The Safety and Efficacy of Combining Saxagliptin and Pioglitazone Therapy in Streptozocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
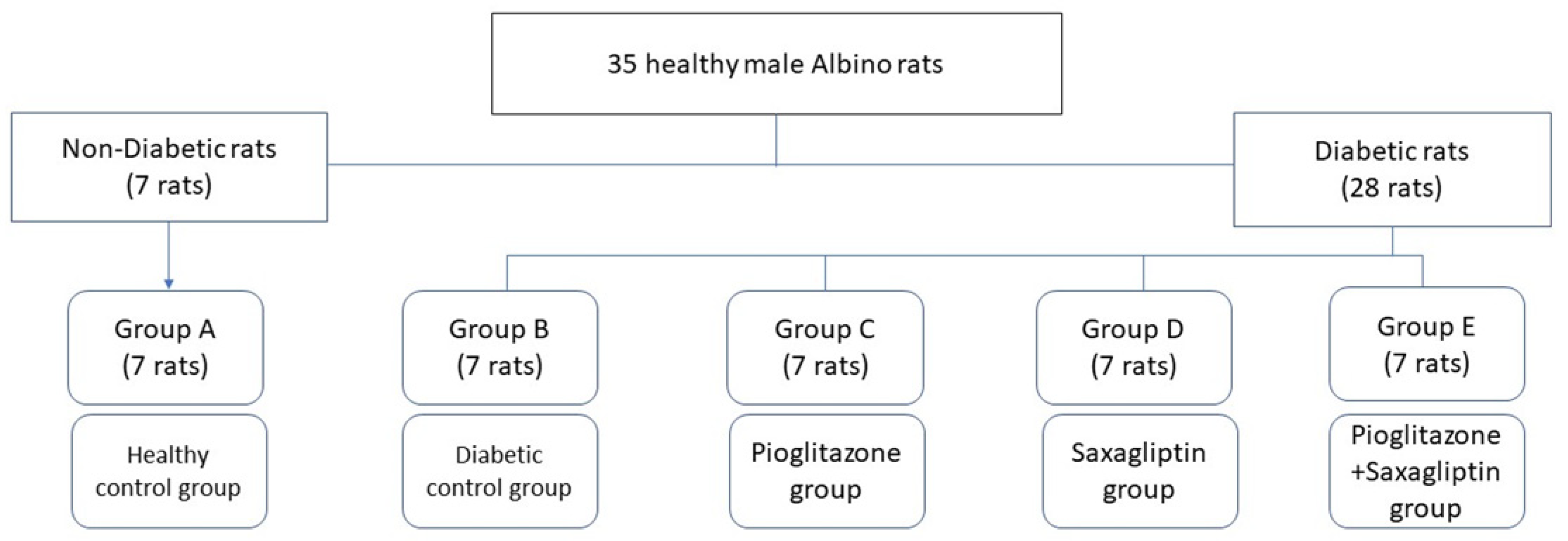
2.2. Experimental Design
2.3. Drugs
2.4. Blood Sampling and Biochemical Analysis
2.5. Glucose Hemostasis Traits and Lipid Profiles
2.6. Liver and Kidney Functions
2.7. Oxidative Stress Parameters
2.8. RNA Extraction and Assaying Gene Expression
2.9. MicroRNA-29a Reverse Transcription and qPCR Detection
2.10. PI3K, PEPCK, and IL-1β mRNA Reverse Transcription and qPCR Detection
2.11. Statistical Analysis
3. Results
3.1. Biochemical Analysis
3.2. Oxidative Stress
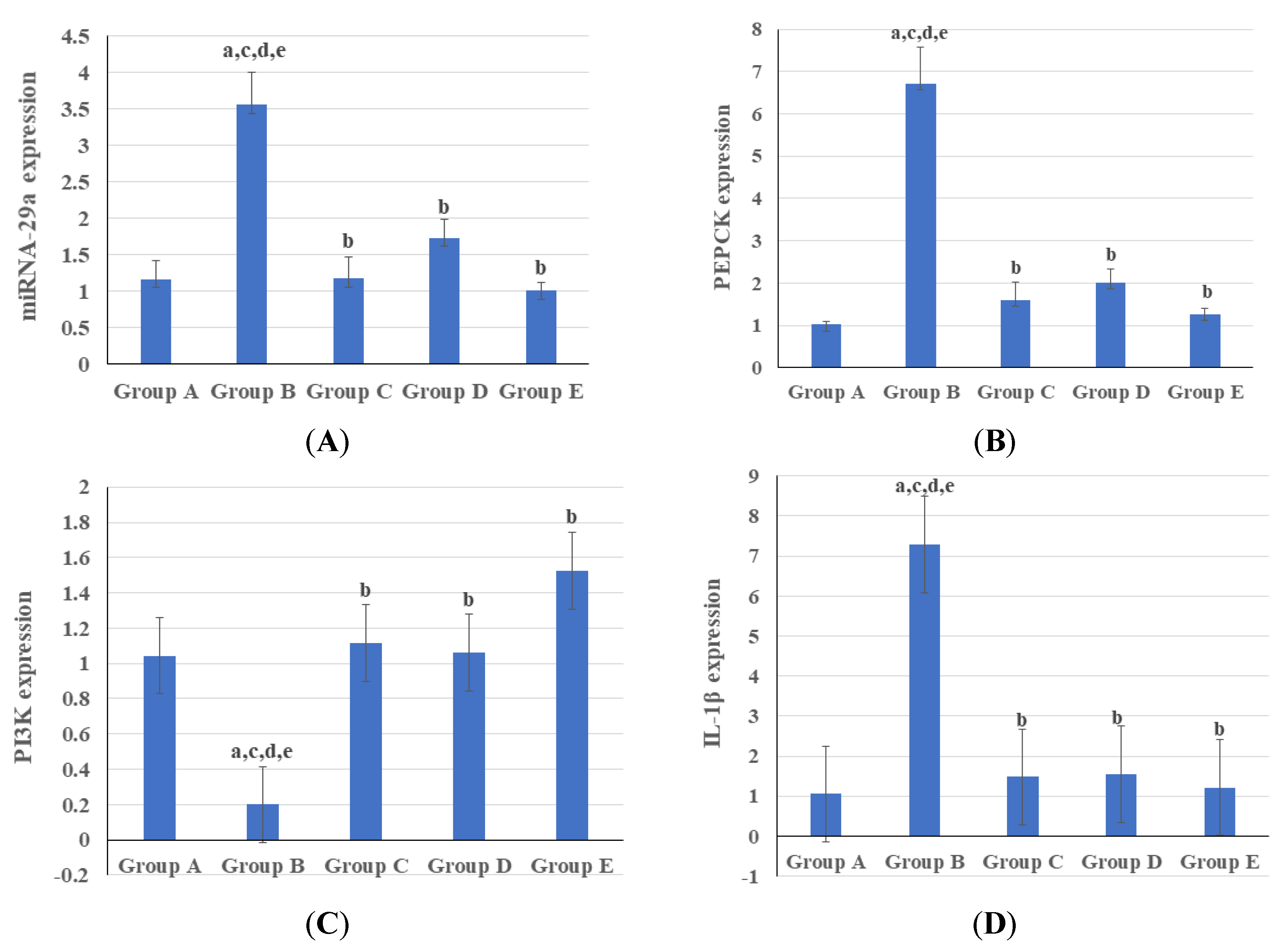
3.3. Genetic and Epigenetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebada, M.A.; Fayed, N.; Fayed, L.; Alkanj, S.; Abdelkarim, A.; Farwati, H.; Hanafy, A.; Negida, A.; Ebada, M.; Noser, Y. Efficacy of Alpha-lipoic Acid in The Management of Diabetes Mellitus: A Systematic Review and Meta-analysis. Iran. J. Pharm. Res. IJPR 2019, 18, 2144–2156. [Google Scholar] [PubMed]
- WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. A J. Br. Diabet. Assoc 1998, 15, 539–553. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative Stress, Insulin Resistance, Dyslipidemia and Type 2 Diabetes Mellitus. World J. Diabetes 2015, 6, 456. Available online: http://www.wjgnet.com/1948-9358/full/v6/i3/456.htm (accessed on 22 January 2023). [CrossRef]
- Bjornstad, P.; Eckel, R.H. Pathogenesis of Lipid Disorders in Insulin Resistance: A Brief Review. Curr. Diabetes Rep. 2018, 18, 127. [Google Scholar] [CrossRef]
- Allah, A.W.; Yahya, M.; Elsaeidy, K.S.; Alkanj, S.; Hamam, K.; El-Saady, M.; Ebada, M.A. Clinical assessment of miRNA-23b as a prognostic factor for various carcinomas: A systematic review and meta-analysis. Meta Gene 2020, 24, 100651. [Google Scholar] [CrossRef]
- Zhao, C.; Lai, S.; Wu, D.; Liu, D.; Zou, X.; Ismail, A.; El-Seedi, H.; Arroo, R.R.; Xiao, J. miRNAs as Regulators of Antidiabetic Effects of Fucoidans. eFood 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Chen, H.; Lan, H.Y.; Roukos, D.H.; Cho, W.C. Application of microRNAs in diabetes mellitus. J. Endocrinol. 2014, 222, R1–R10. [Google Scholar] [CrossRef]
- Zhu, H.; Leung, S.W. Identification of microRNA biomarkers in type 2 diabetes: A meta-analysis of controlled profiling studies. Diabetologia 2015, 58, 900–911. [Google Scholar] [CrossRef]
- Dooley, J.; Garcia-Perez, J.E.; Sreenivasan, J.; Schlenner, S.M.; Vangoitsenhoven, R.; Papadopoulou, A.S.; Tian, L.; Schonefeldt, S.; Serneels, L.; Deroose, C.; et al. The microRNA-29 family dictates the balance between homeostatic and pathological glucose handling in diabetes and obesity. Diabetes 2016, 65, 53–61. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Zhu, L.; Gupta, N.; Chang, Y.; Fang, F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol. Endocrinol. 2007, 21, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Shima, A.; Kuramoto, D.; Kikumoto, D.; Matsui, T.; Michihara, A. Phosphoenolpyruvate Carboxykinase, a Key Enzyme That Controls Blood Glucose, Is a Target of Retinoic Acid Receptor-Related Orphan Receptor α. PLoS ONE 2015, 10, e0137955. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.-P.; Chen, G. Chapter Six—Factors Affecting Insulin-Regulated Hepatic Gene Expression. In Glucose Homeostatis and the Pathogenesis of Diabetes Mellitus [Internet]; Tao, Y.-X., Ed.; Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 165–215. Available online: https://www.sciencedirect.com/science/article/pii/B9780128001011000065 (accessed on 7 February 2023).
- Quinn, P.G.; Yeagley, D. Insulin regulation of PEPCK gene expression: A model for rapid and reversible modulation. Curr. Drug Targets Immune Endocr. Metab. Disord. 2005, 5, 423–437. [Google Scholar] [CrossRef]
- Elo, B.; Villano, C.M.; Govorko, D.; White, L.A. Larval zebrafish as a model for glucose metabolism: Expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J. Mol. Endocrinol. 2007, 38, 433–440. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Simon, A.; van der Meer, J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef]
- Zhao, G.; Dharmadhikari, G.; Maedler, K.; Meyer-Hermann, M. Possible role of interleukin-1β in type 2 diabetes onset and implications for anti-inflammatory therapy strategies. PLoS Comput. Biol. 2014, 10, e1003798. [Google Scholar] [CrossRef]
- Shubrook, J.; Colucci, R.; Guo, A.; Schwartz, F. Saxagliptin: A Selective DPP-4 Inhibitor for the Treatment of Type 2 Diabetes Mellitus. Clin. Med. Insights Endocrinol. Diabetes 2011, 4, CMED-S5114. [Google Scholar] [CrossRef]
- Dave, D.J. Saxagliptin: A dipeptidyl peptidase-4 inhibitor in the treatment of type 2 diabetes mellitus. J. Pharmacol. Pharmacother. 2011, 2, 230–235. [Google Scholar] [CrossRef]
- Orasanu, G.; Ziouzenkova, O.; Devchand, P.R.; Nehra, V.; Hamdy, O.; Horton, E.S.; Plutzky, J. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. J. Am. Coll. Cardiol. 2008, 52, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Coletta, D.K.; Sriwijitkamol, A.; Wajcberg, E.; Tantiwong, P.; Li, M.; Prentki, M.; Madiraju, M.; Jenkinson, C.P.; Cersosimo, E.; Musi, N.; et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: A randomised trial. Diabetologia 2009, 52, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.Z.; Ehssan, N.A.; Ghiet, M.H.; Wahman, L.F. Pioglitazone versus metformin in two rat models of glucose intolerance and diabetes. Pak. J. Pharm. Sci. 2010, 23, 305–312. [Google Scholar] [PubMed]
- Kurtz, C.L.; Peck, B.C.; Fannin, E.E.; Beysen, C.; Miao, J.; Landstreet, S.R.; Ding, S.; Turaga, V.; Lund, P.K.; Turner, S.; et al. MicroRNA-29 fine-tunes the expression of key foxa2- Activated lipid metabolism genes and is dysregulated in animal models of insulin resistance and diabetes. Diabetes 2014, 63, 3141–3148. [Google Scholar] [CrossRef]
- Delić, D.; Wiech, F.; Urquhart, R.; Gabrielyan, O.; Rieber, K.; Rolser, M.; Tsuprykov, O.; Hasan, A.A.; Krämer, B.K.; Baum, P.; et al. Linagliptin and telmisartan induced effects on renal and urinary exosomal miRNA expression in rats with 5/6 nephrectomy. Sci. Rep. 2020, 10, 3373. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, N. Combination therapy with DPP-4 inhibitors and pioglitazone in type 2 diabetes: Theoretical consideration and therapeutic potential. Vasc. Health Risk Manag. 2008, 4, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Hollander, P.; Li, J.; Allen, E.; Chen, R. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J. Clin. Endocrinol. Metab. 2009, 94, 4810–4819. [Google Scholar] [CrossRef]
- Hollander, P.L.; Li, J.; Frederich, R.; Allen, E.; Chen, R. Safety and efficacy of saxagliptin added to thiazolidinedione over 76 weeks in patients with type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2011, 8, 125–135. [Google Scholar] [CrossRef]
- Patel, C.G.; Kornhauser, D.; Vachharajani, N.; Komoroski, B.; Brenner, E.; Handschuh del Corral, M.; Li, L.; Boulton, D.W. Saxagliptin, a potent, selective inhibitor of DPP-4, does not alter the pharmacokinetics of three oral antidiabetic drugs (metformin, glyburide or pioglitazone) in healthy subjects. Diabetes. Obes. Metab. 2011, 13, 604–614. [Google Scholar] [CrossRef]
- Reed, M.J.; Meszaros, K.; Entes, L.J.; Claypool, M.D.; Pinkett, J.G.; Gadbois, T.M.; Reaven, G.M. A new rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metab. Clin. Exp. 2000, 49, 1390–1394. [Google Scholar] [CrossRef]
- Skovsø, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 2014, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Algandaby, M.M.; Alghamdi, H.A.; Ashour, O.M.; Abdel-Naim, A.B.; Ghareib, S.A.; Abdel-Sattar, E.A.; Hajar, A.S. Mechanisms of the antihyperglycemic activity of Retama raetam in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2010, 48, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Su, J.; Luo, Y.; Hu, S.; Tang, L.; Ouyang, S. Advances in Research on Type 2 Diabetes Mellitus Targets and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 13381. [Google Scholar] [CrossRef]
- Gudoor, R.; Suits, A.; Shubrook, J.H. Perfecting the Puzzle of Pathophysiology: Exploring Combination Therapy in the Treatment of Type 2 Diabetes. Diabetology 2023, 4, 379–392. [Google Scholar] [CrossRef]
- Karyekar, C.; Donovan, M.; Allen, E.; Fleming, D.; Ravichandran, S.; Chen, R. Efficacy and safety of saxagliptin combination therapy in US patients with type 2 diabetes. Postgrad. Med. 2011, 123, 63–70. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Sang, Y.; Liu, X.; Liang, J. Comparison of dipeptidyl peptidase-4 inhibitors and pioglitazone combination therapy versus pioglitazone monotherapy in type 2 diabetes: A system review and meta-analysis. Medicine 2018, 97, e12633. [Google Scholar] [CrossRef]
- Petchiappan, V.; Mathew, E.; Jose, J.; Fardan, M.; Chidambaram, Y.; Thangavelu, S. Use of Fixed-dose Combination Therapy with Remogliflozin and Vildagliptin as an Add-on Drug in Improving the Glycemic Control of Type 2 Diabetes Mellitus: An Observational Study. J. Pharmacol. Pharmacother. 2023, 14, 72–78. [Google Scholar] [CrossRef]
- Al-Muzafar, H.M.; Alshehri, F.S.; Amin, K.A. The role of pioglitazone in antioxidant, anti-inflammatory, and insulin sensitivity in a high fat-carbohydrate diet-induced rat model of insulin resistance. Braz. J. Med. Biol. Res. 2021, 54, e10782. [Google Scholar] [CrossRef] [PubMed]
- Yano, W.; Inoue, N.; Ito, S.; Itou, T.; Yasumura, M.; Yoshinaka, Y.; Hagita, S.; Goto, M.; Nakagawa, T.; Inoue, K.; et al. Mechanism of lipid-lowering action of the dipeptidyl peptidase-4 inhibitor, anagliptin, in low-density lipoprotein receptor-deficient mice. J. Diabetes Investig. 2017, 8, 155–160. [Google Scholar] [CrossRef]
- Bolevich, S.; Milosavljevic, I.; Draginic, N.; Andjic, M.; Jeremic, N.; Bolevich, S.; Litvitsky, P.F.; Jakovljevic, V. The effect of the chronic administration of dpp4inhibitors on systemic oxidative stress in rats with diabetes type 2. Serb. J. Exp. Clin. Res. 2019, 20, 199–206. [Google Scholar] [CrossRef]
- Refaat, R.; Sakr, A.; Salama, M.; El Sarha, A. Combination of Vildagliptin and Pioglitazone in Experimental Type 2 Diabetes in Male Rats. Drug Dev. Res. 2016, 77, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. Anti-inflammatory effects of short-term pioglitazone therapy in men with advanced diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2006, 290, F600-5. [Google Scholar] [CrossRef]
- Gumieniczek, A. Effect of the new thiazolidinedione-pioglitazone on the development of oxidative stress in liver and kidney of diabetic rabbits. Life Sci. 2003, 74, 553–562. [Google Scholar] [CrossRef]
- Fukui, T.; Noma, T.; Mizushige, K.; Yasuharu, A.; Kimura, S.; Youichi, A. Dietary troglitazone decreases oxidative stress in early stage type II diabetic rats. Life Sci. 2000, 66, 2043–2049. [Google Scholar] [CrossRef]
- Stanzione, R.; Forte, M.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Busceti, C.L.; Fornai, F.; Rubattu, S. Uncoupling Protein 2 as a Pathogenic Determinant and Therapeutic Target in Cardiovascular and Metabolic Diseases. Curr. Neuropharmacol. 2022, 20, 662–674. [Google Scholar] [CrossRef]
- Chan, S.H.H.; Wu, K.L.H.; Kung, P.S.S.; Chan, J.Y.H. Oral Intake of Rosiglitazone Promotes a Central Antihypertensive Effect Via Upregulation of Peroxisome Proliferator-Activated Receptor-γ and Alleviation of Oxidative Stress in Rostral Ventrolateral Medulla of Spontaneously Hypertensive Rats. Hypertension 2010, 55, 1444–1453. [Google Scholar] [CrossRef]
- Cabrera, J.A.; Ziemba, E.A.; Colbert, R.; Kelly, R.F.; Kuskowski, M.; Arriaga, E.A.; Sluiter, W.; Duncker, D.J.; Ward, H.B.; McFalls, E.O. Uncoupling protein-2 expression and effects on mitochondrial membrane potential and oxidant stress in heart tissue. Transl. Res. 2012, 159, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Itami, S.; Kuroda, M.; Yoshizato, K.; Kawada, N.; Murakami, Y. MiR-29a assists in preventing the activation of human stellate cells and promotes recovery from liver fibrosis in mice. Mol. Ther. 2016, 24, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- Ślusarz, A.; Pulakat, L. The two faces of miR-29. J. Cardiovasc. Med. 2015, 16, 480–490. [Google Scholar]
- Yadollah, S.; Kazemipour, N.; Bakhtiyari, S.; Nazifi, S. Palmitate-induced insulin resistance is attenuated by Pioglitazone and EGCG through reducing the gluconeogenic key enzymes expression in HepG2 cells. J. Med. Life 2017, 10, 244–249. [Google Scholar] [PubMed]
- Kim, T.H.; Lee, J.H.; Chae, Y.N.; Jung, I.H.; Kim, M.K. Additive effects of evogliptin in combination with pioglitazone on fasting glucose control through direct and indirect hepatic effects in diabetic mice. Eur. J. Pharmacol. 2018, 830, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fukunishi, S.; Yokohama, K.; Ohama, H.; Tsuchimoto, Y.; Asai, A.; Tsuda, Y.; Higuchi, K. A long-lasting dipeptidyl peptidase-4 inhibitor, teneligliptin, as a preventive drug for the development of hepatic steatosis in high-fructose diet-fed ob/ob mice. Int. J. Mol. Med. 2017, 39, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Insulin–PI3K signalling: An evolutionarily insulated metabolic driver of cancer. Nat. Rev. Endocrinol. 2020, 16, 276–283. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Yu, N.; Fang, X.; Zhao, D.; Mu, Q.; Zuo, J.; Ma, Y.; Zhang, Y.; Mo, F.; Zhang, D.; Jiang, G.; et al. Anti-diabetic effects of Jiang Tang Xiao Ke granule via PI3K/Akt signalling pathway in type 2 diabetes KKAy mice. PLoS ONE 2017, 12, e0168980. [Google Scholar] [CrossRef]
- Anandharajan, R.; Jaiganesh, S.; Shankernarayanan, N.P.; Viswakarma, R.A.; Balakrishnan, A. In vitro glucose uptake activity of Aegles marmelos and Syzygium cumini by activation of Glut-4, PI3 kinase and PPARγ in L6 myotubes. Phytomedicine 2006, 13, 434–441. [Google Scholar] [CrossRef]
- Khedr, R.M.; Ahmed, A.A.E.; Kamel, R.; Raafat, E.M. Sitagliptin attenuates intestinal ischemia/reperfusion injury via cAMP/PKA, PI3K/Akt pathway in a glucagon-like peptide 1 receptor-dependent manner. Life Sci. 2018, 211, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zaky, D.A.; Abouelfadl, D.M.; Nassar, N.N.; Abdallah, D.M.; Al-Shorbagy, M.Y. The paradox of dipeptidyl peptidase IV inhibition in enterocytic differentiation and epithelial-mesenchymal transition in rat cholestatic sepsis. Toxicol. Appl. Pharmacol. 2020, 394, 114956. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.; Nowell, M.; Chima, R.; Zingarelli, B. Pioglitazone reduces inflammation through inhibition of NF-κB in polymicrobial sepsis. Innate Immun. 2014, 20, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, X.; Chen, Z.; Yao, Q. DPP-4 inhibitor saxagliptin ameliorates oxygen deprivation/reoxygenation-induced brain endothelial injury. Am. J. Transl. Res. 2019, 11, 6316–6325. [Google Scholar]
- Kothari, V.; Galdo, J.A.; Mathews, S.T. Hypoglycemic agents and potential anti-inflammatory activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar]
- Birnbaum, Y.; Bajaj, M.; Qian, J.; Ye, Y. Dipeptidyl peptidase-4 inhibition by Saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ Open Diabetes Res. Care 2016, 4, e000227. [Google Scholar] [CrossRef]
| Gene | Catalog No. | Primers | Annealing Temperature, °C |
|---|---|---|---|
| PI3K | 4448892 | Forward: 5′-GAGCCGAGTTGGAGGAAGCA-3′ Reverse: 5′-CATCCGGGTGTCCATCTGTC-3′ | 60 |
| PEPCK | 4331182 | Forward: 5′-CGTTGGGAGCTAGGAGCAAA-3′ Reverse: 5′-CCCATCAGTGTCAGATGCGA-3′ | 60 |
| Il-1β | 4453320 | Forward: 5′-GGCGGTTCAAGGCATAACAG-3′ Reverse: 5′-TCAGACAGCACGAGGCATTT-3′ | 60 |
| 18S rRNA | 4308329 | Forward: 5′-ACGGACCAGAGCGAAAGCAT-3′ Reverse: 5′-TGTCAATCCTGTCCGTGTCC-3′ | 60 |
| Groups | FBG (mg/dL) | Insulin (µU/mL) | HOMA–IR | QUICKI | TC (mg/dL) | TG (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| Normal control | 89.71 ± 4.21 | 11.51 ± 0.77 | 2.52 ± 0.11 | 0.33 ± 0.002 | 92.65 ± 4.33 | 66.86 ± 3.2 | 43.6 ± 1.78 | 39.51 ± 1.15 |
| Diabetic control | 343.86 ± 10.65 a, c, d, e | 6.31 ± 0.48 a | 5.3 ± 0.27 a, c, d, e | 0.3 ± 0.0019 a, c, e | 173.34 ± 2.95 a, c, d, e | 156.8 ± 6.44 a, c, d, e | 22.38 ± 1.49 a, c, d, e | 136.5 ± 2.06 a, c, d, e |
| Pioglitazone group | 179.86 ± 5.15 a, b, d | 7.06 ± 0.63 a | 3.17 ± 0.36 b | 0.32 ± 0.005 b | 113.63 ± 3.47 a, b, d | 84.08 ± 2.62 a, b | 34.62 ± 1.39 a, b, d | 59.83 ± 1.72 a, b, d, e |
| Saxagliptin group | 210.43 ± 9.02 a, b, c, e | 7.82 ± 1.11 a | 3.92 ± 0.39 a, b | 0.31 ± 0.004 a | 129.35 ± 3.24 a, b, c, e | 98.41 ± 2.86 a, b, e | 28.46 ± 1.1 a, b, c, e | 69.2 ± 0.82 a, b, c, e |
| Saxagliptin + Pioglitazone | 155.29 ± 4.25 a, b, d | 8.04 ± 0.92 a | 3.03 ± 0.27 b | 0.32 ± 0.0037 b | 107.35 ± 3.59 a, b, d | 78.79 ± 2.09 b, d | 38.95 ± 1.27 b, d | 47.58 ± 1.2 a, b, c, d |
| Groups | Urea (mg/dL) | Creatinine (mg/dL) | ALT (U/L) | AST (U/L) | SOD (U/gm Tissue) | Catalase (U/g Tissue) | MDA (nmol/g Tissue) | GSH (mmol/g Tissue) |
|---|---|---|---|---|---|---|---|---|
| Normal control | 41.16 ± 4.19 | 0.73 ± 0.04 | 21.45 ± 1.38 | 59.39 ± 2.35 | 4680.14 ± 307.31 | 121.03 ± 4.74 | 259.34 ±11.29 | 37.0 ± 2.31 |
| Diabetic control | 109.61 ±4.71 a, c, d, e | 1.52 ± 0.11 a, c, d, e | 39.35 ± 1.59 a, c, d, e | 94.91 ± 3.19 a, c, d, e | 2031 ± 193.02 a, c, d, e | 55.16 ± 3.02 a, c, d, e | 429.89 ±11.37 a, c, d, e | 14.02 ± 1.21 a, c, d, e |
| Pioglitazone group | 91.33 ± 3.74 a, b, d, e | 0.9 ± 0.08 b | 25.19 ± 1.31 b | 71.22 ± 3.42 a, b, e | 3502.75 ± 63.59 a, b | 86.65 ± 2.14 a, b, d, e | 339.12 ± 4.93 a, b, d, e | 26.25 ± 0.89 a, b, d, e |
| Saxagliptin group | 70.03 ± 4.72 a, b, c | 0.81 ± 0.05 b | 28.84 ± 0.8 a, b, e | 74.59 ± 1.046 a, b, e | 2972.92 ± 272.97 a, b, e | 72.47 ± 2.45 a, b, c, e | 382.86 ± 5.84 a, b, c, e | 20.16 ± 1.27 a, b, c, e |
| Saxagliptin + Pioglitazone | 61.26 ± 4.12 a, b, c | 0.78 ± 0.07 b | 22.09 ± 1.61 b, d | 60.64 ±. 0.54 b, c, d | 4297.49 ±187.71 b, d | 101.4 ± 2.31 a, b, c, d | 302.2 ± 3.34 a, b, c, d | 33.68 ± 1.06 b, c, d |
| miRNA 29a | PI3K Gene | PEPCK Gene | IL-1β Gene | |||||
|---|---|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | r | p Value | |
| miRNA 29a | −0.49 | 0.003 * | 0.74 | ˂0.001 * | 0.79 | ˂0.001 * | ||
| PI3K gene | −0.49 | 0.003 * | −0.56 | 0.001 * | −0.54 | 0.001 * | ||
| PEPCK gene | 0.74 | ˂0.001 * | −0.56 | 0.001 * | 0.93 | ˂0.001 * | ||
| IL-1β gene | 0.79 | ˂0.001 * | −0.54 | 0.001 * | 0.93 | ˂0.001 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, A.M.; Ashour Ibrahim, I.; Saleh, S.M.; Abo-Elmatty, D.M.; Mesbah, N.M.; Abdel-Hamed, A.R. The Safety and Efficacy of Combining Saxagliptin and Pioglitazone Therapy in Streptozocin-Induced Diabetic Rats. Biomedicines 2023, 11, 3300. https://doi.org/10.3390/biomedicines11123300
Othman AM, Ashour Ibrahim I, Saleh SM, Abo-Elmatty DM, Mesbah NM, Abdel-Hamed AR. The Safety and Efficacy of Combining Saxagliptin and Pioglitazone Therapy in Streptozocin-Induced Diabetic Rats. Biomedicines. 2023; 11(12):3300. https://doi.org/10.3390/biomedicines11123300
Chicago/Turabian StyleOthman, Ahmed Mohamed, Ibrahim Ashour Ibrahim, Samy M. Saleh, Dina M. Abo-Elmatty, Noha M. Mesbah, and Asmaa R. Abdel-Hamed. 2023. "The Safety and Efficacy of Combining Saxagliptin and Pioglitazone Therapy in Streptozocin-Induced Diabetic Rats" Biomedicines 11, no. 12: 3300. https://doi.org/10.3390/biomedicines11123300
APA StyleOthman, A. M., Ashour Ibrahim, I., Saleh, S. M., Abo-Elmatty, D. M., Mesbah, N. M., & Abdel-Hamed, A. R. (2023). The Safety and Efficacy of Combining Saxagliptin and Pioglitazone Therapy in Streptozocin-Induced Diabetic Rats. Biomedicines, 11(12), 3300. https://doi.org/10.3390/biomedicines11123300





