Advantages and Limitations of Diabetic Bone Healing in Mouse Models: A Narrative Review
Abstract
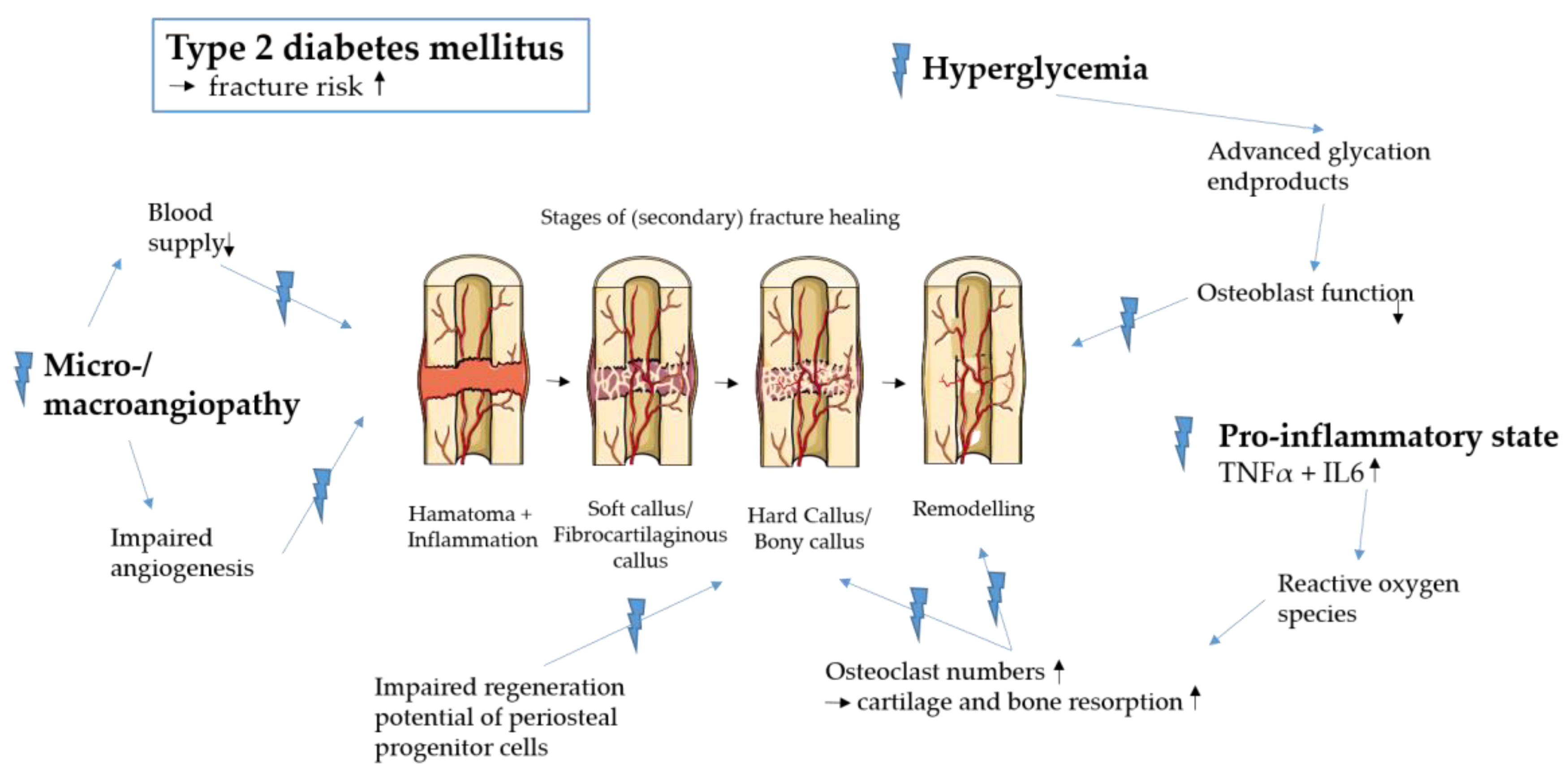
1. Introduction
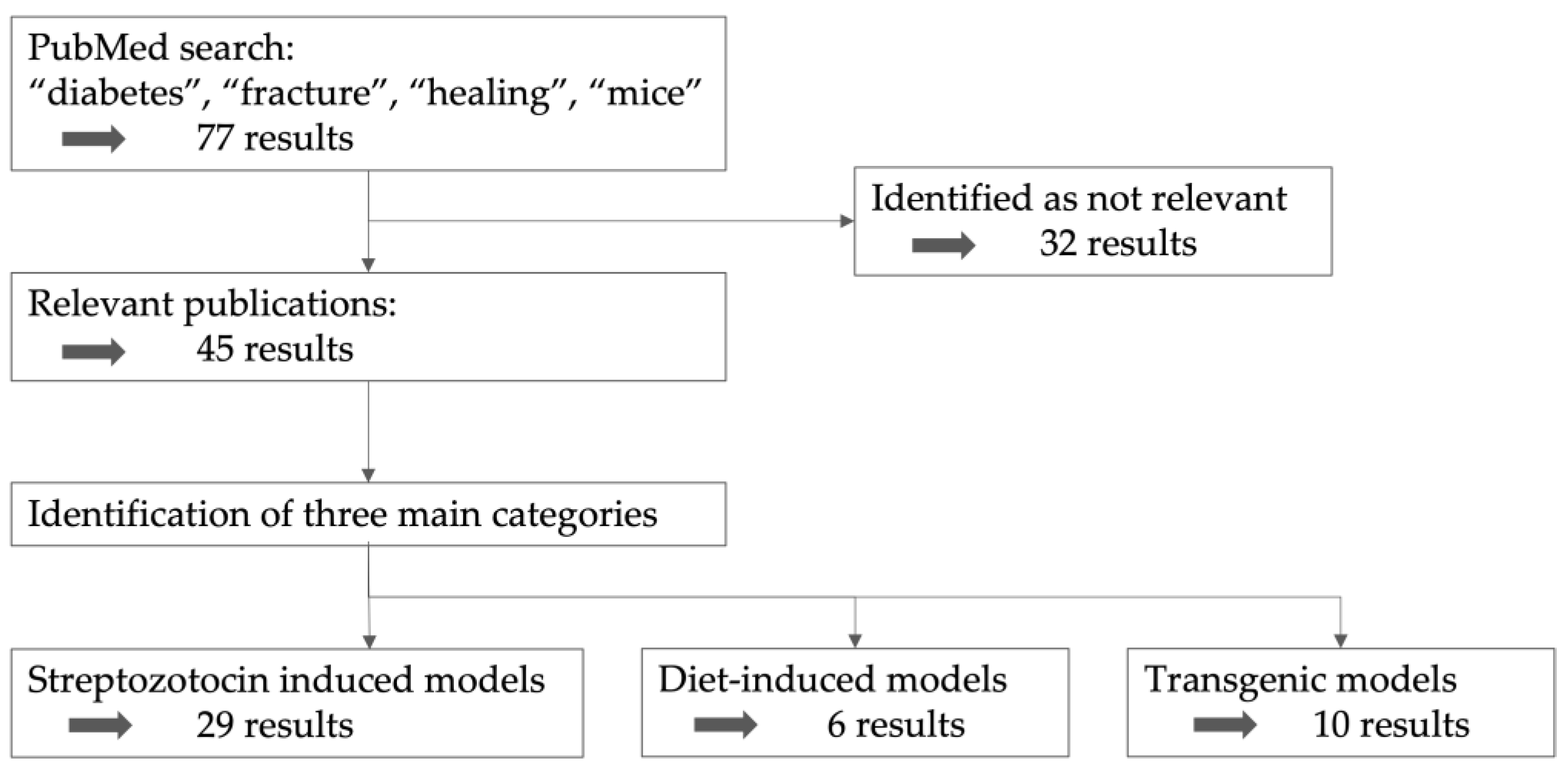
2. Search Strategy
3. Categories of Diabetic Mouse Models
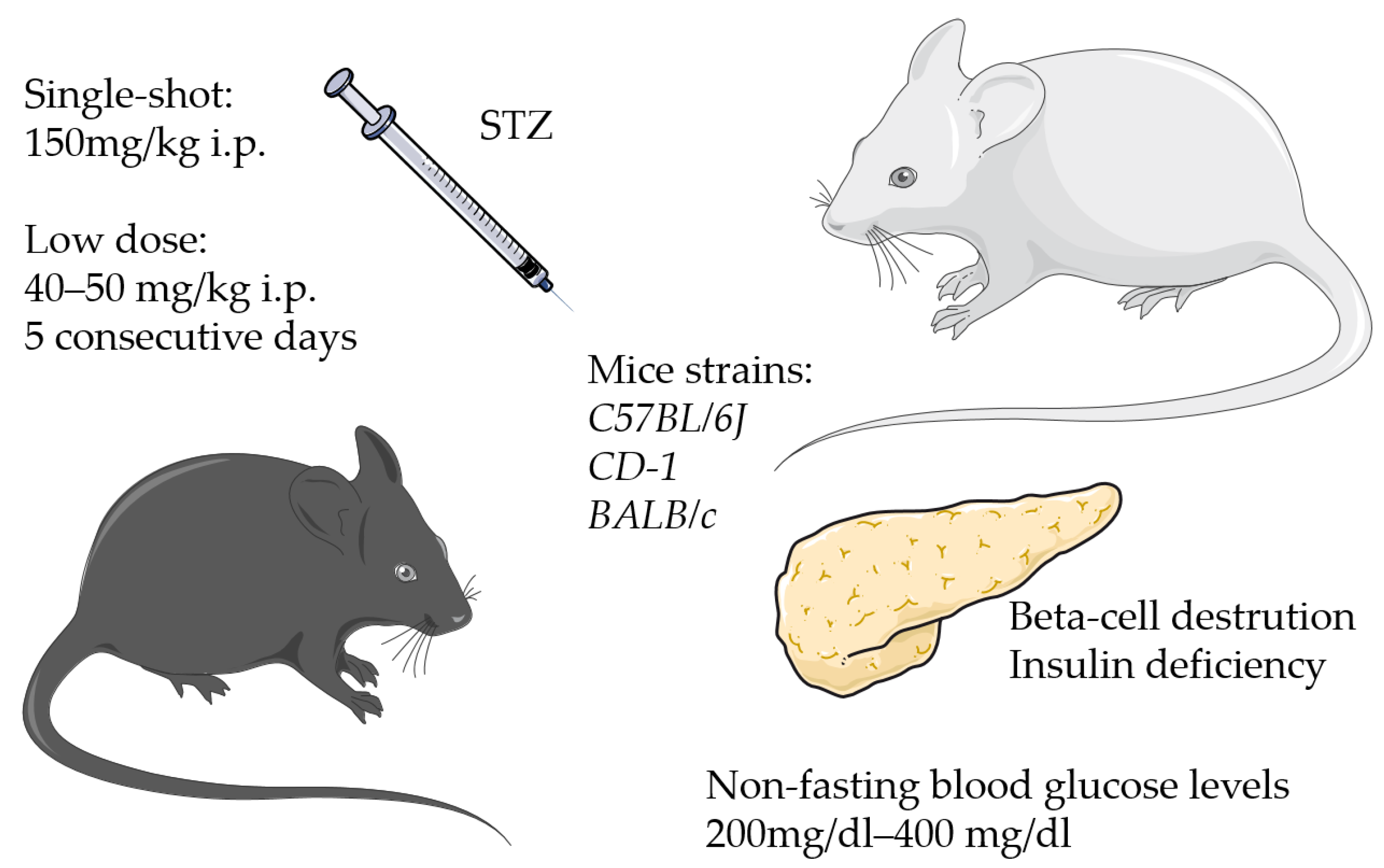
3.1. Streptozotocin-Induced Models
3.2. Diet-Induced Models
3.3. Transgenic Models
| Model | Diabetes Type | Mouse Strain | Diabetes Symptoms | Considered Diabetic | Treatment | Surgery | Fracture Healing | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Medical-induced | STZ * | T1DM | C57BL/6J CD-1 BALB/c | high non-fasting blood glucose level | >200 mg/dL ≥ 400 mg/dL blood glucose level | low dose: 40–50 mg/kg body weight 5 days (i.p.) high dose: single shot 150 mg/kg body weight (i.p.) | 2–4 weeks after injection | delayed fracture healing | [20,24,28,29,54,64] |
| Diet-induced | HFD * | T2DM | C57BL/6J | obesity impaired glucose tolerance hyperglycemia | significant larger AUC * in GTT */ITT * | 45–60% kcal fat | (6-) 8–14 weeks after start of HFD | delayed fracture healing, reduced callus size, reduced torsional rigidity, increased callus adiposity | [28,58,59,60,61,62,63,64] |
| transgenic | ob/ob | T1DM | C57BL/6J Lepob/ob | severe obesity hyperphagia hypometabolism | no testing | - | [55] | ||
| db/db | T1DM | C57BL/6J Lepdb/db | severe obesity hyperphagia hypometabolism polydipsia polyuria | no testing | - | 12–14 weeks old | impaired osteoblast invasion, proliferation, and differentiation; impaired angiogenesis; decreased osteoblast invasion | [65,66,67,68,71] | |
| Akita | T1DM | C57BL/6J (C57BL/6-INS2Akita/J) | hypoinsulinemia | no testing | - | 18 weeks old | reduced callus, less cartilage and bone | [69] | |
| MKR | T1DM | FVB/N | hyperglycemia | Blood glucose > 250 mg/dL | - | 12 weeks | - | [56] |
| DM * | Model | Model Specifi-Cation | Mouse Strain | Sex | Age at Start of Diabetic Treatment | Time Until Intervention/Length of Diet | Testing | Fracture Model | Alterations in Diabetic Fracture Healing | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| T1DM * | STZ * | 40 mg/kg on 5 consecutive days | CD-1 | male | 9 weeks | 3 weeks | blood glucose testing: 300–550 mg/dL (mean 411 mg/dL), considered diabetic: two consecutive measurements > 250 mg/dL | closed transverse tibia fractures, intra-medullary pin | 12 days after fracture: callus size similar, 16 days: significantly smaller callus, 78% more osteoclasts | [20] |
| T1DM | STZ | 150 mg/kg single shot | C57BL/6J (Cg-Tg(Col1a1*2.3-GFP)1Rowe/J) | both | 4–8 weeks | 4 weeks | weight and fasting blood glucose: significant higher fasting blood glucose, no significant weight difference | closed femur fracture, pin fixation | periosteal cell deficient in osteogenic differentiation, reduced population of periosteal mesenchymal progenitors, reduced proliferation capacity | [24] |
| T1DM | STZ | 50 mg/kg on 5 consecutive days | C57BL/6J | male | 12 weeks | 16 days | daily weight and blood glucose levels monitoring for 16 days | open transverse femur fractures, pin stabilization, 0.5 mm fracture gap, clip stabilization | significantly elevated TNF-α, IL-1β, COX2, and NOS-2 levels, delayed bone defect healing | [29] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | C57BL/6J | both | 9 weeks | 3 weeks hyperglycemic | considered diabetic when blood glucose levels exceed 220 mg/dL for 2 consecutive measurements | closed transverse femoral shaft fractures, pin stabilization | inhibition of ciliary gene expression, delayed fracture healing, significantly reduced bone density and mechanical strength, reduced osteoblast marker expression and decreased angiogenesis | [30] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | CD-1 | male | 8 weeks | 3 weeks hyperglycemic | mean glucose values of 25–28 mmol/L (vs. 6–8 mmol/L) | transverse closed femur fractures, pin stabilization | reduced VEGFA expression, reduced angiogenesis in areas of endochondral ossification | [31] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | CD-1 | male | 8 weeks | 3 weeks diabetic | blood glucose levels > 250 mg/dL considered diabetic | transverse closed femur fractures, pin stabilization | increased TNF-α levels and reduced mesenchymal stem cell numbers in new bone areas | [32] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | BALB/c | male | 12 weeks | 1 week, only the diabetic mice where used | blood glucose levels > 270 mg/dL, mean blood glucose level was 300 mg/dL | mid-diaphyseal femur fracture model | no non-diabetic control group | [33] |
| T1DM | STZ | 50 mg/kg on 5 consecutive days | C57BL/6J | n.n. | 8 weeks | 3 weeks diabetic | considered diabetic with blood glucose levels > 220 mg/dL | closed femur fracture, pin fixation | reduced mechanical strength of fracture callus 35 days after fracture | [34] |
| T1DM | STZ | 150 mg/kg single shot | C57BL/6 | male | 6–8 weeks | n.n. | blood glucose levels 250 mg/dL | open tibia fracture, pin stabilization, 2mm diameter defect of the calvarium | [35] | |
| T1DM | STZ | 150 mg/kg single shot | C57BL/6 | male | 12 weeks | 2 weeks | blood glucose levels > 400 mg/dL | closed transverse femur fracture, pin stabilization | 2 and 3 weeks after fracture: smaller callus, significantly reduced osteoclast size but elevated numbers, no alterations ins osteoblast function | [36] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | C57BL/6 | male | 8 weeks | 3 weeks diabetic | considered diabetic with blood glucose levels > 250 mg/dL | transverse tibial and femoral shaft fracture, pin stabilization | three times higher blood glucose levels, decrease in callus and cartilage area, higher TNF-α levels, increase in chondrocyte apoptosis | [37] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | C57BL/6 (Col2α1Cre−.FOXO1L/L) | n.n. | 12–14 weeks | 3 weeks diabetic | considered diabetic with blood glucose levels > 220 mg/dL for two consecutive tests | closed femoral shaft fracture, pin stabilization | three times increase in osteoclasts, two−three times increase in RANKL mRNA and RANKL expressing chondrocytes | [38] |
| T1DM | STZ | 50 mg/kg on 5 consecutive days | C57BL/6J | male | 7 weeks | 3 weeks | considered diabetic with non-fasting blood glucose levels > 300 mg/dL, diabetic: mean 493 mg/dL vs. control: mean 140 mg/dL | drill hole injury, round defect of 1 mm diameter, no stabilization | delayed bone healing at day 7 and 10 | [39] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | CD-1 | male | 8 weeks | 3 weeks diabetic | considered diabetic with blood glucose levels > 250 mg/dL | closed transverse femoral fracture, pin stabilization | significantly reduced callus size at day 16 and 22, on day 10 just missed significance (p = 0.07) | [40] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | C57BL/6J | male | 6–8 weeks | n.n. | considered diabetic with fasting blood glucose levels > 11.1 mmol/L | closed femoral fracture, | significantly reduced bone mineral density, trabecular number/separation/thickness (unfractured bone) | [41] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | CD-1 | male | 8 weeks | 3 weeks hyperglycemic | considered diabetic with blood glucose levels > 250 mg/dL | closed transverse femoral shaft fracture, pin stabilization | upregulation of several chemokines, chondrocytes showed enhanced CCL4 expression | [42] |
| T1DM | STZ | 100 mg/kg on 2 consecutive days | C57BL/6 | male | 10 weeks | 4 weeks | considered diabetic with glucose levels > 290 mg/dL doses, testing 2 weeks after STZ injections | monocortical tibial defect, 0.8 mm in the anterior cortex | low VEGF and Bmp2/4 expression in bone and impaired bone regeneration | [43] |
| T1DM | STZ | 50 mg/kg on 5 consecutive days | C57BL/6 | male | n.n. | 1 week | decrease in body weight, significant increase in glucose levels | femoral mono-cortical bone defect: 4 mm length, 1 mm diameter | delay in bone regeneration, large areas of loose connective tissue within the defects, reduced expression of osteonectin | [44] |
| T1DM | STZ | 50 mg/kg on 4 consecutive days | C57BL/6 | female | 10 weeks | 4 weeks | considered diabetic with non-fasting blood glucose levels > 300 mg/dL, measurement 4 days after last injection, decreased body weight | femoral bone defect, 0.9 mm drill | delayed bone repair (controls healed within 7 days, diabetics not), significantly lower ratio of RANKL/OPG | [45] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | CD-1 | male | 8 weeks | 3 weeks diabetic | considered diabetic with blood glucose levels > 250 mg/dL | closed transverse tibia and femur fracture, pin stabilization | upregulation of 31 out of 38 tested inflammatory gene sets at day 16, significantly increased the number of TNF-α positive proliferative and hypertrophic chondrocytes on day 16 | [46] |
| T1DM | STZ | 50 mg/kg on 5 consecutive days | C57BL6/J | male | 6 weeks | 2 weeks | considered diabetic with blood glucose levels > 300 mg/dL | closed femoral shaft fracture | reduced bone volume to total bone volume ratio and trabecular thickness in lumbar vertebrae, decreased callus mineralization at 6 weeks | [47] |
| T1DM | STZ | 50 mg/kg on 4 consecutive days | C57BL6 | female | 10 weeks | 4 weeks | considered diabetic with non-fasting blood glucose levels > 300 mg/dL, testing 4 days after last injection | femoral bone defect, drill defect, 9 mm diameter of the drill | significantly delayed defect healing at days 7 and 10 | [48] |
| T1DM | STZ | 50 mg/kg on 5 consecutive days | C57BL/6J | both | 8–10 weeks | 4–6 weeks diabetic | considered diabetic with blood glucose levels > 220 mg/dL | closed transverse femur shaft fracture, pin stabilization | enhanced RANK activation in periosteal cells, loss of skeletal stem cells | [49] |
| T1DM | STZ | 50 mg/kg on 5 consecutive days | C57BL/6J | male | 8 weeks | 4 weeks | considered diabetic with blood glucose levels >300 mg/dL, testing 1 week after last injection | closed femoral fracture, nail stabilization | 6 weeks: significant reduction in bone formation, less bone mass, low bone density, porous woven bone, 54% decreased bone volume fraction, 39% decreased bone connectivity density | [50] |
| T1DM | STZ | 50 mg/kg on 5 consecutive days | C57BL/6J | male | 9 weeks | 3 weeks diabetic | considered diabetic with blood glucose levels > 13 mM for 3 consecutive weeks | closed femur shaft fractures, pin stabilization | significantly elevated inflammation-related biomarkers at days 2 and 7, more markers were elevated at day 2 | [51] |
| T1DM | STZ | 50 mg/kg on 5 consecutive days | C57/B6 | male | 8–10 weeks | 3 weeks diabetic | considered diabetic with blood glucose levels > 13 mmol/L for 3 consecutive weeks | closed transverse femur shaft fractures, pin stabilization | no non-diabetic control group | [52] |
| T1DM | STZ | 50 mg/kg on 4 consecutive days | C57BL/6J | female | 8 weeks | 3 weeks | considered diabetic with non-fasting blood glucose levels > 300 mg/dL, decrease in body weight | femoral bone defect, 0.9 mm diameter drill | reduced number of macrophages on day 2 but not day 4, slight increase in TNF-α mRNA levels at the defect site | [53] |
| T1DM | STZ | 40 mg/kg on 5 consecutive days | CD-1 | n.n. | n.n. | 2 weeks diabetic | considered diabetic with blood glucose level > 250 mg/dL | marrow ablation in the proximal tibia | [54] | |
| T1DM/ T2DM * | STZ/ HFD * | 40 mg/kg on 5 consecutive days/ 45% kcal fat | C57BL/6J | male | 8 weeks | 12 weeks | glucose tolerance test, impaired glucose tolerance and hyperinsulinemia in HFD mice | tibial cortical bone defect, 0.8 mm diameter | delayed bone healing, increase in reactive oxygen species, inhibitory effects on osteoblasts | [28] |
| T2DM | HFD | 60% kcal fat | C57BL/6J | male | 5 weeks | 7 weeks | significantly elevated fasting blood glucose levels, pre-diabetic hyperglycemia and glucose intolerance | closed tibial shaft factures, pin stabilization | significantly lower bone strength at day 35, significantly lower bone volume, bone volume density and bone mineral density at days 21 and 35, pathological accumulation of AGEs * in callus leading to increased collagen-fiber crosslink density | [58] |
| T2DM | HFD | 45% kcal fat | C57BL/6J | male | 6 weeks | 14 weeks | glucose tolerance test and insulin tolerance test, significant higher body weight, impaired glucose and insulin tolerance in mice | 2 mm femoral diaphyseal defect, pin stabilization | no significant differences | [59] |
| T2DM | HFD | 45% kcal fat | C57BL/6J | male | 8 weeks | 12 weeks | glucose tolerance test and insulin tolerance test, higher net blood glucose level and total insulin release during the test, significantly larger area under the curve | 2mm intercalary segment from the femoral diaphysis, pin fixation | impaired bone healing | [60] |
| T2DM | HFD | 60% kcal fat | C57BL/6J | 6 weeks | 6–8 weeks | no testing | open transverse osteotomy in the mid-diaphysis of the femur, pin stabilization | significantly lower percentage of cartilaginous callus area in total callus area at 1 week, less bony callus at 2 weeks, callus and fracture lines still visible at 4 weeks | [61] | |
| T2DM | HFD | C57BL/6J | male | 6 weeks | - | 2.4-fold increase in fasting blood glucose | tibia fracture, pin stabilization | Significantly more cartilaginous and adipose tissue in fracture callus, altered osteogenesis and chondrogenesis, decreased blood serum osteocalcin | [62] | |
| T2DM | HFD | 60% kcal fat | C57BL/6J | male | 5 weeks | 12 weeks | increased weight and impaired glucose tolerance | open tibia fractures, pin stabilization | significantly increased fracture callus adiposity at days 21, 28, and 35; significantly decreased woven bone at day 21; significantly reduced torsional rigidity at y 35 | [63] |
| T1DM/ T2DM | TG * (STZ, HFD) | db/db | C57BL/6 | female | 10 weeks and 4 weeks | - | no testing | open femoral shaft fracture, pin stabilization | all models showed significantly reduced strength, db/db: 10-week-old mice: significantly decreased bone mineral density, significantly decreased bone volume/tissue volume; 4-week-old mice: no significant difference in strength | [64] |
| T1DM | TG | ob/ob | C57BL/6 | n.n. | - | 8 weeks | no testing | mid-skull transcortical defects, 3.5 mm drill | diabetic macrophages impair bone regeneration, alterations in vascularization and increased number of adipocytes | [55] |
| T1DM | TG | MKR | FVB/N | male | - | 8 weeks | blood glucose: diabetic: 350 mg/dL vs. non-diabetic: mean 150 mg/dL | closed femoral shaft fracture, pin stabilization and femoral drill hole model, 0.8 mm diameter drill | less callus formation at days 10 and 16 | [56] |
| T1DM | TG | db/db | C57BL/6J | female | - | 12–14 weeks | no testing | 1 mm monocortical tibial defect | reduced bone regeneration, decreased osteoclasts, reduced osteoblastogenesis | [65] |
| T1DM | TG | db/db | C57BL/6J | n.n. | - | 12–16 weeks | no testing | 1 mm monocortical tibial defect | increased activation of TGF-β pathway in callus, significant differences in expression of multiple genes, significantly higher expression of inflammation-associated factors | [66] |
| T1DM | TG | db/db | C57BL/6 | female | - | 12–14 weeks | significant differences in glucose tolerance testing | closed femoral shaft fracture, pin stabilization | no differences at day 3, delayed healing from day 7 on, callus still visible at day 30 vs. healed fracture in controls, poor chondrogenesis, enhanced chondrocyte apoptosis at day 7 and 14 | [67] |
| T1DM | TG | db/db | C57BL/6J | n.n. | - | 12–16 weeks | no testing | 1 mm monocortical tibial defect | impaired osteogenesis | [68] |
| T1DM | TG | Akita | C57BL/6J | male | - | 18 weeks | hyperglycemic at 6 weeks: mean: 496 mg/dL, at 18 weeks: mean: 574 mg/dL | femur fractures | significantly smaller callus with less cartilage and bone area at days 14 and 21, reduced torsional strength | [69] |
| T1DM | TG | Agouti | Agouti | n.n. | - | 8 weeks | described as hyperglycemia, hyperinsulinemia, glucose intolerance, and insulin resistance by 8 weeks of age | mid-shaft tibia osteotomy, two-ring external fixation spanning | no differences in new bone formation | [70] |
| T1DM | TG | db/db | C57BL/6J | n.n. | - | 12 weeks | n.n. | femur shaft fractures | decreased bony callus areas, reduced osteoblast numbers, reduced RANKL | [71] |
4. Comparison of the Bone Fracture/Defect Models Used
5. Discussion
Limitations
6. Which Model to Choose?
7. Conclusions and Recommendation
- High-fat diet: The use of a high-fat diet is known to cause obesity and insulin resistance in mice, similar to the features of T2DM in humans. Prolonged consumption of a high-fat diet helps reproduce the gradual onset of T2DM that typically occurs in older patients.
- Realistic scenario: we emphasize the importance of modeling the gradual evolution of T2DM over time in accordance with the clinical course observed in patients.
- Obesity and type 2 diabetes: High-fat diets are a leading cause of obesity, and a known risk factor for type 2 diabetes. Consequently, the use of this nutritional model allows researchers to study the complex interplay between obesity, diabetes, and fracture healing.
- The chosen mouse model (fracture and diabetic model) should be described meticulously.
- Researchers should explain precisely why they chose the specific models.
- Diabetic testing should be performed with, for example, fasting blood glucose levels, glucose tolerance tests, or insulin tolerance tests.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nailing, I. Nonunion following intramedullary nailing of the femur with and without reaming. Results of a multicenter randomized clinical trial. J. Bone Jt. Surg. Am. 2003, 85, 2093–2096. [Google Scholar]
- Short, W.D.; Steen, E.; Kaul, A.; Wang, X.; Olutoye, O.O.; Vangapandu, H.V.; Templeman, N.; Blum, A.J.; Moles, C.M.; Narmoneva, D.A.; et al. Il-10 promotes endothelial progenitor cell infiltration and wound healing via stat3. FASEB J. 2022, 36, e22298. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE 2018, 13, e0194127. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abujamra, B.A.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; Feskanich, D.; Willett, W.C.; Hu, F. Prospective study of diabetes and risk of hip fracture: The nurses’ health study. Diabetes Care 2006, 29, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; Van Dam, R.M.; Willett, W.C.; Hu, F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 2007, 166, 495–505. [Google Scholar] [CrossRef]
- Hernandez, R.K.; Do, T.P.; Critchlow, C.W.; E Dent, R.; Jick, S.S. Patient-related risk factors for fracture-healing complications in the united kingdom general practice research database. Acta Orthop. 2012, 83, 653–660. [Google Scholar] [CrossRef]
- Ding, Z.; Zeng, W.; Rong, X.; Liang, Z.; Zhou, Z. Do patients with diabetes have an increased risk of impaired fracture healing? A systematic review and meta-analysis. ANZ J. Surg. 2020, 90, 1259–1264. [Google Scholar] [CrossRef]
- Pscherer, S.; Sandmann, G.H.; Ehnert, S.; Nussler, A.K.; Stöckle, U.; Freude, T. Delayed fracture healing in diabetics with distal radius fractures. Acta Chir. Orthop. Traumatol. Cechoslov. 2015, 82, 268–273. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, K.; Coale, M.; Costales, T.; Zerhusen, T., Jr.; Castillo, R.C.; Nascone, J.W.; O’Toole, R.V. Will my tibial fracture heal? Predicting nonunion at the time of definitive fixation based on commonly available variables. Clin. Orthop. Relat. Res. 2016, 474, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef]
- Tanios, M.; Brickman, B.; Cage, E.; Abbas, K.; Smith, C.; Atallah, M.; Baroi, S.; Lecka-Czernik, B. Diabetes and impaired fracture healing: A narrative review of recent literature. Curr. Osteoporos. Rep. 2022, 20, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef]
- Freude, T.; Braun, K.F.; Haug, A.; Pscherer, S.; Stöckle, U.; Nussler, A.K.; Ehnert, S. Hyperinsulinemia reduces osteoblast activity in vitro via upregulation of tgf-β. J. Mol. Med. 2012, 90, 1257–1266. [Google Scholar] [CrossRef]
- Cheng, R.; Ma, J.-X. Angiogenesis in diabetes and obesity. Rev. Endocr. Metab. Disord. 2015, 16, 67–75. [Google Scholar] [CrossRef]
- Catalfamo, D.; Britten, T.; Storch, D.; Calderon, N.; Sorenson, H.; Wallet, S. Hyperglycemia induced and intrinsic alterations in type 2 diabetes-derived osteoclast function. Oral Dis. 2013, 19, 303–312. [Google Scholar] [CrossRef]
- Kayal, R.A.; Tsatsas, D.; A Bauer, M.; Allen, B.; Al-Sebaei, M.O.; Kakar, S.; Leone, C.W.; Morgan, E.F.; Gerstenfeld, L.C.; A Einhorn, T.; et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J. Bone Miner. Res. 2007, 22, 560–568. [Google Scholar] [CrossRef]
- Krakauer, J.C.; Mckenna, M.J.; Buderer, N.F.; Rao, D.S.; Whitehouse, F.W.; Parfitt, A.M. Whitehouse and A. M. Parfitt. Bone loss and bone turnover in diabetes. Diabetes 1995, 44, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kurose, T.; Takizawa, M.; Maruyama, M.; Ushikawa, K.; Kikuyama, M.; Sugimoto, C.; Seino, Y.; Nagamatsu, S.; Ishida, H. Osteoclastic function is accelerated in male patients with type 2 diabetes mellitus: The preventive role of osteoclastogenesis inhibitory factor/osteoprotegerin (ocif/opg) on the decrease of bone mineral density. Diabetes Res. Clin. Pract. 2005, 68, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.B.; Torres, A.M.; Palomino, P.M.; Marty, E.; Saiyed, R.; Cohn, M.; Jo, J.; Warner, S.; E Sroga, G.; King, K.B.; et al. Altered tissue composition, microarchitecture, and mechanical performance in cancellous bone from men with type 2 diabetes mellitus. J. Bone Miner. Res. 2019, 34, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.; Wan, M.; Kalajzic, I.; Sanjay, A. Diabetes impairs periosteal progenitor regenerative potential. Bone 2021, 143, 115764. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45 (Suppl. S2), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.H.; Garcia, P.; Histing, T.; Kristen, A.; Scheuer, C.; Menger, M.D.; Pohlemann, T. Advances in the establishment of defined mouse models for the study of fracture healing and bone regeneration. J. Orthop. Trauma 2009, 23, S31–S38. [Google Scholar] [CrossRef] [PubMed]
- Bonnarens, F.; Einhorn, T.A. Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res. 1984, 2, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Figeac, F.; Tencerova, M.; Ali, D.; Andersen, T.L.; Appadoo, D.R.C.; Kerckhofs, G.; Ditzel, N.; Kowal, J.M.; Rauch, A.; Kassem, M. Impaired bone fracture healing in type 2 diabetes is caused by defective functions of skeletal progenitor cells. Stem Cells 2022, 40, 149–164. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, L.; Ding, Y.; Liu, R.; Guo, Y.; Hettinghouse, A.; Buza, J.; De La Croix, J.; Li, X.; Einhorn, T.A.; et al. Progranulin promotes diabetic fracture healing in mice with type 1 diabetes. Ann. N. Y. Acad. Sci. 2020, 1460, 43–56. [Google Scholar] [CrossRef]
- Chinipardaz, Z.; Liu, M.; Graves, D.; Yang, S. Diabetes impairs fracture healing through disruption of cilia formation in osteoblasts. Bone 2021, 153, 116176. [Google Scholar] [CrossRef]
- Lim, J.C.; Ko, K.I.; Mattos, M.; Fang, M.; Zhang, C.; Feinberg, D.; Sindi, H.; Li, S.; Alblowi, J.; Kayal, R.A.; et al. Tnfα contributes to diabetes impaired angiogenesis in fracture healing. Bone 2017, 99, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.I.; Coimbra, L.S.; Tian, C.; Alblowi, J.; Kayal, R.A.; Einhorn, T.A.; Gerstenfeld, L.C.; Pignolo, R.J.; Graves, D.T. Diabetes reduces mesenchymal stem cells in fracture healing through a tnfα-mediated mechanism. Diabetologia 2015, 58, 633–642. [Google Scholar] [CrossRef]
- Cui, K.; Chen, Y.; Zhong, H.; Wang, N.; Zhou, L.; Jiang, F. Transplantation of il-10-overexpressing bone marrow-derived mesenchymal stem cells ameliorates diabetic-induced impaired fracture healing in mice. Cell Mol. Bioeng. 2020, 13, 155–163. [Google Scholar] [CrossRef]
- Lu, Y.; Alharbi, M.; Zhang, C.; O’Connor, J.P.; Graves, D.T. Deletion of foxo1 in chondrocytes rescues the effect of diabetes on mechanical strength in fracture healing. Bone 2019, 123, 159–167. [Google Scholar] [CrossRef]
- Chen, C.; Lai, C.; Hong, Y.; Lu, J.; Lin, S.; Lee, T.; Chang, L.; Ho, M.; Conway, E.M.; Wu, H.; et al. Thrombomodulin functional domains support osteoblast differentiation and bone healing in diabetes in mice. J. Bone Miner. Res. 2020, 35, 1812–1823. [Google Scholar] [CrossRef]
- Kasahara, T.; Imai, S.; Kojima, H.; Katagi, M.; Kimura, H.; Chan, L.; Matsusue, Y. Malfunction of bone marrow-derived osteoclasts and the delay of bone fracture healing in diabetic mice. Bone 2010, 47, 617–625. [Google Scholar] [CrossRef]
- Kayal, R.A.; Siqueira, M.; Alblowi, J.; McLean, J.; Krothapalli, N.; Faibish, D.; Einhorn, T.A.; Gerstenfeld, L.C.; Graves, D.T. Tnf-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through foxo1. J. Bone Miner. Res. 2010, 25, 1604–1615. [Google Scholar] [CrossRef]
- Alharbi, M.A.; Zhang, C.; Lu, C.; Milovanova, T.N.; Yi, L.; Ryu, J.D.; Jiao, H.; Dong, G.; O’Connor, J.P.; Graves, D.T. Foxo1 deletion reverses the effect of diabetic-induced impaired fracture healing. Diabetes 2018, 67, 2682–2694. [Google Scholar] [CrossRef]
- Aikawa, T.; Matsubara, H.; Ugaji, S.; Shirakawa, J.; Nagai, R.; Munesue, S.; Harashima, A.; Yamamoto, Y.; Tsuchiya, H. Contribution of methylglyoxal to delayed healing of bone injury in diabetes. Mol. Med. Rep. 2017, 16, 403–409. [Google Scholar] [CrossRef]
- Kayal, R.A.; Alblowi, J.; McKenzie, E.; Krothapalli, N.; Silkman, L.; Gerstenfeld, L.; Einhorn, T.A.; Graves, D.T. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone 2009, 44, 357–363. [Google Scholar] [CrossRef]
- Han, J.; Zheng, Q.; Cheng, Y.; Liu, Y.; Bai, Y.; Yan, B.; Guo, S.; Yu, J.; Li, X.; Wang, C. Toll-like receptor 9 (tlr9) gene deletion-mediated fracture healing in type ii diabetic osteoporosis associates with inhibition of the nuclear factor-kappa b (nf-κb) signaling pathway. Bioengineered 2022, 13, 13689–13702. [Google Scholar] [CrossRef]
- Alblowi, J.; Tian, C.; Siqueira, M.F.; Kayal, R.A.; McKenzie, E.; Behl, Y.; Gerstenfeld, L.; Einhorn, T.A.; Graves, D.T. Chemokine expression is upregulated in chondrocytes in diabetic fracture healing. Bone 2013, 53, 294–300. [Google Scholar] [CrossRef]
- Schall, N.; Garcia, J.J.; Kalyanaraman, H.; China, S.P.; Lee, J.J.; Sah, R.L.; Pfeifer, A.; Pilz, R.B. Protein kinase g1 regulates bone regeneration and rescues diabetic fracture healing. JCI Insight 2020, 5, e135355. [Google Scholar] [CrossRef]
- Cignachi, N.P.; Pesquero, J.B.; Oliveira, R.B.; Etges, A.; Campos, M.M. Kinin b1 receptor deletion affects bone healing in type 1 diabetic mice. J. Cell Physiol. 2015, 230, 3019–3028. [Google Scholar] [CrossRef]
- Mao, L.; Kawao, N.; Tamura, Y.; Okumoto, K.; Okada, K.; Yano, M.; Matsuo, O.; Kaji, H. Plasminogen activator inhibitor-1 is involved in impaired bone repair associated with diabetes in female mice. PLoS ONE 2014, 9, e92686. [Google Scholar] [CrossRef]
- Alblowi, J.; Kayal, R.A.; Siqueira, M.; McKenzie, E.; Krothapalli, N.; McLean, J.; Conn, J.; Nikolajczyk, B.; Einhorn, T.A.; Gerstenfeld, L.; et al. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am. J. Pathol. 2009, 175, 1574–1585. [Google Scholar] [CrossRef]
- Yee, C.S.; Xie, L.; Hatsell, S.; Hum, N.; Murugesh, D.; Economides, A.N.; Loots, G.G.; Collette, N.M. Sclerostin antibody treatment improves fracture outcomes in a type I diabetic mouse model. Bone 2016, 82, 122–134. [Google Scholar] [CrossRef]
- Mao, L.; Tamura, Y.; Kawao, N.; Okada, K.; Yano, M.; Okumoto, K.; Kaji, H. Influence of diabetic state and vitamin d deficiency on bone repair in female mice. Bone 2014, 61, 102–108. [Google Scholar] [CrossRef]
- Ko, K.I.; Syverson, A.L.; Kralik, R.M.; Choi, J.; DerGarabedian, B.P.; Chen, C.; Graves, D.T. Diabetes-induced nf-κb dysregulation in skeletal stem cells prevents resolution of inflammation. Diabetes 2019, 68, 2095–2106. [Google Scholar] [CrossRef]
- Park, J.; Yan, G.; Kwon, K.C.; Liu, M.; Gonnella, P.A.; Yang, S.; Daniell, H. Oral delivery of novel human igf-1 bioencapsulated in lettuce cells promotes musculoskeletal cell proliferation, differentiation and diabetic fracture healing. Biomaterials 2020, 233, 119591. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Shaw, G.; Coleman, C.M.; Chen, X.Z.; Connolly, D.; Palamá, E.M.F.; Gentili, C.; Contessotto, P.; Murphy, M.J. Regenerative and anti-inflammatory potential of regularly fed, starved cells and extracellular vesicles in vivo. Cells 2022, 11, 2696. [Google Scholar] [CrossRef]
- Watson, L.; Chen, X.Z.; Ryan, A.E.; Fleming, Á.; Carbin, A.; O’Flynn, L.; Loftus, P.G.; Horan, E.; Connolly, D.; McDonnell, P.; et al. Administration of human non-diabetic mesenchymal stromal cells to a murine model of diabetic fracture repair: A pilot study. Cells 2020, 9, 1394. [Google Scholar] [CrossRef]
- Shimoide, T.; Kawao, N.; Tamura, Y.; Okada, K.; Horiuchi, Y.; Okumoto, K.; Kurashimo, S.; Ishida, M.; Tatsumi, K.; Matsuo, O.; et al. Role of macrophages and plasminogen activator inhibitor-1 in delayed bone repair in diabetic female mice. Endocrinology 2018, 159, 1875–1885. [Google Scholar] [CrossRef]
- Lu, H.; Kraut, D.; Gerstenfeld, L.C.; Graves, D.T. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 2003, 144, 346–352. [Google Scholar] [CrossRef]
- Kang, M.; Thalji, G.; Huang, C.C.; Shirazi, S.; Lu, Y.; Ravindran, S.; Cooper, L.F. Macrophage control of incipient bone formation in diabetic mice. Front. Cell Dev. Biol. 2020, 8, 596622. [Google Scholar] [CrossRef]
- Ding, Y.; Wei, J.; Hettinghouse, A.; Li, G.; Li, X.; Einhorn, T.A.; Liu, C.J. Progranulin promotes bone fracture healing via tnfr pathways in mice with type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2021, 1490, 77–89. [Google Scholar] [CrossRef]
- Shao, X.; Yang, Y.; Tan, Z.; Ding, Y.; Luo, E.; Jing, D.; Cai, J. Amelioration of bone fragility by pulsed electromagnetic fields in type 2 diabetic kk-ay mice involving wnt/β-catenin signaling. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E951–E966. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Soliman, M.; Elfar, J.C.; Lewis, G.S.; Abraham, T.; Kamal, F.; Elbarbary, R.A. Aberrant structure of fibrillar collagen and elevated levels of advanced glycation end products typify delayed fracture healing in the diet-induced obesity mouse model. Bone 2020, 137, 115436. [Google Scholar] [CrossRef]
- Bhatti, F.U.R.; Dadwal, U.C.; Valuch, C.R.; Tewari, N.P.; Awosanya, O.D.; de Andrade Staut, C.; Sun, S.; Mendenhall, S.K.; Perugini, A.J., III; Nagaraj, R.U.; et al. The effects of high fat diet, bone healing, and bmp-2 treatment on endothelial cell growth and function. Bone 2021, 146, 115883. [Google Scholar] [CrossRef]
- Dadwal, U.C.; Staut, C.A.; Tewari, N.P.; Awosanya, O.D.; Mendenhall, S.K.; Valuch, C.R.; Nagaraj, R.U.; Blosser, R.J.; Li, J.; Kacena, M.A. Effects of diet, bmp-2 treatment, and femoral skeletal injury on endothelial cells derived from the ipsilateral and contralateral limbs. J. Orthop. Res. 2022, 40, 439–448. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, J.; Li, Y.; Wang, Y.; Guo, Y.; Tu, Q.; Chen, J.; Wang, C. Adiporon promotes diabetic fracture repair through endochondral ossification-based bone repair by enhancing survival and differentiation of chondrocytes. Exp. Cell Res. 2020, 387, 111757. [Google Scholar] [CrossRef]
- Marin, C.; Tuts, J.; Luyten, F.P.; Vandamme, K.; Kerckhofs, G. Impaired soft and hard callus formation during fracture healing in diet-induced obese mice as revealed by 3D contrast-enhanced computed tomography imaging. Bone 2021, 150, 116008. [Google Scholar] [CrossRef]
- Brown, M.L.; Yukata, K.; Farnsworth, C.W.; Chen, D.G.; Awad, H.; Hilton, M.J.; O’Keefe, R.J.; Xing, L.; Mooney, R.A.; Zuscik, M.J. Delayed fracture healing and increased callus adiposity in a c57bl/6j murine model of obesity-associated type 2 diabetes mellitus. PLoS ONE 2014, 9, e99656. [Google Scholar] [CrossRef][Green Version]
- Tevlin, R.; Seo, E.Y.; Marecic, O.; McArdle, A.; Tong, X.; Zimdahl, B.; Malkovskiy, A.; Sinha, R.; Gulati, G.; Li, X.; et al. Pharmacological rescue of diabetic skeletal stem cell niches. Sci. Transl. Med. 2017, 9, eaag2809. [Google Scholar] [CrossRef]
- Wagner, J.M.; Wallner, C.; Becerikli, M.; Reinkemeier, F.; von Glinski, M.; Sogorski, A.; Huber, J.; Dittfeld, S.; Becker, K.; Lehnhardt, M.; et al. Role of autonomous neuropathy in diabetic bone regeneration. Cells 2022, 11, 612. [Google Scholar] [CrossRef]
- Becerikli, M.; Reinkemeier, F.; Dadras, M.; Wallner, C.; Wagner, J.M.; Drysch, M.; Sogorski, A.; von Glinski, M.; Lehnhardt, M.; Hahn, S.A.; et al. Tgf-beta pathway inhibition as the therapeutic acceleration of diabetic bone regeneration. J. Orthop. Res. 2022, 40, 1810–1826. [Google Scholar] [CrossRef]
- Rőszer, T.; Józsa, T.; Kiss-Tóth, E.D.; De Clerck, N.; Balogh, L. Leptin receptor deficient diabetic (db/db) mice are compromised in postnatal bone regeneration. Cell Tissue Res. 2014, 356, 195–206. [Google Scholar] [CrossRef]
- Wagner, J.M.; Reinkemeier, F.; Wallner, C.; Dadras, M.; Dittfeld, S.; Drysch, M.; Sogorski, A.; von Glinski, M.; Lehnhardt, M.; Behr, B.; et al. Inhibition of pathological increased matrix metalloproteinase (mmp) activity for improvement of bone regeneration in diabetes. Life 2022, 12, 134. [Google Scholar] [CrossRef]
- Hu, P.; McKenzie, J.A.; Buettmann, E.G.; Migotsky, N.; Gardner, M.J.; Silva, M.J. Type 1 diabetic akita mice have low bone mass and impaired fracture healing. Bone 2021, 147, 115906. [Google Scholar] [CrossRef]
- Liu, L.; Aronson, J.; Lecka-Czernik, B. Rosiglitazone disrupts endosteal bone formation during distraction osteogenesis by local adipocytic infiltration. Bone 2013, 52, 247–258. [Google Scholar] [CrossRef]
- Liu, A.; Li, Y.; Wang, Y.; Liu, L.; Shi, H.; Qiu, Y. Exogenous parathyroid hormone-related peptide promotes fracture healing in lepr(−/−) mice. Calcif. Tissue Int. 2015, 97, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qu, C.; Jia, J.; Zhan, Y. Nlrp3 inflammasome inhibitor glyburide expedites diabetic-induced impaired fracture healing. Immunobiology 2019, 224, 786–791. [Google Scholar] [CrossRef]
- Kottaisamy, C.P.D.; Raj, D.S.; Kumar, V.P.; Sankaran, U. Experimental animal models for diabetes and its related complications-a review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef] [PubMed]
- Deeds, M.C.; Anderson, J.M.; Armstrong, A.S.; Gastineau, D.A.; Hiddinga, H.J.; Jahangir, A.; Eberhardt, N.L.; Kudva, Y.C. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Lab. Anim. 2011, 45, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Gurley, S.B.; Clare, S.E.; Snow, K.P.; Hu, A.; Meyer, T.W.; Coffman, T.M. Impact of genetic background on nephropathy in diabetic mice. Am. J. Physiol. Ren. Physiol. 2006, 290, F214–F222. [Google Scholar] [CrossRef]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Waldén, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory t cell population in adipose tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef]
- Histing, T.; Andonyan, A.; Klein, M.; Scheuer, C.; Stenger, D.; Holstein, J.H.; Veith, N.T.; Pohlemann, T.; Menger, M.D. Obesity does not affect the healing of femur fractures in mice. Injury 2016, 47, 1435–1444. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strainsof mice: Kunming, c57bl/6, balb/c and icr. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- Nishikawa, S.; Yasoshima, A.; Doi, K.; Nakayama, H.; Uetsuka, K. Involvement of sex, strain and age factors in high fat diet-induced obesity in c57bl/6j and balb/ca mice. Exp. Anim. 2007, 56, 263–272. [Google Scholar] [CrossRef]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-induced type ii diabetes in c57bl/6j mice. Diabetes 1988, 37, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.; Reifsnyder, P.C.; Malcolm, R.D.; Lucas, C.A.; MacGregor, G.R.; Zhang, W.; Leiter, E.H. Diet-induced obesity in two c57bl/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (nnt) gene. Obesity 2010, 18, 1902–1905. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.H.; Ryan, T.E.; Smith, C.D.; Gilliam, L.A.; Lin, C.T.; Reese, L.R.; Torres, M.J.; Neufer, P.D. A direct comparison of metabolic responses to high-fat diet in c57bl/6j and c57bl/6nj mice. Diabetes 2016, 65, 3249–3261. [Google Scholar] [CrossRef] [PubMed]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G.; et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: Two sides of the same coin. Microbiome 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.L. Obese and diabetes: Two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 1978, 14, 141–148. [Google Scholar] [CrossRef]
- Wallner, C.; Schira, J.; Wagner, J.M.; Schulte, M.; Fischer, S.; Hirsch, T.; Richter, W.; Abraham, S.; Kneser, U.; Lehnhardt, M.; et al. Application of vegfa and fgf-9 enhances angiogenesis, osteogenesis and bone remodeling in type 2 diabetic long bone regeneration. PLoS ONE 2015, 10, e0118823. [Google Scholar] [CrossRef]
- Thomas, T. The complex effects of leptin on bone metabolism through multiple pathways. Curr. Opin. Pharmacol. 2004, 4, 295–300. [Google Scholar] [CrossRef]
- Thomas, T.; Gori, F.; Khosla, S.; Jensen, M.D.; Burguera, B.; Riggs, B.L. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 1999, 140, 1630–1638. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Bava, U.; Lin, C.; Naot, D.; Hill, B.L.; Grey, A.B.; Broom, N.; Myers, D.E.; Nicholson, G.C.; et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J. Endocrinol. 2002, 175, 405–415. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Pennington, C.; Newton, D.; Xie, D.; Isales, C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 2004, 34, 376–383. [Google Scholar] [CrossRef]
- Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.G.; Bannon, T.W.; Noda, M.; et al. Leptin regulation of bone resorption by the sympathetic nervous system and cart. Nature 2005, 434, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Al-Nakkash, L.; Broderick, T.L.; Castro, M.; Plochocki, J.H. Leptin-deficient mice have altered three-dimensional growth plate histomorphometry. Diabetol. Metab. Syndr. 2019, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Tice, M.J.L.; Bailey, S.; Sroga, G.E.; Gallagher, E.J.; Vashishth, D. Non-obese mkr mouse model of type 2 diabetes reveals skeletal alterations in mineralization and material properties. JBMR Plus 2022, 6, e10583. [Google Scholar] [CrossRef] [PubMed]
- Braga Frade, B.; da Cunha Muller, L.D.; Bonfim, D.C. Establishing a diaphyseal femur fracture model in mice. J. Vis. Exp. 2022, 190, 64766. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.H.; Matthys, R.; Histing, T.; Becker, S.C.; Fiedler, M.; Garcia, P.; Meier, C.; Pohlemann, T.; Menger, M.D. Development of a stable closed femoral fracture model in mice. J. Surg. Res. 2009, 153, 71–75. [Google Scholar] [CrossRef]
- Histing, T.; Bremer, P.; Rollmann, M.F.; Herath, S.; Klein, M.; Pohlemann, T.; Menger, M.D.; Fritz, T. A minimally invasive model to analyze endochondral fracture healing in mice under standardized biomechanical conditions. J. Vis. Exp. 2018, 133, 57255. [Google Scholar] [CrossRef]
- Histing, T.; Holstein, J.H.; Garcia, P.; Matthys, R.; Kristen, A.; Claes, L.; Menger, M.D.; Pohlemann, T. Ex vivo analysis of rotational stiffness of different osteosynthesis techniques in mouse femur fracture. J. Orthop. Res. 2009, 27, 1152–1156. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisenbacher, T.C.; Ehnert, S.; Histing, T.; Nüssler, A.K.; Menger, M.M. Advantages and Limitations of Diabetic Bone Healing in Mouse Models: A Narrative Review. Biomedicines 2023, 11, 3302. https://doi.org/10.3390/biomedicines11123302
Maisenbacher TC, Ehnert S, Histing T, Nüssler AK, Menger MM. Advantages and Limitations of Diabetic Bone Healing in Mouse Models: A Narrative Review. Biomedicines. 2023; 11(12):3302. https://doi.org/10.3390/biomedicines11123302
Chicago/Turabian StyleMaisenbacher, Tanja C., Sabrina Ehnert, Tina Histing, Andreas K. Nüssler, and Maximilian M. Menger. 2023. "Advantages and Limitations of Diabetic Bone Healing in Mouse Models: A Narrative Review" Biomedicines 11, no. 12: 3302. https://doi.org/10.3390/biomedicines11123302
APA StyleMaisenbacher, T. C., Ehnert, S., Histing, T., Nüssler, A. K., & Menger, M. M. (2023). Advantages and Limitations of Diabetic Bone Healing in Mouse Models: A Narrative Review. Biomedicines, 11(12), 3302. https://doi.org/10.3390/biomedicines11123302






