Gene Therapy with p14/tBID Induces Selective and Synergistic Apoptosis in Mutant Ras and Mutant p53 Cancer Cells In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Construction of the Modified p14ARFmin Promoter Expression Vectors
2.3. Ap1e-E2Fe-p14ARFmin-MDR1n-p14-tBID Construction
2.4. Luciferase Assay
2.5. Flow Cytometric Analysis for Cell Cycle and Apoptosis
2.6. Western Blot Analyses
2.7. Human CFU-GEMM Stem Cell Assay
2.8. Animal Studies
2.8.1. Human Lung Cancer Xenograft Model
2.8.2. Pancreatic Cancer in the Mut K-Ras/Mut p53 KPC GEMM Mouse Strains and Endpoint Treatment Procedure
2.8.3. Measurement of Tumor Foci
2.8.4. Imaging of KPC Pancreatic Tumors by High-Resolution Ultrasound
2.8.5. Survival Study
3. Results
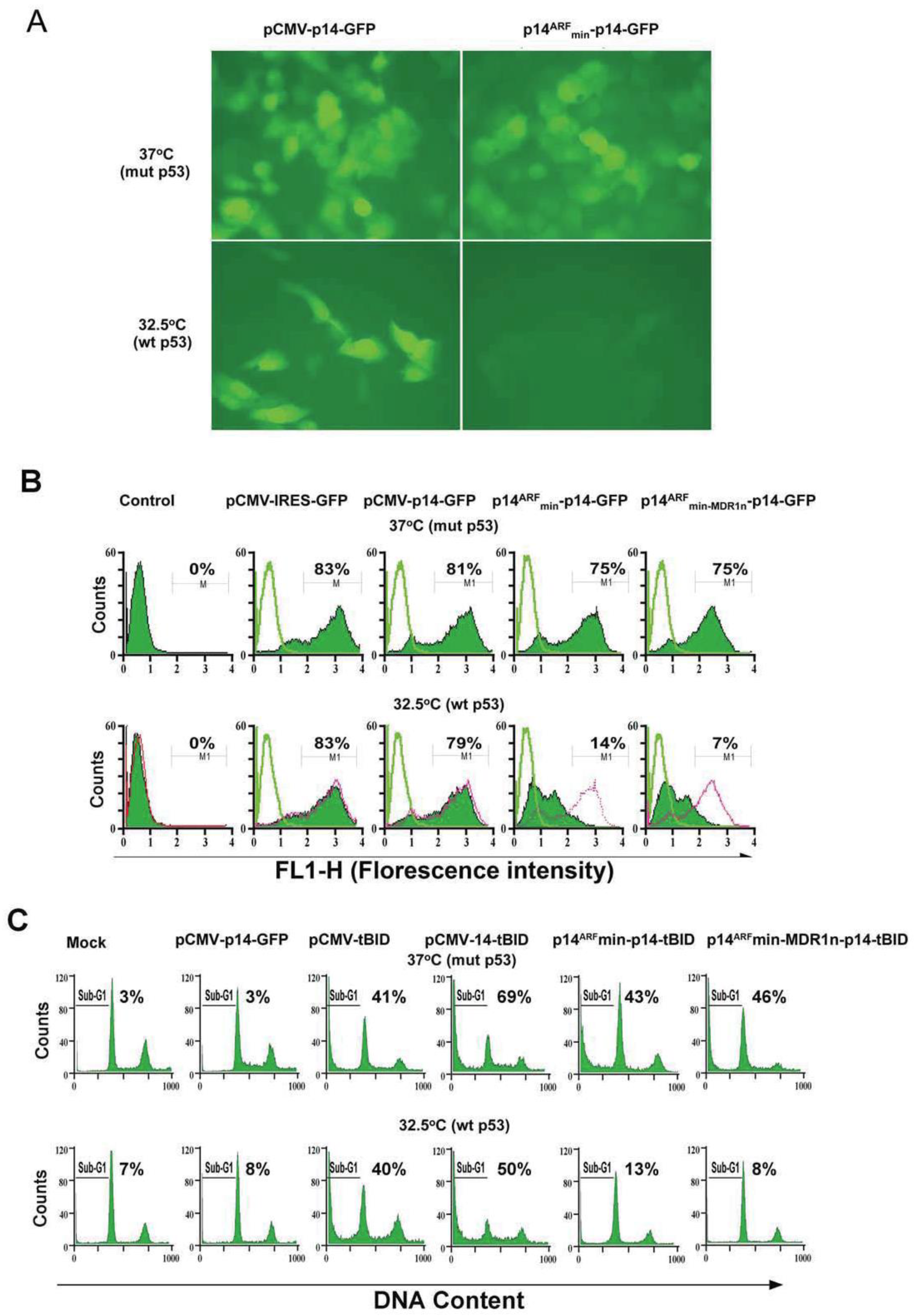
3.1. A Modified p14ARF Promoter Is Specifically Expressed in Mutant Ras and Mutant p53 Cancer Cell Lines
3.2. Bidirectional Control of the Modified p14ARFmin Promoter
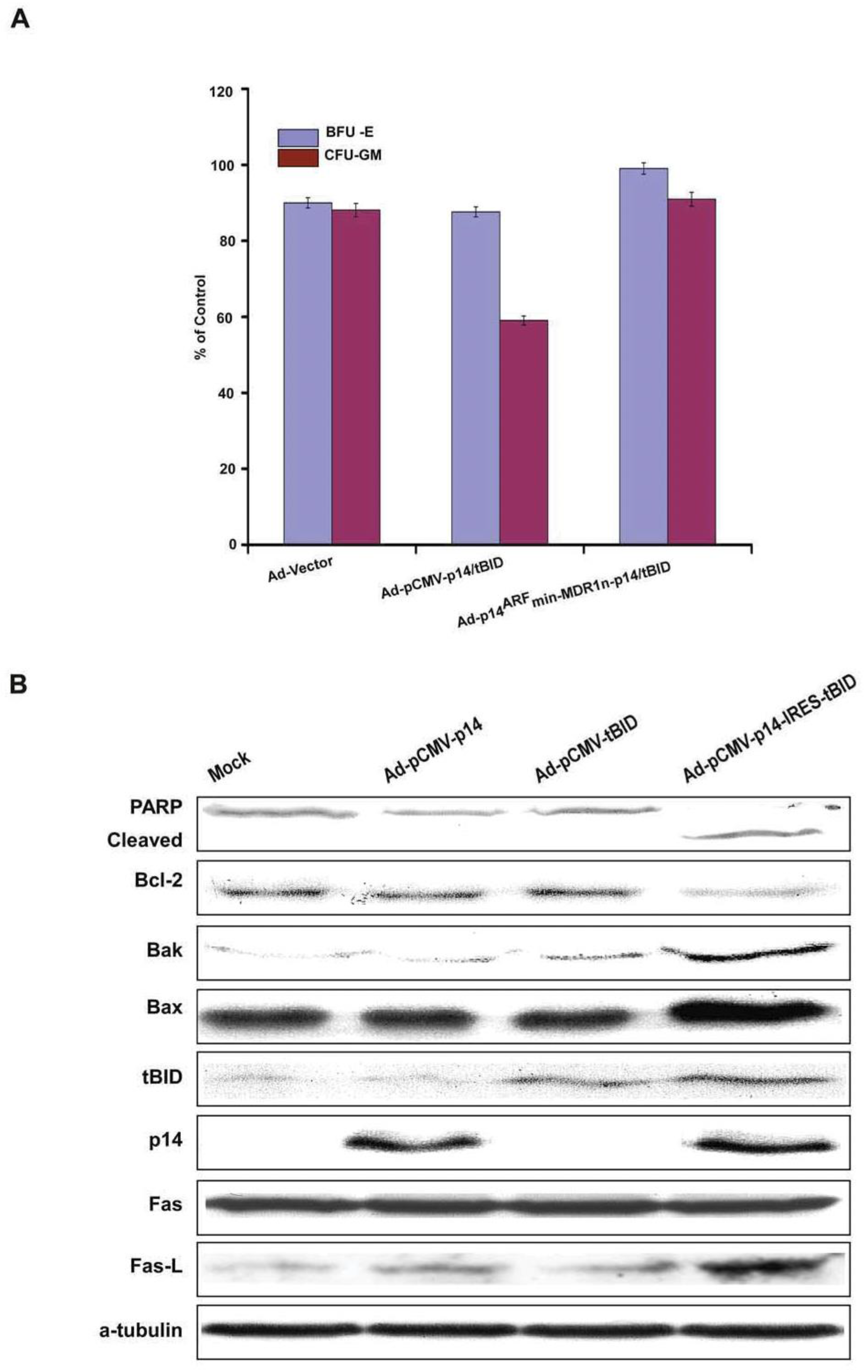
3.3. Bicistronic Gene Products Which Induce Synergistic Apoptosis
3.4. Modified p14ARF-MDR1n-p14ARF-tBID Expression Selectively Killed Cancer Cells with Both p53 and Ras Mutations
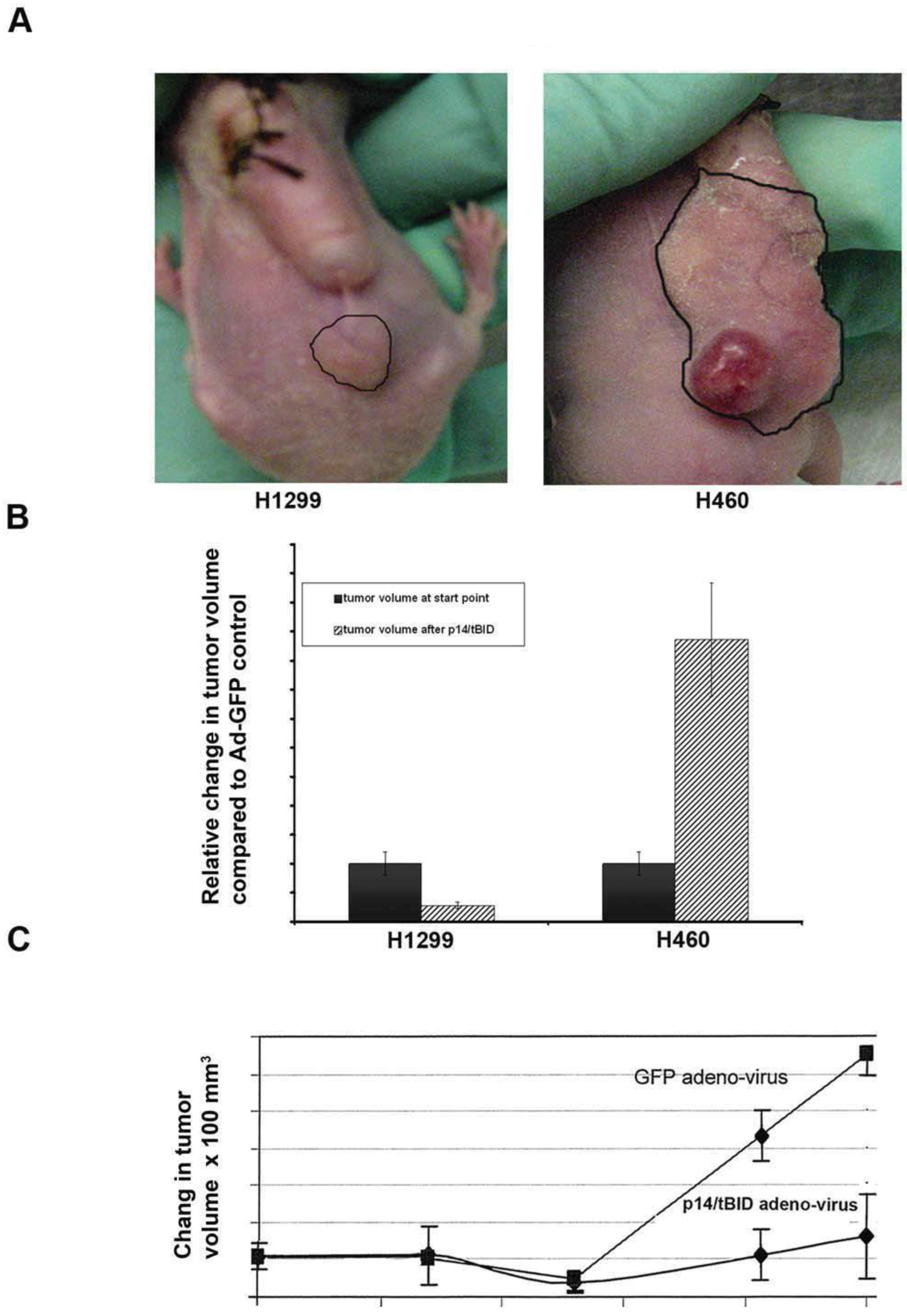
3.5. Animal Studies
3.5.1. Xenograft Models
3.5.2. Pancreatic Cancer in Genetically Engineered Mouse Model (GEMM): Gene Therapy of GEMM (LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx-1-Cre (KPC)) mice with Ad-p14ARFmin-MDR1n-p14-tBID
4. Discussion
5. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.; Yamato, T.; Furukawa, T.; Ohnishi, Y.; Kijima, H.; Horii, A. Characterization of the mutations of the K-ras, p53, p16, and SMAD4 genes in 15 human pancreatic cancer cell lines. Oncol. Rep. 2001, 8, 89–92. [Google Scholar] [CrossRef]
- Slebos, R.J.C.; Hoppin, J.A.; Tolbert, P.E.; Holly, E.A.; Brock, J.W.; Zhang, R.H.; Bracci, P.M.; Foley, J.; Stockton, P.; McGregor, L.M.; et al. K-ras and p53 in Pancreatic Cancer: Association with Medical History, Histopathology, and Environmental Exposures in a Population-based Study. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1223–1232. [Google Scholar]
- Schneider, G.; Schmid, R. Genetic alterations in pancreatic carcinoma. Mol. Cancer 2003, 2, 15. [Google Scholar] [CrossRef]
- Moore, P.S.; Sipos, B.; Orlandini, S.; Sorio, C.; Real, F.X.; Lemoine, N.R.; Gress, T.; Bassi, C.; Kloppel, G.; Kalthoff, H.; et al. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001, 439, 798–802. [Google Scholar] [CrossRef]
- Takeda, S.; Nakao, A.; Miyoshi, K.; Takagi, H. Gene therapy for pancreatic cancer. Semin. Surg. Oncol. 1998, 15, 57–61. [Google Scholar] [CrossRef]
- Midgley, C.A.; Desterro, J.M.; Saville, M.K.; Howard, S.; Sparks, A.; Hay, R.T.; Lane, D.P. An N-terminal p14ARF peptide blocks Mdm2-dependent ubiquitination in vitro and can activate p53 in vivo. Oncogene 2000, 19, 2312–2323. [Google Scholar] [CrossRef]
- Lowe, S.W.; Sherr, C.J. Tumor suppression by Ink4a-Arf: Progress and puzzles. Curr. Opin. Genet. Dev. 2003, 13, 77–83. [Google Scholar] [CrossRef]
- Groth, A.; Weber, J.D.; Willumsen, B.M.; Sherr, C.J.; Roussel, M.F. Oncogenic Ras Induces p19ARF and Growth Arrest in Mouse Embryo Fibroblasts Lacking p21Cip1 and p27Kip1 without Activating Cyclin D-dependent Kinases. J. Biol. Chem. 2000, 275, 27473–27480. [Google Scholar] [CrossRef]
- Bates, S.; Phillips, A.C.; Clark, P.A.; Stott, F.; Peters, G.; Ludwig, R.L.; Vousden, K.H. p14ARF links tumor suppressors RB and p53. Nature 1998, 395, 124–125. [Google Scholar] [CrossRef]
- Robertson, K.D.; Jones, P.A. The Human ARF Cell Cycle Regulatory Gene Promoter Is a CpG Island Which Can Be Silenced by DNA Methylation and Down-Regulated by Wild-Type p53. Mol. Cell. Biol. 1998, 18, 6457–6473. [Google Scholar] [CrossRef]
- Johnson, R.A.; Ince, T.A.; Scotto, K.W. Transcriptional Repression by p53 through Direct Binding to a Novel DNA Element. J. Biol. Chem. 2001, 276, 27716–27720. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Wang, L.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef]
- Olive, K.P.; Tuveson, D.A.; Ruhe, Z.C.; Yin, B.; Willis, N.A.; Bronson, R.T.; Crowley, D.; Jacks, T. Mutant p53 Gain of Function in Two Mouse Models of Li-Fraumeni Syndrome. Cell 2004, 119, 847–860. [Google Scholar] [CrossRef]
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I.; et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013, 62, 112–120. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Mao, Y.; Rosal, R.V.; Senatus, P.B.; Li, Y.; Li, Y.L.; Bruce, J.N.; Brandt-Rauf, P.W.; Fine, R.L. A C-Terminal p53 Palindromic Tetrapeptide Restores Full Apoptotic Potential to Mutant p53 Cancer Cells In Vitro and In Vivo. Proc. Am. Assoc. Cancer Res. 2005, 46, 1723. [Google Scholar]
- Palmero, I.; Pantoja, C.; Serrano, M. p19ARF links the tumour suppressor p53 to Ras. Nature 1998, 395, 125–126. [Google Scholar] [CrossRef]
- Schmitt, C.A.; McCurrach, M.E.; De Stanchina, E.; Wallace-Brodeur, R.R.; Lowe, S.W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999, 13, 2670–2677. [Google Scholar] [CrossRef]
- Dinnen, R.D.; Mao, Y.; Qiu, W.; Cassai, N.; Slavkovich, V.N.; Nichols, G.; Su, G.H.; Brandt-Rauf, P.; Fine, R.L. Redirecting apoptosis to aponecrosis induces selective cytotoxicity to pancreatic cancer cells through increased ROS, decline in ATP levels, and VDAC. Mol. Cancer Ther. 2013, 12, 2792–2803. [Google Scholar] [CrossRef]
- Liu, S.; Bishop, W.R.; Liu, M. Differential effects of cell cycle regulatory protein p21(WAF1/Cip1) on apoptosis and sensitivity to cancer chemotherapy. Drug Resist. Updates 2003, 6, 183–195. [Google Scholar] [CrossRef]
- Kim, M.P.; Li, X.; Deng, J.; Zhang, V.; Dai, B.; Allton, K.L.; Hughes, T.G.; Siangco, C.; Augustine, J.J.; Kang, Y.; et al. Oncogenic KRAS recruits an expensive network through mutant p53 to drive pancreatic cancer mteastasis. Cancer Discov. 2021, 11, 2094–2111. [Google Scholar] [CrossRef]
- Lee, C.S.; Song, I.H.; Lee, A.; Kang, J.; Lee, Y.S.; Lee, I.K.; Song, Y.S.; Lee, S.H. Enhancing the landscape of colorectal cancer using targeted deep sequencing. Sci. Rep. 2021, 11, 8154. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fine, R.L.; Mao, Y.; Garcia-Carracedo, D.; Su, G.H.; Qiu, W.; Hochfeld, U.; Nichols, G.; Li, Y.-L.; Dinnen, R.D.; Raffo, A.; et al. Gene Therapy with p14/tBID Induces Selective and Synergistic Apoptosis in Mutant Ras and Mutant p53 Cancer Cells In Vitro and In Vivo. Biomedicines 2023, 11, 258. https://doi.org/10.3390/biomedicines11020258
Fine RL, Mao Y, Garcia-Carracedo D, Su GH, Qiu W, Hochfeld U, Nichols G, Li Y-L, Dinnen RD, Raffo A, et al. Gene Therapy with p14/tBID Induces Selective and Synergistic Apoptosis in Mutant Ras and Mutant p53 Cancer Cells In Vitro and In Vivo. Biomedicines. 2023; 11(2):258. https://doi.org/10.3390/biomedicines11020258
Chicago/Turabian StyleFine, Robert L., Yuehua Mao, Dario Garcia-Carracedo, Gloria H. Su, Wanglong Qiu, Uri Hochfeld, Gwen Nichols, Yong-Liang Li, Richard D. Dinnen, Anthony Raffo, and et al. 2023. "Gene Therapy with p14/tBID Induces Selective and Synergistic Apoptosis in Mutant Ras and Mutant p53 Cancer Cells In Vitro and In Vivo" Biomedicines 11, no. 2: 258. https://doi.org/10.3390/biomedicines11020258
APA StyleFine, R. L., Mao, Y., Garcia-Carracedo, D., Su, G. H., Qiu, W., Hochfeld, U., Nichols, G., Li, Y.-L., Dinnen, R. D., Raffo, A., & Brandt-Rauf, P. W. (2023). Gene Therapy with p14/tBID Induces Selective and Synergistic Apoptosis in Mutant Ras and Mutant p53 Cancer Cells In Vitro and In Vivo. Biomedicines, 11(2), 258. https://doi.org/10.3390/biomedicines11020258




