Reappraisal of the Lymphatic Drainage System of the Distal Rectum: Functional Lymphatic Flow into the Presacral Space and Its Clinical Implication in Rectal Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. ICG Fluorescence Imaging of Dissected Cadavers
2.2. Intraoperative ICG Fluorescence Imaging
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, S.; Sasaki, J.; Sinclair, G.; Chapuis, P.H.; Bokey, E.L. Understanding the anatomy of lymphatic drainage and the use of blue-dye mapping to determine the extent of lymphadenectomy in rectal cancer surgery: Unresolved issues. Colorectal Dis. 2009, 11, 443–449. [Google Scholar] [CrossRef]
- Heald, R.J. The ‘Holy Plane’ of rectal surgery. J. R. Soc. Med. 1988, 81, 503–508. [Google Scholar] [CrossRef]
- Heald, R.J.; Ryall, R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef]
- Knol, J.; Keller, D.S. Total mesorectal excision technique-past, present, and future. Clin. Colon Rectal Surg. 2020, 33, 134–143. [Google Scholar] [CrossRef]
- Kodeda, K.; Johansson, R.; Zar, N.; Birgisson, H.; Dahlberg, M.; Skullman, S.; Lindmark, G.; Glimelius, B.; Påhlman, L.; Martling, A. Time trends, improvements and national auditing of rectal cancer management over an 18-year period. Colorectal Dis. 2015, 17, O168–O179. [Google Scholar] [CrossRef]
- Hojo, K.; Koyama, Y.; Moriya, Y. Lymphatic spread and its prognostic value in patients with rectal cancer. Am. J. Surg. 1982, 144, 350–354. [Google Scholar] [CrossRef]
- Morikawa, E.; Yasutomi, M.; Shindou, K.; Matsuda, T.; Mori, N.; Hida, J.; Kubo, R.; Kitaoka, M.; Nakamura, M.; Fujimoto, K.; et al. Distribution of metastatic lymph nodes in colorectal cancer by the modified clearing method. Dis. Colon Rectum 1994, 37, 219–223. [Google Scholar] [CrossRef]
- Moriya, Y.; Hojo, K.; Sawada, T.; Koyama, Y. Significance of lateral node dissection for advanced rectal carcinoma at or below the peritoneal reflection. Dis. Colon Rectum 1989, 32, 307–315. [Google Scholar] [CrossRef]
- Takahashi, T.; Ueno, M.; Azekura, K.; Ohta, H. Lateral node dissection and total mesorectal excision for rectal cancer. Dis. Colon Rectum 2000, 43, S59–S68. [Google Scholar] [CrossRef]
- Kim, T.H.; Jeong, S.Y.; Choi, D.H.; Kim, D.Y.; Jung, K.H.; Moon, S.H.; Chang, H.J.; Lim, S.B.; Choi, H.S.; Park, J.G. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann. Surg. Oncol. 2008, 15, 729–737. [Google Scholar] [CrossRef]
- Kusters, M.; Uehara, K.; Velde, C.; Moriya, Y. Is there any reason to still consider lateral lymph node dissection in rectal cancer? Rationale and technique. Clin. Colon Rectal Surg. 2017, 30, 346–356. [Google Scholar]
- Michelassi, F.; Block, G.E. Morbidity and mortality of wide pelvic lymphadenectomy for rectal adenocarcinoma. Dis. Colon Rectum 1992, 35, 1143–1147. [Google Scholar] [CrossRef]
- Ueno, M.; Oya, M.; Azekura, K.; Yamaguchi, T.; Muto, T. Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br. J. Surg. 2005, 92, 756–763. [Google Scholar] [CrossRef]
- Chen, C.H.; Hsieh, M.C.; Hsiao, P.K.; Lin, E.K.; Lu, Y.J.; Wu, S.Y. Tumor location is an independent predictive factor for distant metastasis and metastatic sites of rectal adenocarcinoma in patients receiving total mesorectal excision. J. Cancer 2018, 9, 950–958. [Google Scholar] [CrossRef]
- Hiyoshi, Y.; Miyamoto, Y.; Kiyozumi, Y.; Eto, K.; Nagai, Y.; Iwatsuki, M.; Iwagami, S.; Baba, Y.; Yoshida, N.; Baba, H. Risk factors and prognostic significance of lateral pelvic lymph node metastasis in advanced rectal cancer. Int. J. Clin. Oncol. 2020, 25, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kusters, M.; van de Velde, C.J.; Beets-Tan, R.G.; Akasu, T.; Fujita, S.; Yamamoto, S.; Moriya, Y. Patterns of local recurrence in rectal cancer: A single-center experience. Ann. Surg. Oncol. 2009, 16, 289–296. [Google Scholar] [CrossRef]
- Shinaoka, A.; Koshimune, S.; Yamada, K.; Kumagishi, K.; Suami, H.; Kimata, Y.; Ohtsuka, A. A fresh cadaver study on indocyanine green fluorescence lymphography: A new whole-body imaging technique for investigating the superficial lymphatics. Plast Reconstr. Surg. 2018, 141, 1161–1164. [Google Scholar] [CrossRef]
- Blair, J.B.; Holyoke, E.A.; Best, R.R. A note on the lymphatics of the middle and lower rectum and anus. Anat. Rec. 1950, 108, 635–644. [Google Scholar] [CrossRef]
- Arnaud, J.P.; Bergamaschi, R.; Schloegel, M.; Ollier, J.C.; Haegele, P.; Grob, J.C.; Adloff, M. Progress in the assessment of lymphatic spread in rectal cancer. Rectal endoscopic lymphoscintigraphy. Dis. Colon Rectum 1990, 33, 398–401. [Google Scholar] [CrossRef]
- Sato, K.; Shimoda, H.; Miura, T.; Sakamoto, Y.; Morohashi, H.; Watanabe, S.; Narita, H.; Mitsuhashi, Y.; Umemura, K.; Hakamada, K. Widespread anorectal lymphovascular networks and tissue drainage: Analyses from submucosal India ink injection and indocyanine green fluorescence imaging. Colorectal Dis. 2021, 23, 1334–1345. [Google Scholar] [CrossRef]
- Stelzner, S.; Heinze, T.; Nikolouzakis, T.K.; Torge Mees, S.; Witzigmann, H.; Wedel, T. Perirectal fascial anatomy: New insights into an old problem. Dis. Colon Rectum 2021, 64, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.C. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Havenga, K.; Enker, W.E.; Norstein, J.; Moriya, Y.; Heald, R.J.; van Houwelingen, H.C.; van de Velde, C.J. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: An international analysis of 1411 patients. Eur. J. Surg. Oncol. 1999, 25, 368–374. [Google Scholar] [CrossRef]
- Peeters, K.C.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.K.; Putter, H.; Wiggers, T.; Rutten, H.; Pahlman, L.; Glimelius, B.; Leer, J.W.; et al. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann. Surg. 2007, 246, 693–701. [Google Scholar] [CrossRef]
- Park, J.S.; Park, S.Y.; Kim, H.J.; Cho, S.H.; Kwak, S.G.; Choi, G.S. Long-term oncologic outcomes after neoadjuvant chemoradiation followed by intersphincteric resection with coloanal anastomosis for locally advanced low rectal cancer. Dis. Colon Rectum 2019, 62, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C. Immunological aspects of local radiotherapy: Clinical relevance. Discov. Med. 2010, 9, 119–124. [Google Scholar]
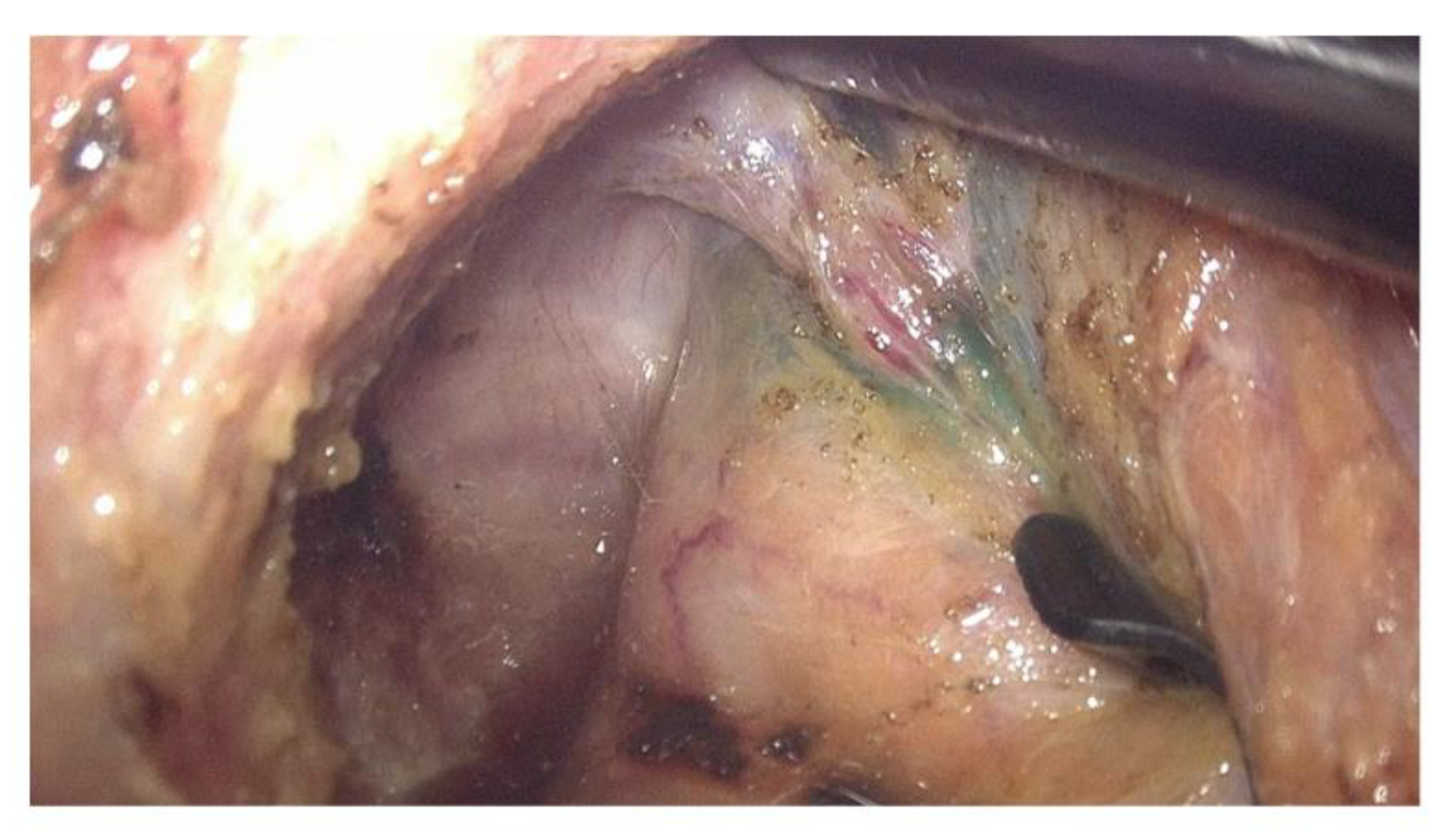
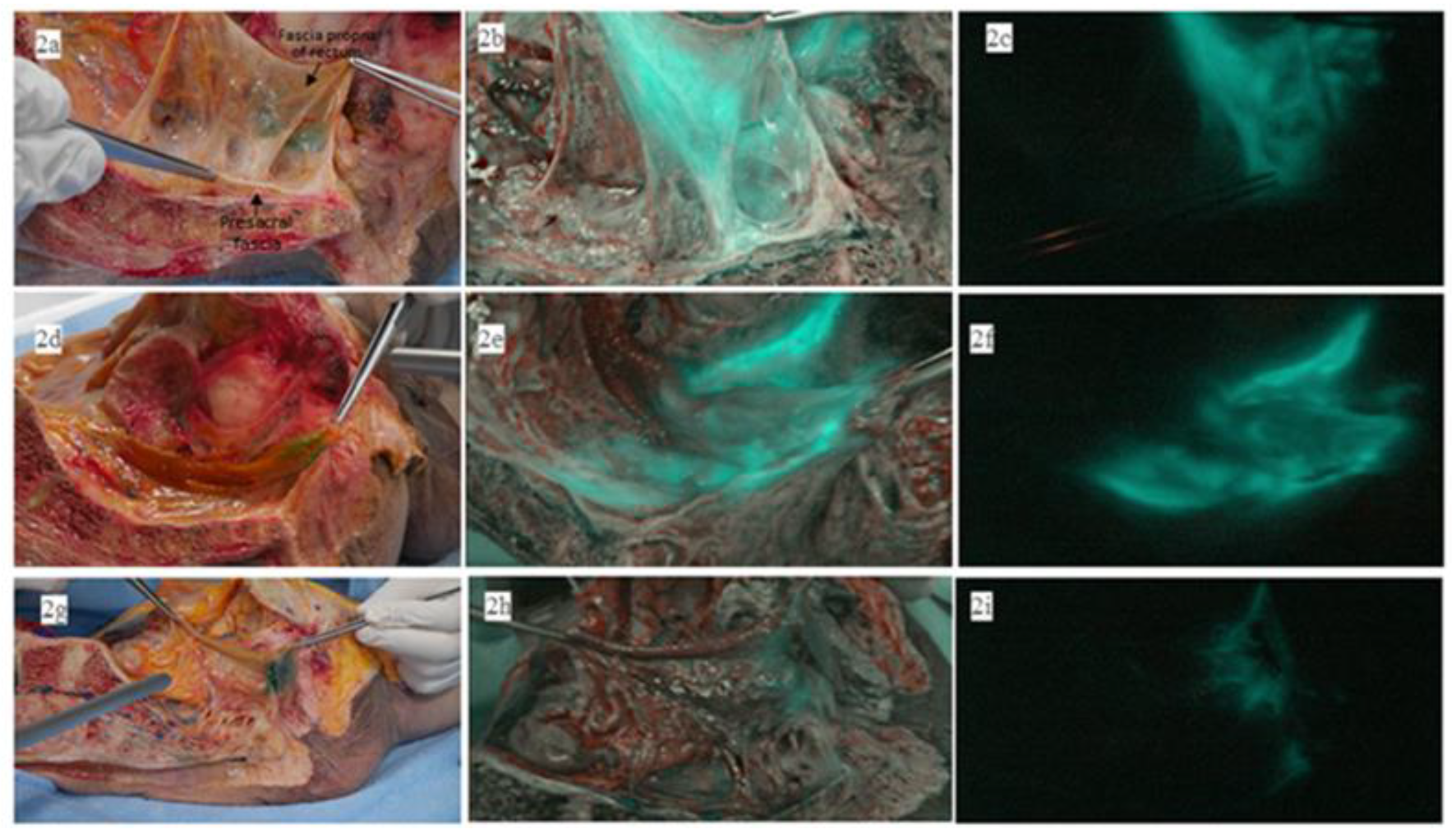



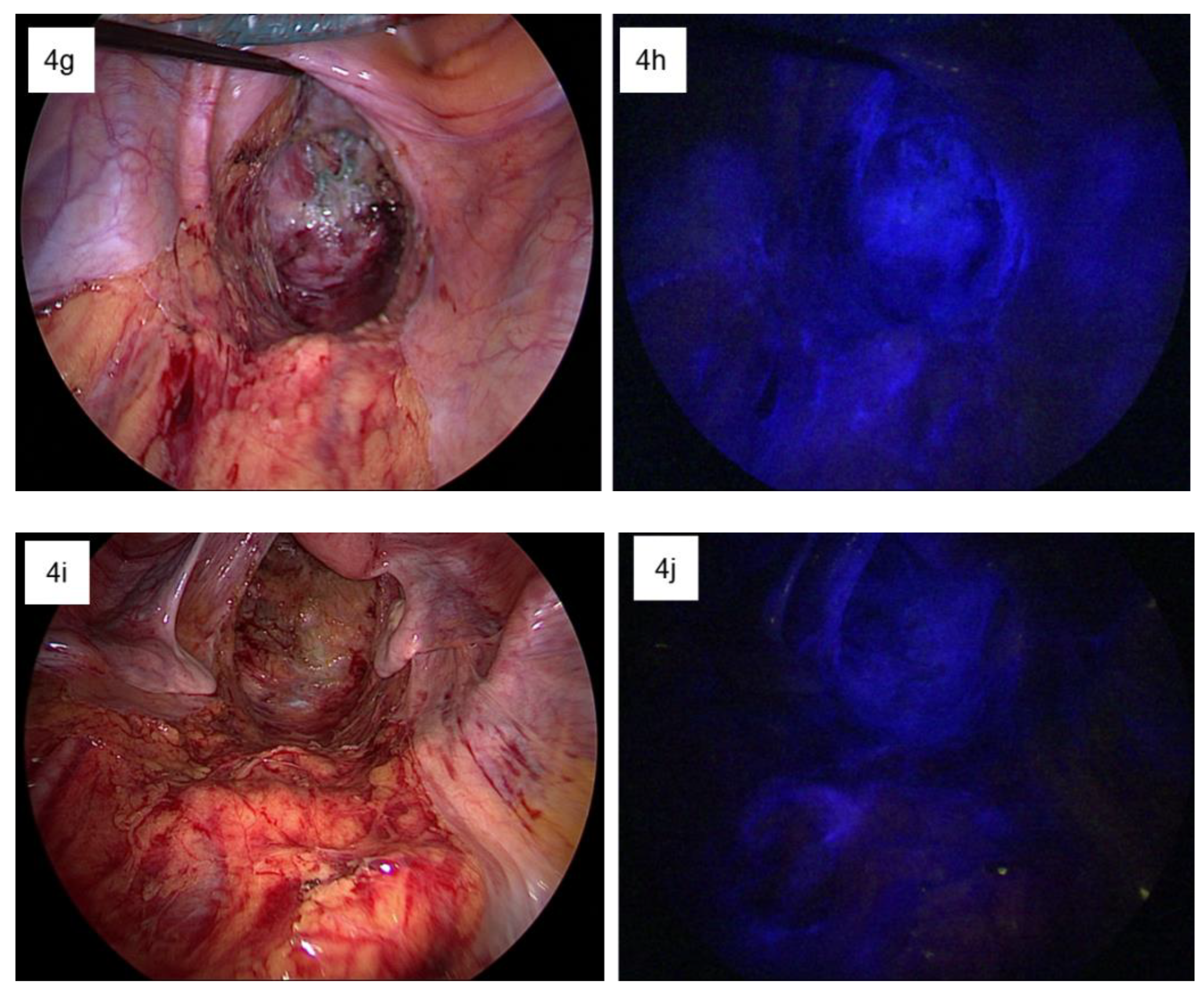
| Patient Number | Sex | Age | Tumor The Location from the Anal Verge (cm) | nCRT | Type of nCRT | Operation | Pathologic TNM Stage (p or yp) | ICG Fluorescent Detection Site | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | N | M | Right Pelvic Side Wall | Left Pelvic Side Wall | Presacral Space | |||||||
| 1 | F | 69 | 6 | Yes | Long course | Lap LAR | 3 | 0 | 0 | - | - | - |
| 2 | M | 46 | 8 | Yes | Long-course | Lap LAR | 3 | 1a | 0 | - | - | - |
| 3 | M | 60 | 10 | No | - | Lap LAR | 3 | 2a | 0 | - | - | - |
| 4 | M | 50 | 2 | Yes | Long-course | Lap ISR | 3 | 1a | 0 | + | + | + |
| 5 | M | 61 | 2 | Yes | Long-course | Lap ISR | 0 | 0 | 0 | - | + | - |
| 6 | M | 68 | 10 | No | - | Lap LAR | 4a | 2a | 0 | + | - | - |
| 7 | M | 66 | 10 | Yes | Long-course | Lap LAR | 3 | 1a | 0 | + | - | - |
| 8 | M | 68 | 10 | No | - | Lap LAR | 2 | 0 | 0 | + | - | - |
| 9 | M | 64 | 7 | Yes | Long-course | Lap LAR | 1 | 0 | 0 | - | - | - |
| 10 | F | 77 | 10 | No | - | Lap LAR | 3 | 0 | 0 | - | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, R.-N.; Cho, H.-M.; Kye, B.-H.; Lee, Y.-S.; Kim, Y.-S. Reappraisal of the Lymphatic Drainage System of the Distal Rectum: Functional Lymphatic Flow into the Presacral Space and Its Clinical Implication in Rectal Cancer Treatment. Biomedicines 2023, 11, 274. https://doi.org/10.3390/biomedicines11020274
Yoo R-N, Cho H-M, Kye B-H, Lee Y-S, Kim Y-S. Reappraisal of the Lymphatic Drainage System of the Distal Rectum: Functional Lymphatic Flow into the Presacral Space and Its Clinical Implication in Rectal Cancer Treatment. Biomedicines. 2023; 11(2):274. https://doi.org/10.3390/biomedicines11020274
Chicago/Turabian StyleYoo, Ri-Na, Hyeon-Min Cho, Bong-Hyeon Kye, Yoon-Suk Lee, and Yi-Suk Kim. 2023. "Reappraisal of the Lymphatic Drainage System of the Distal Rectum: Functional Lymphatic Flow into the Presacral Space and Its Clinical Implication in Rectal Cancer Treatment" Biomedicines 11, no. 2: 274. https://doi.org/10.3390/biomedicines11020274
APA StyleYoo, R.-N., Cho, H.-M., Kye, B.-H., Lee, Y.-S., & Kim, Y.-S. (2023). Reappraisal of the Lymphatic Drainage System of the Distal Rectum: Functional Lymphatic Flow into the Presacral Space and Its Clinical Implication in Rectal Cancer Treatment. Biomedicines, 11(2), 274. https://doi.org/10.3390/biomedicines11020274






