Association of Endotoxemia with Low-Grade Inflammation, Metabolic Syndrome and Distinct Response to Lipopolysaccharide in Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups and Subjects
2.2. Clinical Definitions
2.3. Sampling of Blood for Serum Preparation and Fecal Collection
2.4. Determination of Inflammatory and Endotoxemia Markers
2.5. Indices of Insulin Resistance and NAFLD
2.6. Statistical Analysis
2.6.1. Data Normalization across the Plates
2.6.2. Descriptive Statistics and Comparisons between Two Samples
2.6.3. Correlations and Regression Analysis
3. Results
3.1. Characteristics of Subjects
3.2. Levels of Serum Inflammatory Markers in Patients with T1D Stratified According to MS Presence and Controls
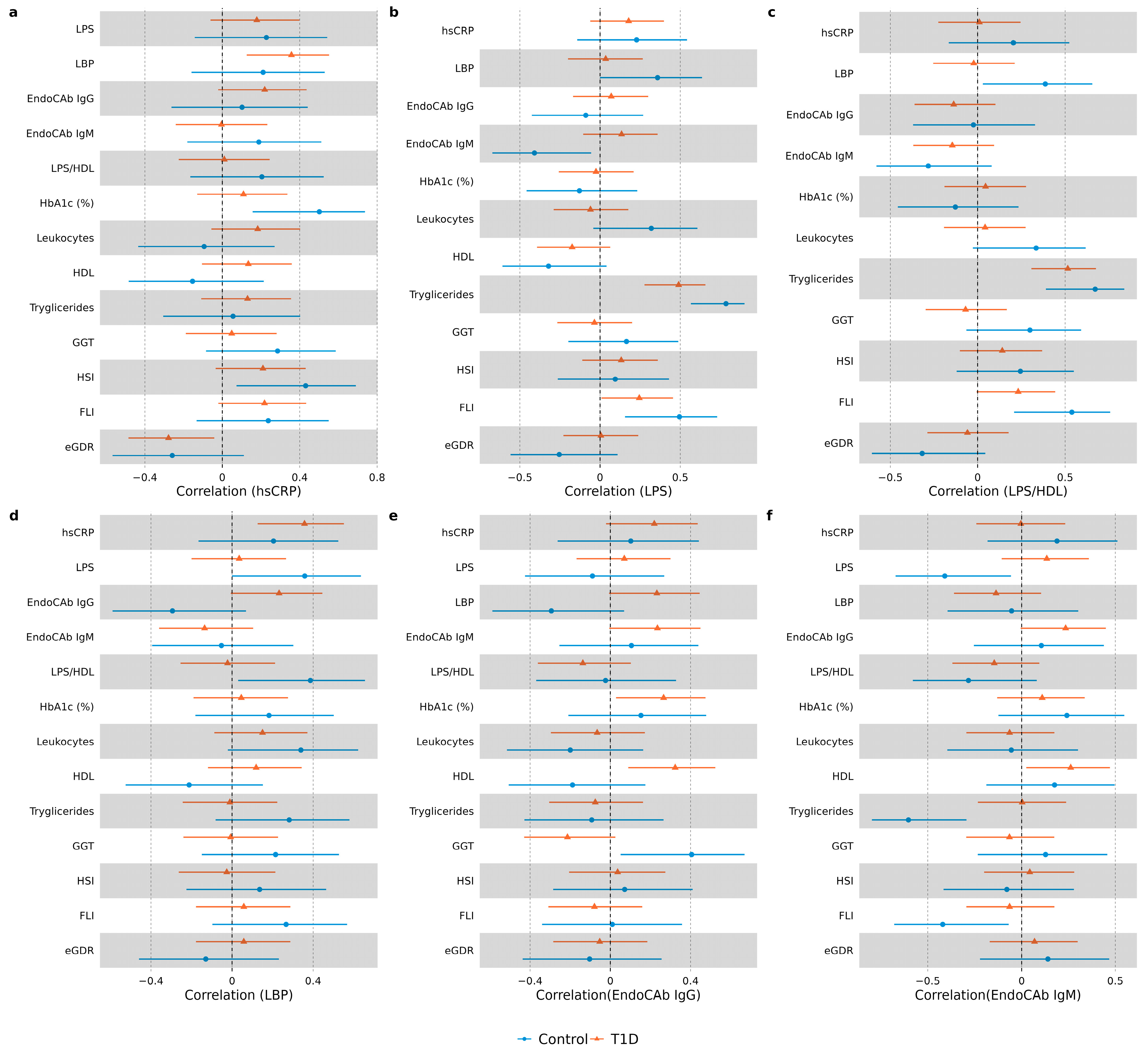
3.3. Correlation Analysis in Study Groups
3.4. Regression Analysis in T1D Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, F.W. The increasing prevalence of autoimmunity and autoimmune diseases: An urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr. Opin. Immunol. 2023, 80, 102266. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Das, S.R.; Gibbons, C.H.; et al. Introduction and Methodology: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S1–S4. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Twigg, S.M.; Flack, J.R. Metabolic syndrome in type 1 diabetes and its association with diabetes complications. Diabet Med. 2021, 38, e14376. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, D.; Deng, H.; Liang, H.; Zheng, X.; Yan, J.; Xu, W.; Liu, X.; Yao, B.; Luo, S.; et al. Association between Metabolic Syndrome and Microvascular Complications in Chinese Adults with Type 1 Diabetes Mellitus. Diabetes Metab. J. 2022, 46, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Chillarón, J.J.; Flores Le-Roux, J.A.; Benaiges, D.; Pedro-Botet, J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism 2014, 63, 181–187. [Google Scholar] [CrossRef]
- Lehto, M.; Groop, P.H. The Gut-Kidney Axis: Putative Interconnections Between Gastrointestinal and Renal Disorders. Front. Endocrinol. 2018, 9, 553. [Google Scholar] [CrossRef]
- Nymark, M.; Pussinen, P.J.; Tuomainen, A.M.; Forsblom, C.; Groop, P.H.; Lehto, M. Serum lipopolysaccharide activity is associated with the progression of kidney disease in finnish patients with type 1 diabetes. Diabetes Care 2009, 32, 1689–1693. [Google Scholar] [CrossRef]
- Riddle, M.; Pencek, R.; Charenkavanich, S.; Lutz, K.; Wilhelm, K.; Porter, L. Randomized comparison of pramlintide or mealtime insulin added to basal insulin treatment for patients with type 2 diabetes. Diabetes Care 2009, 32, 1577–1582. [Google Scholar] [CrossRef][Green Version]
- Gorabi, A.M.; Kiaie, N.; Khosrojerdi, A.; Jamialahmadi, T.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Implications for the role of lipopolysaccharide in the development of atherosclerosis. Trends Cardiovasc. Med. 2022, 32, 525–533. [Google Scholar] [CrossRef]
- Lassenius, M.I.; Ahola, A.J.; Harjutsalo, V.; Forsblom, C.; Groop, P.H.; Lehto, M. Endotoxins are associated with visceral fat mass in type 1 diabetes. Sci. Rep. 2016, 6, 38887. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.A.; Henriksen, P.; Vogt, J.K.; Hansen, T.H.; Ahonen, L.; Suvitaival, T.; Hein Zobel, E.; Frimodt-Møller, M.; Hansen, T.W.; Hansen, T.; et al. Gut microbiota profile and selected plasma metabolites in type 1 diabetes without and with stratification by albuminuria. Diabetologia 2020, 63, 2713–2724. [Google Scholar] [CrossRef] [PubMed]
- Lassenius, M.I.; Fogarty, C.L.; Blaut, M.; Haimila, K.; Riittinen, L.; Paju, A.; Kirveskari, J.; Järvelä, J.; Ahola, A.J.; Gordin, D.; et al. Intestinal alkaline phosphatase at the crossroad of intestinal health and disease—A putative role in type 1 diabetes. J. Intern. Med. 2017, 281, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.A.; Mannerla, M.M.; Frimodt-Møller, M.; Persson, F.; Hansen, T.W.; Lehto, M.; Hörkkö, S.; Blaut, M.; Forsblom, C.; Groop, P.H.; et al. Faecal biomarkers in type 1 diabetes with and without diabetic nephropathy. Sci. Rep. 2021, 11, 15208. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H. Lipopolysaccharide: Basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intestig. Res. 2014, 12, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Mora, V.P.; Loaiza, R.A.; Soto, J.A.; Bohmwald, K.; Kalergis, A.M. Involvement of trained immunity during autoimmune responses. J. Autoimmun. 2023, 137, 102956. [Google Scholar] [CrossRef]
- Schumann, R.R.; Zweigner, J. A novel acute-phase marker: Lipopolysaccharide binding protein (LBP). Clin. Chem. Lab. Med. 1999, 37, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Barclay, G.R. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: A review. Prog. Clin. Biol. Res. 1995, 392, 263–272. [Google Scholar]
- de Groot, P.F.; Belzer, C.; Aydin, Ö.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; van Raalte, D.H.; Scheithauer, T.P.; et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef] [PubMed]
- Lassenius, M.I.; Mäkinen, V.P.; Fogarty, C.L.; Peräneva, L.; Jauhiainen, M.; Pussinen, P.J.; Taskinen, M.R.; Kirveskari, J.; Vaarala, O.; Nieminen, J.K.; et al. Patients with type 1 diabetes show signs of vascular dysfunction in response to multiple high-fat meals. Nutr. Metab. 2014, 11, 28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aravindhan, V.; Mohan, V.; Arunkumar, N.; Sandhya, S.; Babu, S. Chronic Endotoxemia in Subjects with Type-1 Diabetes Is Seen Much before the Onset of Microvascular Complications. PLoS ONE 2015, 10, e0137618. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Katsura, T.; Takahara, M.; Miyashita, K.; Katakami, N.; Matsuoka, T.A.; Kawamori, D.; Shimomura, I. Plasma lipopolysaccharide binding protein level statistically mediates between body mass index and chronic microinflammation in Japanese patients with type 1 diabetes. Diabetol. Int. 2020, 11, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P. NAFLD, MAFLD, and beyond: One or several acronyms for better comprehension and patient care. Intern. Emerg. Med. 2023, 18, 993–1006. [Google Scholar] [CrossRef]
- Nier, A.; Huber, Y.; Labenz, C.; Michel, M.; Bergheim, I.; Schattenberg, J.M. Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 699. [Google Scholar] [CrossRef]
- Bulum, T.; Kolarić, B.; Duvnjak, M.; Duvnjak, L. Alkaline phosphatase is independently associated with renal function in normoalbuminuric type 1 diabetic patients. Ren. Fail. 2014, 36, 372–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Targher, G.; Mantovani, A.; Pichiri, I.; Mingolla, L.; Cavalieri, V.; Mantovani, W.; Pancheri, S.; Trombetta, M.; Zoppini, G.; Chonchol, M.; et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care 2014, 37, 1729–1736. [Google Scholar] [CrossRef]
- Freitag, G.; Munk, A. On Hadamard differentiability in k-sample semiparametric models—With applications to the assessment of structural relationships. J. Multivar. Anal. 2005, 94, 123–158. [Google Scholar] [CrossRef]
- Hall, P.; Lombard, F.; Potgieter, C.J. A New Approach to Function-Based Hypothesis Testing in Location-Scale Families. Technometrics 2013, 55, 215–223. [Google Scholar] [CrossRef]
- Claeskens, G.; Jing, B.-Y.; Peng, L.; Zhou, W. Empirical Likelihood Confidence Regions for Comparison Distributions and ROC Curves. Can. J. Stat./La Rev. Can. De Stat. 2003, 31, 173–190. [Google Scholar] [CrossRef]
- Doksum, K.A.; Sievers, G.L. Plotting with Confidence: Graphical Comparisons of Two Populations. Biometrika 1976, 63, 421–434. [Google Scholar] [CrossRef]
- Valeinis, J.; Cers, E.; Cielens, J. Two-sample problems in statistical data modelling. Math. Model. Anal.-Math. Model Anal. 2010, 15, 137–151. [Google Scholar] [CrossRef]
- Rousselet, G.A.; Pernet, C.R.; Wilcox, R.R. Beyond differences in means: Robust graphical methods to compare two groups in neuroscience. Eur. J. Neurosci. 2017, 46, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Radzeviciene, L.; Zaharenko, L.; Bulum, T.; Skrebinska, S.; Prakapiene, E.; Blaslov, K.; Roso, V.; Rovite, V.; Pirags, V.; et al. Association between symptoms of depression, diabetes complications and vascular risk factors in four European cohorts of individuals with type 1 diabetes—InterDiane Consortium. Diabetes Res. Clin. Pr. 2020, 170, 108495. [Google Scholar] [CrossRef] [PubMed]
- Rovite, V.; Wolff-Sagi, Y.; Zaharenko, L.; Nikitina-Zake, L.; Grens, E.; Klovins, J. Genome Database of the Latvian Population (LGDB): Design, Goals, and Primary Results. J. Epidemiol. 2018, 28, 353–360. [Google Scholar] [CrossRef]
- Sokolovska, J.; Dekante, A.; Baumane, L.; Pahirko, L.; Valeinis, J.; Dislere, K.; Rovite, V.; Pirags, V.; Sjakste, N. Nitric oxide metabolism is impaired by type 1 diabetes and diabetic nephropathy. Biomed. Rep. 2020, 12, 251–258. [Google Scholar] [CrossRef]
- Wadén, J.; Tikkanen, H.; Forsblom, C.; Fagerudd, J.; Pettersson-Fernholm, K.; Lakka, T.; Riska, M.; Groop, P.H. Leisure time physical activity is associated with poor glycemic control in type 1 diabetic women: The FinnDiane study. Diabetes Care 2005, 28, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.V.; Erbey, J.R.; Becker, D.; Arslanian, S.; Orchard, T.J. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 2000, 49, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Sviklāne, L.; Olmane, E.; Dzērve, Z.; Kupčs, K.; Pīrāgs, V.; Sokolovska, J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J. Gastroenterol. Hepatol. 2018, 33, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Cers, E.; Valeinis, J. EL: Two-Sample Empirical Likelihood, R package version, 1.2; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Niehues, T. C-reactive protein and other biomarkers—The sense and non-sense of using inflammation biomarkers for the diagnosis of severe bacterial infection. LymphoSign J. 2018, 5, 35–47. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Erlanson-Albertsson, C.; Stenkula, K.G. The Importance of Food for Endotoxemia and an Inflammatory Response. Int. J. Mol. Sci. 2021, 22, 9562. [Google Scholar] [CrossRef] [PubMed]
- Meilhac, O.; Tanaka, S.; Couret, D. High-Density Lipoproteins Are Bug Scavengers. Biomolecules 2020, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Ayyappan, P.; Harms, R.Z.; Buckner, J.H.; Sarvetnick, N.E. Coordinated Induction of Antimicrobial Response Factors in Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 658. [Google Scholar] [CrossRef]

| Phenotype | No Metabolic Syndrome (n = 43) | Metabolic Syndrome (n = 31) | p |
|---|---|---|---|
| Male/Female, n (%) | 19/24 (44/56) | 9/22 (29/71) | 0.28 |
| Age, years | 38 (32–50) | 45 (36–54) | 0.065 |
| BMI, kg/m2 | 23.5 (21.45–25.65) | 28 (25.4–32.65) | <0.001 |
| Waist/height ratio | 0.457 (0.431–0.497) | 0.56 (0.509–0.621) | <0.001 |
| Smoker, n (%) | 11 (26) | 9 (29) | 0.95 |
| Hypertension, n (%) | 15 (35) | 20 (65) | 0.022 |
| Length of diabetes, years | 20 (11–32) | 24 (17–34) | 0.062 |
| Retinopathy, n (%) | 13 (30) | 19 (61) | 0.015 |
| CVD, n (%) | 3 (7) | 6 (19) | 0.21 |
| Macroalbuminuria, n (%) | 2 (5) | 5 (16) | 0.21 |
| On ACEI/ARB, n (%) | 4 (9) | 11 (35) | 0.013 |
| On lipid-lowering medication, n (%) | 5 (12) | 12 (39) | 0.014 |
| Autoimmune thyroid disease, n (%) | 9 (21) | 10 (32) | 0.41 |
| Hemoglobin A1C, % | 7.3 (6.8–9.0) | 8.4 (7.4–9.3) | 0.087 |
| Hemoglobin A1C, mmol/mol | 53 (47–67) | 64 (54–64) | 0.087 |
| Estimated glomerular filtration rate, mL/min/1.73m2 | 114 (104–119) | 98 (74–108) | <0.001 |
| Albumin/creatinine ratio in urine, mg/mmol | 0.38 (0.19–1.05) | 0.97 (0.37–2.34) | 0.072 |
| Total cholesterol, mmol/L | 4.72 (4.29–5.18) | 5.51 (4.89–6.04) | 0.006 |
| Low-density lipoproteins, mmol/L | 2.68 (2.08–3.24) | 3.22 (2.47–3.75) | 0.02 |
| High-density lipoproteins, mmol/L | 1.71 (1.38–2.01) | 1.44 (1.21–1.73) | 0.012 |
| Triglycerides, mmol/L | 1.02 (0.79–1.18) | 1.72 (1.30–2.11) | <0.001 |
| Alanine aminotransaminase, U/L | 19 (134–26) | 21 (17–28) | 0.087 |
| Aspartate aminotransferase, U/L | 22 (19–28) | 25 (17–31) | 0.879 |
| Gamma-glutamyltransferase, U/L | 15 (13–22) | 18 (15–26) | 0.212 |
| Bilirubin, µmol/L | 10.3 (8.0–13.1) | 8.4 (6.3–10.6) | 0.015 |
| Hemoglobin, g/L | 141 (134–149) | 139 (126–150) | 0.321 |
| Erythrocytes, 10 × 12/L | 4.7 (4.5–4.9) | 4.7 (4.4–5.1) | 0.583 |
| Leukocytes, 10 × 9/L | 6.2 (5.2–7.5) | 6.1 (5.0–7.1) | 0.576 |
| Thrombocytes, 10 × 9/L | 262 (238–280) | 254 (226–295) | 0.518 |
| eGDR | 4.9 (2.8–6.7) | 2.1 (1.0–3.3) | 0.001 |
| FLI | 13.2 (6.0–22.1) | 54.0 (30.1–78.9) | <0.001 |
| HSI | 31.2 (28.8–33.7) | 39.6 (34.5–42.0) | <0.001 |
| Phenotype | No Metabolic Syndrome (n = 43) | Metabolic Syndrome (n = 31) | p |
|---|---|---|---|
| hsCRP, mg/L | 0.80 (0.49–1.53) | 1.23 (0.38–2.17) | 0.41 |
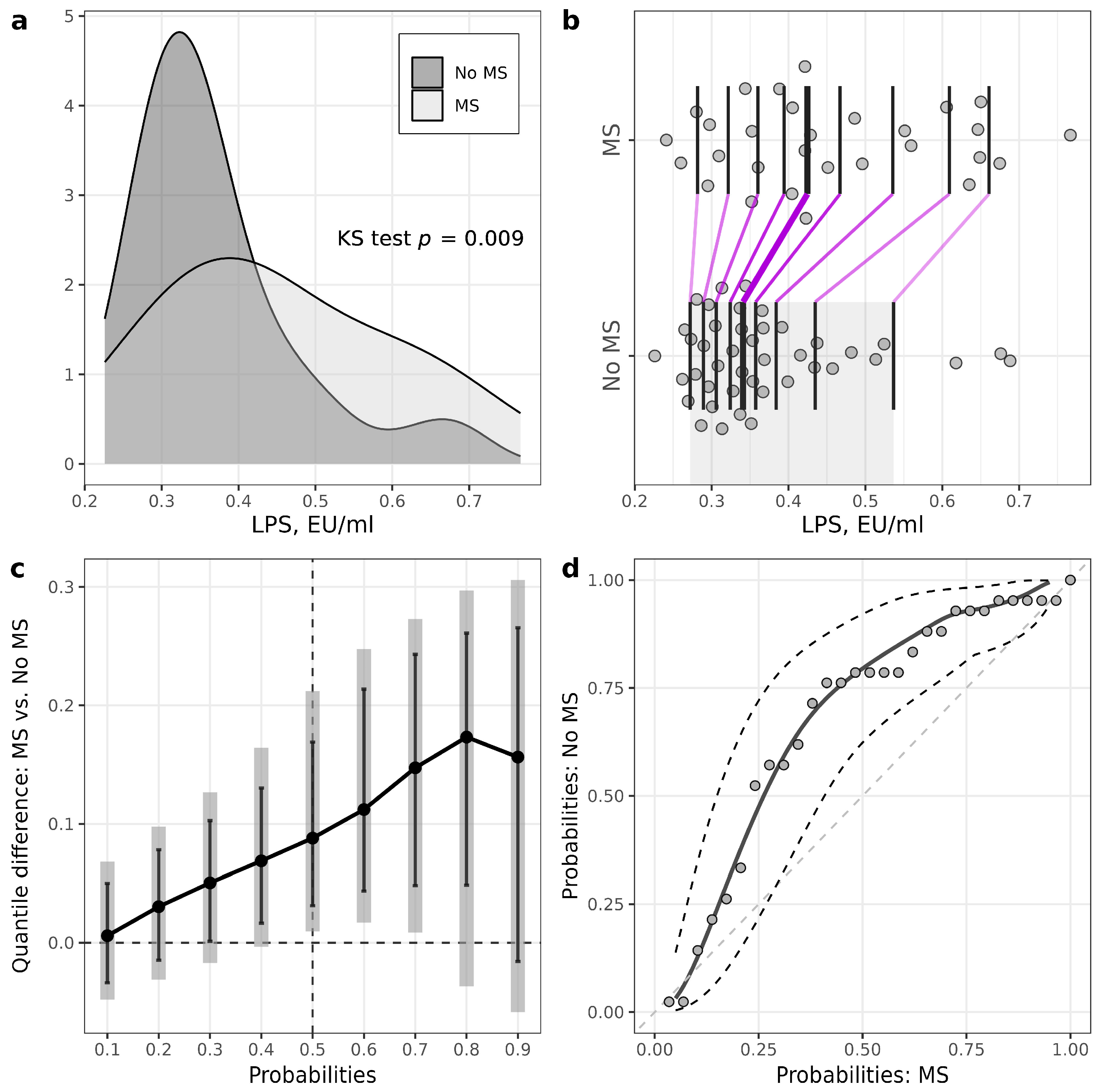
| LPS, EU/mL | 0.34 (0.30–0.40) | 0.42 (0.35–0.56) | 0.009 |
| LPS/HDL ratio | 0.22 (0.17–0.26) | 0.28 (0.24–0.38) | 0.001 |
| EndoCAb IgG, GMU/mL | 89.5 (67.1–150.2) | 96.0 (59.1–141.6) | 0.84 |
| EndoCAb IgM, MMU/mL | 46.6 (34.2–85.2) | 43.7 (31.3–60.4) | 0.33 |
| LBP, µg/mL | 11.1 (7.9–16.3) | 11.1 (7.9–13.3) | 0.89 |
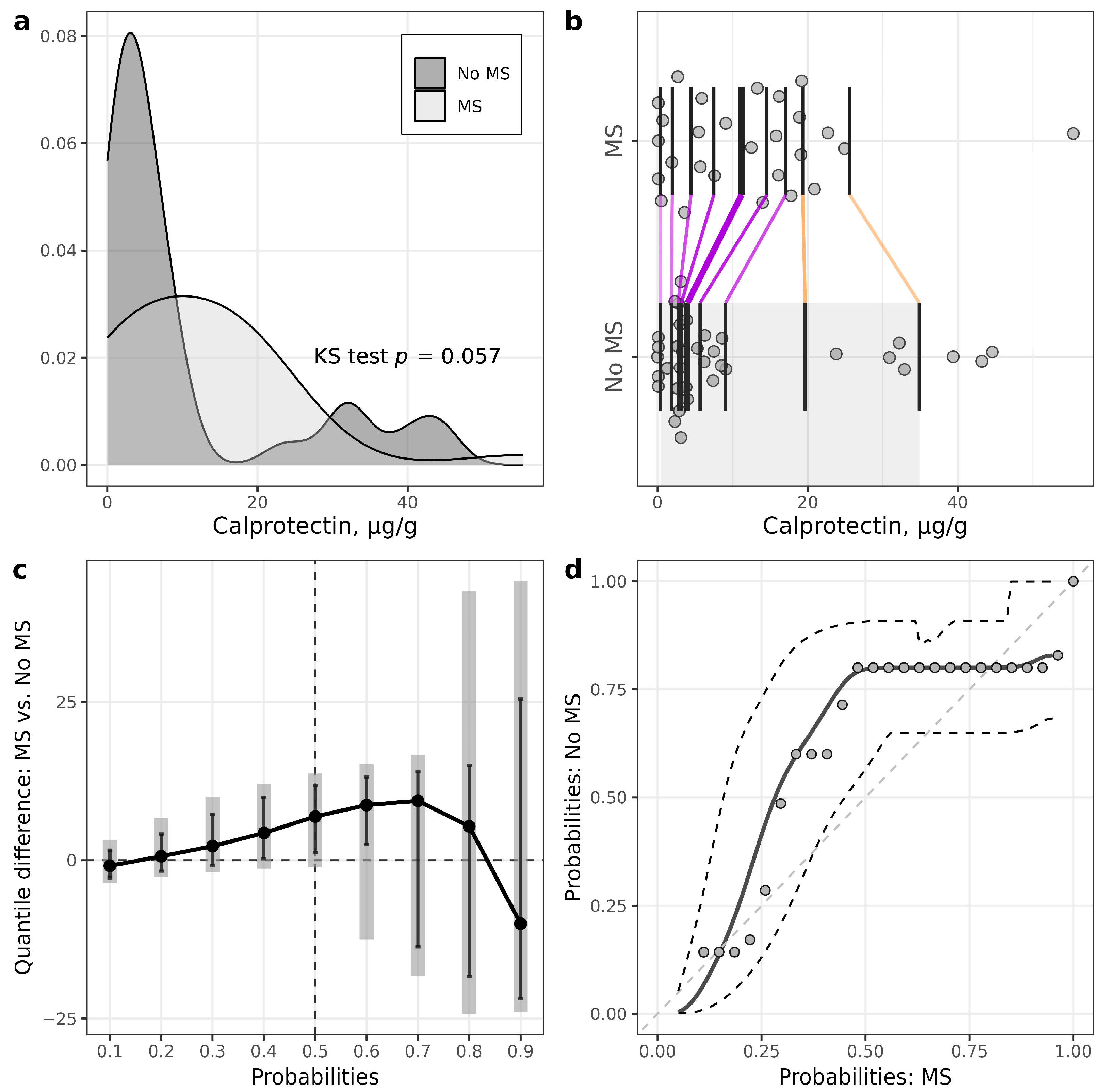
| Calprotectin, µg/g | 3.8 (2.7–8.6) | 12.5 (3.2–18.4) | 0.18 |
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Predictor | p | Predictor | OR (95% CI) | p | |
| LBP | 0.30 (0.09; 0.51) | 0.005 | LBP | 0.92 (0.45; 1.90) | 0.82 |
| EndoCAb IgG | 0.29 (0.07; 0.50) | 0.008 | EndoCAb IgG | 1.67 (0.81; 3.45) | 0.16 |
| EndoCAb IgM | −0.06 (−0.27; 0.16) | 0.61 | EndoCAb IgM | 0.32 (0.11; 0.93) | 0.036 |
| LPS/HDL | 0.19 (−0.03; 0.41) | 0.084 | LPS/HDL | 6.5 (2.1; 20.0) | 0.001 |
| hsCRP | - | - | hsCRP | 0.57 (0.26; 1.25) | 0.16 |
| Sex (female) | 0.23 (−0.22; 0.68) | 0.31 | Sex (female) | 2.7 (0.6; 12.7) | 0.21 |
| Diabetes duration | 0.24 (−0.01; 0.49) | 0.059 | Diabetes duration | 3.4 (1.2; 9.8) | 0.021 |
| BMI | 0.10 (−0.13; 0.32) | 0.39 | BMI | 2.3 (1.1; 4.9) | 0.025 |
| R2 = 0.32, F(7, 60) = 3.97, p = 0.001 | AIC = 72.8, Nagelkerke R2 = 0.56 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedulovs, A.; Pahirko, L.; Jekabsons, K.; Kunrade, L.; Valeinis, J.; Riekstina, U.; Pīrāgs, V.; Sokolovska, J. Association of Endotoxemia with Low-Grade Inflammation, Metabolic Syndrome and Distinct Response to Lipopolysaccharide in Type 1 Diabetes. Biomedicines 2023, 11, 3269. https://doi.org/10.3390/biomedicines11123269
Fedulovs A, Pahirko L, Jekabsons K, Kunrade L, Valeinis J, Riekstina U, Pīrāgs V, Sokolovska J. Association of Endotoxemia with Low-Grade Inflammation, Metabolic Syndrome and Distinct Response to Lipopolysaccharide in Type 1 Diabetes. Biomedicines. 2023; 11(12):3269. https://doi.org/10.3390/biomedicines11123269
Chicago/Turabian StyleFedulovs, Aleksejs, Leonora Pahirko, Kaspars Jekabsons, Liga Kunrade, Jānis Valeinis, Una Riekstina, Valdis Pīrāgs, and Jelizaveta Sokolovska. 2023. "Association of Endotoxemia with Low-Grade Inflammation, Metabolic Syndrome and Distinct Response to Lipopolysaccharide in Type 1 Diabetes" Biomedicines 11, no. 12: 3269. https://doi.org/10.3390/biomedicines11123269
APA StyleFedulovs, A., Pahirko, L., Jekabsons, K., Kunrade, L., Valeinis, J., Riekstina, U., Pīrāgs, V., & Sokolovska, J. (2023). Association of Endotoxemia with Low-Grade Inflammation, Metabolic Syndrome and Distinct Response to Lipopolysaccharide in Type 1 Diabetes. Biomedicines, 11(12), 3269. https://doi.org/10.3390/biomedicines11123269







