premature senescence phenotype in their differentiated progeny. Cell Cycle 2012, 11, 3260–3269. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, C.K.; Yang, S.C.; Hsu, S.C.; Lin, H.; Chang, F.P.; Kuo, T.C.; Shen, C.N.; Chiang, P.M.; Hsiao, M.; et al. Elimination of undifferentiated human embryonic stem cells by cardiac glycosides. Sci. Rep. 2017, 7, 5289. [Google Scholar] [CrossRef]
- Deryabin, P.I.; Shatrova, A.N.; Borodkina, A.V. Apoptosis resistance of senescent cells is an intrinstic barrier for senolysis induced by cardiac glycosides. Cell. Mol. Life Sci. 2021, 78, 7757–7776. [Google Scholar] [CrossRef]
- Cerella, C.; Muller, F.; Gaigneaux, A.; Radogna, F.; Viry, E.; Chateauvieux, S.; Dicato, M.; Diederich, M. Early downregulation of Mcl-1 regulates apoptosis triggered by cardiac glycoside UNBS1450. Cell Death Dif. 2015, 6, e1782. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Wang, L.; Cai, W.; Han, B.; Zhang, J.; Yu, B.; Chen, M. Ouabain exhibited strong anticancer effects in melanoma cells via induction of apoptosis, G2/M phase arrest, and migration inhibition. Onco Targets Ther. 2021, 14, 1261–1273. [Google Scholar] [CrossRef]
- Vereninov, A.A.; Rubashkin, A.A.; Goryachaya, T.S.; Moshkov, A.V.; Rozanov, Y.M.; Shirokova, A.V.; Strelkova, E.G.; Lang, F.; Yurinskaya, V.E. Pump and channel K+(Rb+) fluxes in apoptosis of human lymphoid cell line U937. Cell. Physiol. Biochem. 2008, 22, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kondratskyi, A.; Kondratska, K.; Skryma, R.; Prevarskaya, N. Ion channels in the regulation of apoptosis. Biochim. Biophys. Acta 2015, 1848, 2531–2546. [Google Scholar] [CrossRef] [PubMed]
- Bortner, C.D.; Cidlowski, J.A. Ions, the movement of water and the apoptotic volume decrease. Front. Cell Dev. Biol. 2020, 8, 611211. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Flinck, M.; Pardo, L.A. The interplay between dysregulated ion transport and mitochondrial architecture as a dangerous liaison in cancer. Int. J. Mol. Sci. 2021, 22, 5209. [Google Scholar] [CrossRef] [PubMed]
- Garlid, K.D.; Paucek, P. Mitochondrial potassium transport: The K+ cycle. Biochim. Biophys. Acta 2003, 1606, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Kaasik, A.; Safiulina, D.; Zharkovsky, A.; Veksler, V. Regulation of mitochondrial matrix volume. Am. J. Physiol. Cell Physiol. 2007, 292, C157–C163. [Google Scholar] [CrossRef]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef]
- Nowikovsky, K.; Schweyen, R.J.; Bernardi, P. Pathophysiology of mitochondrial volume homeostasis: Potassium transport and permeability transition. Biochim. Biophys. Acta 2009, 1787, 345–350. [Google Scholar] [CrossRef]
- Shimizu, S.; Narita, M.; Tsujimoto, Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 1999, 399, 483–487. [Google Scholar] [CrossRef]
- Harris, M.; Thompson, C.B. The role of the Bcl-2 family in the regulation of outer -mitochondrial membrane permeability. Cell Death Differ. 2000, 7, 1182–1191. [Google Scholar] [CrossRef]
- Tsujimoto, Y.J. Cell death regulation by the Bcl-2 protein family in the mitochondria. Cell Physiol. 2003, 195, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta 2006, 1757, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Roufayel, R.; Younes, K.; Al-Sabi, A.; Murshid, N. BH3-only proteins Noxa and Puma are key regulators of induced apoptosis. Life 2022, 12, 256. [Google Scholar] [CrossRef] [PubMed]


| Symbol | Primer Sequence | Amplification Conditions | PCR Product Size (bp) | Accession Number |
|---|---|---|---|---|
| BAX | F: 5′-GGGTTGTCGCCCTTTTCT-3′ R: 5′-CAGCCCATGATGGTTCTGATCAG-3′ | 93 °C, 20 s, 59–62 °C, 30 s 72 °C, 30 s | 91 | XM_047439168.1 |
| Bcl-Xl | F: 5′-GCTTGGATGGCCACTTACCT-3′ R: 5′-GGGAGGGTAGAGTGGATGGT-3′ | 93 °C, 20 s, 59–62 °C, 30 s 72 °C, 30 s | 231 | NM_001317919.2 |
| GAPDH | F: 5’-GAGGTCAATGAAGGGGTCAT-3′ R: 5’-AGTCAACGGATTTGGTCGTA-3′ | 93 °C, 20 s, 59–62 °C, 30 s 72 °C, 30 s | 100 | NM_001357943.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shatrova, A.N.; Domnina, A.P.; Pugovkina, N.A.; Alekseenko, L.L.; Marakhova, I.I. Mesenchymal Stem/Stromal Cells in Three-Dimensional Cell Culture: Ion Homeostasis and Ouabain-Induced Apoptosis. Biomedicines 2023, 11, 301. https://doi.org/10.3390/biomedicines11020301
Shatrova AN, Domnina AP, Pugovkina NA, Alekseenko LL, Marakhova II. Mesenchymal Stem/Stromal Cells in Three-Dimensional Cell Culture: Ion Homeostasis and Ouabain-Induced Apoptosis. Biomedicines. 2023; 11(2):301. https://doi.org/10.3390/biomedicines11020301
Chicago/Turabian StyleShatrova, Alla N., Alisa P. Domnina, Natalia A. Pugovkina, Larisa L. Alekseenko, and Irina I. Marakhova. 2023. "Mesenchymal Stem/Stromal Cells in Three-Dimensional Cell Culture: Ion Homeostasis and Ouabain-Induced Apoptosis" Biomedicines 11, no. 2: 301. https://doi.org/10.3390/biomedicines11020301
APA StyleShatrova, A. N., Domnina, A. P., Pugovkina, N. A., Alekseenko, L. L., & Marakhova, I. I. (2023). Mesenchymal Stem/Stromal Cells in Three-Dimensional Cell Culture: Ion Homeostasis and Ouabain-Induced Apoptosis. Biomedicines, 11(2), 301. https://doi.org/10.3390/biomedicines11020301





