Oral Surgery and Osteoradionecrosis in Patients Undergoing Head and Neck Radiation Therapy: An Update of the Current Literature
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Optimal Timing for Dento-Alveolar Surgery in Cases of Head and Neck RT
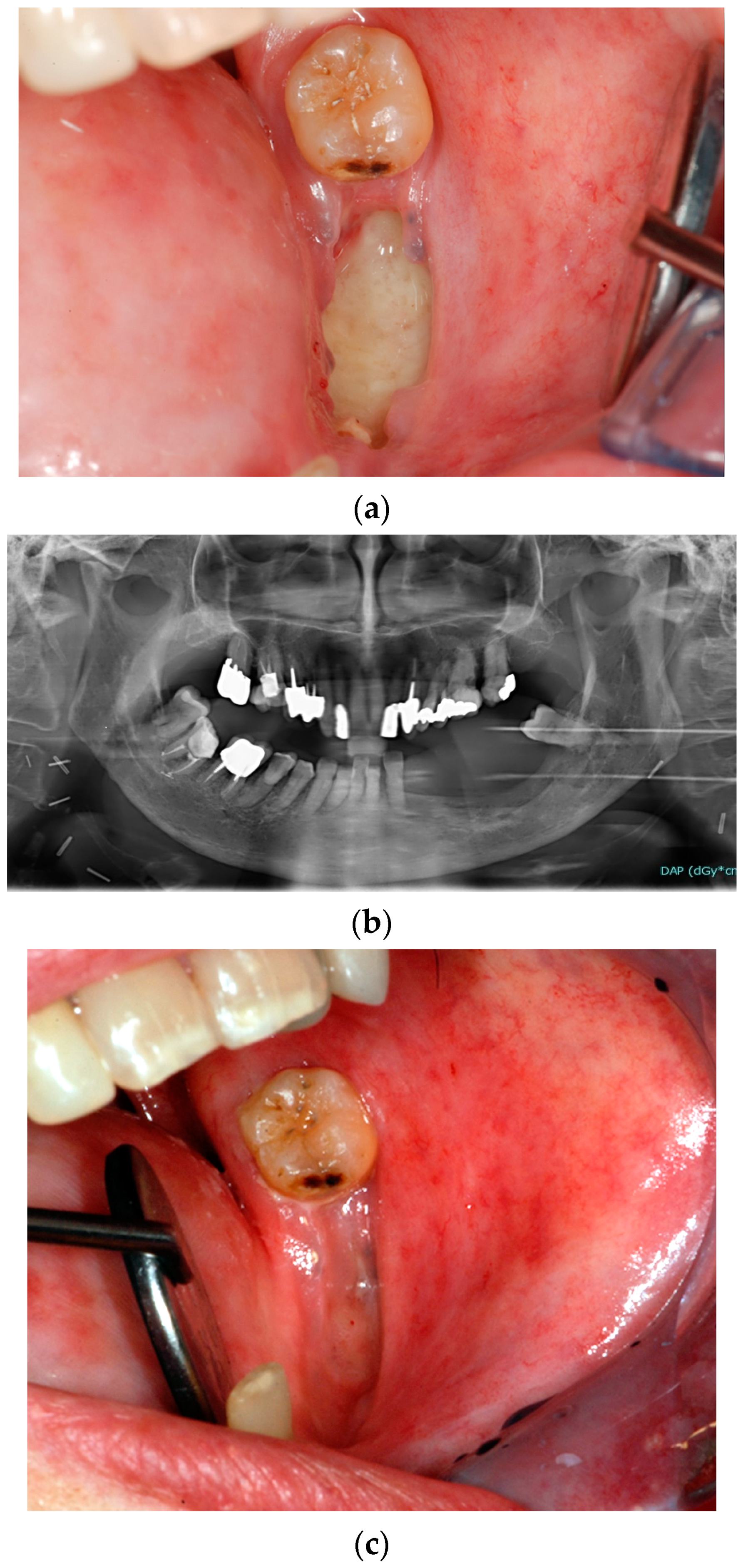
3.1.1. Dental Extractions
3.1.2. Implant Placement in Patients Receiving Head and Neck RT
3.2. Preventive Strategies for Reducing the Risk of ORN Related to Dental Extractions
3.2.1. Pentoxifylline and Tocopherol
| PubMedID | First Author | Year | Study Design | No. of Included Studies | No. of Included Patients | Mean Delay RT—Implant Placement | Implant Survival | Other Risk Factors Associated with Reduced Implant Survival | Follow-Up Time | Conclusions/Reccomandations |
|---|---|---|---|---|---|---|---|---|---|---|
| 31612191 | Di Carlo, S. [71] | 2019 | Retrospective study | / | 17 | 14 | 90.50% | / | >12 m | Better outcomes when the implant was placed at least after 14 months and not loaded until at least 6 months after placement. |
| 34903387 | Shokouhi, B. [59] | 2022 | Systematic review and meta-analysis | 7 | 441 | 6–18 m | / | RT doses > 50 Gy Implant placed in the maxilla | 1–14 y | Implant survival is significantly lower in RT compared with non-RT patients (p < 0.001). Implant placement should be delayed by at least six months following RT. |
| 34255187 | Schiegnitz, E. [62] | 2021 | Retrospective study | / | 164 | 43.6 m | 87.3% (5 y), 80.0% (10 y) at time of surgery—92.5% (5 y), 89.5 (10 y) after oncological treatment | Implant placed in augmented and irradiated bone | 37–49 m | A successful and safe rehabilitation of the irradiated oral cancer patient with high implant survival rates is possible for either secondary or primary placed implants. |
| 33278135 | Veld, MI. [80] | 2021 | Systematic review | 10 | / | / | 90.4–100% | / | 12–174 m | Slightly higher survival of immediately placed implants compared with postponed placed implants (p = 0.81). RT vs. non-RT showed a better survival of immediately placed implants not having received RT (p = 0.10). |
| 31898358 | Koudougou, C. [56] | 2020 | Literature review | 4 | 341 | / | 82–96.7% | / | 29–60 m | The outcomes for implant survival rates appear to be positive for irradiated implants. All mandibular implants were selected for this review. |
| 27034761 | Shugaa-Addin, B. [63] | 2016 | Literature review | 18 | 1175 | / | 74.4–97% | Maxillary implants RT doses > 70 Gy | 0.5–10 | Dental implants may be affected by RT, especially when they are placed in the maxilla, in grafted bone, or after radiation; however, they remain a functional option for the rehabilitation of HNC patients. |
| 20701621 | Korfage, A. [57] | 2010 | Prospective study | / | 50 | Time of surgery | 89.40% | / | 5y | Oral cancer patients can benefit from implants placed during ablative surgery, with a high survival rate of the implants, a high percentage of rehabilitated patients, and a high denture satisfaction up to 5 years after treatment. |
| 25926008 | Zen Filho, EV. [68] | 2016 | Systematic review | 8 | 331 | 1–20 m | / | RT doses > 50 Gy | 1–168 m | The placement of implants in the irradiated bone is viable, and head and neck RT should not be considered as an absolute contraindication for dental rehabilitation with implants. |
| 23742098 | Piardi Claudy, M. [70] | 2013 | Systematic review and meta-analysis | 10 | 39 | / | 13.6% (risk of failure) | Placement of dental implants between 6 and 12 months post-RT | 1–170 m | Placing implants in the bone within a period shorter than 12 months after RT may result in a higher risk of failure. |
3.2.2. HBO Therapy and ORN Prevention
4. Study Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBO | hyperbaric oxygen |
| HNC | head and neck cancer |
| HPV | human papillomavirus |
| IMRT | intensity-modulated RT |
| MRONJ | medication-related ORN of the jaw |
| ORN | osteoradionecrosis |
| PENTO | pentoxifylline and tocopherol |
| RIF | radiation-induced fibrosis |
| RT | radiation therapy |
| 3DCRT | three-dimensional conformal RT |
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Petti, S. Lifestyle risk factors for oral cancer. Oral Oncol. 2009, 45, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Saito, S.; Uppaluri, R. Immunotherapy for Head and Neck Cancer: A Paradigm Shift From Induction Chemotherapy to Neoadjuvant Immunotherapy. Front. Oncol. 2021, 11, 727433. [Google Scholar] [CrossRef] [PubMed]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCNN) Head and Neck Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 15 October 2023).
- Taku, N.; Wang, L.; Garden, A.S.; Rosenthal, D.I.; Gunn, G.B.; Morrison, W.H.; Fuller, C.D.; Phan, J.; Reddy, J.P.; Moreno, A.C.; et al. Proton Therapy for HPV-Associated Oropharyngeal Cancers of the Head and Neck: A De-Intensification Strategy. Curr. Treat. Options Oncol. 2021, 22, 54. [Google Scholar] [CrossRef]
- Nadella, K.R.; Kodali, R.M.; Guttikonda, L.K.; Jonnalagadda, A. Osteoradionecrosis of the Jaws: Clinico-Therapeutic Management: A Literature Review and Update. J. Maxillofac. Oral Surg. 2015, 14, 891–901. [Google Scholar] [CrossRef]
- Ben-David, M.A.; Diamante, M.; Radawski, J.D.; Vineberg, K.A.; Stroup, C.; Murdoch-Kinch, C.A.; Zwetchkenbaum, S.R.; Eisbruch, A. Lack of Osteoradionecrosis of the Mandible After Intensity-Modulated Radiotherapy for Head and Neck Cancer: Likely Contributions of Both Dental Care and Improved Dose Distributions. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 396–402. [Google Scholar] [CrossRef]
- Beumer, J.; Harrison, R.; Sanders, B.; Kurrasch, M. Postradiation dental extractions: A review of the literature and a report of 72 episodes. Head Neck Surg. 1983, 6, 581–586. [Google Scholar] [CrossRef]
- Mücke, T.; Koschinski, J.; Wagenpfeil, S.; Wolff, K.D.; Kanatas, A.; Mitchell, D.A.; Deppe, H.; Kesting, M.R. Functional outcome after different oncological interventions in head and neck cancer patients. J. Cancer Res. Clin. Oncol. 2012, 138, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Tsai, C.J.; Lee, R.S.; Freymiller, H.; Kadempour, A.; Varthis, S.; Sax, A.Z.; Rosen, E.B.; Yom, S.H.K.; Randazzo, J.; et al. The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT): The Memorial Sloan Kettering Cancer Center experience. Oral Oncol. 2017, 64, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, H.P.; Davies, G.R.; Boyd, N.M. Predictive factors for osteoradionecrosis of the jaws: A retrospective study. Head Neck 2018, 40, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Renda, L.; Tsai, T.Y.; Huang, J.J.; Ito, R.; Hsieh, W.C.; Kao, H.K.; Hung, S.Y.; Huang, Y.; Huang, Y.C.; Chang, Y.L.; et al. A nomogram to predict osteoradionecrosis in oral cancer after marginal mandibulectomy and radiotherapy. Laryngoscope 2020, 130, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lajolo, C.; Rupe, C.; Gioco, G.; Troiano, G.; Patini, R.; Petruzzi, M.; Micciche’, F.; Giuliani, M. Osteoradionecrosis of the jaws due to teeth extractions during and after radiotherapy: A systematic review. Cancers 2021, 13, 5798. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. A new concept in the treatment of osteoradionecrosis. J. Oral Maxillofac. Surg. 1983, 41, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Lefaix, J.L. The radiation-induced fibroatrophic process: Therapeutic perspective via the antioxidant pathway. Radiother. Oncol. 2004, 73, 119–131. [Google Scholar] [CrossRef]
- Delanian, S.; Lefaix, J.L. Current Management for Late Normal Tissue Injury: Radiation-Induced Fibrosis and Necrosis. Semin. Radiat. Oncol. 2007, 17, 99–107. [Google Scholar] [CrossRef]
- Duarte, V.M.; Liu, Y.F.; Rafizadeh, S.; Tajima, T.; Nabili, V.; Wang, M.B. Comparison of dental health of patients with head and neck cancer receiving IMRT vs. conventional radiation. Otolaryngol.-Head Neck Surg. 2014, 150, 81–86. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Orecchia, R. Radiotherapy-induced mandibular bone complications. Cancer Treat. Rev. 2002, 28, 65–74. [Google Scholar] [CrossRef]
- Singh, A.; Kitpanit, S.; Neal, B.; Yorke, E.; White, C.; Yom, S.H.K.; Randazzo, J.D.; Wong, R.J.; Huryn, J.M.; Tsai, C.J.; et al. Osteoradionecrosis of the Jaw Following Proton Radiation Therapy for Patients With Head and Neck Cancer. JAMA Otolaryngol.-Head Neck Surg. 2023, 149, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.-W.; Yu, T.-P.; Kao, Y.-S. A systematic review and meta-analysis of osteoradionecrosis following proton therapy in patients with head and neck cancer. Oral Oncol. 2024, 148, 106649. [Google Scholar] [CrossRef] [PubMed]
- Rice, N.; Polyzois, I.; Ekanayake, K.; Omer, O.; Stassen, L.F.A. The management of osteoradionecrosis of the jaws—A review. Surgeon 2015, 13, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.W.; Robinson, A.; Hong, C. Dental Evaluation Prior to Cancer Therapy. Front. Oral Health 2022, 3, 876941. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.C.; Nabil, S.; Fauzi, A.A.; Yunus, S.S.M.; Ngeow, W.C.; Ramli, R. Predictors of osteoradionecrosis following irradiated tooth extraction. Radiat. Oncol. 2021, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.; Dorna Mojdami, Z.; Oladega, A.; Hope, A.; Glogauer, M. Clinical practice guidelines for dental management prior to radiation for head and neck cancer. Oral Oncol. 2021, 123, 105604. [Google Scholar] [CrossRef] [PubMed]
- Abed, H. National and international guidelines on the replacement of missing teeth with dentures for head and neck cancer patients post-radiotherapy: A rapid review. Saudi Dent. J. 2023, 35, 125–132. [Google Scholar] [CrossRef]
- Pippi, R. Post-surgical clinical monitoring of soft tissue wound healing in periodontal and implant surgery. Int. J. Med. Sci. 2017, 14, 721–728. [Google Scholar] [CrossRef]
- Epstein, J.B.; Rea, G.; Wong, F.L.W.; Spinelli, J.; Stevenson-Moore, P. Osteonecrosis: Study of the relationship of dental extractions in patients receiving radiotherapy. Head Neck Surg. 1987, 10, 48–54. [Google Scholar] [CrossRef]
- Strohl, M.P.; Chen, J.P.; Ha, P.K.; Seth, R.; Yom, S.S.; Heaton, C.M. Can Early Dental Extractions Reduce Delays in Postoperative Radiation for Patients With Advanced Oral Cavity Carcinoma? J. Oral Maxillofac. Surg. 2019, 77, 2215–2220. [Google Scholar] [CrossRef]
- Balermpas, P.; van Timmeren, J.E.; Knierim, D.J.; Guckenberger, M.; Ciernik, I.F. Dental extraction, intensity-modulated radiotherapy of head and neck cancer, and osteoradionecrosis: A systematic review and meta-analysis. Strahlenther. Und Onkol. 2022, 198, 219–228. [Google Scholar] [CrossRef]
- Lajolo, C.; Gioco, G.; Rupe, C.; Troiano, G.; Cordaro, M.; Lucchese, A.; Paludetti, G.; Giuliani, M. Tooth extraction before radiotherapy is a risk factor for developing osteoradionecrosis of the jaws: A systematic review. Oral Dis. 2021, 27, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Wadhwa Soni, B.; Gupta, A.; Ghoshal, S. Time required for prophylactic oral care in head and neck cancer patients scheduled for radiotherapy: A single center, prospective cohort study. Head Neck 2023, 45, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Costa Normando, A.G.; Pérez-de-Oliveira, M.E.; Guerra, E.N.S.; Lopes, M.A.; Rocha, A.C.; Brandão, T.B.; Prado-Ribeiro, A.C.; Gueiros, L.A.M.; Epstein, J.B.; Migliorati, C.A.; et al. To extract or not extract teeth prior to head and neck radiotherapy? A systematic review and meta-analysis. Support. Care Cancer 2022, 30, 8745–8759. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, O.; DeLong, H.R.; Ziegler, K.M.; Pilcher, L.; Pahlke, S.; Tampi, M.P.; O’Brien, K.K.; Patton, L.L.; Agrawal, N.; Hofstede, T.M.; et al. Effect of preradiation dental intervention on incidence of osteoradionecrosis in patients with head and neck cancer. J. Am. Dent. Assoc. 2022, 153, 931–942.e32. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, S.; Bhatia, N.; McDowell, L.; Fua, T.; McCullough, M.; Celentano, A.; Yap, T. Timing of dental extractions in patients undergoing radiotherapy and the incidence of osteoradionecrosis: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2021, 59, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; Kanani, R.; Romeed, S.A. An audit into the timing of dental extractions pre-head and neck radiotherapy and the prevalence of osteoradionecrosis. Br. Dent. J. 2022, 232, 628. [Google Scholar] [CrossRef]
- King, R.; Li, C.; Lowe, D.; Rogers, S.N. An audit of dental assessments including orthopantomography and timing of dental extractions before radiotherapy for head and neck cancer. Br. Dent. J. 2022, 232, 38–43. [Google Scholar] [CrossRef]
- See Toh, Y.L.; Soong, Y.L.; Chim, Y.X.; Tan, L.T.; Lye, W.K.; Teoh, K.H. Dental extractions for preradiation dental clearance and incidence of osteoradionecrosis in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. J. Investig. Clin. Dent. 2018, 9, e12295. [Google Scholar] [CrossRef]
- Marciani, R.D.; Ownby, H.E. Osteoradionecrosis of the jaws. J. Oral Maxillofac. Surg. 1986, 44, 218–223. [Google Scholar] [CrossRef]
- Wanifuchi, S.; Akashi, M.; Ejima, Y.; Shinomiya, H.; Minamikawa, T.; Furudoi, S.; Otsuki, N.; Sasaki, R.; Nibu, K.; Komori, T. Cause and occurrence timing of osteoradionecrosis of the jaw: A retrospective study focusing on prophylactic tooth extraction. Oral Maxillofac. Surg. 2016, 20, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Beech, N.M.; Porceddu, S.; Batstone, M.D. Radiotherapy-associated dental extractions and osteoradionecrosis. Head Neck 2017, 39, 128–132. [Google Scholar] [CrossRef]
- Marx, R.E.; Johnson, R.P. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg. Oral Med. Oral Pathol. 1987, 64, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Reher, P.; Sousa, A.A.; Harris, M. Osteoradionecrosis of the jaws-a current overview-part 2: Dental management and therapeutic options for treatment. Oral Maxillofac. Surg. 2010, 14, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.J.; Leung, C.M.; Chang, H.S.; Wu, C.N.; Chen, W.L.; Chen, G.J.; Lai, Y.C.; Huang, W.C. Jaw osteoradionecrosis and dental extraction after head and neck radiotherapy: A nationwide population-based retrospective study in Taiwan. Oral Oncol. 2016, 56, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Nabil, S.; Samman, N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2011, 40, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-H.; Liu, C.-J.; Chao, T.-F.; Chen, T.-J.; Hu, Y.-W. Risk factors for and the role of dental extractions in osteoradionecrosis of the jaws: A national-based cohort study. Head Neck 2017, 39, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Lye, K.W.; Wee, J.; Gao, F.; Neo, P.S.H.; Soong, Y.L.; Poon, C.Y. The effect of prior radiation therapy for treatment of nasopharyngeal cancer on wound healing following extractions: Incidence of complications and risk factors. Int. J. Oral Maxillofac. Surg. 2007, 36, 315–320. [Google Scholar] [CrossRef]
- Lee, I.J.; Koom, W.S.; Lee, C.G.; Kim, Y.B.; Yoo, S.W.; Keum, K.C.; Kim, G.E.; Choi, E.C.; Cha, I.H. Risk Factors and Dose–Effect Relationship for Mandibular Osteoradionecrosis in Oral and Oropharyngeal Cancer Patients. Int. J. Radiat. Oncol. 2009, 75, 1084–1091. [Google Scholar] [CrossRef]
- Bonan, P.R.F.; Lopes, M.A.; Pires, F.R.; Almeida, O.P. de Dental management of low socioeconomic level patients before radiotherapy of the head and neck with special emphasis on the prevention of osteoradionecrosis. Braz. Dent. J. 2006, 17, 336–342. [Google Scholar] [CrossRef]
- Liao, P.H.; Lin, C.; Huang, J.Y.; Lin, H.M.; Kuo, T.J. Association between tooth extraction during radiotherapy and the risk of osteoradionecrosis in patients with head and neck cancers. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Somay, E.; Yilmaz, B.; Topkan, E.; Pehlivan, B.; Selek, U. Radiotherapy and Dental Implant Applications in Patients with Head and Neck Cancer; Exon Publications: Brisbane, Australia, 2023. [Google Scholar]
- Schoen, P.J.; Reintsema, H.; Raghoebar, G.M.; Vissink, A.; Roodenburg, J.L.N. The use of implant retained mandibular prostheses in the oral rehabilitation of head and neck cancer patients. A review and rationale for treatment planning. Oral Oncol. 2004, 40, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Kende, P.P.; Ranganath, S.; Landge, J.S.; Sarda, A.; Wadewale, M.; Patil, A.; Singhavi, H.R. Survival of Dental Implants on Irradiated Jaws: A Systematic Review and Meta-analysis. J. Maxillofac. Oral Surg. 2022, 21, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Koudougou, C.; Bertin, H.; Lecaplain, B.; Badran, Z.; Longis, J.; Corre, P.; Hoornaert, A. Postimplantation radiation therapy in head and neck cancer patients: Literature review. Head Neck 2020, 42, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Korfage, A.; Schoen, P.J.; Raghoebar, G.M.; Roodenburg, J.L.N.; Vissink, A.; Reintsema, H. Benefits of dental implants installed during ablative tumour surgery in oral cancer patients: A prospective 5-year clinical trial. Clin. Oral Implants Res. 2010, 21, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Ozen, J.; Dirican, B.; Oysul, K.; Beyzadeoglu, M.; Ucok, O.; Beydemir, B. Dosimetric evaluation of the effect of dental implants in head and neck radiotherapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2005, 99, 743–747. [Google Scholar] [CrossRef]
- Shokouhi, B.; Cerajewska, T. Radiotherapy and the survival of dental implants: A systematic review. Br. J. Oral Maxillofac. Surg. 2022, 60, 422–429. [Google Scholar] [CrossRef]
- Nooh, N. Dental Implant Survival in Irradiated Oral Cancer Patients: A Systematic Review of the Literature. Int. J. Oral Maxillofac. Implant. 2013, 28, 1233–1242. [Google Scholar] [CrossRef]
- Camolesi, G.C.V.; Veronese, H.R.M.; Celestino, M.A.; Blum, D.F.C.; Márquez-Zambrano, J.A.; Carmona-Pérez, F.A.; Jara-Venegas, T.A.; Pellizzon, A.C.A.; Bernaola-Paredes, W.E. Survival of osseointegrated implants in head and neck cancer patients submitted to multimodal treatment: A systematic review and meta-analysis. Support. Care Cancer 2023, 31, 641. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Reinicke, K.; Sagheb, K.; König, J.; Al-Nawas, B.; Grötz, K.A. Dental implants in patients with head and neck cancer—A systematic review and meta-analysis of the influence of radiotherapy on implant survival. Clin. Oral Implants Res. 2022, 33, 967–999. [Google Scholar] [CrossRef]
- Shugaa-Addin, B.; Al-Shamiri, H.M.; Al-Maweri, S.; Tarakji, B. The effect of radiotherapy on survival of dental implants in head and neck cancer patients. J. Clin. Exp. Dent. 2016, 8, e194–e200. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Meraw, S.; Al-Hezaimi, K.; Wang, H.-L. The Influence of Radiation Therapy on Dental Implantology. Implant Dent. 2013, 22, 31–38. [Google Scholar] [CrossRef]
- Marx, R.E.; Ehler, W.J.; Tayapongsak, P.; Pierce, L.W. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am. J. Surg. 1990, 160, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Dholam, K.P.; Gurav, S.V. Dental implants in irradiated jaws: A literature review. J. Cancer Res. Ther. 2012, 8, S85–S93. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Hezaimi, K.; Al-Rasheed, A.; Almas, K.; Romanos, G.E. Implant survival rate after oral cancer therapy: A review. Oral Oncol. 2010, 46, 854–859. [Google Scholar] [CrossRef]
- Zen Filho, E.V.; Tolentino, E.d.S.; Santos, P.S.S. Viability of dental implants in head and neck irradiated patients: A systematic review. Head Neck 2016, 38, E2229–E2240. [Google Scholar] [CrossRef]
- Granstrom, G.; Tjellstrom, A.; Granström, G.; Tjellström, A. Effects of irradiation on osseointegration before and after implant placement: A report of three cases. Int. J. Oral Maxillofac. Implants 1997, 12, 547–551. [Google Scholar]
- Claudy, M.P.; Miguens, S.A.Q.; Celeste, R.K.; Camara Parente, R.; Hernandez, P.A.G.; da Silva, A.N. Time interval after radiotherapy and dental implant failure: Systematic review of observational studies and meta-analysis. Clin. Implant Dent. Relat. Res. 2015, 17, 402–411. [Google Scholar] [CrossRef]
- Di Carlo, S.; De Angelis, F.; Ciolfi, A.; Quarato, A.; Piccoli, L.; Pompa, G.; Brauner, E. Timing for implant placement in patients treated with radiotherapy of head and neck. Clin. Ter. 2019, 170, E345–E351. [Google Scholar] [CrossRef]
- El-Rabbany, M.; Duchnay, M.; Raziee, H.R.; Zych, M.; Tenenbaum, H.; Shah, P.S.; Azarpazhooh, A. Interventions for preventing osteoradionecrosis of the jaws in adults receiving head and neck radiotherapy. Cochrane Database Syst. Rev. 2019, 2019, CD011559. [Google Scholar] [CrossRef]
- Batstone, M.D.; Cosson, J.; Marquart, L.; Acton, C. Platelet rich plasma for the prevention of osteoradionecrosis. A double blinded randomized cross over controlled trial. Int. J. Oral Maxillofac. Surg. 2012, 41, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Horiot, J.C.; Schraub, S.; Bone, M.C.; Bain, Y.; Ramadier, J.; Chaplain, G.; Nabid, N.; Thevenot, B.; Bransfield, D. Dental preservation in patients irradiated for head and neck tumours: A 10-year experience with topical fluoride and a randomized trial between two fluoridation methods. Radiother. Oncol. 1983, 1, 77–82. [Google Scholar] [CrossRef]
- Marx, R.E.; Johnson, R.P.; Kline, S.N. Prevention of osteoradionecrosis: A randomized prospective clinical trial of hyperbaric oxygen versus penicillin. J. Am. Dent. Assoc. 1985, 111, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Schoen, P.J.; Raghoebar, G.M.; Bouma, J.; Reintsema, H.; Vissink, A.; Sterk, W.; Roodenburg, J.L.N. Rehabilitation of oral function in head and neck cancer patients after radiotherapy with implant-retained dentures: Effects of hyperbaric oxygen therapy. Oral Oncol. 2007, 43, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.V.; Gomes, T.P.; da Silva, F.M.; Chambrone, L.; Marques, M.M.; Palma, L.F. Autologous platelet concentrates in extraction sockets for the prevention of osteoradionecrosis: A systematic review of controlled clinical trials. Oral Maxillofac. Surg. 2022, 26, 555–561. [Google Scholar] [CrossRef]
- Lombardi, N.; Varoni, E.; Villa, G.; Salis, A.; Lodi, G. Pentoxifylline and tocopherol for prevention of osteoradionecrosis in patients who underwent oral surgery: A clinical audit. Spec. Care Dent. 2023, 43, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Depondt, J.; Lefaix, J.L. Major healing of refractory mandible osteoradionecrosis after treatment combining pentoxifylline and tocopherol: A phase II trial. Head Neck 2005, 27, 114–123. [Google Scholar] [CrossRef]
- In ‘t Veld, M.; Schulten, E.A.J.M.; Leusink, F.K.J. Immediate dental implant placement and restoration in the edentulous mandible in head and neck cancer patients: A systematic review and meta-analysis. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 126–137. [Google Scholar] [CrossRef]
- Banjar, A.; Patel, V.; Abed, H. Pentoxifylline and tocopherol (vitamin E) with/without clodronate for the management of osteoradionecrosis: A scoping review. Oral Dis. 2021, 29, 29–39. [Google Scholar] [CrossRef]
- Arqueros-Lemus, M.; Mariño-Recabarren, D.; Niklander, S.; Martínez-Flores, R.; Moraga, V. Pentoxifylline and tocopherol for the treatment of osteoradionecrosis of the jaws. A systematic review. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e293–e300. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lombardi, N.; Villa, G.; Pispero, A.; Sardella, A.; Lodi, G. Conservative management of medication-related osteonecrosis of the jaws (Mronj): A retrospective cohort study. Antibiotics 2021, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Paiva, G.L.A.; de Campos, W.G.; Rocha, A.C.; Júnior, C.A.L.; Migliorati, C.A.; dos Santos Silva, A.R. Can the prophylactic use of pentoxifylline and tocopherol before dental extractions prevent osteoradionecrosis? A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 136, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Gadiwalla, Y.; Sassoon, I.; Sproat, C.; Kwok, J.; McGurk, M. Prophylactic use of pentoxifylline and tocopherol in patients who require dental extractions after radiotherapy for cancer of the head and neck. Br. J. Oral Maxillofac. Surg. 2016, 54, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Goutam, M.; Singh, M.; Kharat, N.; Singh, V.; Vyas, S.; Singh, H. Prophylactic use of pentoxifylline and tocopherol in patients undergoing dental extractions following radiotherapy for head and neck cancer. Niger. J. Surg. 2017, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Annamaraju, P.; Baradhi, K. Pentoxifylline. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Thom, S.R. Hyperbaric oxygen: Its mechanisms and efficacy. Plast. Reconstr. Surg. 2011, 127, 131S–141S. [Google Scholar] [CrossRef] [PubMed]
- Gevorgyan, A.; Wong, K.; Poon, I.; Blanas, N.; Enepekides, D.J.; Higgins, K.M. Osteoradionecrosis of the mandible: A case series at a single institution. J. Otolaryngol.-Head Neck Surg. 2013, 42, 46. [Google Scholar] [CrossRef]
- Dang, B.; Gamage, S.; Sethi, S.; Jensen, E.D.; Sambrook, P.; Goss, A. The role of hyperbaric oxygen in osteoradionecrosis—A prophylactic insight. Aust. Dent. J. 2023, 68, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Morrish, R.B.; Chan, E.; Silverman, S.; Meyer, J.; Fu, K.K.; Greenspan, D. Osteonecrosis in patients irradiated for head and neck carcinoma. Cancer 1981, 47, 1980–1983. [Google Scholar] [CrossRef]
- Wong, J.; Wood, R.; McLean, M. Conservative management of osteoradionecrosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1997, 84, 16–21. [Google Scholar] [CrossRef]
- Oh, H.K.; Chambers, M.S.; Martin, J.W.; Lim, H.J.; Park, H.J. Osteoradionecrosis of the Mandible: Treatment Outcomes and Factors Influencing the Progress of Osteoradionecrosis. J. Oral Maxillofac. Surg. 2009, 67, 1378–1386. [Google Scholar] [CrossRef]
- Shaw, R.J.; Butterworth, C.J.; Silcocks, P.; Tesfaye, B.T.; Bickerstaff, M.; Jackson, R.; Kanatas, A.; Nixon, P.; McCaul, J.; Praveen, P.; et al. HOPON (Hyperbaric Oxygen for the Prevention of Osteoradionecrosis): A Randomized Controlled Trial of Hyperbaric Oxygen to Prevent Osteoradionecrosis of the Irradiated Mandible After Dentoalveolar Surgery. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Hanna, G.J.; Margalit, D.N.; Chau, N.; Goguen, L.A.; Marty, F.M.; Rabinowits, G.; Schoenfeld, J.D.; Sonis, S.T.; Thomas, T.; et al. The Use of Hyperbaric Oxygen for the Prevention and Management of Osteoradionecrosis of the Jaw: A Dana-Farber/Brigham and Women’s Cancer Center Multidisciplinary Guideline. Oncologist 2017, 22, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G.W.; Gunsolley, J.C.; Abubaker, O.; Laskin, D.M. Efficacy of Pre- and Postirradiation Hyperbaric Oxygen Therapy in the Prevention of Postextraction Osteoradionecrosis: A Systematic Review. J. Oral Maxillofac. Surg. 2010, 68, 2653–2660. [Google Scholar] [CrossRef] [PubMed]
- Curi, M.M.; Condezo, A.F.B.; Ribeiro, K.D.C.B.; Cardoso, C.L. Long-term success of dental implants in patients with head and neck cancer after radiation therapy. Int. J. Oral Maxillofac. Surg. 2018, 47, 783–788. [Google Scholar] [CrossRef]
- August, M.; Bast, B.; Jackson, M.; Perrott, D. Use of the fixed mandibular implant in oral cancer patients: A retrospective study. J. Oral Maxillofac. Surg. 1998, 56, 297–301. [Google Scholar] [CrossRef]
- Cotic, J.; Jamsek, J.; Kuhar, M.; Hren, N.I.; Kansky, A.; Özcan, M.; Jevnikar, P. Implant-prosthetic rehabilitation after radiation treatment in head and neck cancer patients: A case-series report of outcome. Radiol. Oncol. 2016, 50, 94–100. [Google Scholar] [CrossRef]
- Benites Condezo, A.F.; Araujo, R.Z.; Koga, D.H.; Curi, M.M.; Cardoso, C.L. Hyperbaric oxygen therapy for the placement of dental implants in irradiated patients: Systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2021, 59, 625–632. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrao, G.; Mazzola, G.C.; Lombardi, N.; Marvaso, G.; Pispero, A.; Baruzzi, E.; Decani, S.; Tarozzi, M.; Bergamaschi, L.; Lorubbio, C.; et al. Oral Surgery and Osteoradionecrosis in Patients Undergoing Head and Neck Radiation Therapy: An Update of the Current Literature. Biomedicines 2023, 11, 3339. https://doi.org/10.3390/biomedicines11123339
Corrao G, Mazzola GC, Lombardi N, Marvaso G, Pispero A, Baruzzi E, Decani S, Tarozzi M, Bergamaschi L, Lorubbio C, et al. Oral Surgery and Osteoradionecrosis in Patients Undergoing Head and Neck Radiation Therapy: An Update of the Current Literature. Biomedicines. 2023; 11(12):3339. https://doi.org/10.3390/biomedicines11123339
Chicago/Turabian StyleCorrao, Giulia, Giovanni Carlo Mazzola, Niccolò Lombardi, Giulia Marvaso, Alberto Pispero, Elisa Baruzzi, Sem Decani, Marco Tarozzi, Luca Bergamaschi, Chiara Lorubbio, and et al. 2023. "Oral Surgery and Osteoradionecrosis in Patients Undergoing Head and Neck Radiation Therapy: An Update of the Current Literature" Biomedicines 11, no. 12: 3339. https://doi.org/10.3390/biomedicines11123339
APA StyleCorrao, G., Mazzola, G. C., Lombardi, N., Marvaso, G., Pispero, A., Baruzzi, E., Decani, S., Tarozzi, M., Bergamaschi, L., Lorubbio, C., Repetti, I., Starzyńska, A., Alterio, D., Ansarin, M., Orecchia, R., D’Amore, F., Franchini, R., Nicali, A., Castellarin, P., ... Jereczek-Fossa, B. A. (2023). Oral Surgery and Osteoradionecrosis in Patients Undergoing Head and Neck Radiation Therapy: An Update of the Current Literature. Biomedicines, 11(12), 3339. https://doi.org/10.3390/biomedicines11123339








