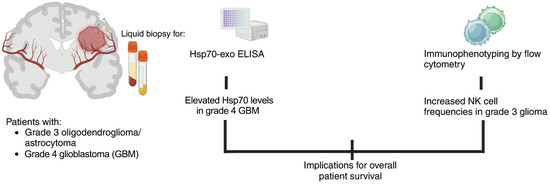
Biomarkers in Adult-Type Diffuse Gliomas: Elevated Levels of Circulating Vesicular Heat Shock Protein 70 Serve as a Biomarker in Grade 4 Glioblastoma and Increase NK Cell Frequencies in Grade 3 Glioma
Abstract
1. Introduction
2. Materials and Methods
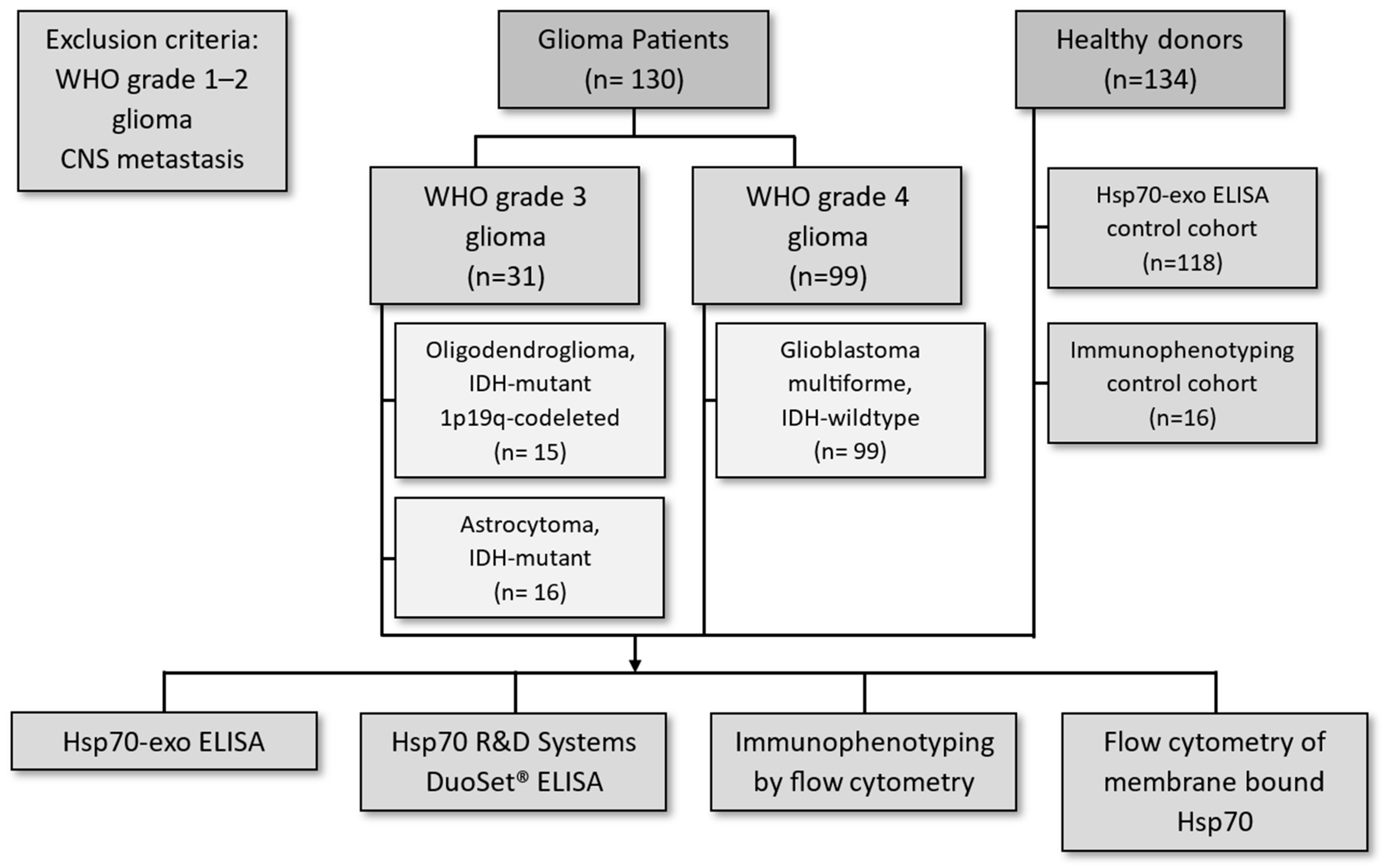
2.1. Patients
2.2. Immunohistochemical Analysis of IDH1 R132H Point Mutation
2.3. Measurement of Intracellular Hsp70 by Immunohistochemistry on Tumor Sections
2.4. Measurement of Free and Vesicular Hsp70 in the Blood Using the Hsp70-exo ELISA
2.5. Measurement of Free Hsp70 in the Blood Using the R&D Systems DuoSet® ELISA
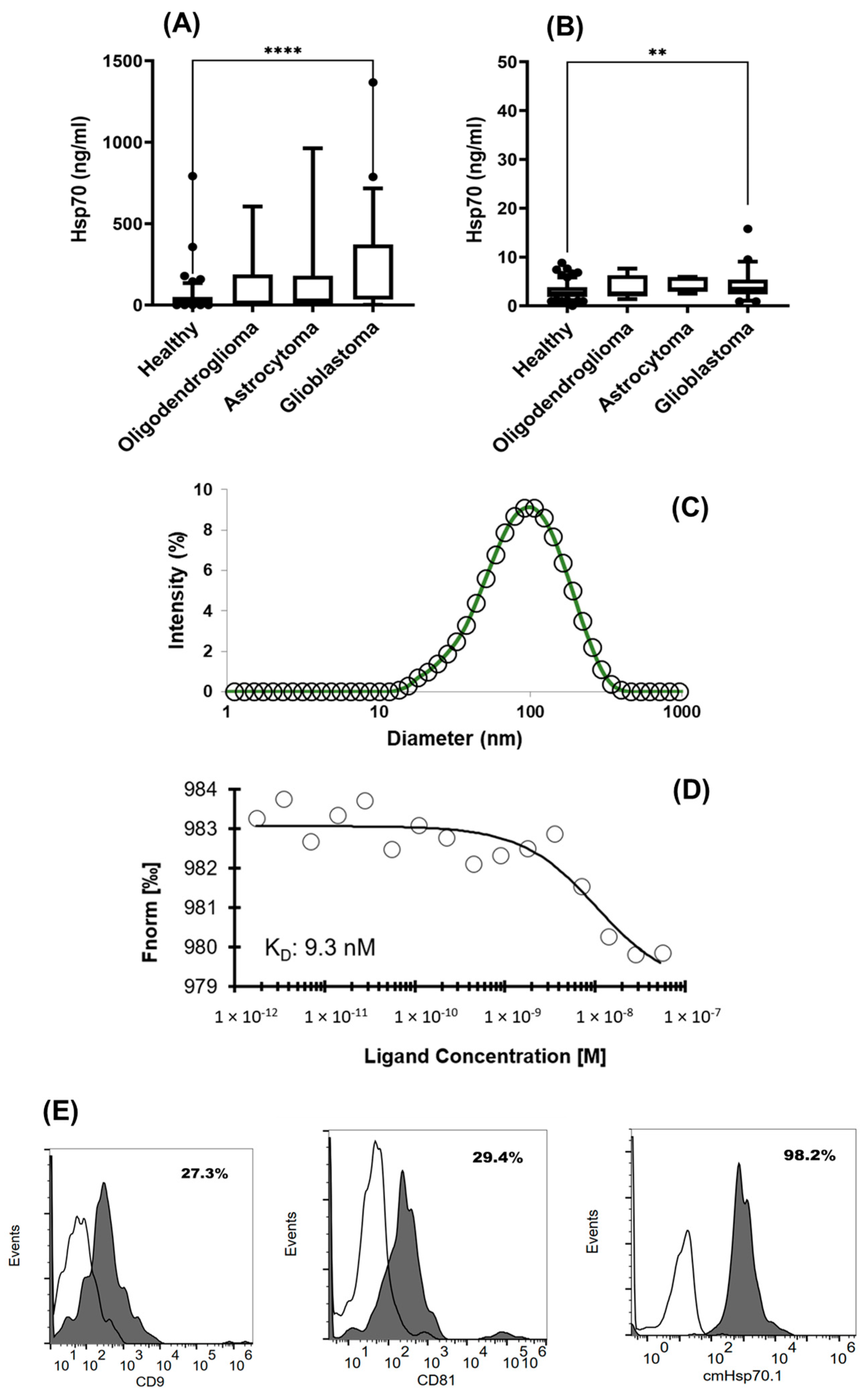
2.6. Exosome Characterization
2.7. Peripheral Blood Immunophenotyping by Multiparameter Flow Cytometry
2.8. Flow Cytometry of Membrane-Bound Hsp70 on Viable Primary Brain Tumor Cells, Astrocytes, and Glioblastoma Cell Cultures
2.9. Statistical Analysis
3. Results
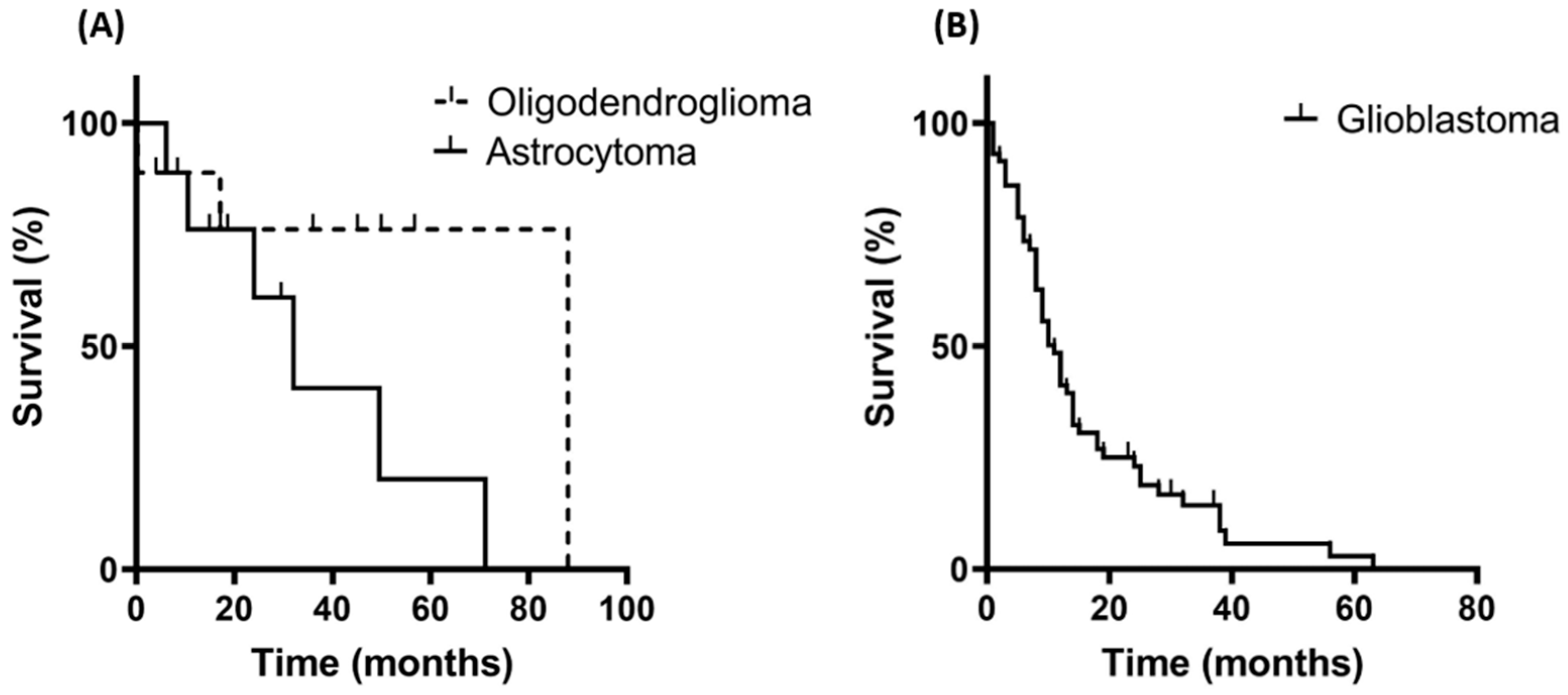
3.1. Patient Characteristics and Overall Survival
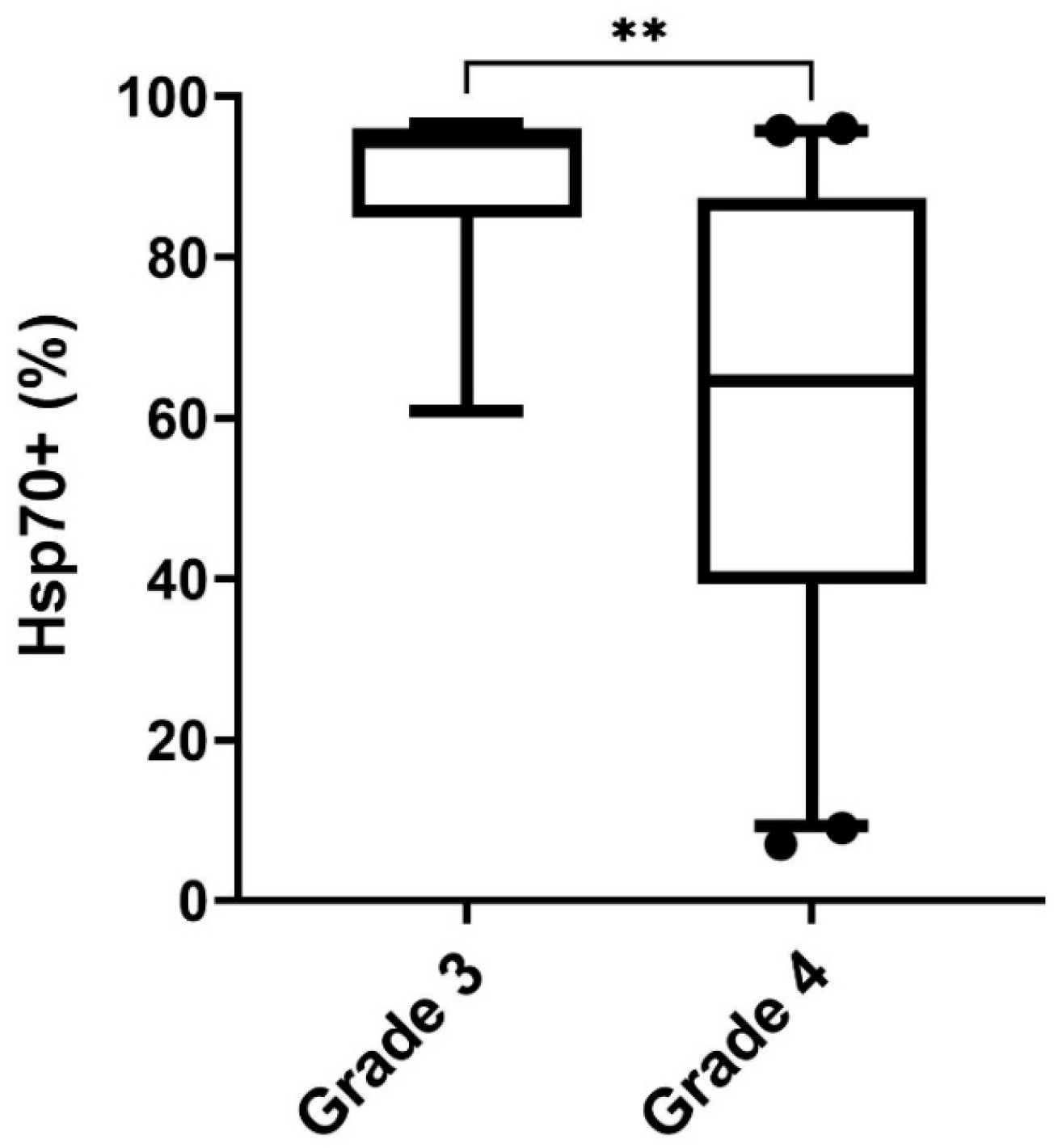
3.2. Comparison of Intracellular and Circulating Hsp70 Levels in Patients with Oligodendroglioma, Astrocytoma, and Glioblastoma (GBM) and Characterization of Extracellular Vesicles
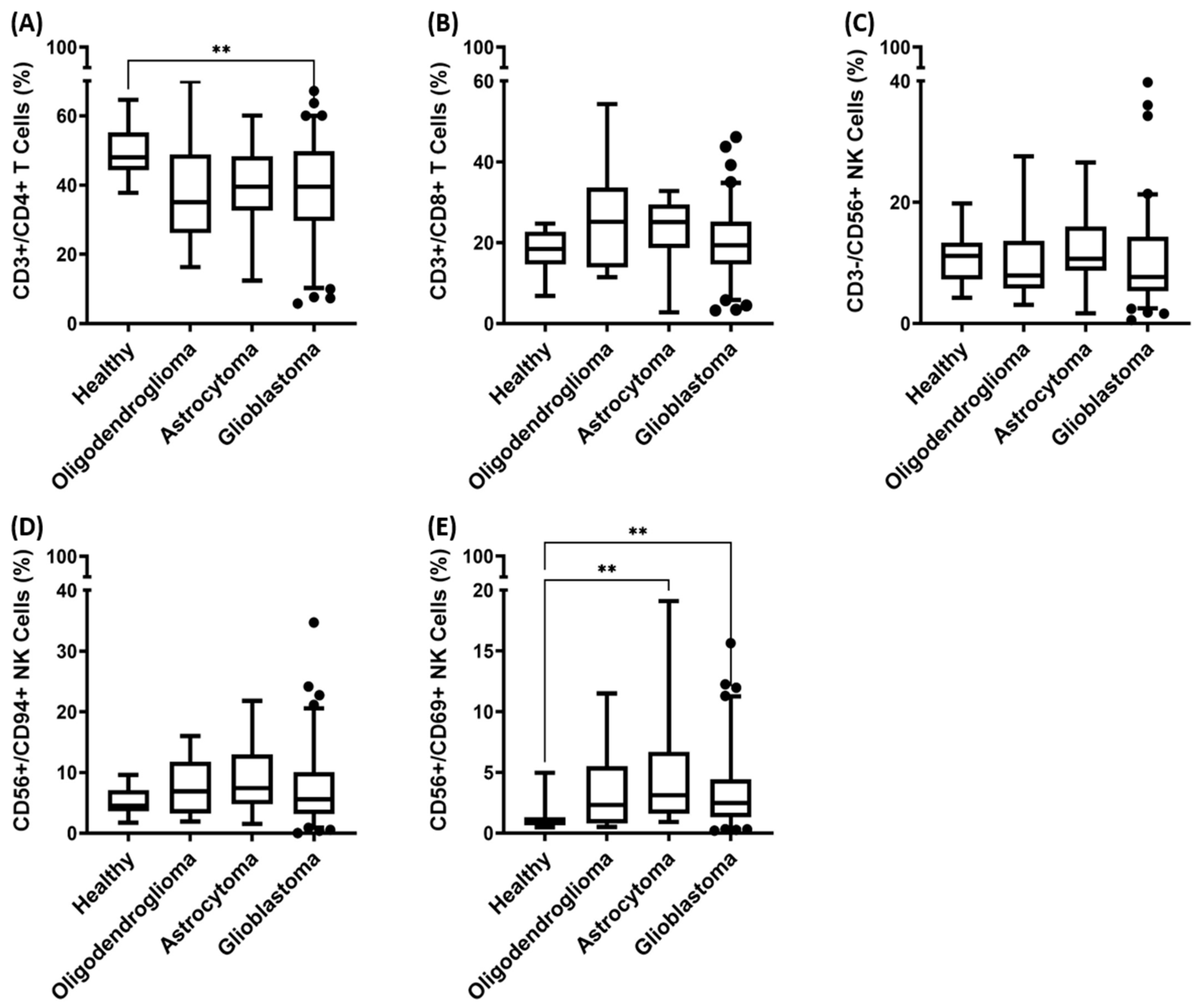
3.3. Immunophenotyping of Major Lymphocyte Subpopulations in the Peripheral Blood of Patients with Oligodendroglioma, Astrocytoma, and Glioblastoma (GBM)
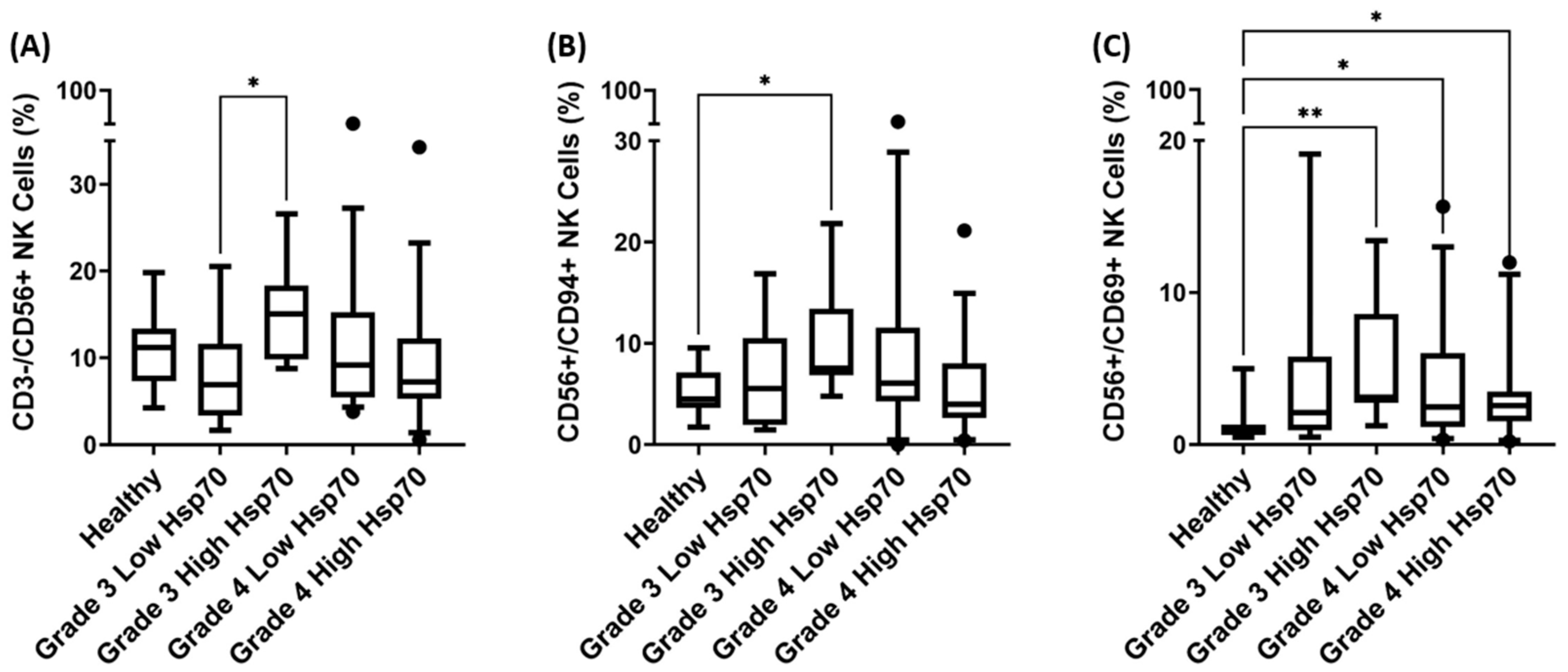
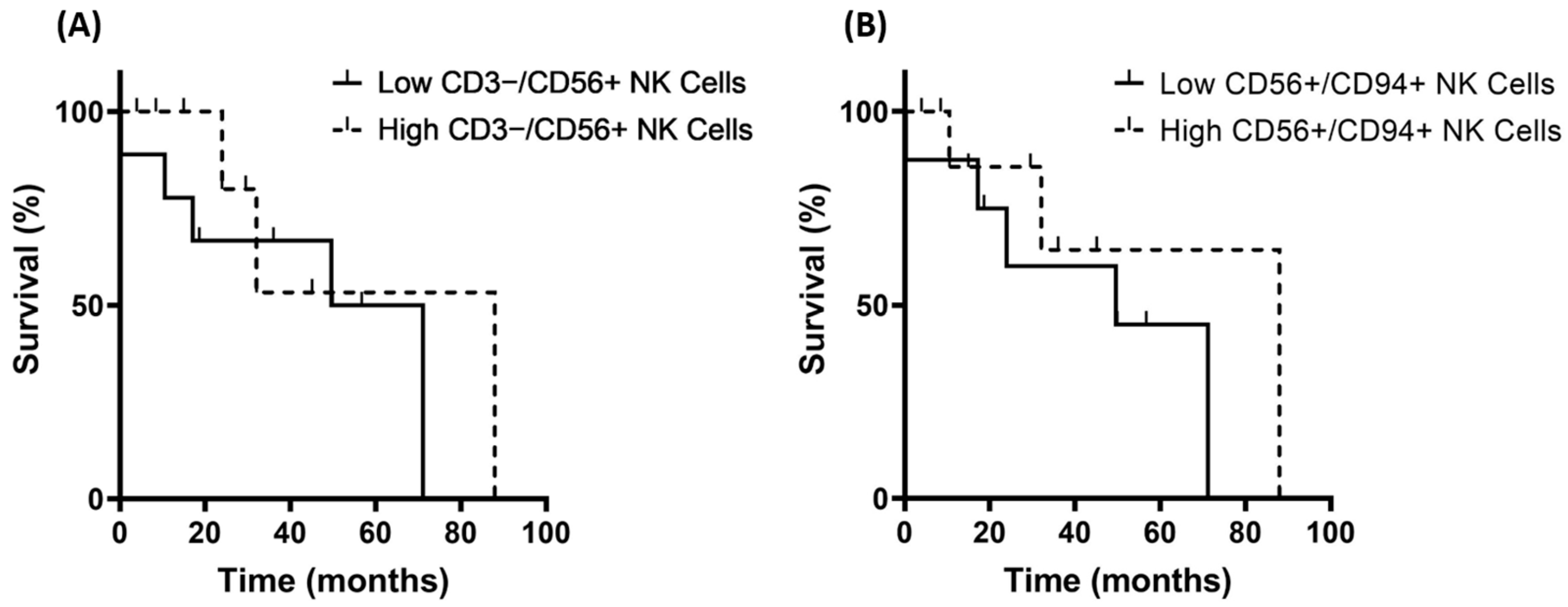
3.4. Correlation of Hsp70low/Hsp70high Levels and Frequencies of NK Cell Subpopulations in Patients with Grade 3 and Grade 4 Gliomas
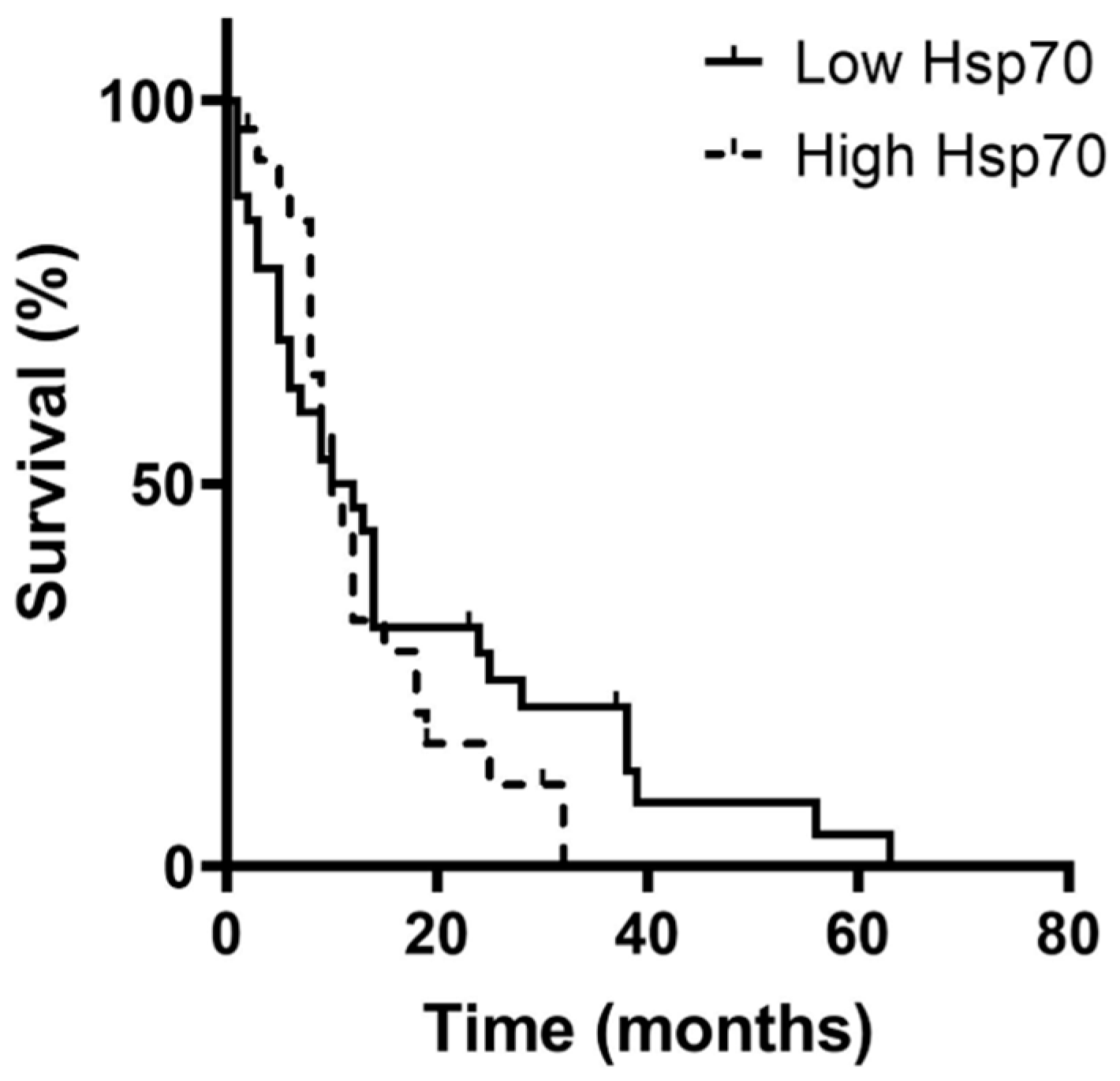
3.5. Correlation of Hsp70low/Hsp70high Levels, Lymphocyte Frequencies, and Overall Survival in Patients with Oligodendroglioma, Astrocytoma, and Glioblastoma (GBM)
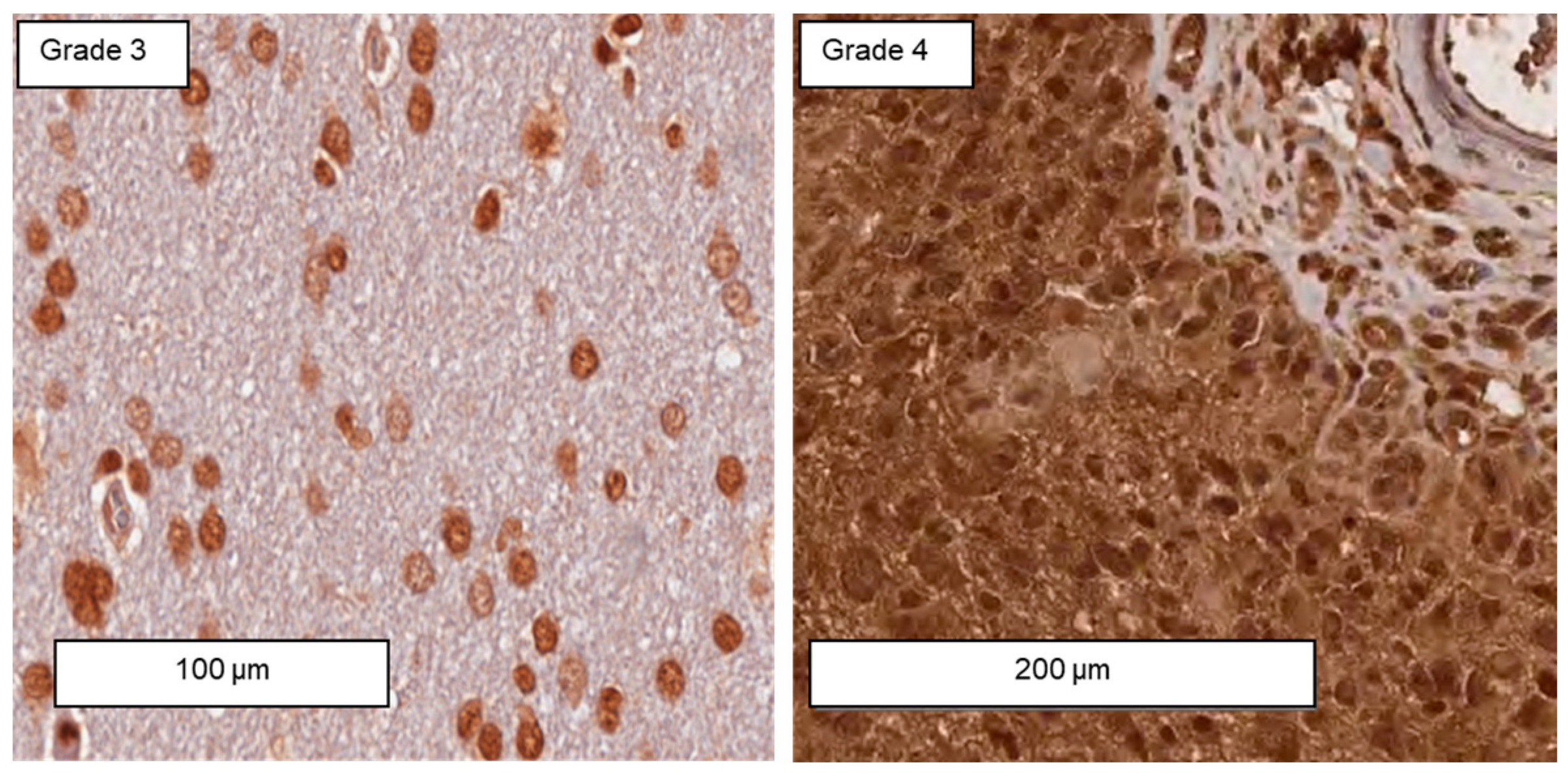
3.6. Differences in the Expression of Membrane-Bound Hsp70 in Grade 3 and Grade 4 Gliomas, Astrocytes, and Cultured Glioblastoma Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, B.T.; Huse, J.T. Classification of Adult-Type Diffuse Gliomas: Impact of the World Health Organization 2021 Update. Brain Pathol. 2022, 32, e13062. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Witthayanuwat, S.; Pesee, M.; Supaadirek, C.; Supakalin, N.; Thamronganantasakul, K.; Krusun, S. Survival Analysis of Glioblastoma Multiforme. Asian Pac. J. Cancer Prev. 2018, 19, 2613–2617. [Google Scholar] [CrossRef]
- Aum, D.J.; Kim, D.H.; Beaumont, T.L.; Leuthardt, E.C.; Dunn, G.P.; Kim, A.H. Molecular and Cellular Heterogeneity: The Hallmark of Glioblastoma. Neurosurg. Focus FOC 2014, 37, E11. [Google Scholar] [CrossRef]
- DeCordova, S.; Shastri, A.; Tsolaki, A.G.; Yasmin, H.; Klein, L.; Singh, S.K.; Kishore, U. Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma. Front. Immunol. 2020, 11, 1402. [Google Scholar] [CrossRef]
- Silantyev, A.S.; Falzone, L.; Libra, M.; Gurina, O.I.; Kardashova, K.S.; Nikolouzakis, T.K.; Nosyrev, A.E.; Sutton, C.W.; Mitsias, P.D.; Tsatsakis, A. Current and Future Trends on Diagnosis and Prognosis of Glioblastoma: From Molecular Biology to Proteomics. Cells 2019, 8, 863. [Google Scholar] [CrossRef]
- Delgado-Martín, B.; Medina, M.Á. Advances in the Knowledge of the Molecular Biology of Glioblastoma and Its Impact in Patient Diagnosis, Stratification, and Treatment. Adv. Sci. 2020, 7, 1902971. [Google Scholar] [CrossRef] [PubMed]
- Tamai, S.; Ichinose, T.; Nakada, M. Liquid Biomarkers in Glioma. Brain Tumor Pathol. 2023, 40, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Małusecka, E.; Zborek, A.; Krzyzowska-Gruca, S.; Krawczyk, Z. Expression of Heat Shock Proteins HSP70 and HSP27 in Primary Non-Small Cell Lung Carcinomas. An Immunohistochemical Study. Anticancer Res. 2001, 21, 1015–1021. [Google Scholar] [PubMed]
- Zimmermann, M.; Nickl, S.; Lambers, C.; Hacker, S.; Mitterbauer, A.; Hoetzenecker, K.; Rozsas, A.; Ostoros, G.; Laszlo, V.; Hofbauer, H.; et al. Discrimination of Clinical Stages in Non-Small Cell Lung Cancer Patients by Serum HSP27 and HSP70: A Multi-Institutional Case–Control Study. Clin. Chim. Acta 2012, 413, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.S.; Han, H.S.; Choi, H.K.; Lee, Y.J.; KIM, Y.-J.; HAN, M.-Y.; PARK, Y.-M. Differential, Stage-dependent Expression of Hsp70, Hsp110 and Bcl-2 in Colorectal Cancer. J. Gastroenterol. Hepatol. 2003, 18, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Gráf, L.; Barabás, L.; Madaras, B.; Garam, N.; Maláti, É.; Horváth, L.; Prohászka, Z.; Horváth, Z.; Kocsis, J. High Serum Hsp70 Level Predicts Poor Survival in Colorectal Cancer: Results Obtained in an Independent Validation Cohort. Cancer Biomark. 2018, 23, 539–547. [Google Scholar] [CrossRef]
- Abe, M.; Manola, J.B.; Oh, W.K.; Parslow, D.L.; George, D.J.; Austin, C.L.; Kantoff, P.W. Plasma Levels of Heat Shock Protein 70 in Patients with Prostate Cancer: A Potential Biomarker for Prostate Cancer. Clin. Prostate Cancer 2004, 3, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gurshaney, S.; Adagunodo, Y.; Gage, E.; Qadri, S.; Sharma, M.; Malik, S.; Manne, U.; Singh, U.P.; Singh, R. Hsp70 and Gama-Semino Protein as Possible Prognostic Marker of Prostate Cancer. Front. Biosci. Landmark Ed. 2018, 23, 1987. [Google Scholar]
- Lobinger, D.; Gempt, J.; Sievert, W.; Barz, M.; Schmitt, S.; Nguyen, H.T.; Stangl, S.; Werner, C.; Wang, F.; Wu, Z.; et al. Potential Role of Hsp70 and Activated NK Cells for Prediction of Prognosis in Glioblastoma Patients. Front. Mol. Biosci. 2021, 8, 669366. [Google Scholar] [CrossRef]
- Werner, C.; Stangl, S.; Salvermoser, L.; Schwab, M.; Shevtsov, M.; Xanthopoulos, A.; Wang, F.; Dezfouli, A.B.; Thölke, D.; Ostheimer, C. Hsp70 in Liquid Biopsies—A Tumor-Specific Biomarker for Detection and Response Monitoring in Cancer. Cancers 2021, 13, 3706. [Google Scholar] [CrossRef]
- Elstner, A.; Stockhammer, F.; Nguyen-Dobinsky, T.-N.; Nguyen, Q.L.; Pilgermann, I.; Gill, A.; Guhr, A.; Zhang, T.; von Eckardstein, K.; Picht, T.; et al. Identification of Diagnostic Serum Protein Profiles of Glioblastoma Patients. J. Neuro-Oncol. 2011, 102, 71–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mayer, M.P.; Bukau, B. Hsp70 Chaperones: Cellular Functions and Molecular Mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Radons, J. The Human HSP70 Family of Chaperones: Where Do We Stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Sharp, F.R.; Zhan, X.; Liu, D.-Z. Heat Shock Proteins in the Brain: Role of Hsp70, Hsp 27, and HO-1 (Hsp32) and Their Therapeutic Potential. Transl. Stroke Res. 2013, 4, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Denis-Larose, C.; Massie, B. Role of the Human Heat Shock Protein Hsp70 in Protection against Stress-Induced Apoptosis. Mol. Cell. Biol. 1997, 17, 5317–5327. [Google Scholar] [CrossRef]
- Roufayel, R.; Kadry, S. Molecular Chaperone HSP70 and Key Regulators of Apoptosis-a Review. Curr. Mol. Med. 2019, 19, 315–325. [Google Scholar] [CrossRef]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L.; et al. Monitoring HSP70 Exosomes in Cancer Patients’ Follow up: A Clinical Prospective Pilot Study. J. Extracell. Vesicles 2020, 9, 1766192. [Google Scholar] [CrossRef]
- Vostakolaei, M.A.; Hatami-Baroogh, L.; Babaei, G.; Molavi, O.; Kordi, S.; Abdolalizadeh, J. Hsp70 in Cancer: A Double Agent in the Battle between Survival and Death. J. Cell. Physiol. 2021, 236, 3420–3444. [Google Scholar] [CrossRef]
- Gehrmann, M.; Liebisch, G.; Schmitz, G.; Anderson, R.; Steinem, C.; De Maio, A.; Pockley, G.; Multhoff, G. Tumor-Specific Hsp70 Plasma Membrane Localization Is Enabled by the Glycosphingolipid Gb3. PLoS ONE 2008, 3, e1925. [Google Scholar] [CrossRef]
- Nylandsted, J.; Gyrd-Hansen, M.; Danielewicz, A.; Fehrenbacher, N.; Lademann, U.; Høyer-Hansen, M.; Weber, E.; Multhoff, G.; Rohde, M.; Jäättelä, M. Heat Shock Protein 70 Promotes Cell Survival by Inhibiting Lysosomal Membrane Permeabilization. J. Exp. Med. 2004, 200, 425–435. [Google Scholar] [CrossRef]
- Elmallah, M.I.; Cordonnier, M.; Vautrot, V.; Chanteloup, G.; Garrido, C.; Gobbo, J. Membrane-Anchored Heat-Shock Protein 70 (Hsp70) in Cancer. Cancer Lett. 2020, 469, 134–141. [Google Scholar] [CrossRef]
- Multhoff, G. Heat Shock Protein 70 (Hsp70): Membrane Location, Export and Immunological Relevance. Methods 2007, 43, 229–237. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Huang, W. A 14-Mer Peptide from HSP70 Protein Is the Critical Epitope Which Enhances NK Activity against Tumor Cells in Vivo. Immunol. Investig. 2007, 36, 233–246. [Google Scholar] [CrossRef]
- Multhoff, G.; Seier, S.; Stangl, S.; Sievert, W.; Shevtsov, M.; Werner, C.; Pockley, A.G.; Blankenstein, C.; Hildebrandt, M.; Offner, R.; et al. Targeted Natural Killer Cell–Based Adoptive Immunotherapy for the Treatment of Patients with NSCLC after Radiochemotherapy: A Randomized Phase II Clinical Trial. Clin. Cancer Res. 2020, 26, 5368–5379. [Google Scholar] [CrossRef]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef]
- Gunther, S.; Ostheimer, C.; Stangl, S.; Specht, H.M.; Mozes, P.; Jesinghaus, M.; Vordermark, D.; Combs, S.E.; Peltz, F.; Jung, M.P.; et al. Correlation of Hsp70 Serum Levels with Gross Tumor Volume and Composition of Lymphocyte Subpopulations in Patients with Squamous Cell and Adeno Non-Small Cell Lung Cancer. Front. Immunol. 2015, 6, 556. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The Biology of Human Natural Killer-Cell Subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Vulpis, E.; Soriani, A.; Cerboni, C.; Santoni, A.; Zingoni, A. Cancer Exosomes as Conveyors of Stress-Induced Molecules: New Players in the Modulation of NK Cell Response. Int. J. Mol. Sci. 2019, 20, 611. [Google Scholar] [CrossRef]
- Zingoni, A.; Fionda, C.; Borrelli, C.; Cippitelli, M.; Santoni, A.; Soriani, A. Natural Killer Cell Response to Chemotherapy-Stressed Cancer Cells: Role in Tumor Immunosurveillance. Front. Immunol. 2017, 8, 1194. [Google Scholar] [CrossRef]
- Vuletić, A.; Mirjačić Martinović, K.; Tišma Miletić, N.; Zoidakis, J.; Castellvi-Bel, S.; Čavić, M. Cross-Talk between Tumor Cells Undergoing Epithelial to Mesenchymal Transition and Natural Killer Cells in Tumor Microenvironment in Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 750022. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K. Role of Tumor Microenvironment in Tumorigenesis. J. Cancer 2017, 8, 761. [Google Scholar] [CrossRef]
- Erices, J.I.; Bizama, C.; Niechi, I.; Uribe, D.; Rosales, A.; Fabres, K.; Navarro-Martínez, G.; Torres, Á.; San Martín, R.; Roa, J.C. Glioblastoma Microenvironment and Invasiveness: New Insights and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 7047. [Google Scholar] [CrossRef]
- Bikfalvi, A.; da Costa, C.A.; Avril, T.; Barnier, J.-V.; Bauchet, L.; Brisson, L.; Cartron, P.F.; Castel, H.; Chevet, E.; Chneiweiss, H. Challenges in Glioblastoma Research: Focus on the Tumor Microenvironment. Trends Cancer 2023, 9, 9–27. [Google Scholar] [CrossRef]
- Komarova, E.Y.; Suezov, R.V.; Nikotina, A.D.; Aksenov, N.D.; Garaeva, L.A.; Shtam, T.A.; Zhakhov, A.V.; Martynova, M.G.; Bystrova, O.A.; Istomina, M.S.; et al. Hsp70-Containing Extracellular Vesicles Are Capable of Activating of Adaptive Immunity in Models of Mouse Melanoma and Colon Carcinoma. Sci. Rep. 2021, 11, 21314. [Google Scholar] [CrossRef]
- Shin, D.-W.; Lee, S.; Song, S.W.; Cho, Y.H.; Hong, S.H.; Kim, J.H.; Kim, H.S.; Park, J.E.; Nam, S.J.; Kim, Y.-H. Survival Outcome and Prognostic Factors in Anaplastic Oligodendroglioma: A Single-Institution Study of 95 Cases. Sci. Rep. 2020, 10, 20162. [Google Scholar] [CrossRef]
- Reuss, D.E.; Mamatjan, Y.; Schrimpf, D.; Capper, D.; Hovestadt, V.; Kratz, A.; Sahm, F.; Koelsche, C.; Korshunov, A.; Olar, A.; et al. IDH Mutant Diffuse and Anaplastic Astrocytomas Have Similar Age at Presentation and Little Difference in Survival: A Grading Problem for WHO. Acta Neuropathol. 2015, 129, 867–873. [Google Scholar] [CrossRef]
- Dong, X.; Noorbakhsh, A.; Hirshman, B.R.; Zhou, T.; Tang, J.A.; Chang, D.C.; Carter, B.S.; Chen, C.C. Survival Trends of Grade I, II, and III Astrocytoma Patients and Associated Clinical Practice Patterns between 1999 and 2010: A SEER-Based Analysis. Neuro-Oncol. Pract. 2016, 3, 29–38. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.M.; Barnholtz-Sloan, J.S. Epidemiology of Gliomas. In Current Understanding and Treatment of Gliomas; Raizer, J., Parsa, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–14. ISBN 978-3-319-12048-5. [Google Scholar]
- Wang, Z.; Gou, W.; Liu, M.; Sang, W.; Chu, H.; Zhang, W. Expression of P53 and HSP70 in Chronic Hepatitis, Liver Cirrhosis, and Early and Advanced Hepatocellular Carcinoma Tissues and Their Diagnostic Value in Hepatocellular Carcinoma: An Immunohistochemical Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 3209. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the Blood–Brain Barrier Really Disrupted in All Glioblastomas? A Critical Assessment of Existing Clinical Data. Neuro-Oncology 2018, 20, 184–191. [Google Scholar] [CrossRef]
- Gao, G.; Liu, S.; Yao, Z.; Zhan, Y.; Chen, W.; Liu, Y. The Prognostic Significance of Hsp70 in Patients with Colorectal Cancer Patients: A PRISMA-Compliant Meta-Analysis. BioMed Res. Int. 2021, 2021, 5526327. [Google Scholar] [CrossRef]
- Bauer, K.; Nitsche, U.; Slotta-Huspenina, J.; Drecoll, E.; von Weyhern, C.H.; Rosenberg, R.; Höfler, H.; Langer, R. High HSP27 and HSP70 Expression Levels Are Independent Adverse Prognostic Factors in Primary Resected Colon Cancer. Cell. Oncol. 2012, 35, 197–205. [Google Scholar] [CrossRef]
- Binder, R.J. Heat-Shock Protein-Based Vaccines for Cancer and Infectious Disease. Expert Rev. Vaccines 2008, 7, 383–393. [Google Scholar] [CrossRef]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK Cell–Cancer Cycle: Advances and New Challenges in NK Cell–Based Immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef]
- Hudspeth, K.; Silva-Santos, B.; Mavilio, D. Natural Cytotoxicity Receptors: Broader Expression Patterns and Functions in Innate and Adaptive Immune Cells. Front. Immunol. 2013, 4, 69. [Google Scholar] [CrossRef]
- Firouzi, J.; Hajifathali, A.; Azimi, M.; Parvini, N.; Ghaemi, F.; Asl, N.S.; Asl, A.A.H.; Safa, M.; Ebrahimi, M. Hsp70, in Combination with IL-15 and PD-1 Blocker, Interferes with the Induction of Cytotoxic NK Cells in Relapsed Acute Myeloid Leukemia Patients. Cell J. 2023, 25, 92. [Google Scholar]
- Sharifzad, F.; Mardpour, S.; Mardpour, S.; Fakharian, E.; Taghikhani, A.; Sharifzad, A.; Kiani, S.; Heydarian, Y.; Łos, M.J.; Azizi, Z.; et al. HSP70/IL-2 Treated NK Cells Effectively Cross the Blood Brain Barrier and Target Tumor Cells in a Rat Model of Induced Glioblastoma Multiforme (GBM). Int. J. Mol. Sci. 2020, 21, 2263. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Li, S.; Kotlabova, K.; Dickinson, A.M. Influence of in Vitro IL-2 or IL-15 Alone or in Combination with Hsp 70 Derived 14-Mer Peptide (TKD) on the Expression of NK Cell Activatory and Inhibitory Receptors on Peripheral Blood T Cells, B Cells and NKT Cells. PLoS ONE 2016, 11, e0151535. [Google Scholar] [CrossRef]
- Fontana, A.; Hengartner, H.; de Tribolet, N.; Weber, E. Glioblastoma Cells Release Interleukin 1 and Factors Inhibiting Interleukin 2-Mediated Effects. J. Immunol. 1984, 132, 1837–1844. [Google Scholar] [CrossRef]
- Masood, A.; Kayani, M.; Batool, S. Targetting Interleukins Involved in Glioblastoma—A New Pharmacological Approach. JLBSR 2020, 1, 82–88. [Google Scholar] [CrossRef]
- Zhu, V.F.; Yang, J.; LeBrun, D.G.; Li, M. Understanding the Role of Cytokines in Glioblastoma Multiforme Pathogenesis. Cancer Lett. 2012, 316, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Tarassishin, L.; Lim, J.; Weatherly, D.B.; Angeletti, R.H.; Lee, S.C. Interleukin-1-Induced Changes in the Glioblastoma Secretome Suggest Its Role in Tumor Progression. J. Proteom. 2014, 99, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Himes, B.T.; Geiger, P.A.; Ayasoufi, K.; Bhargav, A.G.; Brown, D.A.; Parney, I.F. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front. Oncol. 2021, 11, 770561. [Google Scholar] [CrossRef] [PubMed]
- Nduom, E.K.; Weller, M.; Heimberger, A.B. Immunosuppressive Mechanisms in Glioblastoma. Neuro-Oncology 2015, 17, vii9–vii14. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wöhrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M.; et al. Programmed Death Ligand 1 Expression and Tumor-Infiltrating Lymphocytes in Glioblastoma. Neuro-Oncology 2015, 17, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Agoff, S.N.; Hou, J.; Linzer, D.I.; Wu, B. Regulation of the Human Hsp70 Promoter by P53. Science 1993, 259, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Zylicz, M.; King, F.W.; Wawrzynow, A. Hsp70 Interactions with the P53 Tumour Suppressor Protein. EMBO J. 2001, 20, 4634–4638. [Google Scholar] [CrossRef] [PubMed]
- Zagzag, D.; Salnikow, K.; Chiriboga, L.; Yee, H.; Lan, L.; Ali, M.A.; Garcia, R.; Demaria, S.; Newcomb, E.W. Downregulation of Major Histocompatibility Complex Antigens in Invading Glioma Cells: Stealth Invasion of the Brain. Lab. Investig. 2005, 85, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, R.; Cai, T.; Chen, Z.; Lan, M.; Zou, T.; Wang, B.; Wang, Q.; Zhao, Y.; Cai, Y. Effects of Immune Cells and Cytokines on Inflammation and Immunosuppression in the Tumor Microenvironment. Int. Immunopharmacol. 2020, 88, 106939. [Google Scholar] [CrossRef]
- Martinez-Lage, M.; Lynch, T.M.; Bi, Y.; Cocito, C.; Way, G.P.; Pal, S.; Haller, J.; Yan, R.E.; Ziober, A.; Nguyen, A.; et al. Immune Landscapes Associated with Different Glioblastoma Molecular Subtypes. Acta Neuropathol. Commun. 2019, 7, 203. [Google Scholar] [CrossRef]
- Kmiecik, J.; Poli, A.; Brons, N.H.C.; Waha, A.; Eide, G.E.; Enger, P.Ø.; Zimmer, J.; Chekenya, M. Elevated CD3+ and CD8+ Tumor-Infiltrating Immune Cells Correlate with Prolonged Survival in Glioblastoma Patients despite Integrated Immunosuppressive Mechanisms in the Tumor Microenvironment and at the Systemic Level. J. Neuroimmunol. 2013, 264, 71–83. [Google Scholar] [CrossRef]
| Parameters | Grade 3 Glioma | Grade 4 Glioma | |
|---|---|---|---|
| Tumor histology | Oligodendroglioma | Astrocytoma | GBM |
| Number of patients (n) | 15 | 16 | 99 |
| Gender (f/m) | 6/9 | 7/9 | 26/73 |
| Age range | 29–75 | 29–81 | 25–94 |
| Median age | 54 | 49 | 65 |
| Isocitrate dehydrogenase status (IDH) | IDH mutant | IDH mutant | IDH wildtype |
| 1p19q status | Codeleted | Non-codeleted | - |
| Therapy regimen | Maximal resection + RT + sequential PC(V) | Maximal resection + RT + sequential or simultaneous TMZ | Maximal resection + RT + simultaneous and sequential TMZ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lennartz, P.; Thölke, D.; Bashiri Dezfouli, A.; Pilz, M.; Lobinger, D.; Messner, V.; Zanth, H.; Ainslie, K.; Kafshgari, M.H.; Rammes, G.; et al. Biomarkers in Adult-Type Diffuse Gliomas: Elevated Levels of Circulating Vesicular Heat Shock Protein 70 Serve as a Biomarker in Grade 4 Glioblastoma and Increase NK Cell Frequencies in Grade 3 Glioma. Biomedicines 2023, 11, 3235. https://doi.org/10.3390/biomedicines11123235
Lennartz P, Thölke D, Bashiri Dezfouli A, Pilz M, Lobinger D, Messner V, Zanth H, Ainslie K, Kafshgari MH, Rammes G, et al. Biomarkers in Adult-Type Diffuse Gliomas: Elevated Levels of Circulating Vesicular Heat Shock Protein 70 Serve as a Biomarker in Grade 4 Glioblastoma and Increase NK Cell Frequencies in Grade 3 Glioma. Biomedicines. 2023; 11(12):3235. https://doi.org/10.3390/biomedicines11123235
Chicago/Turabian StyleLennartz, Philipp, Dennis Thölke, Ali Bashiri Dezfouli, Mathias Pilz, Dominik Lobinger, Verena Messner, Hannah Zanth, Karen Ainslie, Morteza Hasanzadeh Kafshgari, Gerhard Rammes, and et al. 2023. "Biomarkers in Adult-Type Diffuse Gliomas: Elevated Levels of Circulating Vesicular Heat Shock Protein 70 Serve as a Biomarker in Grade 4 Glioblastoma and Increase NK Cell Frequencies in Grade 3 Glioma" Biomedicines 11, no. 12: 3235. https://doi.org/10.3390/biomedicines11123235
APA StyleLennartz, P., Thölke, D., Bashiri Dezfouli, A., Pilz, M., Lobinger, D., Messner, V., Zanth, H., Ainslie, K., Kafshgari, M. H., Rammes, G., Ballmann, M., Schlegel, M., Foulds, G. A., Pockley, A. G., Schmidt-Graf, F., & Multhoff, G. (2023). Biomarkers in Adult-Type Diffuse Gliomas: Elevated Levels of Circulating Vesicular Heat Shock Protein 70 Serve as a Biomarker in Grade 4 Glioblastoma and Increase NK Cell Frequencies in Grade 3 Glioma. Biomedicines, 11(12), 3235. https://doi.org/10.3390/biomedicines11123235