Does COVID-19 Infection during Pregnancy Increase the Appearance of Congenital Gastrointestinal Malformations in Neonates?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Limitations in Our Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Mocanu, V.; Bhagwani, D.; Sharma, A.; Borza, C.; Rosca, C.I.; Stelian, M.; Bhagwani, S.; Haidar, L.; Kshtriya, L.; Kundnani, N.R.; et al. COVID-19 and the Human Eye: Conjunctivitis, a Lone COVID-19 Finding—A Case-Control Study. Med. Princ. Pract. 2022, 31, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rosca, C.I.; Branea, H.S.; Sharma, A.; Nicoras, V.A.; Borza, C.; Lighezan, D.F.; Morariu, S.I.; Kundnani, N.R. Rhythm Disturbances in Post-Acute COVID-19 Syndrome in Young Men without Pre-Existing Known Cardiovascular Disease—A Case Series. Biomedicines 2023, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Poon, L.C. Novel coronavirus infection and pregnancy. Ultrasound Obstet. Gynecol. 2020, 55, 435–437. [Google Scholar] [CrossRef]
- Sánchez-García, J.C.; Moreno, N.P.C.; Tovar-Gálvez, M.I.; Cortés-Martín, J.; Liñán-González, A.; Olmedo, L.A.; Rodríguez-Blanque, R. COVID-19 in Pregnant Women, Maternal—Fetal Involvement, and Vertical Mother-to-Child Transmission: A Systematic Review. Biomedicines 2022, 10, 2554. [Google Scholar] [CrossRef]
- Prabhudas, M.; Bonney, E.; Caron, K.; Dey, S.; Erlebacher, A.; Fazleabas, A.; Fisher, S.; Golos, T.; Matzuk, M.; McCune, J.M.; et al. Immune mechanisms at the maternal-fetal interface: Perspectives and challenges. Nat. Immunol. 2015, 16, 328–334. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J. COVID-19 and Pregnancy. Infect. Dis. Clin. N. Am. 2022, 36, 423–433. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Zhu, Z.; Liu, Y. Coronavirus disease 2019 (COVID-19) and pregnancy: A systematic review. J. Matern. Neonatal Med. 2020, 35, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Zamaniyan, M.; Ebadi, A.; Aghajanpoor, S.; Rahmani, Z.; Haghshenas, M.; Azizi, S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat. Diagn. 2020, 40, 1759–1761. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jun, L.; Nawsherwan; Siddique, R.; Li, Y.; Han, G.; Xue, M.; Nabi, G.; Liu, J. Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin. Microbiol. Infect. 2020, 26, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Dolk, H.; Loane, M.; Garne, E. The prevalence of congenital anomalies in Europe. Adv. Exp. Med. Biol. 2010, 686, 349–364. [Google Scholar] [PubMed]
- He, R.; Zhang, J.; Mao, Y.; Degomme, O.; Zhang, W.-H. Preparedness and Responses Faced during the COVID-19 Pandemic in Belgium: An Observational Study and Using the National Open Data. Int. J. Environ. Res. Public Health 2020, 17, 7985. [Google Scholar] [CrossRef] [PubMed]
- Bin Nisar, Y.; Aurangzeb, B.; Dibley, M.J.; Alam, A. Qualitative exploration of facilitating factors and barriers to use of antenatal care services by pregnant women in urban and rural settings in Pakistan. BMC Pregnancy Childbirth 2016, 16, 42. [Google Scholar]
- Patra, C.; Sarkar, S.; Dasgupta, M.; Nayek, K.; Karmakar, P. Prevalence of congenital anomalies in neonates and associated risk factors in a tertiary care hospital in eastern India. J. Clin. Neonatol. 2013, 2, 131–134. [Google Scholar] [CrossRef]
- Boyle, M.I.; Jespersgaard, C.; Brøndum-Nielsen, K.; Bisgaard, A.M.; Tümer, Z. Cornelia de Lange syndrome. Clin. Genet. 2015, 88, 1–12. [Google Scholar] [CrossRef]
- Liao, Y.; Wen, H.; Ouyang, S.; Yuan, Y.; Bi, J.; Guan, Y.; Fu, Q.; Yang, X.; Guo, W.; Huang, Y.; et al. Routine first-trimester ultrasound screening using a standardized anatomical protocol. Am. J. Obstet. Gynecol. 2020, 224, 396.e1–396.e15. [Google Scholar] [CrossRef]
- Menon, P.; Binu, V.; Rao, K.L.N.; Suri, V. Trends in referral pattern of antenatally diagnosed surgical abnormalities in a tertiary care center in North India. J. Indian Assoc. Pediatr. Surg. 2018, 23, 198–202. [Google Scholar] [CrossRef]
- Goldshtein, I.; Steinberg, D.M.; Kuint, J.; Chodick, G.; Segal, Y.; Ben David, S.S.; Ben-Tov, A. Association of BNT162b2 COVID-19 Vaccination During Pregnancy With Neonatal and Early Infant Outcomes. JAMA Pediatr. 2022, 176, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.C.H.; Kan, A.S.Y.; Chung, P.H.; Shek, N.W.M.; Chan, I.H.Y.; Wong, K.K.Y. Antenatal counselling of congenital surgical anomalies: A decade of experience in a local tertiary centre. J. Paediatr. Child Health 2021, 57, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Hon, K.; Leong, K. Rubella (German measles) revisited. Hong Kong Med. J. 2019, 25, 134–141. [Google Scholar] [CrossRef]
- Hoffman, M.C.; Freedman, R.; Law, A.J.; Clark, A.M.; Hunter, S.K. Maternal nutrients and effects of gestational COVID-19 infection on fetal brain development. Clin. Nutr. ESPEN 2021, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Faizan, I.; Abdullah, M.; Ali, S.; Naqvi, I.H.; Ahmed, A.; Parveen, S. Zika Virus-Induced Microcephaly and Its Possible Molecular Mechanism. Intervirology 2016, 59, 152–158. [Google Scholar] [CrossRef]
- Luteijn, J.M.; Brown, M.J.; Dolk, H. Influenza and congenital anomalies: A systematic review and meta-analysis. Hum. Reprod. 2013, 29, 809–823. [Google Scholar] [CrossRef]
- Zafeiri, A.; Raja, E.A.; Mitchell, R.T.; Hay, D.C.; Bhattacharya, S.; A Fowler, P. Maternal over-the-counter analgesics use during pregnancy and adverse perinatal outcomes: Cohort study of 151 141 singleton pregnancies. BMJ Open 2022, 12, e048092. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020, 222, 415–426. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Taheri, M.; Mazaheripour, Z.; Abbasi-Khameneh, F. The incidence of congenital anomalies in newborns before and during the COVID-19 pandemic. Ital. J. Pediatr. 2022, 48, 174. [Google Scholar] [CrossRef]
- Brandibur, T.E.; Manea, A.M.; Sharma, A.; Kundnani, N.R.; Popoiu, M.C.; Ahmad, B.; Dahdal, D.S.; Cioboata, D.; Lungu, N.; Doandes, F.M.; et al. Macronutrients Management for Growth in Neonates with Congenital Gastrointestinal Malformation. Experiment 2022, 28, e938106. [Google Scholar] [CrossRef]
- Ben, X.-M. Nutritional management of newborn infants: Practical guidelines. World J. Gastroenterol. 2008, 14, 6133–6139. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://old.ms.ro/?pag=181 (accessed on 20 June 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 June 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bland, M. An Introduction to Medical Statistics, 3rd ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Yu, W.; Hu, X.; Cao, B. Viral Infections During Pregnancy: The Big Challenge Threatening Maternal and Fetal Health. Matern. Med. 2021, 4, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Ravaldi, C.; Wilson, A.; Ricca, V.; Homer, C.; Vannacci, A. Pregnant women voice their concerns and birth expectations during the COVID-19 pandemic in Italy. Women Birth 2020, 34, 335–343. [Google Scholar] [CrossRef]
- Ayaz, R.; Hocaoğlu, M.; Günay, T.; Yardımcı, O.D.; Turgut, A.; Karateke, A. Anxiety and depression symptoms in the same pregnant women before and during the COVID-19 pandemic. JPME 2020, 48, 965–970. [Google Scholar] [CrossRef]
- Tomfohr-Madsen, L.M.; Racine, N.; Giesbrecht, G.F.; Lebel, C.; Madigan, S. Depression and anxiety in pregnancy during COVID-19: A rapid review and meta-analysis. Psychiatry Res. 2021, 300, 113912. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Erdei, C.; Mittal, L. Risk factors for depression, anxiety, and PTSD symptoms in perinatal women during the COVID-19 Pandemic. Psychiatry Res. 2021, 295, 113552. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2017, 15, 230–240. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Cheng, Z.; Zhong, Y.; Chen, X.; Song, W. Triglyceride glucose-body mass index and the risk of diabetes: A general population-based cohort study. Lipids Health Dis. 2021, 20, 99. [Google Scholar] [CrossRef]
- Conklin, A.I.; Guo, S.X.; Tam, A.C.; Richardson, C.G. Gender, stressful life events and interactions with sleep: A systematic review of determinants of adiposity in young people. BMJ Open 2018, 8, e019982. [Google Scholar] [CrossRef]
- Fortin, O.; Mulkey, S.B. Neurodevelopmental outcomes in congenital and perinatal infections. Curr. Opin. Infect. Dis. 2023, 36, 405–413. [Google Scholar] [CrossRef]
- Rosca, C.I.; Sharma, A.; Nisulescu, D.-D.; Otiman, G.; Duda-Seiman, D.-M.; Morariu, S.I.; Lighezan, D.F.; Kundnani, N.R. Prevalence of Cardio-Embolic Brain Complications in Permanent and Paroxysmal Atrial Fibrillation Patients. Healthcare 2023, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- MacDonell, N.; Hancox, R.J. Childhood and Adolescent Television Viewing and Metabolic Syndrome in Mid-Adulthood. Pediatrics 2023, 152, e2022060768. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Christodorescu, R.; Agbariah, A.; Duda-Seiman, D.; Dahdal, D.; Man, D.; Kundnani, N.R.; Cretu, O.M.; Dragan, S. Cardiovascular Risk Prediction Parameters for Better Management in Rheumatic Diseases. Healthcare 2022, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Rosca, C.I.; Lighezan, D.F.; Nisulescu, D.-D.; Sharma, A.; Neagu, M.N.; Nistor, D.; Georgescu, D.; Kundnani, N.R. Metabolic Syndrome: A Strange Companion of Atrial Fibrillation; A Blessing in Disguise from the Neuropsychiatric Point of View. Biomedicines 2023, 11, 2012. [Google Scholar] [CrossRef]
- Villarreal, S.E.G.; Pacheco, S.M.S.; Alaniz, F.V.; Conde, M.I.B.; Pacheco, J.M.S.; Vazquez, K.C.C.; Leal, A.C.S.; Maldonado, O.A.T.; Contreras, J.A.R.; Cosain, E.I.H.; et al. Risk Factors Associated with COVID-19 Mortality in the State of Durango, Mexico. Int. J. Med. Sci. 2023, 20, 993–999. [Google Scholar] [CrossRef]
- Rosca, C.I.; Kundnani, N.R.; Tudor, A.; Rosca, M.-S.; Nicoras, V.-A.; Otiman, G.; Ciurariu, E.; Ionescu, A.; Stelian, M.; Sharma, A.; et al. Benefits of prescribing low-dose digoxin in atrial fibrillation. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211051955. [Google Scholar] [CrossRef]
- Kundnani, N.R.; Rosca, C.I.; Sharma, A.; Tudor, A.; Rosca, M.S.; Nisulescu, D.D.; Branea, H.S.; Mocanu, V.; Crisan, D.C.; Buzas, D.R.; et al. Selecting the right anticoagulant for stroke prevention in atrial fibrillation. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4499–4505. [Google Scholar]
- Liu, E.N.; Yang, J.H.; Patel, L.; Arora, J.; Gooding, A.; Ellis, R.; Graves, J.S. Longitudinal analysis and treatment of neuropsychiatric symptoms in post-acute sequelae of COVID-19. J. Neurol. 2023, 270, 4661–4672. [Google Scholar] [CrossRef]
- Bauer, K.A.; Puzniak, L.A.; Yu, K.C.; Klinker, K.P.; Watts, J.A.; Moise, P.A.; Finelli, L.; Gupta, V. Association of SARS-CoV-2 status and antibiotic-resistant bacteria with inadequate empiric therapy in hospitalized patients: A US multicenter cohort evaluation (July 2019–October 2021). BMC Infect Dis. 2023, 23, 490. [Google Scholar] [CrossRef]
- Turdybekova, Y.G.; Kopobayeva, I.L.; Kamyshanskiy, Y.K.; Turmukhambetova, A.A. Comparative clinical and placental pathologic characteristics in pregnancies with and without SARS-CoV-2 infection. J. Perinat. Med. 2023, 51, 1179–1188. [Google Scholar] [CrossRef]
- Garabedian, C.; Vaast, P.; Bigot, J.; Sfeir, R.; Michaud, L.; Gottrand, F.; Verpillat, P.; Coulon, C.; Subtil, D.; Houfflin Debarge, V. Esophageal atresia: Prevalence, prenatal diagnosis and prognosis. J. Gynecol. Obstet. Biol. Reprod. 2014, 43, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Reppucci, M.; Kaizer, A.; Prendergast, C.; Acker, S.; Mandell, E.; Euser, A.; Diaz-Miron, J. Incidence of congenital complications related to COVID-19 infection during pregnancy. J. Neonatal-Perinatal Med. 2023, 16, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Tárnok, A.; Méhes, K. Gastrointestinal Malformations, Associated Congenital Abnormalities, and Intrauterine Growth. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Doandes, F.M.; Manea, A.-M.; Lungu, N.; Cioboata, D.; Brandibur, T.; Costescu, O.; Hudisteanu, A.; Boia, E.R.; Boia, M. Clinical, biological and electroencephalographic monitoring of newborns with neurological risk in the Neonatal Intensive Care Unit. Exp. Ther. Med. 2021, 22, 760. [Google Scholar] [CrossRef] [PubMed]
- Luxi, N.; Giovanazzi, A.; Capuano, A.; Crisafulli, S.; Cutroneo, P.M.; Fantini, M.P.; Ferrajolo, C.; Moretti, U.; Poluzzi, E.; Raschi, E.; et al. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug Saf. 2021, 44, 1247–1269. [Google Scholar] [CrossRef] [PubMed]
- Goldshtein, I.; Nevo, D.; Steinberg, D.M.; Rotem, R.S.; Gorfine, M.; Chodick, G.; Segal, Y. Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women. JAMA 2021, 326, 728–735. [Google Scholar] [CrossRef]
- Ekingen, G.; Ceran, C.; Guvenc, B.H.; Tuzlaci, A.; Kahraman, H. Early enteral feeding in newborn surgical patients. Nutrition 2005, 21, 142–146. [Google Scholar] [CrossRef]
- Jorgensen, S.C.; Tabbara, N.; Burry, L. A review of COVID-19 therapeutics in pregnancy and lactation. Obstet. Med. 2022, 15, 225–232. [Google Scholar] [CrossRef]
- Domnicu, A.; Mogoi, M.; Manea, A.; Boia, E.R.; Boia, M. Clinical Factors Associated with COVID-19 Severity in Chronic Hospitalized Infants and Toddlers: Data from a Center in the West Part of Romania. Healthcare 2022, 10, 808. [Google Scholar] [CrossRef]



| % or N (%) | |
|---|---|
| Rural/Urban | 50%/50% |
| Primipara (P/MP) | 46%/54% |
| COVID-19 Status: | |
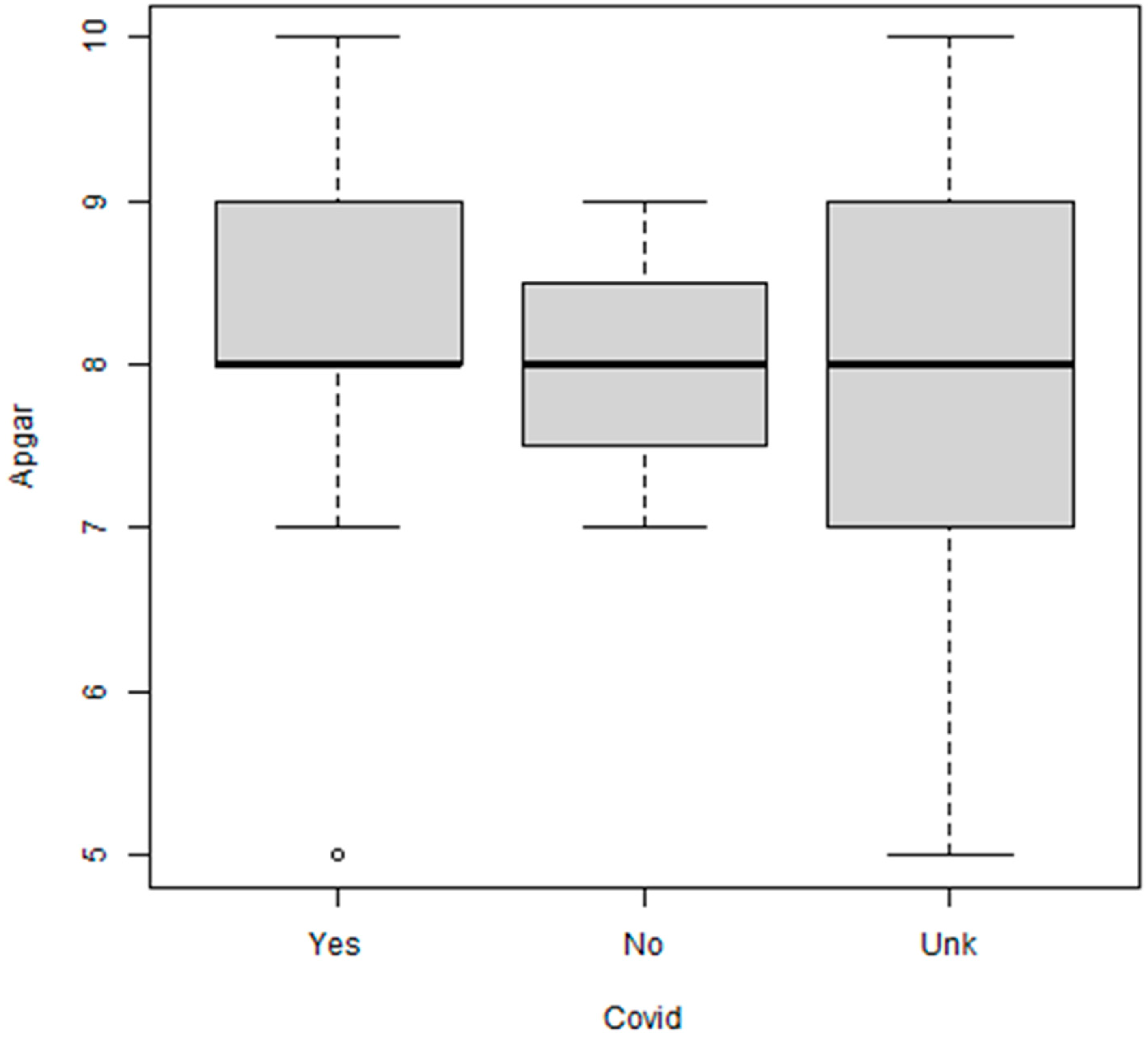
| Yes | 14 (29.8%) |
| No | 8 (17%) |
| Unknown | 25 (53.2%) |
| COVID-19 Vaccine (Yes/No) | 13%/87% |
| Vegan (Yes/No) | 9%/91% |
| Sex (M/F) | 61%/39% |
| Apgar Score at One Minute: <9 vs. ≥9 | 63%/37% |
| Nutrition (Diverse/Formula) | 87%/13% |
| Median (IQR) | |
|---|---|
| 37 (36.0–38.0) weeks | Gestational Age |
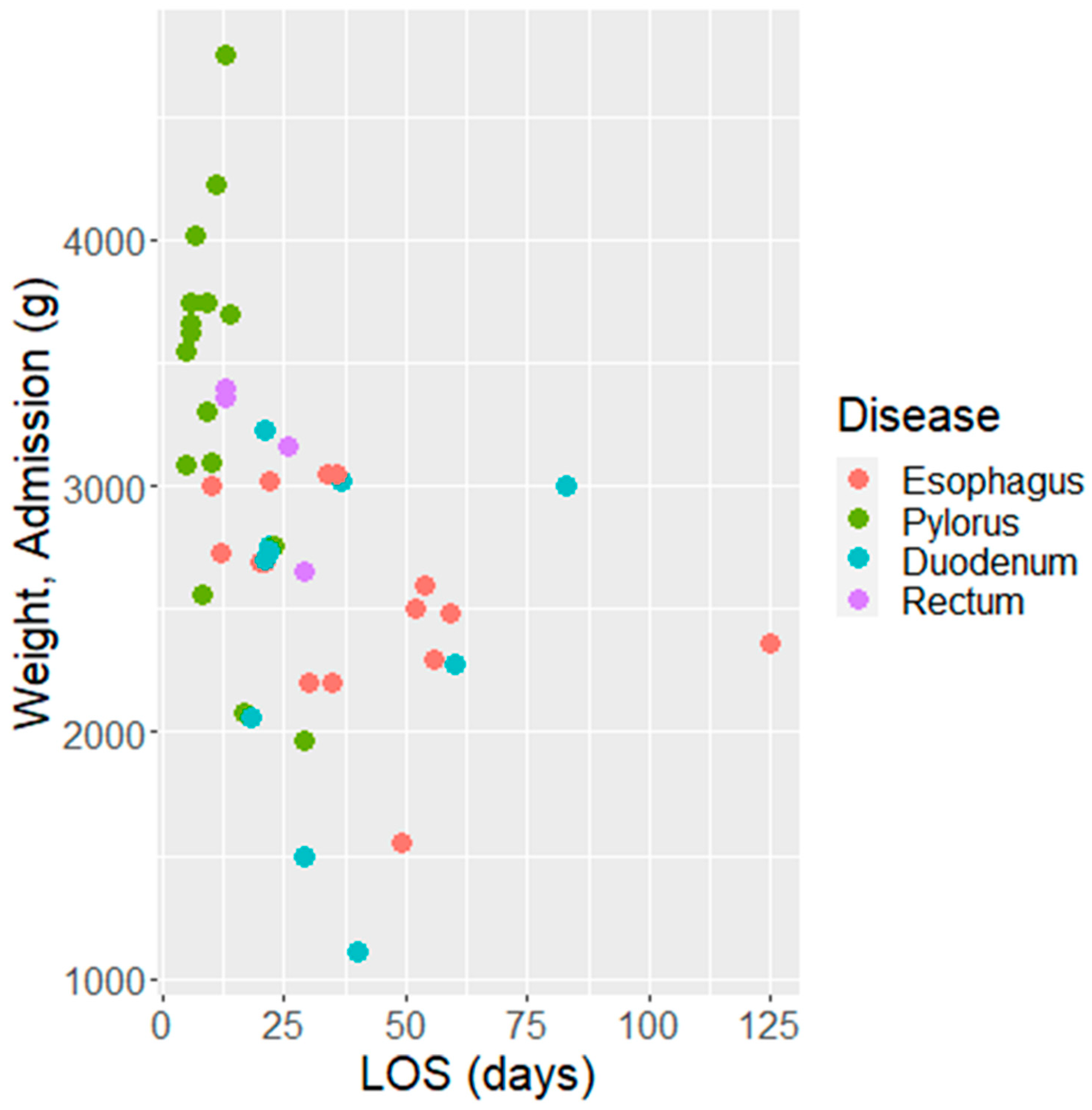
| 2750 (2190–3200) g | Birth Weight |
| 2760 (2390–3282.5) g | Admission Weight |
| 3135 (2600–3500) g | Discharge Weight |
| 8 (7–9) | Apgar score |
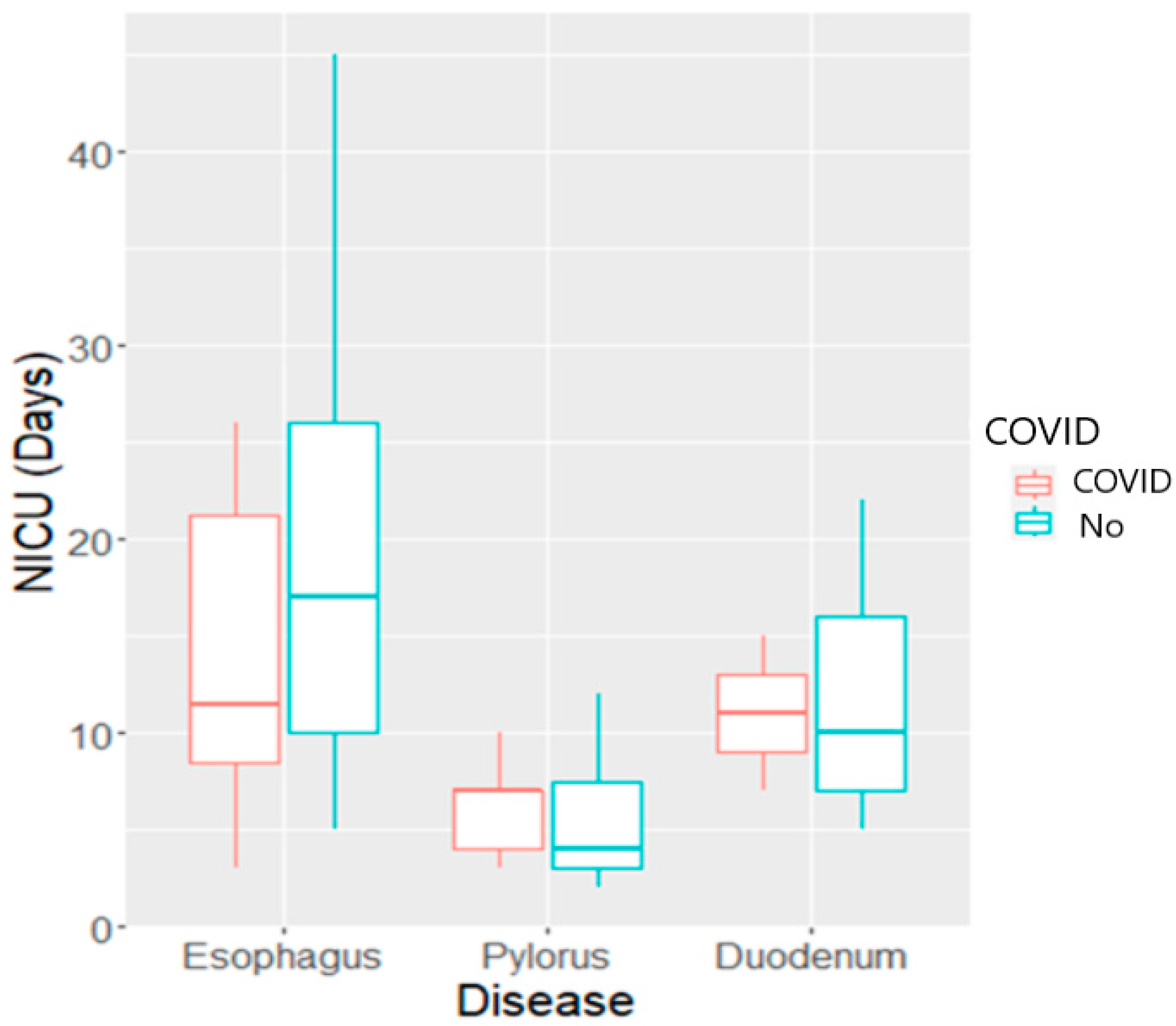
| 8 (5–13) days | LOS (ICU) |
| 11.5 (5–21.75) days | LOS (post- ICU) |
| 21.5 (11.25–35.75) days | LOS (Total) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandibur, T.E.; Kundnani, N.R.; Boia, M.; Nistor, D.; Velimirovici, D.M.; Mada, L.; Manea, A.M.; Boia, E.R.; Neagu, M.N.; Popoiu, C.M. Does COVID-19 Infection during Pregnancy Increase the Appearance of Congenital Gastrointestinal Malformations in Neonates? Biomedicines 2023, 11, 3105. https://doi.org/10.3390/biomedicines11123105
Brandibur TE, Kundnani NR, Boia M, Nistor D, Velimirovici DM, Mada L, Manea AM, Boia ER, Neagu MN, Popoiu CM. Does COVID-19 Infection during Pregnancy Increase the Appearance of Congenital Gastrointestinal Malformations in Neonates? Biomedicines. 2023; 11(12):3105. https://doi.org/10.3390/biomedicines11123105
Chicago/Turabian StyleBrandibur, Timea Elisabeta, Nilima Rajpal Kundnani, Marioara Boia, Daciana Nistor, Daniel Milan Velimirovici, Leonard Mada, Aniko Maria Manea, Eugen Radu Boia, Marioara Nicula Neagu, and Calin Marius Popoiu. 2023. "Does COVID-19 Infection during Pregnancy Increase the Appearance of Congenital Gastrointestinal Malformations in Neonates?" Biomedicines 11, no. 12: 3105. https://doi.org/10.3390/biomedicines11123105
APA StyleBrandibur, T. E., Kundnani, N. R., Boia, M., Nistor, D., Velimirovici, D. M., Mada, L., Manea, A. M., Boia, E. R., Neagu, M. N., & Popoiu, C. M. (2023). Does COVID-19 Infection during Pregnancy Increase the Appearance of Congenital Gastrointestinal Malformations in Neonates? Biomedicines, 11(12), 3105. https://doi.org/10.3390/biomedicines11123105







