Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine
Abstract
1. Introduction
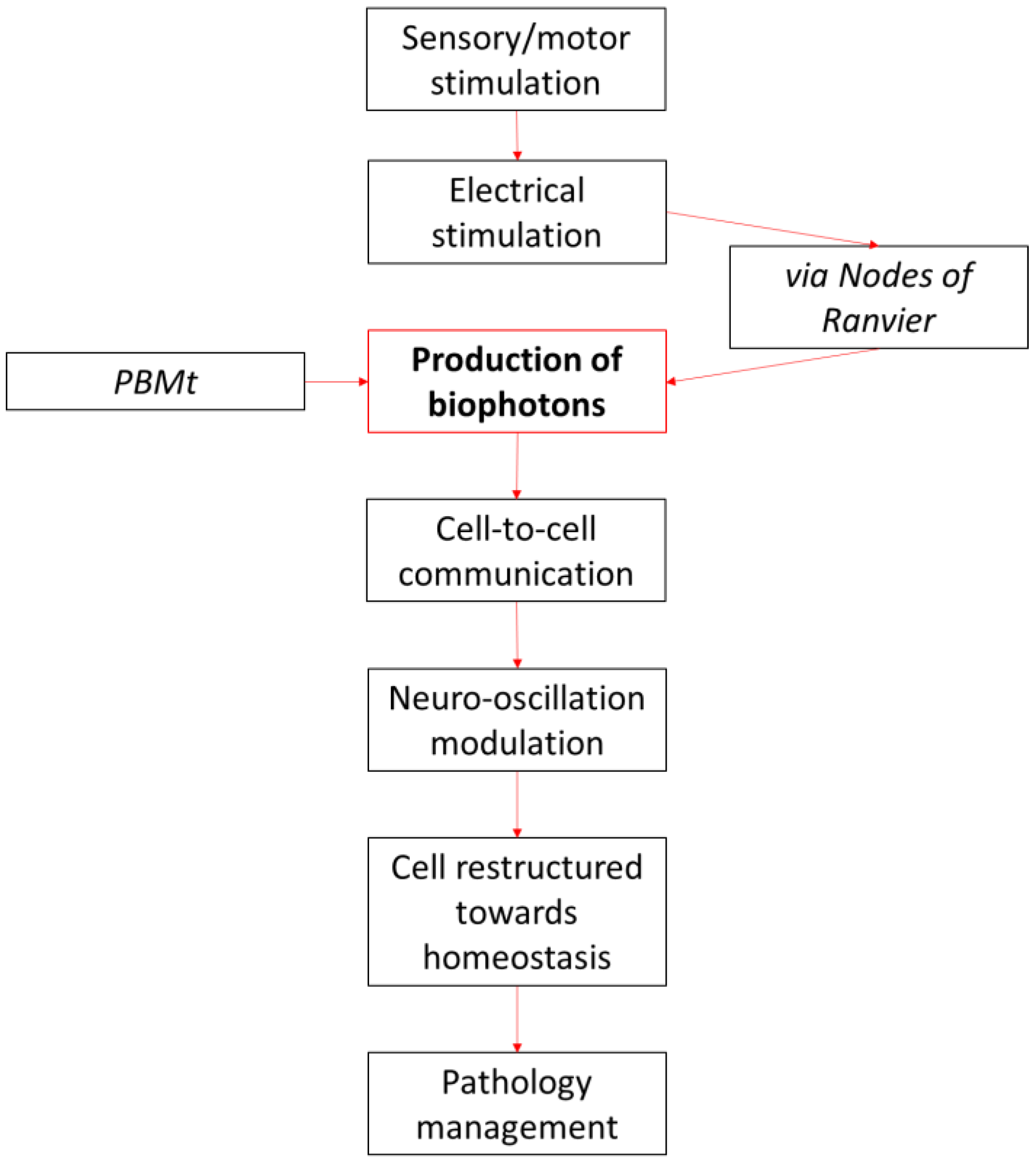
2. Biophotons and PBM
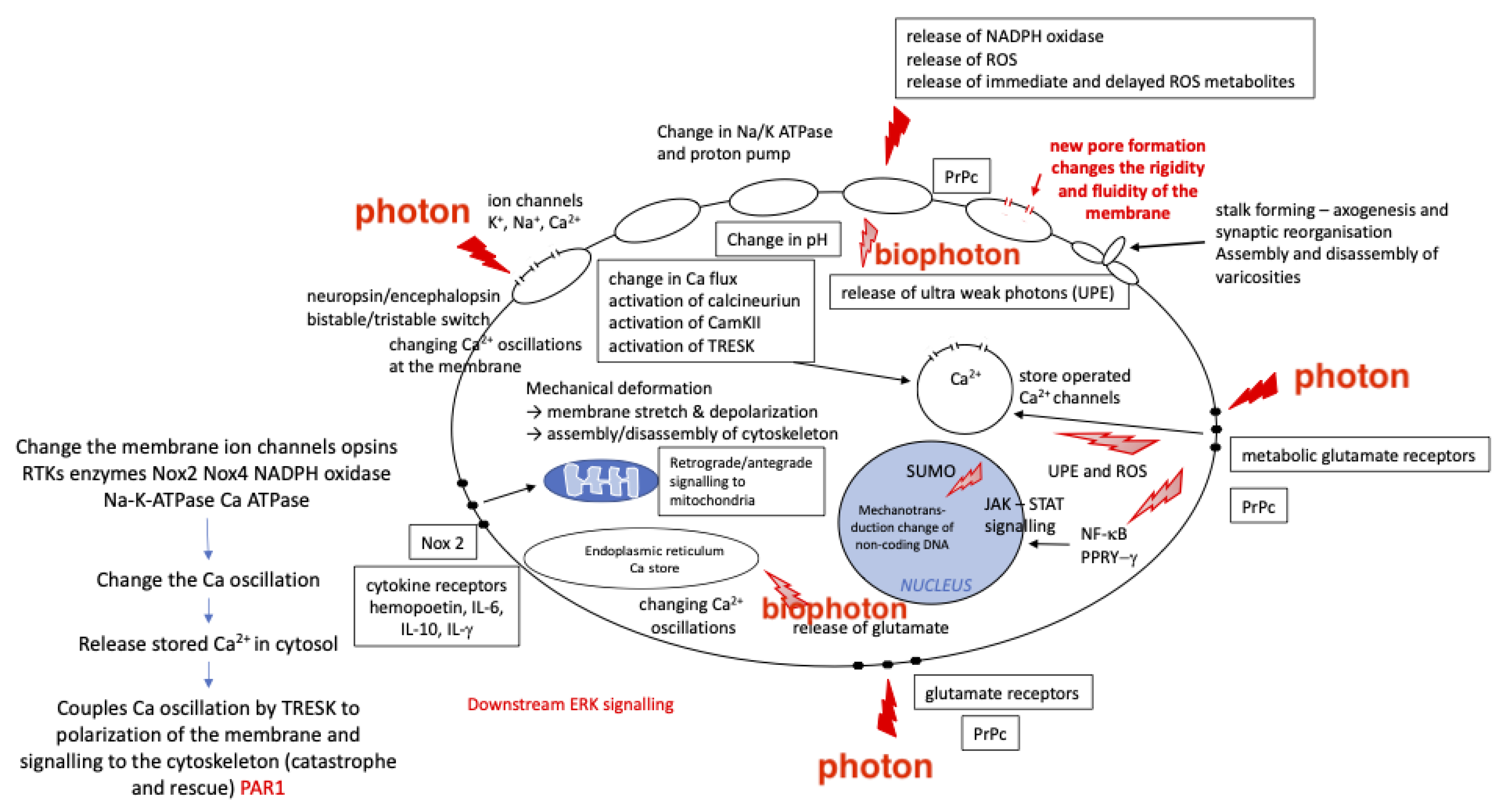
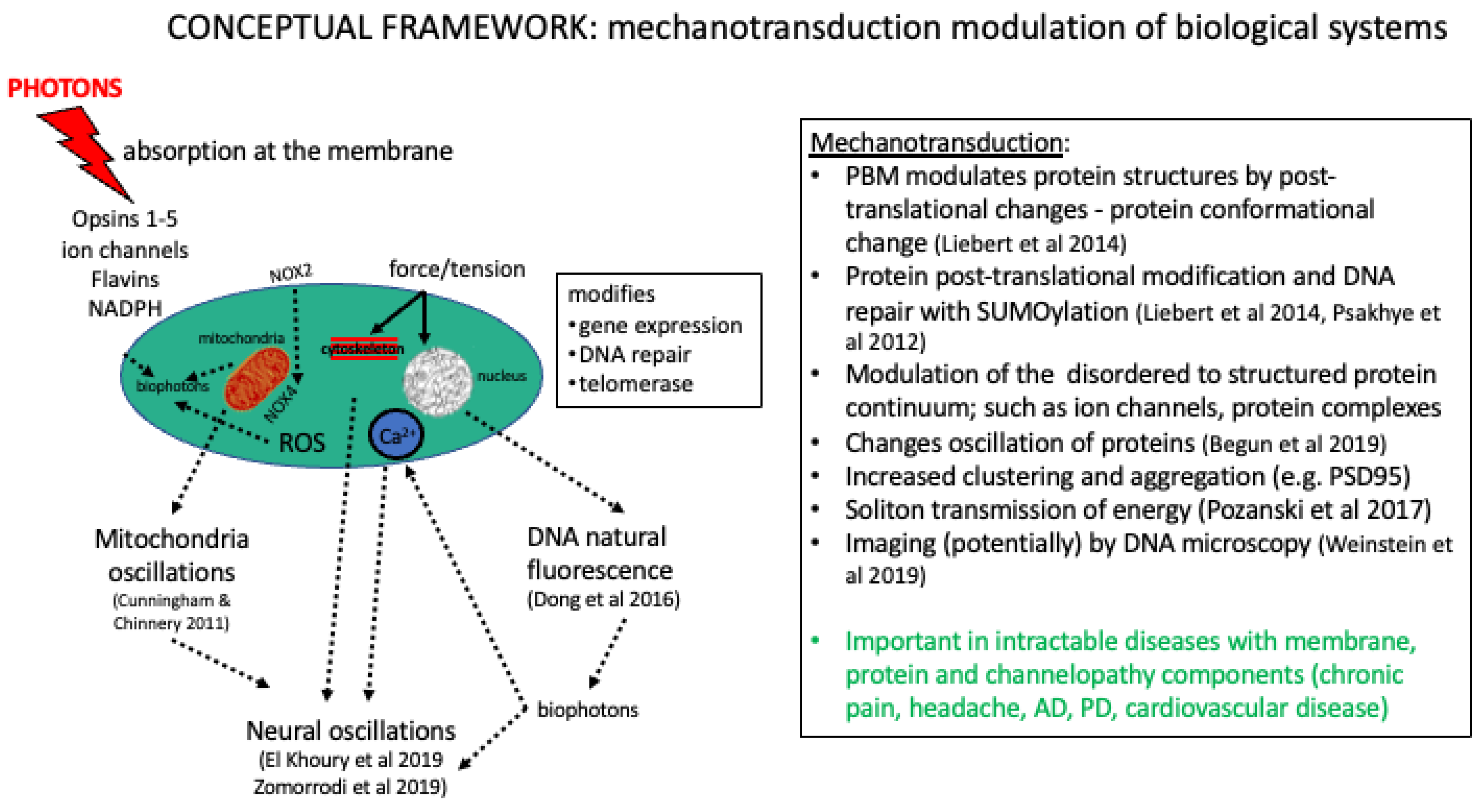
3. PBMt and Mechanotransduction
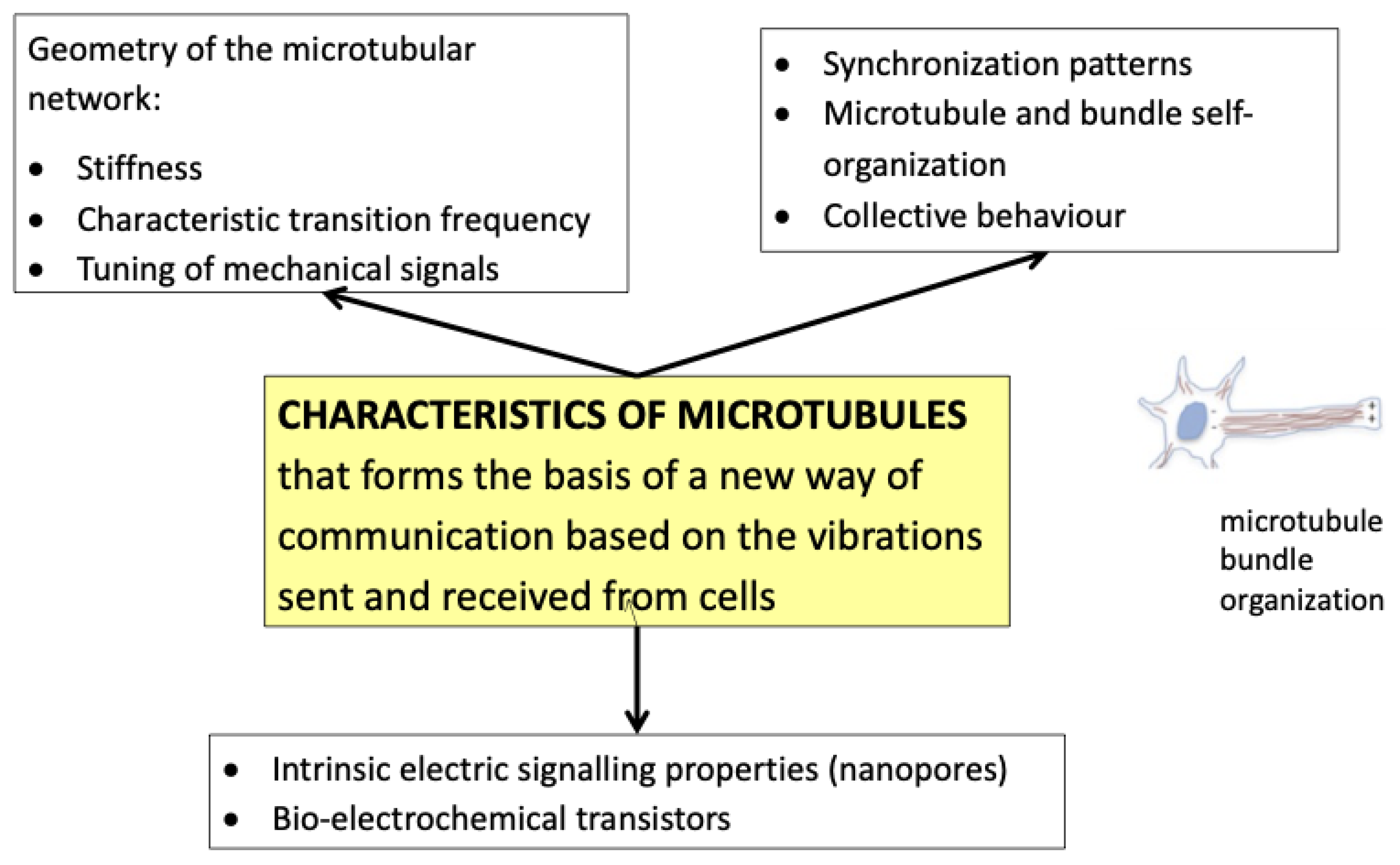
3.1. PBMt Modulation of the Cytoskeleton
3.2. PBMt Modulation of Ion Channels
3.3. PBMt Modulation of the Microbiome
3.4. PBMt and Glymphatic Clearance
4. PBMt and Photophysical Mechanisms
4.1. Cell-to-Cell Oscillation
4.2. Wavelength Specificity and Protein Interactions—Photophysical Resonance
4.3. Fluorescent/Auto-Fluorescent Proteins
5. Current Clinical Applications
5.1. Resonance
5.2. Neutrophils
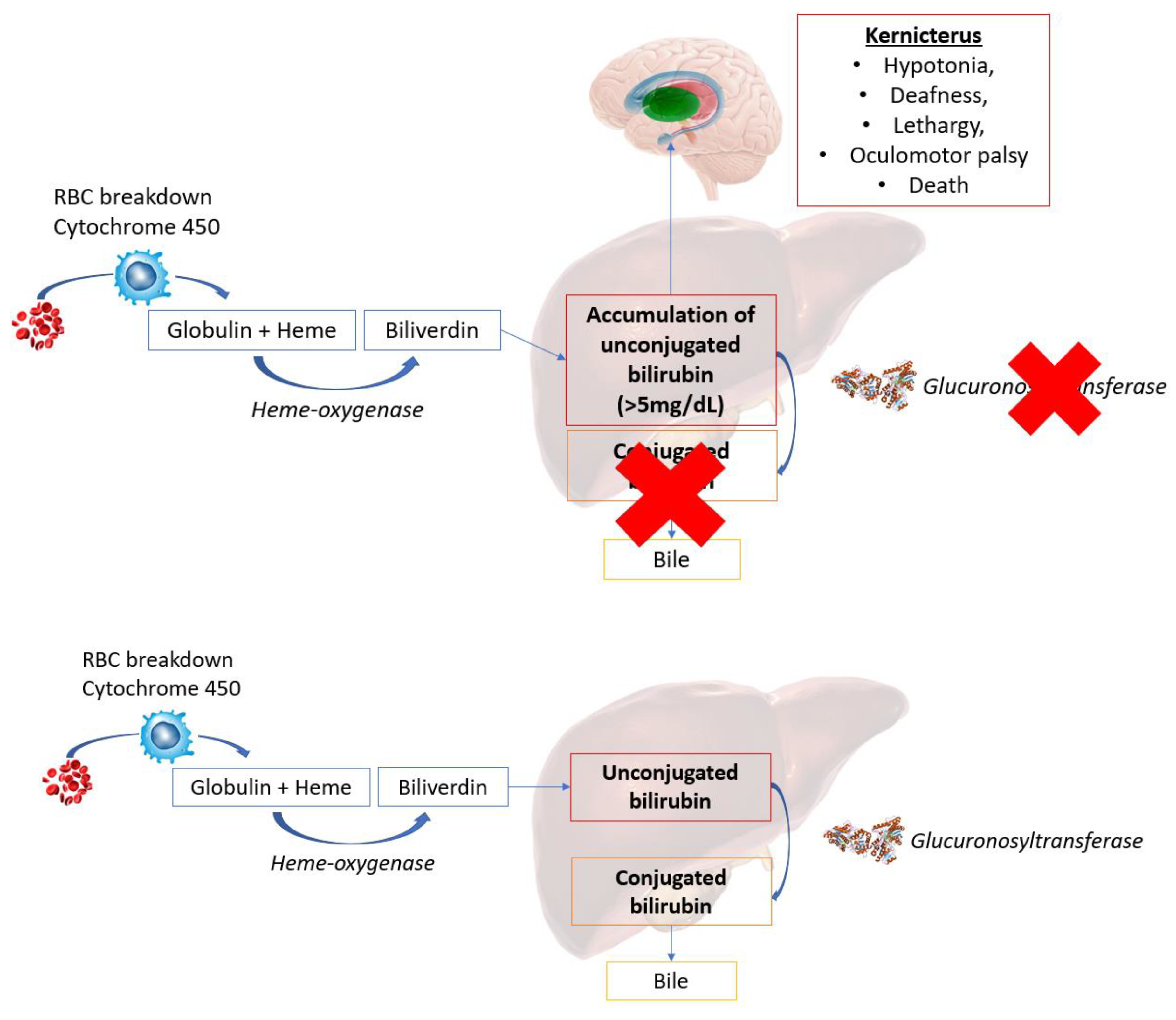
5.3. Channelopathies
5.4. Analgesia and Anaesthetic Effects
5.5. Wounds and Aging
6. PBMt and Neuro Oscillatory Networks: Clinical Implications
7. Future Implications of Photophysical PBMt Mechanisms Applied to Clinical Therapy
8. PBMt and Precision Medicine
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AQP | Aquaporin |
| ATP | Adenosine triphosphate |
| PBMt | Photobiomodulation therapy |
| CASP-9 | Caspase-9 |
| CSF | Cerebrospinal fluid |
| DRG | Dorsal root gangion |
| EM | Elctromagnetic |
| GABA | gamma aminobutyric acid |
| H2O2 | Hydrogen peroxide |
| JAK-STAT | Janus kinases-signal transducer and activator of transcription proteins |
| KHz | Kilohertz |
| L-DOPA | Levodopa |
| LED | Light-emitting diode |
| LFOs | Low-frequency oscillations |
| MAP2 | Microtubule-associated protein 2 |
| MMP | Mitochondrial membrane potential |
| NIR | Near-infrared |
| NO | nitric oxide |
| PD | Parkinson’s disease |
| REM | Rapid eye-movement |
| ROS | Reactive oxygen species |
| RRM | Resonant recognition model |
| SPD | Spectral power density |
| THz | Terahertz |
| TRESK | TWIK-related spinal cord potassium channel |
| TRP | Transient receptor potential |
| TWIK | the weakly inward rectifying K channel |
| UDP | 5′-diphosphglucuronosyltransferase 1-A1 |
| UPE | Ultra-weak photon emissions |
| VE | vascular endothelial |
References
- Hamblin, M.R. Photobiomodulation or low-level laser therapy. J. Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Pigatto, G.R.; Silva, C.S.; Parizotto, N.A. Photobiomodulation therapy reduces acute pain and inflammation in mice. J. Photochem. Photobiol. B 2019, 196, 111513. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Kiat, H. The history of light therapy in hospital physiotherapy and medicine with emphasis on Australia: Evolution into novel areas of practice. Physiother. Theory Pract. 2021, 37, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 2010, 62, 607–610. [Google Scholar] [CrossRef]
- Lima, P.L.V.; Pereira, C.V.; Nissanka, N.; Arguello, T.; Gavini, G.; Maranduba, C.; Diaz, F.; Moraes, C.T. Photobiomodulation enhancement of cell proliferation at 660 nm does not require cytochrome c oxidase. J. Photochem. Photobiol. B 2019, 194, 71–75. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Afanasyeva, N.I. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 2005, 36, 307–314. [Google Scholar] [CrossRef]
- Yang, W.-Z.; Chen, J.-Y.; Yu, J.-T.; Zhou, L.-W. Effects of Low Power Laser Irradiation on Intracellular Calcium and Histamine Release in RBL-2H3 Mast Cells. Photochem. Photobiol. 2007, 83, 979–984. [Google Scholar] [CrossRef]
- Benedicenti, S.; Pepe, I.M.; Angiero, F.; Benedicenti, A. Intracellular ATP Level Increases in Lymphocytes Irradiated with Infrared Laser Light of Wavelength 904 nm. Photomed. Laser Surg. 2008, 26, 451–453. [Google Scholar] [CrossRef]
- Chen, A.C.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef]
- Lavi, R.; Ankri, R.; Sinyakov, M.; Eichler, M.; Friedmann, H.; Shainberg, A.; Breitbart, H.; Lubart, R. The Plasma Membrane is Involved in the Visible Light–Tissue Interaction. Photomed. Laser Surg. 2012, 30, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lavi, R.; Shainberg, A.; Shneyvays, V.; Hochauser, E.; Isaac, A.; Zinman, T.; Friedmann, H.; Lubart, R. Detailed analysis of reactive oxygen species induced by visible light in various cell types. Lasers Surg. Med. 2010, 42, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Tafur, J.; Mills, P.J. Low-intensity light therapy: Exploring the role of redox mechanisms. Photomed. Laser Surg. 2008, 26, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef]
- Pope, N.J.; Denton, M.L. Differential effects of 808-nm light on electron transport chain enzymes in isolated mitochondria: Implications for photobiomodulation initiation. Mitochondrion 2022, 68, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological effects of low level laser therapy. J. Lasers Med. Sci. 2014, 5, 58–62. [Google Scholar]
- Tessitore, A.; Russo, A.; Giordano, A.; Conte, F.; Corbo, D.; De Stefano, M.; Cirillo, S.; Cirillo, M.; Esposito, F.; Tedeschi, G. Disrupted default mode network connectivity in migraine without aura. J. Headache Pain 2013, 14, 89. [Google Scholar] [CrossRef]
- Lim, M.; Kim, J.S.; Kim, D.J.; Chung, C.K. Increased Low- and High-Frequency Oscillatory Activity in the Prefrontal Cortex of Fibromyalgia Patients. Front. Hum. Neurosci. 2016, 10, 111. [Google Scholar] [CrossRef]
- Halje, P.; Brys, I.; Mariman, J.J.; da Cunha, C.; Fuentes, R.; Petersson, P. Oscillations in cortico-basal ganglia circuits: Implications for Parkinson’s disease and other neurologic and psychiatric conditions. J. Neurophysiol. 2019, 122, 203–231. [Google Scholar] [CrossRef]
- Rombouts, S.A.R.B.; Barkhof, F.; Goekoop, R.; Stam, C.J.; Scheltens, P. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: An fMRI study. Hum. Brain Mapp. 2005, 26, 231–239. [Google Scholar] [CrossRef]
- Liebert, A.; Krause, A.; Goonetilleke, N.; Bicknell, B.; Kiat, H. A Role for Photobiomodulation in the Prevention of Myocardial Ischemic Reperfusion Injury: A Systematic Review and Potential Molecular Mechanisms. Sci. Rep. 2017, 7, 42386. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of light parameters and photobiomodulation efficacy: Dive into complexity. J. Biomed. Opt. 2018, 23, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gurwitsch, V.; Gurwitsch, L. Mitogenetic Emission; Gos. Med. Izdat. Gosudarstvo Medical Izdatelstvo: Moscow, Russia, 1932. [Google Scholar]
- Popp, F.A.; Nagl, W.; Li, K.H.; Scholz, W.; Weingärtner, O.; Wolf, R. Biophoton emission. New evidence for coherence and DNA as source. Cell Biophys. 1984, 6, 33–52. [Google Scholar] [CrossRef]
- Kert, J.; Rose, L. Low Level Laser Therapy; p-LaserSystem Internationa: Veksoe, Denmark, 1989; p. 240. [Google Scholar]
- Albrecht-Buehler, G. Rudimentary form of cellular “vision”. Proc. Natl. Acad. Sci. USA 1992, 89, 8288–8292. [Google Scholar] [CrossRef] [PubMed]
- Laakso, L.; Richardson, C.; Cramond, T. Quality of light—Is laser necessary for effective photobiostimulation? Aust. J. Physiother. 1993, 39, 87–92. [Google Scholar] [CrossRef]
- Amano, T.; Kobayashi, M.; Devaraj, B.; Inaba, H. Ultraweak biophoton emission imaging of transplanted bladder cancer. Urol. Res. 1995, 23, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Cosic, I. The Resonant Recognition Model of Bio-molecular Interactions: Possibility of electromagnetic resonance. Pol. J. Med. Phys. Eng. 2001, 7, 73–87. [Google Scholar]
- Voeikov, V.L.; Asfaramov, R.; Bouravleva, E.V.; Novikov, C.N.; Vilenskaya, N.D. Biophoton research in blood reveals its holistic properties. Indian J. Exp. Biol. 2003, 41, 473–482. [Google Scholar]
- Amat, A.; Rigau, J.; Waynant, R.W.; Ilev, I.K.; Anders, J.J. The electric field induced by light can explain cellular responses to electromagnetic energy: A hypothesis of mechanism. J. Photochem. Photobiol. B Biol. 2006, 82, 152–160. [Google Scholar] [CrossRef]
- Chow, R.; David, M.; Armati, P. 830 nm laser irradiation induces varicosity formation, reduces mitochondrial membrane potential and blocks fast axonal flow in small and medium diameter rat dorsal root dorsal root ganglion: Implications for the analgesic effects of 830nm laser. J. Peripher. Nerv. Syst. 2007, 12, 28–39. [Google Scholar] [CrossRef]
- Mathew, M.; Amat-Roldan, I.; Andrés, R.; Santos, S.I.; Artigas, D.; Soriano, E.; Loza-Alvarez, P. Signalling effect of NIR pulsed lasers on axonal growth. J. Neurosci. Methods 2010, 186, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Dai, J. Biophotons as neural communication signals demonstrated by in situ biophoton autography. Photochem. Photobiol. Sci. 2010, 9, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Bókkon, I.; Salari, V.; Tuszynski, J.; Antal, I. Estimation of the number of biophotons involved in the visual perception of a single-object image: Biophoton intensity can be considerably higher inside cells than outside. J. Photochem. Photobiol. B Biol. 2010, 100, 160–166. [Google Scholar] [CrossRef]
- Minke, B. The history of the Drosophila TRP channel: The birth of a new channel superfamily. J. Neurogenet. 2010, 24, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Hanczyc, P.; Samoc, M.; Norden, B. Multiphoton absorption in amyloid protein fibres. Nat. Photon. 2013, 7, 969–972. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Adams, R. Protein conformational modulation by photons: A mechanism for laser treatment effects. Med. Hypotheses 2014, 82, 275–281. [Google Scholar] [CrossRef]
- Niggli, H. Biophotons: Ultraweak light impulses regulate life processes in aging. J. Gerontol. Geriat. Res. 2014, 3, 143. [Google Scholar] [CrossRef]
- Tang, R.; Dai, J. Spatiotemporal imaging of glutamate-induced biophotonic activities and transmission in neural circuits. PLoS ONE 2014, 9, e85643. [Google Scholar] [CrossRef]
- Budagovsky, A.V.; Solovykh, N.V.; Budagovskaya, O.N.; Budagovsky, I.A. Cell response to quasi-monochromatic light with different coherence. Quantum Electron. 2015, 45, 351. [Google Scholar] [CrossRef]
- Shi, L.; Galvez, E.J.; Alfano, R.R. Photon entanglement through brain tissue. Sci. Rep. 2016, 6, 37714. [Google Scholar] [CrossRef]
- Cosic, I.; Cosic, D. The treatment of crigler-najjar syndrome by blue light as explained by resonant recognition model. EPJ Nonlinear Biomed. Phys. 2016, 4, 9. [Google Scholar] [CrossRef]
- Poznanski, R.; Cacha, L.; Al-Wesabi, Y.; Ali, J.; Bahadoran, M.; Yupapin, P.; Yunus, J. Solitonic conduction of electrotonic signals in neuronal branchlets with polarized microstructure. Sci. Rep. 2017, 7, 2746. [Google Scholar] [CrossRef] [PubMed]
- Cantero, M.d.R.; Villa Etchegoyen, C.; Perez, P.L.; Scarinci, N.; Cantiello, H.F. Bundles of brain microtubules generate electrical oscillations. Sci. Rep. 2018, 8, 11899. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.S.; Winlow, W. The Soliton and the Action Potential–Primary Elements Underlying Sentience. Front. Physiol. 2018, 9, 779. [Google Scholar] [CrossRef]
- Fekrazad, R. Photons Harmony for Cell Communication. Photomed. Laser Surg. 2018, 36, 177–178. [Google Scholar] [CrossRef]
- Santana-Blank, L.; Rodríguez-Santana, E. Photobiomodulation in Light of Our Biological Clock’s Inner Workings; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2018; Volume 36, pp. 119–121. [Google Scholar]
- Facchin, F.; Canaider, S.; Tassinari, R.; Zannini, C.; Bianconi, E.; Taglioli, V.; Olivi, E.; Cavallini, C.; Tausel, M.; Ventura, C. Physical energies to the rescue of damaged tissues. World J. Stem Cells 2019, 11, 297–321. [Google Scholar] [CrossRef]
- Zomorrodi, R.; Loheswaran, G.; Pushparaj, A.; Lim, L. Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: A pilot exploratory study. Sci. Rep. 2019, 9, 6309. [Google Scholar] [CrossRef]
- Wang, X.; Dmochowski, J.P.; Zeng, L.; Kallioniemi, E.; Husain, M.; Gonzalez-Lima, F.; Liu, H. Transcranial photobiomodulation with 1064-nm laser modulates brain electroencephalogram rhythms. Neurophotonics 2019, 6, 025013. [Google Scholar] [CrossRef]
- Pope, N.J.; Powell, S.M.; Wigle, J.C.; Denton, M.L. Wavelength-and irradiance-dependent changes in intracellular nitric oxide level. J. Biomed. Opt. 2020, 25, 085001. [Google Scholar] [CrossRef]
- Esmaeilpour, T.; Fereydouni, E.; Dehghani, F.; Bókkon, I.; Panjehshahin, M.R.; Császár-Nagy, N.; Ranjbar, M.; Salari, V. An Experimental Investigation of Ultraweak Photon Emission from Adult Murine Neural Stem Cells. Sci. Rep. 2020, 10, 463. [Google Scholar] [CrossRef]
- Sordillo, P.P.; Sordillo, L.A. The mystery of chemotherapy brain: Kynurenines, tubulin and biophoton release. Anticancer Res. 2020, 40, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Habibalahi, A.; Moghari, M.D.; Campbell, J.M.; Anwer, A.G.; Mahbub, S.B.; Gosnell, M.; Saad, S.; Pollock, C.; Goldys, E.M. Non-invasive real-time imaging of reactive oxygen species (ROS) using auto-fluorescence multispectral imaging technique: A novel tool for redox biology. Redox Biol. 2020, 34, 101561. [Google Scholar] [CrossRef] [PubMed]
- Zangari, A.; Micheli, D.; Galeazzi, R.; Tozzi, A.; Balzano, V.; Bellavia, G.; Caristo, M.E. Photons detected in the active nerve by photographic technique. Sci. Rep. 2021, 11, 3022. [Google Scholar] [CrossRef] [PubMed]
- Staelens, M.; Di Gregorio, E.; Kalra, A.P.; Le, H.T.; Hosseinkhah, N.; Karimpoor, M.; Lim, L.; Tuszyński, J.A. Near-Infrared Photobiomodulation of Living Cells, Tubulin, and Microtubules. Front. Med. Technol. 2022, 4, 871196. [Google Scholar] [CrossRef] [PubMed]
- Korneev, A.; Begun, A.; Liubimov, S.; Kachlishvili, K.; Molochkov, A.; Niemi, A.J.; Maisuradze, G.G. Exploring Structural Flexibility and Stability of α-Synuclein by the Landau–Ginzburg–Wilson Approach. J. Phys. Chem. B 2022, 126, 6878–6890. [Google Scholar] [CrossRef]
- Moro, C.; Liebert, A.; Hamilton, C.; Pasqual, N.; Jeffery, G.; Stone, J.; Mitrofanis, J. The code of light: Do neurons generate light to communicate and repair? Neural Regen. Res. 2022, 17, 1251. [Google Scholar]
- Moro, C.; Valverde, A.; Dole, M.; Hoh Kam, J.; Hamilton, C.; Liebert, A.; Bicknell, B.; Benabid, A.-L.; Magistretti, P.; Mitrofanis, J. The effect of photobiomodulation on the brain during wakefulness and sleep. Front. Neurosci. 2022, 16, 942536. [Google Scholar] [CrossRef] [PubMed]
- Wijk, R.V.; Wijk, E.P. An introduction to human biophoton emission. Komplement. Kl. Nat. 2005, 12, 77–83. [Google Scholar] [CrossRef]
- Suzuki, K.; Nagai, T. Recent progress in expanding the chemiluminescent toolbox for bioimaging. Curr. Opin. Biotechnol. 2017, 48, 135–141. [Google Scholar] [CrossRef]
- Ortega-Ojeda, F.; Calcerrada, M.; Ferrero, A.; Campos, J.; Garcia-Ruiz, C. Measuring the Human Ultra-Weak Photon Emission Distribution Using an Electron-Multiplying, Charge-Coupled Device as a Sensor. Sensors 2018, 18, 1152. [Google Scholar] [CrossRef]
- Kobayashi, K.; Okabe, H.; Kawano, S.; Hidaka, Y.; Hara, K. Biophoton emission induced by heat shock. PLoS ONE 2014, 9, e105700. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Cui, Y.; Yamagata, A.; Niigaki, M.; Hirohata, T.; Oishi, N.; Watanabe, Y. Activity-dependent neural tissue oxidation emits intrinsic ultraweak photons. Biochem. Biophys. Res. Commun. 2001, 285, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.C.R.; Schoeman, J.C.; Winden, L.J.V.; Červinková, K.; Ramautar, R.; Van Wijk, E.P.A.; Cifra, M.; Berger, R.; Hankemeier, T.; Greef, J.V. Ultra-weak photon emission as a dynamic tool for monitoring oxidative stress metabolism. Sci. Rep. 2017, 7, 1229. [Google Scholar] [CrossRef] [PubMed]
- Tafur, J.; Van Wijk, E.P.; Van Wijk, R.; Mills, P.J. Biophoton detection and low-intensity light therapy: A potential clinical partnership. Photomed. Laser Surg. 2010, 28, 23–30. [Google Scholar] [CrossRef]
- Cerdeira, C.D.; Lima Brigagão, M.R.P.; Carli, M.L.; de Souza Ferreira, C.; de Oliveira Isac Moraes, G.; Hadad, H.; Costa Hanemann, J.A.; Hamblin, M.R.; Sperandio, F.F. Low-level laser therapy stimulates the oxidative burst in human neutrophils and increases their fungicidal capacity. J. Biophotonics 2016, 9, 1180–1188. [Google Scholar] [CrossRef]
- Biasibetti, M.; Rojas, D.B.; Hentschke, V.S.; Moura, D.J.; Karsten, M.; Wannmacher, C.M.; Saffi, J.; Dal Lago, P. The influence of low-level laser therapy on parameters of oxidative stress and DNA damage on muscle and plasma in rats with heart failure. Lasers Med. Sci. 2014, 29, 1895–1906. [Google Scholar] [CrossRef]
- Kumar, S.; Boone, K.; Tuszyński, J.; Barclay, P.; Simon, C. Possible existence of optical communication channels in the brain. Sci. Rep. 2016, 6, 36508. [Google Scholar] [CrossRef]
- Margineanu, D.G.; Schoffeniels, E. Molecular events and energy changes during the action potential. Proc. Natl. Acad. Sci. USA 1977, 74, 3810–3813. [Google Scholar] [CrossRef]
- Fraser, A.; Frey, A.H. Electromagnetic emission at micron wavelengths from active nerves. Biophys. J. 1968, 8, 731–734. [Google Scholar] [CrossRef]
- Tang, R.; Dai, J. Biophoton signal transmission and processing in the brain. J. Photochem. Photobiol. B 2014, 139, 71–75. [Google Scholar] [CrossRef]
- Cifra, M.; Pospíšil, P. Ultra-weak photon emission from biological samples: Definition, mechanisms, properties, detection and applications. J. Photochem. Photobiol. B 2014, 139, 2–10. [Google Scholar] [CrossRef]
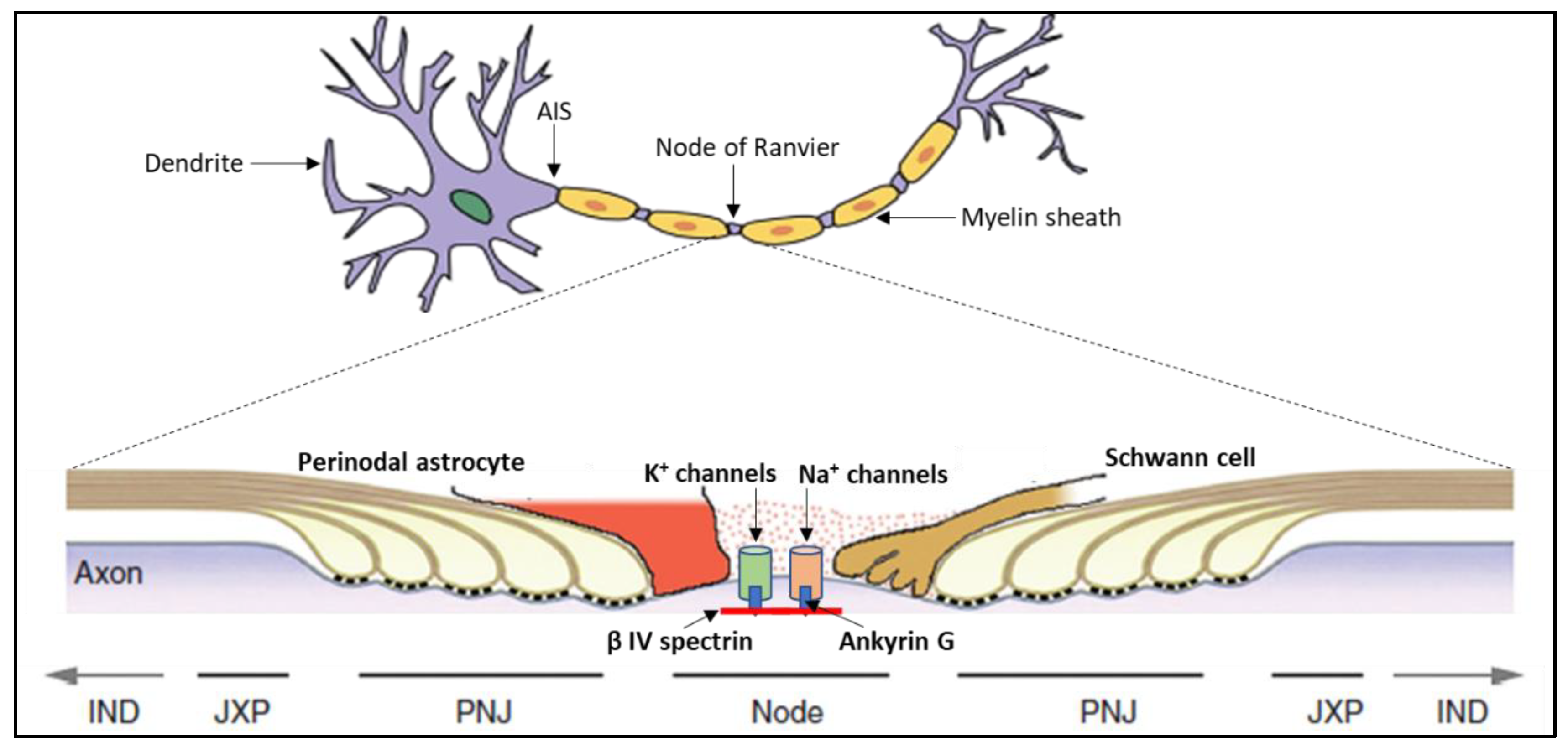
- Zangari, A.; Micheli, D.; Galeazzi, R.; Tozzi, A. Node of Ranvier as an Array of Bio-Nanoantennas for Infrared Communication in Nerve Tissue. Sci. Rep. 2018, 8, 539. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.G.; Homma, K.; Villarreal, S.; Richter, C.P.; Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 2012, 3, 736. [Google Scholar] [CrossRef] [PubMed]
- Cifra, M.; Fields, J.Z.; Farhadi, A. Electromagnetic cellular interactions. Prog. Biophys. Mol. Biol. 2011, 105, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P.; Prasad, A.; Rác, M. Role of reactive oxygen species in ultra-weak photon emission in biological systems. J. Photochem. Photobiol. B 2014, 139, 11–23. [Google Scholar] [CrossRef]
- Zarkeshian, P.; Kumar, S.; Tuszynski, J.; Barclay, P.; Simon, C. Are there optical communication channels in the brain? Front. Biosci. 2018, 23, 1407–1421. [Google Scholar] [CrossRef]
- Tessaro, L.W.E.; Dotta, B.T.; Persinger, M.A. Bacterial biophotons as non-local information carriers: Species-specific spectral characteristics of a stress response. MicrobiologyOpen 2019, 8, e00761. [Google Scholar] [CrossRef]
- Black, J.A.; Waxman, S.G. The perinodal astrocyte. Glia 1988, 1, 169–183. [Google Scholar] [CrossRef]
- Butt, A.M.; Duncan, A.; Berry, M. Astrocyte associations with nodes of Ranvier: Ultrastructural analysis of HRP-filled astrocytes in the mouse optic nerve. J. Neurocytol. 1994, 23, 486–499. [Google Scholar] [CrossRef]
- Butt, A.M.; Colquhoun, K.; Tutton, M.; Berry, M. Three-dimensional morphology of astrocytes and oligodendrocytes in the intact mouse optic nerve. J. Neurocytol. 1994, 23, 469–485. [Google Scholar] [CrossRef]
- FITZHUGH, R. Computation of impulse initiation and saltatory conduction in a myelinated nerve fiber. Biophys. J. 1962, 2, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, C.; Xu, Y.; Ma, J. Model of electrical activity in cardiac tissue under electromagnetic induction. Sci. Rep. 2016, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.D.; Chow, R.T.; Bicknell, B.T.; Varigos, E. Neuroprotective Effects Against POCD by Photobiomodulation: Evidence from Assembly/Disassembly of the Cytoskeleton. J. Exp. Neurosci. 2016, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Holanda, V.M.; Chavantes, M.C.; Wu, X.; Anders, J.J. The mechanistic basis for photobiomodulation therapy of neuropathic pain by near infrared laser light. Lasers Surg. Med. 2017, 49, 516–524. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, M.; Tong, L.; Morse, T.M.; McDougal, R.A.; Ding, H.; Chan, D.; Cai, Y.; Grutzendler, J. PLD3 affects axonal spheroids and network defects in Alzheimer’s disease. Nature 2022, 612, 328–337. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B. The role of channelopathies in pain and the implications for laser treatment. In Proceedings of the 9th World Association for Laser Therapy Congress, WALT Gold Coast, Australia, 27–30 September 2012. [Google Scholar]
- Mortimer, P.M.; Mc Intyre, S.A.; Thomas, D.C. Beyond the Extra Respiration of Phagocytosis: NADPH Oxidase 2 in Adaptive Immunity and Inflammation. Front. Immunol. 2021, 12, 733918. [Google Scholar] [CrossRef]
- Rizzo, N.R.; Hank, N.C.; Zhang, J. Detecting presence of cardiovascular disease through mitochondria respiration as depicted through biophotonic emission. Redox Biol. 2016, 8, 11–17. [Google Scholar] [CrossRef]
- Andreu, N.; Zelmer, A.; Wiles, S. Noninvasive biophotonic imaging for studies of infectious disease. FEMS Microbiol. Rev. 2011, 35, 360–394. [Google Scholar] [CrossRef]
- El Khoury, H.; Mitrofanis, J.; Henderson, L.A. Exploring the Effects of Near Infrared Light on Resting and Evoked Brain Activity in Humans Using Magnetic Resonance Imaging. Neuroscience 2019, 422, 161–171. [Google Scholar] [CrossRef]
- Khoury, H.E.; Mitrofanis, J.; Henderson, L.A. Does photobiomodulation influence the resting-state brain networks in young human subjects? Exp. Brain Res. 2021, 239, 435–449. [Google Scholar] [CrossRef]
- Buendía, D.; Guncay, T.; Oyanedel, M.; Lemus, M.; Weinstein, A.; Ardiles, Á.O.; Marcos, J.; Fernandes, A.; Zângaro, R.; Muñoz, P. The Transcranial Light Therapy Improves Synaptic Plasticity in the Alzheimer’s Disease Mouse Model. Brain Sci. 2022, 12, 1272. [Google Scholar] [CrossRef] [PubMed]
- Zuccolo, E.; Kheder, D.A.; Lim, D.; Perna, A.; Nezza, F.D.; Botta, L.; Scarpellino, G.; Negri, S.; Martinotti, S.; Soda, T.; et al. Glutamate triggers intracellular Ca(2+) oscillations and nitric oxide release by inducing NAADP- and InsP(3) -dependent Ca(2+) release in mouse brain endothelial cells. J. Cell. Physiol. 2019, 234, 3538–3554. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Bicknell, B.; Laakso, E.L.; Heller, G.; Jalilitabaei, P.; Tilley, S.; Mitrofanis, J.; Kiat, H. Improvements in clinical signs of Parkinson’s disease using photobiomodulation: A prospective proof-of-concept study. BMC Neurol. 2021, 21, 256. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Palmiter, R.D.; Xia, Z. Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson’s disease model. J. Cell Biol. 2011, 192, 873–882. [Google Scholar] [CrossRef]
- Cunningham, M.O.; Chinnery, P.F. Mitochondria and cortical gamma oscillations: Food for thought? Brain 2011, 134, 330–332. [Google Scholar] [CrossRef]
- Dong, B.; Almassalha, L.M.; Stypula-Cyrus, Y.; Urban, B.E.; Chandler, J.E.; Nguyen, T.-Q.; Sun, C.; Zhang, H.F.; Backman, V. Superresolution intrinsic fluorescence imaging of chromatin utilizing native, unmodified nucleic acids for contrast. Proc. Natl. Acad. Sci. USA 2016, 113, 9716–9721. [Google Scholar] [CrossRef] [PubMed]
- Psakhye, I.; Jentsch, S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 2012, 151, 807–820. [Google Scholar] [CrossRef]
- Begun, A.; Molochkov, A.; Niemi, A.J. Protein tertiary structure and the myoglobin phase diagram. Sci. Rep. 2019, 9, 10819. [Google Scholar] [CrossRef]
- Weinstein, J.A.; Regev, A.; Zhang, F. DNA microscopy: Optics-free spatio-genetic imaging by a stand-alone chemical reaction. Cell 2019, 178, 229–241.e16. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- Blatt, A.; Elbaz-Greener, G.A.; Tuby, H.; Maltz, L.; Siman-Tov, Y.; Ben-Aharon, G.; Copel, L.; Eisenberg, I.; Efrati, S.; Jonas, M.; et al. Low-Level Laser Therapy to the Bone Marrow Reduces Scarring and Improves Heart Function Post-Acute Myocardial Infarction in the Pig. Photomed. Laser Surg. 2016, 34, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Kumbalasiri, T.; Provencio, I. Melanopsin and other novel mammalian opsins. Exp. Eye Res. 2005, 81, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Terakita, A. The opsins. Genome Biol. 2005, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, D.; Schwarz, W. TRPV Channels in Mast Cells as a Target for Low-Level-Laser Therapy. Cells 2014, 3, 662–673. [Google Scholar] [CrossRef]
- Oron, A.; Oron, U.; Streeter, J.; de Taboada, L.; Alexandrovich, A.; Trembovler, V.; Shohami, E. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J. Neurotrauma 2007, 24, 651–656. [Google Scholar] [CrossRef]
- Wu, Q.; Xuan, W.; Ando, T.; Xu, T.; Huang, L.; Huang, Y.Y.; Dai, T.; Dhital, S.; Sharma, S.K.; Whalen, M.J.; et al. Low-level laser therapy for closed-head traumatic brain injury in mice: Effect of different wavelengths. Lasers Surg. Med. 2012, 44, 218–226. [Google Scholar] [CrossRef]
- De Taboada, L.; Yu, J.; El-Amouri, S.; Gattoni-Celli, S.; Richieri, S.; McCarthy, T.; Streeter, J.; Kindy, M.S. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J. Alzheimer’s Dis. 2011, 23, 521–535. [Google Scholar] [CrossRef]
- Chen, X.; Xue, B.; Wang, J.; Liu, H.; Shi, L.; Xie, J. Potassium Channels: A Potential Therapeutic Target for Parkinson’s Disease. Neurosci. Bull. 2018, 34, 341–348. [Google Scholar] [CrossRef]
- Meng, C.; He, Z.; Xing, D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: Implications for Alzheimer’s disease. J. Neurosci. 2013, 33, 13505–13517. [Google Scholar] [CrossRef]
- Xuan, W.; Agrawal, T.; Huang, L.; Gupta, G.K.; Hamblin, M.R. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J. Biophotonics 2015, 8, 502–511. [Google Scholar] [CrossRef]
- Pascher, T.; Chesick, J.P.; Winkler, J.R.; Gray, H.B. Protein folding triggered by electron transfer. Science 1996, 271, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Roediger, B.; Armati, P.J. Oxidative stress induces axonal beading in cultured human brain tissue. Neurobiol. Dis. 2003, 13, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Konar, A.; Kumar, A.; Maloney, B.; Lahiri, D.K.; Thakur, M.K. A serine protease KLK8 emerges as a regulator of regulators in memory: Microtubule protein dependent neuronal morphology and PKA-CREB signaling. Sci. Rep. 2018, 8, 9928. [Google Scholar] [CrossRef]
- Cartelli, D.; Aliverti, A.; Barbiroli, A.; Santambrogio, C.; Ragg, E.M.; Casagrande, F.V.; Cantele, F.; Beltramone, S.; Marangon, J.; De Gregorio, C.; et al. α-Synuclein is a Novel Microtubule Dynamase. Sci. Rep. 2016, 6, 33289. [Google Scholar] [CrossRef] [PubMed]
- Oliveira da Silva, M.I.; Liz, M.A. Linking Alpha-Synuclein to the Actin Cytoskeleton: Consequences to Neuronal Function. Front. Cell Dev. Biol. 2020, 8, 787. [Google Scholar] [CrossRef] [PubMed]
- Toba, S.; Jin, M.; Yamada, M.; Kumamoto, K.; Matsumoto, S.; Yasunaga, T.; Fukunaga, Y.; Miyazawa, A.; Fujita, S.; Itoh, K.; et al. Publisher Correction: Alpha-synuclein facilitates to form short unconventional microtubules that have a unique function in the axonal transport. Sci. Rep. 2018, 8, 8019. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, A.; Lovisa, B.; Perrin, J.; Wagnières, G.; van den Bergh, H.; Tardy, Y.; Lashuel, H.A. Photobiomodulation Suppresses Alpha-Synuclein-Induced Toxicity in an AAV-Based Rat Genetic Model of Parkinson’s Disease. PLoS ONE 2015, 10, e0140880. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Luján, R.; Watanabe, M.; Adelman, J.P.; Maylie, J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat. Neurosci. 2008, 11, 170–177. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Tessier, C.; Nagpal, J.; Clarke, G.; O’Driscoll, C.M.; Cryan, J.F. Microbial-Derived Metabolites Induce Actin Cytoskeletal Rearrangement and Protect Blood-Brain Barrier Function. Iscience 2022, 25, 105648. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Johnstone, D.M.; Gordon, L.C.; Kiat, H.; Hamblin, M.R. “Photobiomics”: Can Light, Including Photobiomodulation, Alter the Microbiome? Photobiomodul. Photomed. Laser Surg. 2019, 37, 681–693. [Google Scholar] [CrossRef]
- Stefanis, L. α-Synuclein in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2011, 2, a009399. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Moon, J.C.; Shin, S.Y.; Son, H.; Jung, Y.J.; Kim, N.H.; Kim, Y.M.; Jang, M.K.; Lee, J.R. Functional characterization of alpha-synuclein protein with antimicrobial activity. Biochem. Biophys. Res. Commun. 2016, 478, 924–928. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Jin, H.; Serrano, G.E.; Anantharam, V.; Kanthasamy, A.; Adler, C.H.; Beach, T.G.; Kanthasamy, A.G. Blinded RT-QuIC Analysis of alpha-Synuclein Biomarker in Skin Tissue from Parkinson’s Disease Patients. Mov. Disord. 2020, 35, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xia, Y.; Wan, F.; Ma, K.; Guo, X.; Kou, L.; Yin, S.; Han, C.; Liu, L.; Huang, J.; et al. New Perspectives on Roles of Alpha-Synuclein in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 370. [Google Scholar] [CrossRef]
- Mollenhauer, B.; El-Agnaf, O.M.A.; Marcus, K.; Trenkwalder, C.; Schlossmacher, M.G. Quantification of α-synuclein in cerebrospinal fluid as a biomarker candidate: Review of the literature and considerations for future studies. Biomark. Med. 2010, 4, 683–699. [Google Scholar] [CrossRef]
- Barbour, R.; Kling, K.; Anderson, J.P.; Banducci, K.; Cole, T.; Diep, L.; Fox, M.; Goldstein, J.M.; Soriano, F.; Seubert, P.; et al. Red Blood Cells Are the Major Source of Alpha-Synuclein in Blood. Neurodegener. Dis. 2008, 5, 55–59. [Google Scholar] [CrossRef]
- Zou, W.; Pu, T.; Feng, W.; Lu, M.; Zheng, Y.; Du, R.; Xiao, M.; Hu, G. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated alpha-synuclein. Transl. Neurodegener. 2019, 8, 7. [Google Scholar] [CrossRef]
- Salehpour, F.; Khademi, M.; Bragin, D.E.; DiDuro, J.O. Photobiomodulation Therapy and the Glymphatic System: Promising Applications for Augmenting the Brain Lymphatic Drainage System. Int. J. Mol. Sci. 2022, 23, 2975. [Google Scholar] [CrossRef]
- Reinhart, F.; Massri, N.E.; Torres, N.; Chabrol, C.; Molet, J.; Johnstone, D.M.; Stone, J.; Benabid, A.L.; Mitrofanis, J.; Moro, C. The behavioural and neuroprotective outcomes when 670nm and 810nm near infrared light are applied together in MPTP-treated mice. Neurosci. Res. 2017, 117, 42–47. [Google Scholar] [CrossRef]
- Luo, G.-Y.; Sun, L.; Lie, T.C.-Y. Aquaporin-1-Mediated Effects of Low Level He-Ne Laser Irradiation on Human Erythrocytes. Int. J. Photoenergy 2012, 2012, 5. [Google Scholar] [CrossRef]
- Pelletier-Aouizerate, M.; Zivic, Y. Early cases of acute infectious respiratory syndrome treated with photobiomodulation, diagnosis and intervention: Two case reports. Clin. Case Rep. 2021, 9, 2429–2437. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Pla, V.; Giannetto, M.; Vinitsky, H.S.; Staeger, F.F.; Metcalfe, T.; Nguyen, R.; Benrais, A.; Nedergaard, M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Postnov, D.; Lavrova, A.; Fedosov, I.; Borisova, E.; Nikolenko, V.; Penzel, T.; Kurths, J.; Tuchin, V. Biophotonic strategies of measurement and stimulation of the cranial and the extracranial lymphatic drainage function. IEEE J. Sel. Top. Quantum Electron. 2020, 27, 1–13. [Google Scholar] [CrossRef]
- Zinchenko, E.; Navolokin, N.; Shirokov, A.; Khlebtsov, B.; Dubrovsky, A.; Saranceva, E.; Abdurashitov, A.; Khorovodov, A.; Terskov, A.; Mamedova, A.; et al. Pilot study of transcranial photobiomodulation of lymphatic clearance of beta-amyloid from the mouse brain: Breakthrough strategies for non-pharmacologic therapy of Alzheimer’s disease. Biomed. Opt. Express 2019, 10, 4003–4017. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Dubrovsky, A.; Klimova, M.; Agranovich, I.; Terskov, A.; Shirokov, A.; Vinnik, V.; Kuzmina, A.; Lezhnev, N.; et al. Photobiomodulation of lymphatic drainage and clearance: Perspective strategy for augmentation of meningeal lymphatic functions. Biomed. Opt. Express 2020, 11, 725–734. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Klimova, M.; Dubrovsky, A.; Shirokov, A.; Fomin, A.; Terskov, A.; Agranovich, I.; Mamedova, A.; Khorovodov, A.; et al. Photostimulation of cerebral and peripheral lymphatic functions. Transl. Biophotonics 2020, 2, e201900036. [Google Scholar] [CrossRef]
- Zinchenko, E.; Klimova, M.; Mamedova, A.; Agranovich, I.; Blokhina, I.; Antonova, T.; Terskov, A.; Shirokov, A.; Navolokin, N.; Morgun, A.; et al. Photostimulation of Extravasation of Beta-Amyloid through the Model of Blood-Brain Barrier. Electronics 2020, 9, 1056. [Google Scholar] [CrossRef]
- Li, D.-Y.; Liu, S.-J.; Yu, T.-T.; Liu, Z.; Sun, S.-L.; Bragin, D.; Navolokin, N.; Kurths, J.; Glushkovskaya-Semyachkina, O.; Zhu, D. Photostimulation of lymphatic clearance of red blood cells from the mouse brain after intraventricular hemorrhage. bioRxiv 2020. [Google Scholar] [CrossRef]
- Saucedo, C.L.; Courtois, E.C.; Wade, Z.S.; Kelley, M.N.; Kheradbin, N.; Barrett, D.W.; Gonzalez-Lima, F. Transcranial laser stimulation: Mitochondrial and cerebrovascular effects in younger and older healthy adults. Brain Stimul. 2021, 14, 440–449. [Google Scholar] [CrossRef]
- Uozumi, Y.; Nawashiro, H.; Sato, S.; Kawauchi, S.; Shima, K.; Kikuchi, M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg. Med. 2010, 42, 566–576. [Google Scholar] [CrossRef]
- Karu, T.I. Cellular and Molecular Mechanisms of Photobiomodulation (Low-Power Laser Therapy). IEEE J. Sel. Top. Quantum Electron. 2014, 20, 143–148. [Google Scholar] [CrossRef]
- Yan, W.; Chow, R.; Armati, P.J. Inhibitory effects of visible 650-nm and infrared 808-nm laser irradiation on somatosensory and compound muscle action potentials in rat sciatic nerve: Implications for laser-induced analgesia. J. Peripher. Nerv. Syst. 2011, 16, 130–135. [Google Scholar] [CrossRef]
- Cohen, M.X. Where Does EEG Come From and What Does It Mean? Trends Neurosci. 2017, 40, 208–218. [Google Scholar] [CrossRef]
- Timofeev, I.; Bazhenov, M.; Seigneur, J.; Sejnowski, T. Neuronal Synchronization and Thalamocortical Rhythms in Sleep, Wake and Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Metz, A.J.; Klein, S.D.; Scholkmann, F.; Wolf, U. Continuous coloured light altered human brain haemodynamics and oxygenation assessed by systemic physiology augmented functional near-infrared spectroscopy. Sci. Rep. 2017, 7, 10027. [Google Scholar] [CrossRef]
- Flyktman, A.; Manttari, S.; Nissila, J.; Timonen, M.; Saarela, S. Transcranial light affects plasma monoamine levels and expression of brain encephalopsin in the mouse. J. Exp. Biol. 2015, 218, 1521–1526. [Google Scholar] [CrossRef]
- El Massri, N.; Cullen, K.M.; Stefani, S.; Moro, C.; Torres, N.; Benabid, A.L.; Mitrofanis, J. Evidence for encephalopsin immunoreactivity in interneurones and striosomes of the monkey striatum. Exp. Brain Res. 2018, 236, 955–961. [Google Scholar] [CrossRef]
- Basar, E.; Basar-Eroglu, C.; Guntekin, B.; Yener, G.G. Brain’s alpha, beta, gamma, delta, and theta oscillations in neuropsychiatric diseases: Proposal for biomarker strategies. Suppl. Clin. Neurophysiol. 2013, 62, 19–54. [Google Scholar] [CrossRef]
- Nimmrich, V.; Draguhn, A.; Axmacher, N. Neuronal Network Oscillations in Neurodegenerative Diseases. NeuroMol. Med. 2015, 17, 270–284. [Google Scholar] [CrossRef]
- Buzsaki, G.; Wang, X.J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Gu, H.; Yang, Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J. Neurosci. 2013, 33, 18566–18573. [Google Scholar] [CrossRef]
- Lauritzen, M. Pathophysiology of the migraine aura. Brain 1994, 117, 199–210. [Google Scholar] [CrossRef]
- Hodkinson, D.J.; Wilcox, S.L.; Veggeberg, R.; Noseda, R.; Burstein, R.; Borsook, D.; Becerra, L. Increased Amplitude of Thalamocortical Low-Frequency Oscillations in Patients with Migraine. J. Neurosci. 2016, 36, 8026–8036. [Google Scholar] [CrossRef]
- He, Z.G.; Liu, B.W.; Xiang, H.B. Cross interaction of melanocortinergic and dopaminergic systems in neural modulation. Int. J. Physiol. Pathophysiol. Pharmacol. 2015, 7, 152–157. [Google Scholar]
- Yang, J.; Ye, M.; Tian, C.; Yang, M.; Wang, Y.; Shu, Y. Dopaminergic modulation of axonal potassium channels and action potential waveform in pyramidal neurons of prefrontal cortex. J. Physiol. 2013, 591, 3233–3251. [Google Scholar] [CrossRef]
- Dang, L.C.; O’Neil, J.P.; Jagust, W.J. Dopamine supports coupling of attention-related networks. J. Neurosci. 2012, 32, 9582–9587. [Google Scholar] [CrossRef]
- Murugan, N.J.; Rouleau, N.; Karbowski, L.M.; Persinger, M.A. Biophotonic markers of malignancy: Discriminating cancers using wavelength-specific biophotons. Biochem. Biophys. Rep. 2018, 13, 7–11. [Google Scholar] [CrossRef]
- Cosic, I. Macromolecular bioactivity: Is it resonant interaction between macromolecules?—Theory and applications. IEEE Trans. Biomed. Eng. 1994, 41, 1101–1114. [Google Scholar] [CrossRef]
- Cosic, I.; Cosic, D.; Lazar, K. Tesla, Bioresonances and Resonant Recognition Model. In Proceedings of the 2nd International Congress Nikola Tesla, Belgrade, Serbia, 2 June 2017. [Google Scholar]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef]
- Maiello, M.; Losiewicz, O.M.; Bui, E.; Spera, V.; Hamblin, M.R.; Marques, L.; Cassano, P. Transcranial Photobiomodulation with Near-Infrared Light for Generalized Anxiety Disorder: A Pilot Study. Photobiomodul. Photomed. Laser Surg. 2019, 37, 644–650. [Google Scholar] [CrossRef]
- Foo, A.S.C.; Soong, T.W.; Yeo, T.T.; Lim, K.-L. Mitochondrial Dysfunction and Parkinson’s Disease—Near-Infrared Photobiomodulation as a Potential Therapeutic Strategy. Front. Aging Neurosci. 2020, 12, 89. [Google Scholar] [CrossRef]
- Surre, J.; Saint-Ruf, C.; Collin, V.; Orenga, S.; Ramjeet, M.; Matic, I. Strong increase in the autofluorescence of cells signals struggle for survival. Sci. Rep. 2018, 8, 12088. [Google Scholar] [CrossRef]
- Billinton, N.; Knight, A.W. Seeing the wood through the trees: A review of techniques for distinguishing green fluorescent protein from endogenous autofluorescence. Anal. Biochem. 2001, 291, 175–197. [Google Scholar] [CrossRef]
- Croce, A.C.; Bottiroli, G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 2461. [Google Scholar] [CrossRef]
- Monici, M.; Pratesi, R.; Bernabei, P.A.; Caporale, R.; Ferrini, P.R.; Croce, A.C.; Balzarini, P.; Bottiroli, G. Natural fluorescence of white blood cells: Spectroscopic and imaging study. J. Photochem. Photobiol. B 1995, 30, 29–37. [Google Scholar] [CrossRef]
- Croce, A.C.; Spano, A.; Locatelli, D.; Barni, S.; Sciola, L.; Bottiroli, G. Dependence of fibroblast autofluorescence properties on normal and transformed conditions. Role of the metabolic activity. Photochem. Photobiol. 1999, 69, 364–374. [Google Scholar] [CrossRef]
- Croce, A.C.; De Simone, U.; Freitas, I.; Boncompagni, E.; Neri, D.; Cillo, U.; Bottiroli, G. Human liver autofluorescence: An intrinsic tissue parameter discriminating normal and diseased conditions. Lasers Surg. Med. 2010, 42, 371–378. [Google Scholar] [CrossRef]
- Croce, A.C.; Ferrigno, A.; Bottiroli, G.; Vairetti, M. Autofluorescence-based optical biopsy: An effective diagnostic tool in hepatology. Liver Int. 2018, 38, 1160–1174. [Google Scholar] [CrossRef]
- Croce, A.C.; Ferrigno, A.; Santin, G.; Vairetti, M.; Bottiroli, G. Bilirubin: An autofluorescence bile biomarker for liver functionality monitoring. J. Biophotonics 2014, 7, 810–817. [Google Scholar] [CrossRef]
- Croce, A.C.; Palladini, G.; Ferrigno, A.; Vairetti, M. Autofluorescence Label-Free Imaging of the Liver Reticular Structure. Methods Mol. Biol. 2023, 2566, 29–35. [Google Scholar] [CrossRef]
- Cheon, H.; Yang, H.J.; Lee, S.H.; Kim, Y.A.; Son, J.H. Terahertz molecular resonance of cancer DNA. Sci. Rep. 2016, 6, 37103. [Google Scholar] [CrossRef]
- Myšková, J.; Rybakova, O.; Brynda, J.; Khoroshyy, P.; Bondar, A.; Lazar, J. Directionality of light absorption and emission in representative fluorescent proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 32395–32401. [Google Scholar] [CrossRef]
- Gailite, L.; Valenzuela-Palomo, A.; Sanoguera-Miralles, L.; Rots, D.; Kreile, M.; Velasco, E.A. UGT1A1 Variants c.864+5G>T and c.996+2_996+5del of a Crigler-Najjar Patient Induce Aberrant Splicing in Minigene Assays. Front. Genet. 2020, 11, 169. [Google Scholar] [CrossRef]
- Cao, Z.; Lin, C.T.; Chuang, C.H.; Lai, K.L.; Yang, A.C.; Fuh, J.L.; Wang, S.J. Resting-state EEG power and coherence vary between migraine phases. J. Headache Pain 2016, 17, 102. [Google Scholar] [CrossRef]
- Lofredi, R.; Neumann, W.J.; Bock, A.; Horn, A.; Huebl, J.; Siegert, S.; Schneider, G.H.; Krauss, J.K.; Kuhn, A.A. Dopamine-dependent scaling of subthalamic gamma bursts with movement velocity in patients with Parkinson’s disease. eLife 2018, 7, e31895. [Google Scholar] [CrossRef]
- Mably, A.J.; Colgin, L.L. Gamma oscillations in cognitive disorders. Curr. Opin. Neurobiol. 2018, 52, 182–187. [Google Scholar] [CrossRef]
- Mendonca-de-Souza, M.; Monteiro, U.M.; Bezerra, A.S.; Silva-de-Oliveira, A.P.; Ventura-da-Silva, B.R.; Barbosa, M.S.; de Souza, J.A.; Criado, E.C.; Ferrarezi, M.C.; Alencar Gde, A.; et al. Resilience in migraine brains: Decrease of coherence after photic stimulation. Front. Hum. Neurosci. 2012, 6, 207. [Google Scholar] [CrossRef]
- Sarnthein, J.; Jeanmonod, D. High thalamocortical theta coherence in patients with Parkinson’s disease. J. Neurosci. 2007, 27, 124–131. [Google Scholar] [CrossRef]
- Schwartz, S.; Kessler, R.; Gaughan, T.; Buckley, A.W. Electroencephalogram Coherence Patterns in Autism: An Updated Review. Pediatr. Neurol. 2017, 67, 7–22. [Google Scholar] [CrossRef]
- Shin, Y.W.; O’Donnell, B.F.; Youn, S.; Kwon, J.S. Gamma oscillation in schizophrenia. Psychiatry Investig. 2011, 8, 288–296. [Google Scholar] [CrossRef]
- Swann, N.C.; de Hemptinne, C.; Miocinovic, S.; Qasim, S.; Wang, S.S.; Ziman, N.; Ostrem, J.L.; San Luciano, M.; Galifianakis, N.B.; Starr, P.A. Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J. Neurosci. 2016, 36, 6445–6458. [Google Scholar] [CrossRef]
- De Lima, F.M.; Aimbire, F.; Miranda, H.; de Paula Vieira, R.; de Oliveira, A.P.L.; Albertini, R. Low-level laser therapy attenuates the myeloperoxidase activity and inflammatory mediator generation in lung inflammation induced by gut ischemia and reperfusion: A dose-response study. J. Lasers Med. Sci. 2014, 5, 63. [Google Scholar] [PubMed]
- Ekpenyong, A.E.; Toepfner, N.; Chilvers, E.R.; Guck, J. Mechanotransduction in neutrophil activation and deactivation. Biochim Biophys Acta 2015, 1853, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.; Chahel, H.; Lord, J.M. Ageing and the neutrophil: No appetite for killing? Immunology 2000, 100, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, P.; Tridetti, J.; Nguyen, M.L.; Wéra, O.; Jiang, Z.; Gustin, M.; Donneau, A.F.; Oury, C.; Lancellotti, P. Neutrophil Phenotypes in Coronary Artery Disease. J. Clin. Med. 2020, 9, 1602. [Google Scholar] [CrossRef] [PubMed]
- Gaul, D.S.; Stein, S.; Matter, C.M. Neutrophils in cardiovascular disease. Eur. Heart J. 2017, 38, 1702–1704. [Google Scholar] [CrossRef]
- Vitte, J.; Michel, B.F.; Bongrand, P.; Gastaut, J.L. Oxidative stress level in circulating neutrophils is linked to neurodegenerative diseases. J. Clin. Immunol. 2004, 24, 683–692. [Google Scholar] [CrossRef]
- Muñoz-Delgado, L.; Macías-García, D.; Jesús, S.; Martín-Rodríguez, J.F.; Labrador-Espinosa, M.; Jiménez-Jaraba, M.V.; Adarmes-Gómez, A.; Carrillo, F.; Mir, P. Peripheral Immune Profile and Neutrophil-to-Lymphocyte Ratio in Parkinson’s Disease. Mov. Disord. 2021, 36, 2426–2430. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Tarelli, R. Parkinson’s disease and systemic inflammation. Parkinsons. Dis. 2011, 2011, 436813. [Google Scholar] [CrossRef]
- Pott Godoy, M.C.; Tarelli, R.; Ferrari, C.C.; Sarchi, M.I.; Pitossi, F.J. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 2008, 131, 1880–1894. [Google Scholar] [CrossRef]
- Reusch, N.; De Domenico, E.; Bonaguro, L.; Schulte-Schrepping, J.; Baßler, K.; Schultze, J.L.; Aschenbrenner, A.C. Neutrophils in COVID-19. Front. Immunol. 2021, 12, 652470. [Google Scholar] [CrossRef]
- Nejatifard, M.; Asefi, S.; Jamali, R.; Hamblin, M.R.; Fekrazad, R. Probable positive effects of the photobiomodulation as an adjunctive treatment in COVID-19: A systematic review. Cytokine 2021, 137, 155312. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Liu, T.C.; Li, Y.; Guo, H.; Yao, L.B. Signal transduction pathways involved in low intensity He-Ne laser-induced respiratory burst in bovine neutrophils: A potential mechanism of low intensity laser biostimulation. Lasers Surg. Med. 2001, 29, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.R.; Brendzel, A.M.; Lint, T.F. Chemiluminescence spectra of human myeloperoxidase and polymorphonuclear leukocytes. Infect. Immun. 1977, 17, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Garriga, J.; Laumet, G.; Chen, S.R.; Zhang, Y.; Madzo, J.; Issa, J.J.; Pan, H.L.; Jelinek, J. Nerve Injury-Induced Chronic Pain Is Associated with Persistent DNA Methylation Reprogramming in Dorsal Root Ganglion. J. Neurosci. 2018, 38, 6090–6101. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Ren, K.; Dubner, R. Epigenetic regulation of persistent pain. Transl. Res. 2015, 165, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Aberrant DNA methylation as a cancer-inducing mechanism. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, G.; Brown, R. Aberrant DNA methylation in cancer: Potential clinical interventions. Expert Rev. Mol. Med. 2002, 4, 1–17. [Google Scholar] [CrossRef]
- Baylin, S.B.; Esteller, M.; Rountree, M.R.; Bachman, K.E.; Schuebel, K.; Herman, J.G. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum. Mol. Genet. 2001, 10, 687–692. [Google Scholar] [CrossRef]
- Lakshman, R.; Finn, A. Neutrophil disorders and their management. J. Clin. Pathol. 2001, 54, 7–19. [Google Scholar] [CrossRef]
- Leiding, J.W. Neutrophil Evolution and Their Diseases in Humans. Front. Immunol. 2017, 8, 1009. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Wooding, D.J.; Ryu, M.H.; Li, H.; Alexis, N.E.; Pena, O.; Carlsten, C.; Network, C.R.R. Acute air pollution exposure alters neutrophils in never-smokers and at-risk humans. Eur. Respir. J. 2020, 55, 1901495. [Google Scholar] [CrossRef]
- Liu, J.; Pang, Z.; Wang, G.; Guan, X.; Fang, K.; Wang, Z.; Wang, F. Advanced Role of Neutrophils in Common Respiratory Diseases. J. Immunol. Res. 2017, 2017, 6710278. [Google Scholar] [CrossRef]
- De Souza Costa, M.; Teles, R.H.G.; Dutra, Y.M.; Neto, J.C.R.M.; de Brito, T.V.; de Sousa Nunes Queiroz, F.F.; do Vale, D.B.N.; de Souza, L.K.M.; Silva, I.S.; Dos Reis Barbosa, A.L.; et al. Photobiomodulation reduces neutrophil migration and oxidative stress in mice with carrageenan-induced peritonitis. Lasers Med. Sci. 2018, 33, 1983–1990. [Google Scholar] [CrossRef]
- Dotta, B.T.; Buckner, C.A.; Cameron, D.; Lafrenie, R.F.; Persinger, M.A. Biophoton emissions from cell cultures: Biochemical evidence for the plasma membrane as the primary source. Gen. Physiol. Biophys. 2011, 30, 301–309. [Google Scholar] [CrossRef]
- Niggli, H.J.; Tudisco, S.; Privitera, G.; Applegate, L.A.; Scordino, A.; Musumeci, F. Laser-ultraviolet-A-induced ultraweak photon emission in mammalian cells. J. Biomed. Opt. 2005, 10, 024006. [Google Scholar] [CrossRef]
- Knoll, R.; Hoshijima, M.; Chien, K. Cardiac mechanotransduction and implications for heart disease. J. Mol. Med. 2003, 81, 750–756. [Google Scholar] [CrossRef]
- Magi, S.; Lariccia, V.; Maiolino, M.; Amoroso, S.; Gratteri, S. Sudden cardiac death: Focus on the genetics of channelopathies and cardiomyopathies. J. Biomed. Sci. 2017, 24, 56. [Google Scholar] [CrossRef]
- Lyon, R.C.; Zanella, F.; Omens, J.H.; Sheikh, F. Mechanotransduction in cardiac hypertrophy and failure. Circ. Res. 2015, 116, 1462–1476. [Google Scholar] [CrossRef]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53–62. [Google Scholar] [CrossRef]
- Kim, J.C.; Son, M.J.; Woo, S.H. Regulation of cardiac calcium by mechanotransduction: Role of mitochondria. Arch. Biochem. Biophys. 2018, 659, 33–41. [Google Scholar] [CrossRef]
- Bruegmann, T.; Beiert, T.; Vogt, C.C.; Schrickel, J.W.; Sasse, P. Optogenetic termination of atrial fibrillation in mice. Cardiovasc. Res. 2018, 114, 713–723. [Google Scholar] [CrossRef]
- Nussinovitch, U.; Gepstein, L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat. Biotechnol. 2015, 33, 750–754. [Google Scholar] [CrossRef]
- Heo, J.Y.; Nam, M.H.; Yoon, H.H.; Kim, J.; Hwang, Y.J.; Won, W.; Woo, D.H.; Lee, J.A.; Park, H.J.; Jo, S.; et al. Aberrant Tonic Inhibition of Dopaminergic Neuronal Activity Causes Motor Symptoms in Animal Models of Parkinson’s Disease. Curr. Biol. 2020, 30, 276–291.e9. [Google Scholar] [CrossRef]
- Chow, R.T.; Armati, P.J. Photobiomodulation: Implications for anesthesia and pain relief. Photomed. Laser Surg. 2016, 34, 599–609. [Google Scholar] [CrossRef]
- Teng, C.; Egger, S.; Blinman, P.L.; Vardy, J.L. Evaluating laser photobiomodulation for chemotherapy-induced peripheral neuropathy: A randomised phase II trial. Support. Care Cancer 2023, 31, 1–11. [Google Scholar] [CrossRef]
- Chow, R.T.; Johnson, M.I.; Lopes-Martins, R.A.; Bjordal, J.M. Efficacy of low-level laser therapy in the management of neck pain: A systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 2009, 374, 1897–1908. [Google Scholar] [CrossRef]
- Gabel, C.P.; Petrie, S.R.; Mischoulon, D.; Hamblin, M.R.; Yeung, A.; Sangermano, L.; Cassano, P. A case control series for the effect of photobiomodulation in patients with low back pain and concurrent depression PBM for Low Back Pain and Depression. Laser Ther. 2018, 27, 167–173. [Google Scholar] [CrossRef]
- Ramezani, F.; Razmgir, M.; Tanha, K.; Nasirinezhad, F.; Neshastehriz, A.; Bahrami-Ahmadi, A.; Hamblin, M.R.; Janzadeh, A. Photobiomodulation for spinal cord injury: A systematic review and meta-analysis. Physiol. Behav. 2020, 224, 112977. [Google Scholar] [CrossRef]
- Tsai, C.-M. ‘Optoanesthesia’: The Application of Transcranial Photobiomodulation to General Anesthesia. Open J. Anesthesiol. 2022, 12, 289–300. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Radwan, N.M.; Ibrahim, K.M.; Khedr, M.E.; El Aziz, M.A.; Khadrawy, Y.A. Effect of three different intensities of infrared laser energy on the levels of amino acid neurotransmitters in the cortex and hippocampus of rat brain. Photomed. Laser Surg. 2008, 26, 479–488. [Google Scholar] [CrossRef]
- Tsai, C.M.; Chang, S.F.; Chang, H. Transcranial photobiomodulation attenuates pentylenetetrazole-induced status epilepticus in peripubertal rats. J. Biophotonics 2020, 13, e202000095. [Google Scholar] [CrossRef]
- Tsai, C.M.; Chang, S.F.; Li, C.C.; Chang, H. Transcranial photobiomodulation (808 nm) attenuates pentylenetetrazole-induced seizures by suppressing hippocampal neuroinflammation, astrogliosis, and microgliosis in peripubertal rats. Neurophotonics 2022, 9, 015006. [Google Scholar] [CrossRef]
- Vogel, D.D.S.; Ortiz-Villatoro, N.N.; de Freitas, L.; Aimbire, F.; Scorza, F.A.; Albertini, R.; Scorza, C.A. Repetitive transcranial photobiomodulation but not long-term omega-3 intake reduces epileptiform discharges in rats with stroke-induced epilepsy. J. Biophotonics 2021, 14, e202000287. [Google Scholar] [CrossRef]
- Tsai, C.M.; Chang, S.F.; Chang, H. Transcranial photobiomodulation add-on therapy to valproic acid for pentylenetetrazole-induced seizures in peripubertal rats. BMC Complement. Med. Ther. 2022, 22, 81. [Google Scholar] [CrossRef]
- Craddock, T.J.; St George, M.; Freedman, H.; Barakat, K.H.; Damaraju, S.; Hameroff, S.; Tuszynski, J.A. Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: Implications for side effects of general anesthesia. PLoS ONE 2012, 7, e37251. [Google Scholar] [CrossRef]
- Emerson, D.J.; Weiser, B.P.; Psonis, J.; Liao, Z.; Taratula, O.; Fiamengo, A.; Wang, X.; Sugasawa, K.; Smith, A.B.; Eckenhoff, R.G.; et al. Direct modulation of microtubule stability contributes to anthracene general anesthesia. J. Am. Chem. Soc. 2013, 135, 5389–5398. [Google Scholar] [CrossRef]
- Pan, J.Z.; Xi, J.; Tobias, J.W.; Eckenhoff, M.F.; Eckenhoff, R.G. Halothane binding proteome in human brain cortex. J. Proteome Res. 2007, 6, 582–592. [Google Scholar] [CrossRef]
- Craddock, T.J.; Hameroff, S.R.; Ayoub, A.T.; Klobukowski, M.; Tuszynski, J.A. Anesthetics act in quantum channels in brain microtubules to prevent consciousness. Curr. Top. Med. Chem. 2015, 15, 523–533. [Google Scholar] [CrossRef]
- Craddock, T.J.A.; Kurian, P.; Preto, J.; Sahu, K.; Hameroff, S.R.; Klobukowski, M.; Tuszynski, J.A. Anesthetic Alterations of Collective Terahertz Oscillations in Tubulin Correlate with Clinical Potency: Implications for Anesthetic Action and Post-Operative Cognitive Dysfunction. Sci. Rep. 2017, 7, 9877. [Google Scholar] [CrossRef]
- Zamani, A.R.N.; Saberianpour, S.; Geranmayeh, M.H.; Bani, F.; Haghighi, L.; Rahbarghazi, R. Modulatory effect of photobiomodulation on stem cell epigenetic memory: A highlight on differentiation capacity. Lasers Med. Sci. 2020, 35, 299–306. [Google Scholar] [CrossRef]
- Martins, M.D.; Silveira, F.M.; Martins, M.A.T.; Almeida, L.O.; Bagnato, V.S.; Squarize, C.H.; Castilho, R.M. Photobiomodulation therapy drives massive epigenetic histone modifications, stem cells mobilization and accelerated epithelial healing. J. Biophotonics 2021, 14, e202000274. [Google Scholar] [CrossRef]
- Cardoso, F.D.S.; Mansur, F.C.B.; Lopes-Martins, R.B.; Gonzalez-Lima, F.; Gomes da Silva, S. Transcranial Laser Photobiomodulation Improves Intracellular Signaling Linked to Cell Survival, Memory and Glucose Metabolism in the Aged Brain: A Preliminary Study. Front. Cell. Neurosci. 2021, 15, 683127. [Google Scholar] [CrossRef]
- De Farias Gabriel, A.; Wagner, V.P.; Correa, C.; Webber, L.P.; Pilar, E.F.S.; Curra, M.; Carrard, V.C.; Martins, M.A.T.; Martins, M.D. Photobiomodulation therapy modulates epigenetic events and NF-κB expression in oral epithelial wound healing. Lasers Med. Sci. 2019, 34, 1465–1472. [Google Scholar] [CrossRef]
- Hamilton, C.L.; El Khoury, H.; Hamilton, D.; Nicklason, F.; Mitrofanis, J. “Buckets”: Early Observations on the Use of Red and Infrared Light Helmets in Parkinson’s Disease Patients. Photobiomodul. Photomed. Laser Surg. 2019, 37, 615–622. [Google Scholar] [CrossRef]
- Ptáček, L.J. The place of migraine as a channelopathy. Curr. Opin. Neurol. 1998, 11, 217–226. [Google Scholar] [CrossRef]
- Kim, J.B. Channelopathies. Korean J. Pediatr. 2014, 57, 1–18. [Google Scholar] [CrossRef]
- Lascano, A.M.; Korff, C.M.; Picard, F. Seizures and Epilepsies due to Channelopathies and Neurotransmitter Receptor Dysfunction: A Parallel between Genetic and Immune Aspects. Mol. Syndromol. 2016, 7, 197–209. [Google Scholar] [CrossRef]
- Curatolo, M. Pharmacological and Interventional Management of Pain After Whiplash Injury. J. Orthop. Sports Phys. Ther. 2016, 46, 845–850. [Google Scholar] [CrossRef]
- Curatolo, M.; Petersen-Felix, S.; Arendt-Nielsen, L.; Giani, C.; Zbinden, A.M.; Radanov, B.P. Central hypersensitivity in chronic pain after whiplash injury. Clin. J. Pain 2001, 17, 306–315. [Google Scholar] [CrossRef]
- Greiner, P.; Houdek, P.; Sládek, M.; Sumová, A. Early rhythmicity in the fetal suprachiasmatic nuclei in response to maternal signals detected by omics approach. PLoS Biol. 2022, 20, e3001637. [Google Scholar] [CrossRef] [PubMed]
- Leisman, G.; Machado, C.; Machado, Y.; Chinchilla-Acosta, M. Effects of Low-Level Laser Therapy in Autism Spectrum Disorder. Adv. Exp. Med. Biol. 2018, 1116, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tian, Y.; Nie, J.; Xu, J.; Liu, D. Red light and the sleep quality and endurance performance of Chinese female basketball players. J. Athl. Train. 2012, 47, 673–678. [Google Scholar] [CrossRef]
- Naeser, M.A.; Zafonte, R.; Krengel, M.H.; Martin, P.I.; Frazier, J.; Hamblin, M.R.; Knight, J.A.; Meehan, W.P., 3rd; Baker, E.H. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: Open-protocol study. J. Neurotrauma 2014, 31, 1008–1017. [Google Scholar] [CrossRef]
- Naeser, M.A.; Martin, P.I.; Ho, M.D.; Krengel, M.H.; Bogdanova, Y.; Knight, J.A.; Yee, M.K.; Zafonte, R.; Frazier, J.; Hamblin, M.R.; et al. Transcranial, Red/Near-Infrared Light-Emitting Diode Therapy to Improve Cognition in Chronic Traumatic Brain Injury. Photomed. Laser Surg. 2016, 34, 610–626. [Google Scholar] [CrossRef]
- Özcan, G.G.; Lim, S.; Leighton, P.L.A.; Allison, W.T.; Rihel, J. Sleep is bi-directionally modified by amyloid beta oligomers. eLife 2020, 9, e53995. [Google Scholar] [CrossRef]
- Dang-Vu, T.T. Neuronal oscillations in sleep: Insights from functional neuroimaging. Neuromol. Med. 2012, 14, 154–167. [Google Scholar] [CrossRef]
- Buskila, Y.; Bellot-Saez, A.; Morley, J.W. Generating Brain Waves, the Power of Astrocytes. Front. Neurosci. 2019, 13, 1125. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. Mitochondrial oscillations in physiology and pathophysiology. Adv. Exp. Med. Biol. 2008, 641, 98–117. [Google Scholar] [CrossRef]
- Dupont, G.; Combettes, L.; Bird, G.S.; Putney, J.W. Calcium oscillations. Cold Spring Harb. Perspect. Biol. 2011, 3, a004226. [Google Scholar] [CrossRef]
- Wang, X.; Wanniarachchi, H.; Wu, A.; Gonzalez-Lima, F.; Liu, H. Transcranial photobiomodulation and thermal stimulation induce distinct topographies of EEG alpha and beta power changes in healthy humans. Sci. Rep. 2021, 11, 18917. [Google Scholar] [CrossRef] [PubMed]
- Stephan, W.; Banas, L.J.; Hamblin, M.R. Treatment Efficacy of Photobiomodulation for Moderate and Advanced Dementia or Alzheimer’s Disease: Case Studies. Adv. Alzheimer’s Dis. 2022, 11, 39–47. [Google Scholar] [CrossRef]
- Chao, L.L.; Barlow, C.; Karimpoor, M.; Lim, L. Changes in brain function and structure after self-administered home photobiomodulation treatment in a concussion case. Front. Neurol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Almansour, N.M.; Pirogova, E.; Coloe, P.J.; Cosic, I.; Istivan, T.S. A bioactive peptide analogue for myxoma virus protein with a targeted cytotoxicity for human skin cancer in vitro. J. Biomed. Sci. 2012, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Al-Rayahi, I.A.; Sanyi, R.H. The overlapping roles of antimicrobial peptides and complement in recruitment and activation of tumor-associated inflammatory cells. Front. Immunol. 2015, 6, 2. [Google Scholar] [CrossRef]
- McCormick, T.S.; Weinberg, A. Epithelial cell-derived antimicrobial peptides are multifunctional agents that bridge innate and adaptive immunity. Periodontol. 2000 2010, 54, 195–206. [Google Scholar] [CrossRef]
- Tripodi, N.; Feehan, J.; Husaric, M.; Kiatos, D.; Sidiroglou, F.; Fraser, S.; Apostolopoulos, V. Good, better, best? The effects of polarization on photobiomodulation therapy. J. Biophotonics 2020, 13, e201960230. [Google Scholar] [CrossRef]
- Del Rocío Cantero, M.; Gutierrez, B.C.; Cantiello, H.F. Actin filaments modulate electrical activity of brain microtubule protein two-dimensional sheets. Cytoskeleton 2020, 77, 167–177. [Google Scholar] [CrossRef]
- Brouhard, G.J.; Rice, L.M. Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef]
- Downing, K.H.; Nogales, E. New insights into microtubule structure and function from the atomic model of tubulin. Eur. Biophys. J. 1998, 27, 431–436. [Google Scholar] [CrossRef]
| Authors | Title | Contribution |
|---|---|---|
| Gurwitsch 1932 [23] | Mitogenetic Emission | Release of biophotons |
| Popp et al., 1984 [24] | Biophoton emission. New evidence for coherence and DNA as source | Release of biophotons |
| Kert & Rose 1989 [25] | Low level laser therapy | Clinical applications of PBM |
| Albrecht-Buehler 1992 [26] | Rudimentary form of cellular “vision” | Release of biophotons |
| Laakso et al., 1993 [27] | Quality of light—is laser necessary for effective photobiostimulation? | PBM coherence |
| Amano et al., 1995 [28] | Ultraweak biophoton emission imaging of transplanted bladder cancer | Biophotons for diagnosis |
| Cosic 2001 [29] | The Resonant Recognition Model of Bio-molecular Interactions: possibility of electromagnetic resonance | Resonant oscillation theory |
| Voeikov et al., 2003 [30] | Biophoton research in blood reveals its holistic properties | Release of biophotons |
| Amat et al., 2006 [31] | The electric field induced by light can explain cellular responses to electromagnetic energy: A hypothesis of mechanism | PBM coherence |
| Chow et al., 2007 [32] | 830 nm laser irradiation induces varicosity formation, reduces mitochondrial membrane potential and blocks fast axonal flow in small and medium diameter rat dorsal root dorsal root ganglion: implications for the analgesic effects of 830 nm laser. | PBM modulation of cytoskeleton |
| Mathew et al., 2010 [33] | Signalling effect of NIR pulsed lasers on axonal growth | Biophoton signaling |
| Sun et al., 2010 [34] | Biophotons as neural communication signals demonstrated by in situ biophoton autography. | Communication with biophotons |
| Bokkon et al., 2010 [35] | Estimation of the number of biophotons involved in the visual perception of a single-object image: Biophoton intensity can be considerably higher inside cells than outside | Communication with biophotons from periphery to brain |
| Minke 2010 [36] | The history of the Drosophila TRP channel: the birth of a new channel superfamily | Photon activation of neuronal ion channels |
| Lavi et al., 2012 [11] | The Plasma Membrane is Involved in the Visible Light–Tissue Interaction | PBM membrane interactions |
| Hanczyc et al., 2013 [37] | Multiphoton absorption in amyloid protein fibres | Photons for diagnosis |
| Liebert et al., 2014 [38] | Protein conformational modulation by photons: A mechanism for laser treatment effects | Biophoton theory for PBM |
| Niggli 2014 [39] | Biophotons: ultraweak light impulses regulate life processes in aging | Biophotons for diagnosis |
| Tang & Dai 2014 [40] | Spatiotemporal imaging of glutamate-induced biophotonic activities and transmission in neural circuits | Communication with biophotons in the brain |
| Budagovsky et al., 2015 [41] | Cell response to quasi-monochromatic light with different coherence | Oscillation theory |
| Shi et al., 2016 [42] | Photon entanglement through brain tissue | Quantum entanglement theory |
| Cosic & Cosic 2016 [43] | The treatment of Crigler-Najjar syndrome by blue light as explained by resonant recognition model | Clinical application of resonance theory |
| Poznanski et al., 2017 [44] | Solitonic conduction of electrotonic signals in neuronal branchlets with polarized microstructure | Soliton and nerve theory |
| Cantero et al., 2018 [45] | Bundles of brain microtubules generate electrical oscillations | Photon modification of microtubules in neurons |
| Johnson & Winlow 2018 [46] | The Soliton and the Action Potential—Primary Elements Underlying Sentience | Soliton and nerve theory |
| Fekrazad 2018 [47] | Photons Harmony for Cell Communication | Biophotons and PBM |
| Santana-Blank & Rodríguez-Santana [48] | Photobiomodulation in Light Our Biological Clock’s Inner Workings | PBM and circadian oscillations |
| Facchin et al., 2019 [49] | Physical energies to the rescue of damaged tissues | Biophotons and PBM |
| Zomorrodi et al., 2019 [50] | Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: a pilot exploratory study | PBM and neural oscillations |
| Wang et al., 2019 [51] | Transcranial photobiomodulation with 1064-nm laser modulates brain electroencephalogram rhythms | PBM and neural oscillations |
| Lima et al., 2019 [6] | Photobiomodulation enhancement of cell proliferation at 660 nm does not require cytochrome c oxidase | PBM photophysical mechanisms |
| Pope et al., 2020 [52] | Wavelength-and irradiance-dependent changes in intracellular nitric oxide level | PBM photophysical mechanisms |
| Esmaeilpour et al., 2020 [53] | An Experimental Investigation of Ultraweak Photon Emission from Adult Murine Neural Stem Cells | Biophotons for diagnosis |
| Sordillo & Sordillo 2020 [54] | The mystery of chemotherapy brain: kynurenines, tubulin and biophoton release | Clinical application of biophotons |
| Mahbub et al., 2020 [55] | Non-invasive real-time imaging of reactive oxygen species (ROS) using auto-fluorescence multispectral imaging technique: A novel tool for redox biology | Autofluorescence |
| Zangari et al., 2021 [56] | Photons detected in the active nerve by photographic technique | Biophotons and nerve theory |
| Staelens et al., 2022 [57] | Near-Infrared Photobiomodulation of Living Cells, Tubulin, and Microtubules | Photon modification of microtubules in neurons |
| Korneev et al., 2022 [58] | Exploring Structural Flexibility and Stability of α-Synuclein by the Landau–Ginzburg–Wilson Approach | Solitons |
| Moro et al., 2022 [59] | The code of light: do neurons generate light to communicate and repair? | Biophoton communication |
| Moro et al., 2022 [60] | The effect of photobiomodulation on the brain during wakefulness and sleep | Biophoton and circadian rhythms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebert, A.; Capon, W.; Pang, V.; Vila, D.; Bicknell, B.; McLachlan, C.; Kiat, H. Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine. Biomedicines 2023, 11, 237. https://doi.org/10.3390/biomedicines11020237
Liebert A, Capon W, Pang V, Vila D, Bicknell B, McLachlan C, Kiat H. Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine. Biomedicines. 2023; 11(2):237. https://doi.org/10.3390/biomedicines11020237
Chicago/Turabian StyleLiebert, Ann, William Capon, Vincent Pang, Damien Vila, Brian Bicknell, Craig McLachlan, and Hosen Kiat. 2023. "Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine" Biomedicines 11, no. 2: 237. https://doi.org/10.3390/biomedicines11020237
APA StyleLiebert, A., Capon, W., Pang, V., Vila, D., Bicknell, B., McLachlan, C., & Kiat, H. (2023). Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine. Biomedicines, 11(2), 237. https://doi.org/10.3390/biomedicines11020237








