Improvement of Symptoms and Cardiac Magnetic Resonance Abnormalities in Patients with Post-Acute Sequelae of SARS-CoV-2 Cardiovascular Syndrome (PASC-CVS) after Guideline-Oriented Therapy
Abstract
:1. Introduction
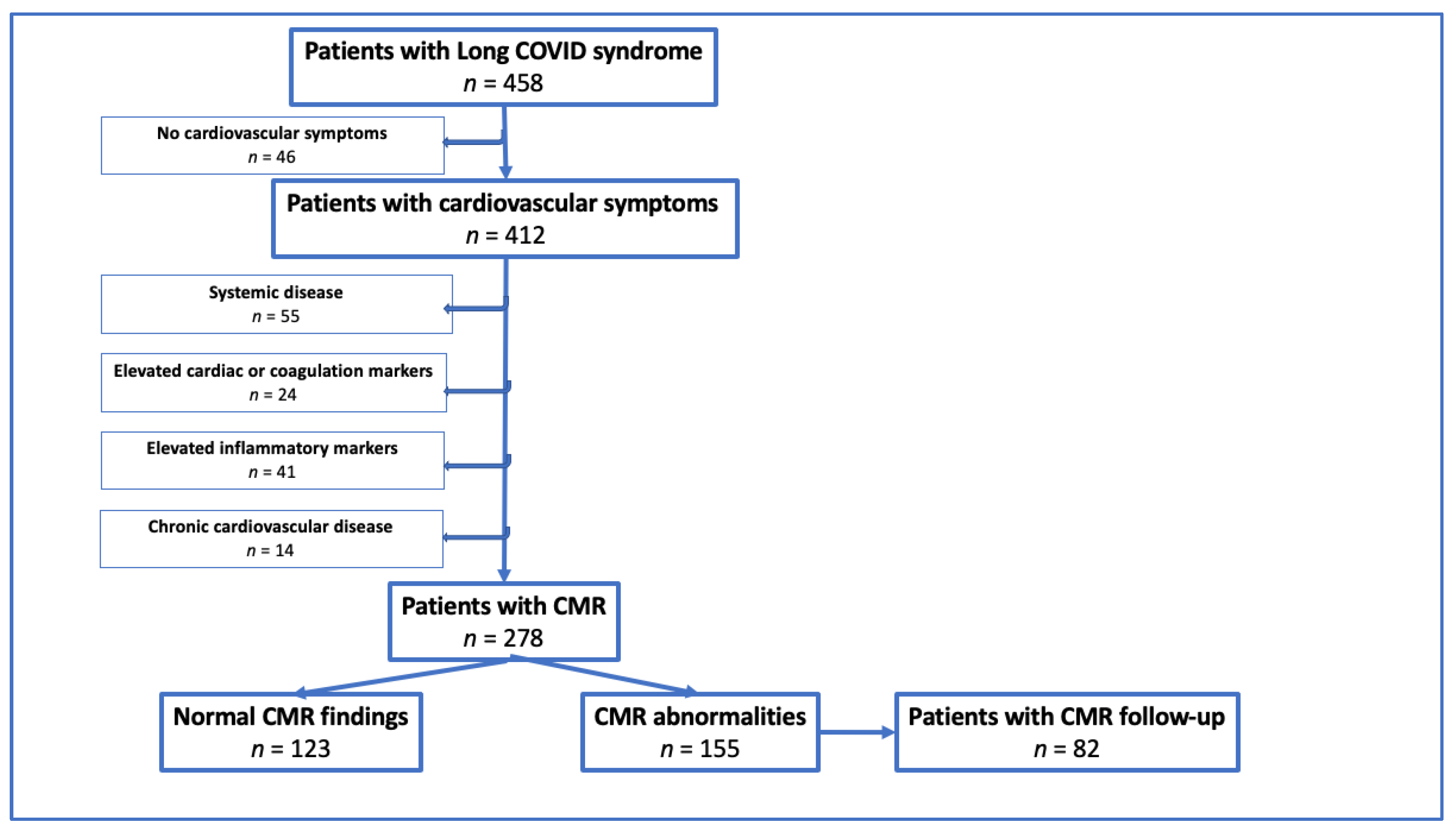
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Clinical Data
2.4. Laboratory Data
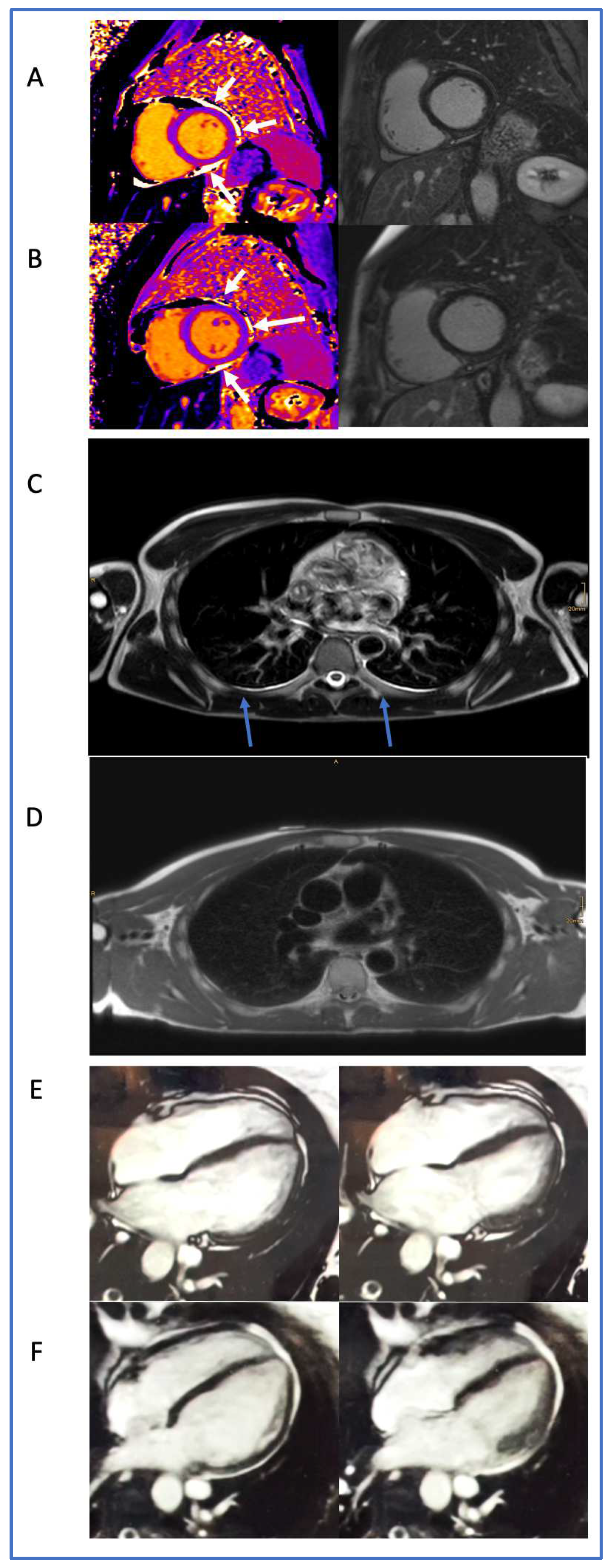
2.5. CMR Acquisition and Analysis
2.6. Definitions
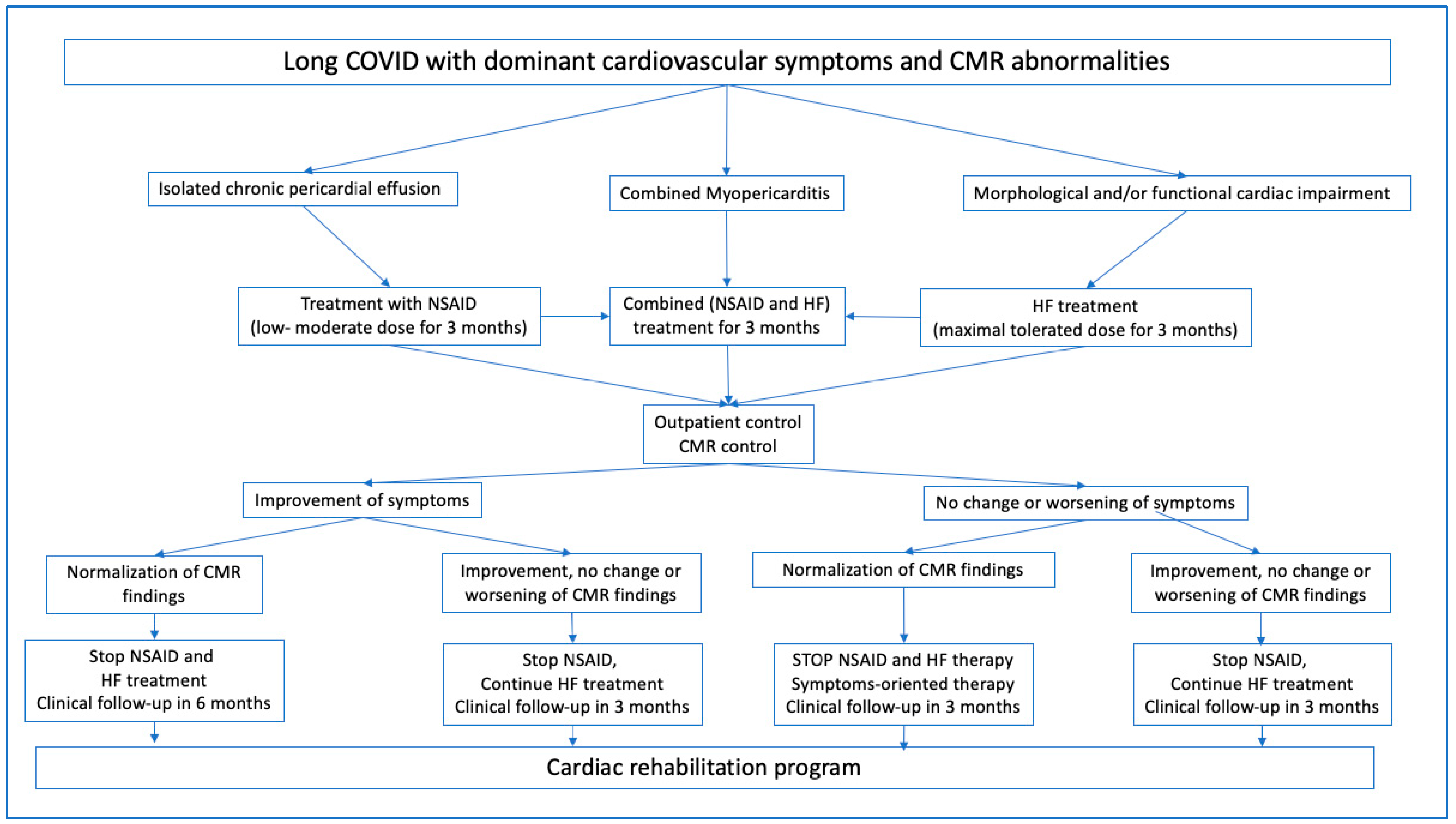
2.7. Treatments
2.8. Statistics
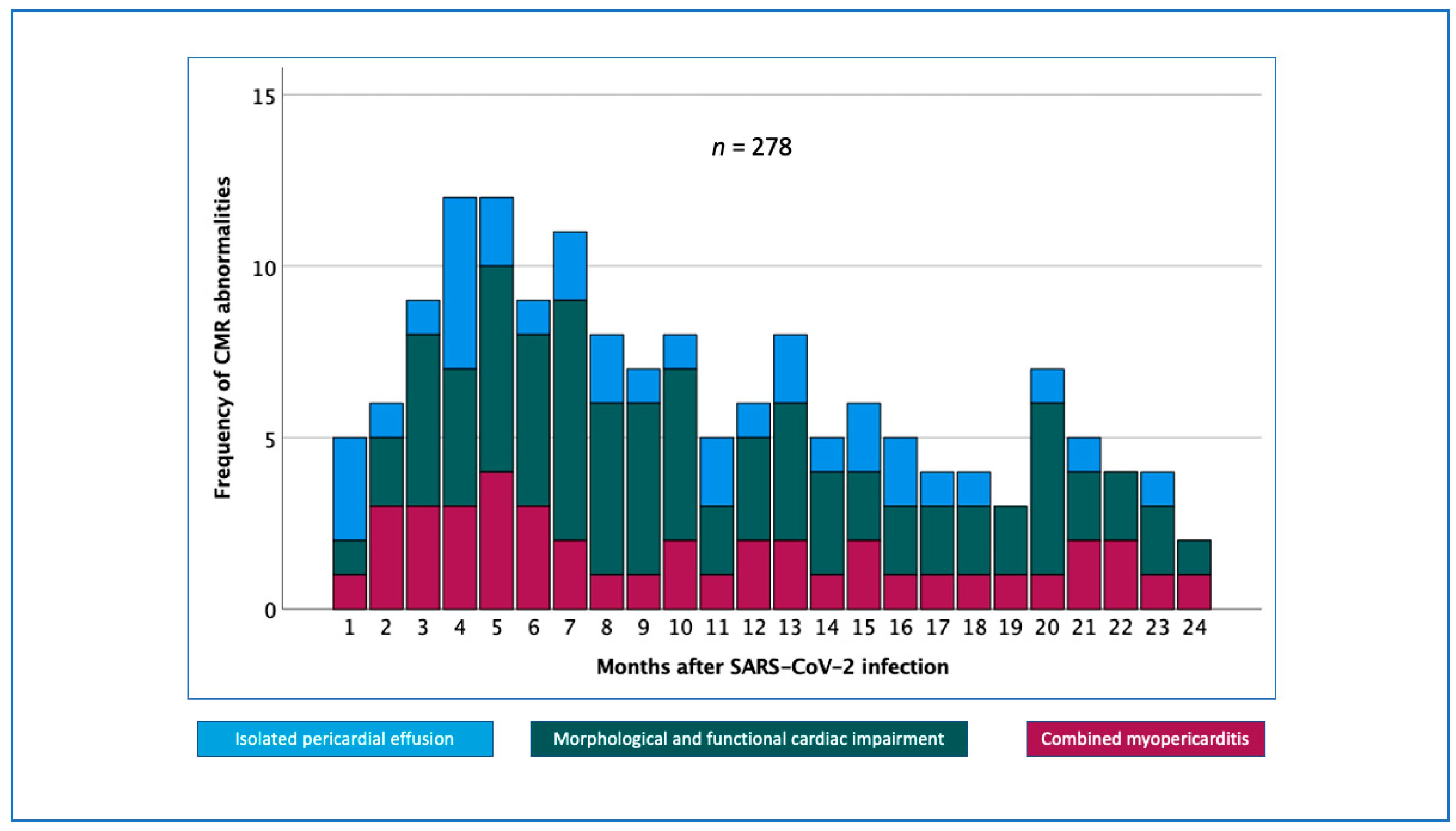
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gyongyosi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.; Camici, G.G.; Martins, P.D.C.; Ferdinandy, P.; Fontana, M.; Girao, H.; Gnecchi, M.; et al. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: A joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc. Res. 2023, 119, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Writing, C.; Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K., 3rd; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Martin, S.; Shchendrygina, A.; Hoffmann, J.; Ka, M.M.; Giokoglu, E.; Vanchin, B.; Holm, N.; Karyou, A.; Laux, G.S.; et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022, 28, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Vidula, M.K.; Rajewska-Tabor, J.; Cao, J.J.; Kang, Y.; Craft, J.; Mei, W.; Chandrasekaran, P.S.; Clark, D.E.; Poenar, A.M.; Gorecka, M.; et al. Myocardial Injury on CMR in Patients With COVID-19 and Suspected Cardiac Involvement. JACC Cardiovasc. Imaging 2023, 16, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Plein, S.; Wong, T.C.; Tao, Q.; Raisi-Estabragh, Z.; Jain, S.S.; Han, Y.; Ojha, V.; Bluemke, D.A.; Hanneman, K.; et al. Cardiovascular magnetic resonance for evaluation of cardiac involvement in COVID-19: Recommendations by the Society for Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reason. 2023, 25, 21. [Google Scholar] [CrossRef]
- Imazio, M.; Gaita, F.; LeWinter, M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA 2015, 314, 1498–1506. [Google Scholar] [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Lip, G.Y.H. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur. J. Clin. Investig. 2021, 51, e13679. [Google Scholar] [CrossRef]
- Mitiku, T.Y.; Heidenreich, P.A. A small pericardial effusion is a marker of increased mortality. Am. Heart J. 2011, 161, 152–157. [Google Scholar] [CrossRef]
- Zavada, J.; Dixon, W.G.; Askling, J.; EULAR Study Group on Longitudinal Observational Registers and Drug Studies. Launch of a checklist for reporting longitudinal observational drug studies in rheumatology: A EULAR extension of STROBE guidelines based on experience from biologics registries. Ann. Rheum. Dis. 2014, 73, 628. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.E.; Niehaus, W.N.; Whiteson, J.; Azola, A.; Baratta, J.M.; Fleming, T.K.; Kim, S.Y.; Naqvi, H.; Sampsel, S.; Silver, J.K.; et al. Response to letter to the editor regarding “Multi-Disciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in patients with Post-Acute sequelae of SARS-CoV-2 infection (PASC)”. PM&R 2021, 13, 1439–1440. [Google Scholar] [CrossRef]
- Haslacher, H.; Gerner, M.; Hofer, P.; Jurkowitsch, A.; Hainfellner, J.; Kain, R.; Wagner, O.F.; Perkmann, T. Usage Data and Scientific Impact of the Prospectively Established Fluid Bioresources at the Hospital-Based MedUni Wien Biobank. Biopreserv. Biobank. 2018, 16, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reason. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Kelle, S.; Bucciarelli-Ducci, C.; Judd, R.M.; Kwong, R.Y.; Simonetti, O.; Plein, S.; Raimondi, F.; Weinsaft, J.W.; Wong, T.C.; Carr, J. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J. Cardiovasc. Magn. Reason. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reason. 2020, 22, 19. [Google Scholar] [CrossRef]
- Kammerlander, A.A.; Marzluf, B.A.; Zotter-Tufaro, C.; Aschauer, S.; Duca, F.; Bachmann, A.; Knechtelsdorfer, K.; Wiesinger, M.; Pfaffenberger, S.; Greiser, A.; et al. T1 Mapping by CMR Imaging: From Histological Validation to Clinical Implication. JACC Cardiovasc. Imaging 2016, 9, 14–23. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P. The 2015 ESC Guidelines on the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 2873–2874. [Google Scholar] [CrossRef]
- Chiabrando, J.G.; Bonaventura, A.; Vecchie, A.; Wohlford, G.F.; Mauro, A.G.; Jordan, J.H.; Grizzard, J.D.; Montecucco, F.; Berrocal, D.H.; Brucato, A.; et al. Management of Acute and Recurrent Pericarditis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 76–92. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.G.; Rigante, D.; Sicignano, L.L.; Verrecchia, E.; De Vito, F.; Gasbarrini, A.; Manna, R. Therapeutic management of idiopathic recurrent serositis: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3352–3359. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, Y.; Hader, S.N.; Beyer, A.M. Differential impacts of COVID-19 variants on human microvascular function. Cardiovasc. Res. 2023, 119, e115–e117. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Fonarow, G.C. Coronavirus Disease 2019 (COVID-19) and the Heart—Is Heart Failure the Next Chapter? JAMA Cardiol. 2020, 5, 1216–1217. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Sundaramoorthy, E.; Leonard, M.; Mak, R.; Liao, J.; Fulzele, A.; Bennett, E.J. ZNF598 and RACK1 Regulate Mammalian Ribosome-Associated Quality Control Function by Mediating Regulatory 40S Ribosomal Ubiquitylation. Mol. Cell. 2017, 65, 751–760.e4. [Google Scholar] [CrossRef]
- Losada, I.; Gonzalez-Moreno, J.; Roda, N.; Ventayol, L.; Borjas, Y.; Dominguez, F.J.; Fernandez-Baca, V.; Garcia-Gasalla, M.; Payeras, A. Polyserositis: A diagnostic challenge. Intern. Med. J. 2018, 48, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Shanghavi, S.; Viner, T. Polyserositis secondary to COVID-19: The diagnostic dilemma. BMJ Case Rep. 2021, 14, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Delmonaco, A.G.; Carpino, A.; Raffaldi, I.; Pruccoli, G.; Garrone, E.; Del Monte, F.; Riboldi, L.; Licciardi, F.; Urbino, A.F.; Parodi, E. First diagnosis of multisystem inflammatory syndrome in children (MIS-C): An analysis of PoCUS findings in the ED. Ultrasound J. 2021, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Lazaros, G.; Imazio, M.; Tsioufis, P.; Lazarou, E.; Vlachopoulos, C.; Tsioufis, C. Chronic Pericardial Effusion: Causes and Management. Can. J. Cardiol. 2023, 39, 1121–1131. [Google Scholar] [CrossRef]
- Lamontagne, F.; Stegemann, M.; Agarwal, A.; Agoritsas, T.; Siemieniuk, R.; Rochwerg, B.; Bartoszko, J.; Askie, L.; Macdonald, H.; Al-Maslamani, M.; et al. A living WHO guideline on drugs to prevent COVID-19. BMJ 2021, 372, n526. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, Y.; Hashimoto, Y.; Motokura, T. Frequent co-reactivation of Epstein-Barr virus in patients with cytomegalovirus viremia under immunosuppressive therapy and/or chemotherapy. J. Int. Med. Res. 2020, 48, 300060520972880. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Song, J.; Liu, H.; Zheng, H.; Chen, C. Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 2021, 11, 10902. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Lukovic, D.; Mester-Tonczar, J.; Zlabinger, K.; Einzinger, P.; Spannbauer, A.; Schweiger, V.; Schefberger, K.; Samaha, E.; Bergler-Klein, J.; et al. Effect of monovalent COVID-19 vaccines on viral interference between SARS-CoV-2 and several DNA viruses in patients with long-COVID syndrome. NPJ Vaccines 2023, 8, 145. [Google Scholar] [CrossRef]
- Yar, A.; Uusitalo, V.; Vaara, S.M.; Holmstrom, M.; Vuorinen, A.M.; Helio, T.; Paakkanen, R.; Kivisto, S.; Syvaranta, S.; Hastbacka, J. Cardiac magnetic resonance -detected myocardial injury is not associated with long-term symptoms in patients hospitalized due to COVID-19. PLoS ONE 2023, 18, e0282394. [Google Scholar] [CrossRef]

| Clinical Characteristics and Laboratory Findings | n = 278 |
|---|---|
| Time between COVID-19 infection and CMR (days) | 328 ± 214 |
| Number of patients with at least one COVID-19 vaccine (prior to or after COVID-19 infection) | 227 (81.7%) |
| Anti-spike protein titer (AU/mL) | 1546 ± 1093 |
| Female sex n (%) | 196 (70.5%) |
| Age (years) | 43 ± 13 |
| Body mass index (kg/m2) | 25.2 ± 5.2 |
| Diabetes mellitus n (%) | 7 (2.5%) |
| Hypertension n (%) | 44 (15.8%) |
| Hyperlipidemia n (%) | 39 (14.0%) |
| Smoking n (%) | 9 (3.2%) |
| Systolic blood pressure (mmHg) | 129 ± 17 |
| Diastolic blood pressure (mmHg) | 82 ± 11 |
| Heart rate (bpm) | 73 ± 12 |
| Cumulative ECG abnormalities n (%) | 66 (23.7%) |
| Cardiac arrhythmias n (%) | 13 (4.7%) |
| Conduction abnormalities n (%) | 59 (21.2%) |
| QRS width (ms) | 89.2 ± 12.7 |
| Leukocyte (G/L) | 7.0 ± 2.1 |
| Fibrinogen (mg/dL) | 313 ± 67 |
| D-dimer (ug/mL) | 0 (0;0.37) |
| Cardiolipin IgG (U/mL) | 1.2 (0;1.7) |
| Cardiolipin IgM (U/mL) | 1.4 (0;2.3) |
| Creatine kinase (U/L) | 89 (62;122) |
| Creatine kinase myocardial subfraction (U/L) | 14.1 (12;18.5) |
| Troponin T (ng/L) | 0 (0;6) |
| NT-proBNP (pg/mL) | 44.0 (23.7;82.0) |
| C-reactive protein (mg/dL) | 0.08 (0.04;0.20) |
| Rheumafactor latex (IU/mL) | 0.0 (0.0;0.0) |
| Alpha 1 antitrypsin (mg/dL) | 138 ± 24.2 |
| Interleukin-6 (pg/mL) | 0 (0;2.14) |
| Procalcitonin (ng/mL) | 0.03 (0;0.04) |
| Transferrin (mg/dL) | 267.5 ± 45.6 |
| Transferrin saturation (%) | 24.3 ± 10.4 |
| CMR Findings | n = 278 |
|---|---|
| No abnormalities | 123 (44.2%) |
| Cumulative CMR abnormalities n (%) | 155 (55.8%) |
| Isolated pericardial effusion (without functional impairment) n (%) | 34/278 (12.2%) |
| Morphological and functional impairment n (%) | 79/278 (28.4%) |
| Combined myopericarditis n (%) | 42/278 (15.1%) |
| Pericardial effusion (w/wo functional impairment) n (%) | 72 (25.9%) |
| Reduced LVF n (%) | 39 (14.0%) |
| Reduced RVF n (%) | 55 (19.8%) |
| Biventricular enlargement n (%) | 21 (7.6%) |
| Isolated LV enlargement n (%) | 56 (20.1%) |
| Isolated RV enlargement n (%) | 47 (16.9%) |
| Myocardial edema n (%) | 9 (3.2%) |
| T1 increase n (%) | 5 (1.8%) |
| Nonischemic late gadolinium enhancement | 35 (12.6%) |
| Pleural effusion n (%) | 16 (5.8%) |
| CMR LV EF (%) | 59 ± 7 |
| CMR LV EDV (mL) | 142 ± 36 |
| CMR LV ESV (mL) | 60 ± 20 |
| CMR RV EF (%) | 55 ± 6 |
| CMR RV EDV (mL) | 152 ± 39 |
| CMR RV ESV (mL) | 70 ± 25 |
| CMR Findings Pre- and Post-Treatment | Baseline (n = 82) | Follow-up (n = 82) | p Value |
|---|---|---|---|
| Cumulative CMR abnormalities n (%) | 82 (100%) | 53 (64.6%) | <0.001 |
| CMR phenotype n (%) | <0.001 | ||
| No abnormalities n (%) | 29 (35.4%) | ||
| Isolated pericardial effusion (without functional impairment) n (%) | 19 (23.2%) | 22 (26.8%) | |
| Morphological and functional impairment n (%) | 29 (35.4%) | 20 (24.4%) | |
| Combined myopericarditis n (%) | 34 (41.5%) | 11 (13.4%) | |
| Pericardial effusion (w/wo functional impairment) n (%) | 51 (62.2%) | 32 (39.0%) | 0.005 |
| Reduced LVF n (%) | 25 (30.5%) | 4 (4.9%) | <0.001 |
| Reduced RVF n (%) | 27 (32.9%) | 11 (13.4%) | 0.039 |
| Biventricular enlargement n (%) | 14 (17.1%) | 9 (11.0%) | |
| Isolated LV enlargement n (%) | 29 (35.4%) | 16 (19.5%) | 0.035 |
| Isolated RV enlargement n (%) | 18 (22.0%) | 16 (19.5%) | |
| Myocardial edema n (%) | 8 (9.8%) | 1 (1.2%) | 0.034 |
| T1 increase n (%) | 1 (1.2%) | 0 (0%) | |
| Nonischemic late gadolinium enhancement n (%) | 20 (24.4%) | 16 (19.5%) | |
| Pleural effusion n (%) | 5 (6.2%) | 8 (9.8%) | |
| Pericardial effusion (mm) * | 4 (3;5.75) | 2 (0;3) | <0.001 |
| CMR LV EF (%) | 57 ± 7 | 59 ± 5 | 0.034 |
| CMR LV EDV (mL) | 150 ± 39 | 151 ± 36 | |
| CMR LV ESV (mL) | 65 ± 21 | 64 ± 20 | |
| CMR RV EF (%) | 53 ± 7 | 557 ± 7 | |
| CMR RV EDV (mL) | 161 ± 44 | 167 ± 44 | |
| CMR RV ESV (mL) | 76 ± 25 | 76 ± 29 |
| Subgroups of CMR Phenotypes/Follow-up CMR Findings after Treatment | Normalized | Improved | Unchanged | Worsened | Total |
|---|---|---|---|---|---|
| Isolated pericardial effusion (without functional impairment) n (%) | 6 (31.6%) | 5 (26.3%) | 5 (26.3%) | 3 (15.8%) | 19 (100%) |
| Morphological and functional impairment n (%) | 12 (41.4%) | 7 (24.1%) | 8 (27.6%) | 2 (6.9%) | 29 (100%) |
| Combined myopericarditis n (%) | 11 (32.4%) | 22 (64.7%) | 1 (2.9%) | 0 (0%) | 34 (100%) |
| Total | 29 (35.4%) | 34 (41.5%) | 14 (17.1%) | 5 (6.1%) | 82 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyöngyösi, M.; Hasimbegovic, E.; Han, E.; Zlabinger, K.; Spannbauer, A.; Riesenhuber, M.; Hamzaraj, K.; Bergler-Klein, J.; Hengstenberg, C.; Kammerlander, A.; et al. Improvement of Symptoms and Cardiac Magnetic Resonance Abnormalities in Patients with Post-Acute Sequelae of SARS-CoV-2 Cardiovascular Syndrome (PASC-CVS) after Guideline-Oriented Therapy. Biomedicines 2023, 11, 3312. https://doi.org/10.3390/biomedicines11123312
Gyöngyösi M, Hasimbegovic E, Han E, Zlabinger K, Spannbauer A, Riesenhuber M, Hamzaraj K, Bergler-Klein J, Hengstenberg C, Kammerlander A, et al. Improvement of Symptoms and Cardiac Magnetic Resonance Abnormalities in Patients with Post-Acute Sequelae of SARS-CoV-2 Cardiovascular Syndrome (PASC-CVS) after Guideline-Oriented Therapy. Biomedicines. 2023; 11(12):3312. https://doi.org/10.3390/biomedicines11123312
Chicago/Turabian StyleGyöngyösi, Mariann, Ena Hasimbegovic, Emilie Han, Katrin Zlabinger, Andreas Spannbauer, Martin Riesenhuber, Kevin Hamzaraj, Jutta Bergler-Klein, Christian Hengstenberg, Andreas Kammerlander, and et al. 2023. "Improvement of Symptoms and Cardiac Magnetic Resonance Abnormalities in Patients with Post-Acute Sequelae of SARS-CoV-2 Cardiovascular Syndrome (PASC-CVS) after Guideline-Oriented Therapy" Biomedicines 11, no. 12: 3312. https://doi.org/10.3390/biomedicines11123312
APA StyleGyöngyösi, M., Hasimbegovic, E., Han, E., Zlabinger, K., Spannbauer, A., Riesenhuber, M., Hamzaraj, K., Bergler-Klein, J., Hengstenberg, C., Kammerlander, A., Kastl, S., Loewe, C., & Beitzke, D. (2023). Improvement of Symptoms and Cardiac Magnetic Resonance Abnormalities in Patients with Post-Acute Sequelae of SARS-CoV-2 Cardiovascular Syndrome (PASC-CVS) after Guideline-Oriented Therapy. Biomedicines, 11(12), 3312. https://doi.org/10.3390/biomedicines11123312








