The Prognosis Performance of a Neutrophil- and Lymphocyte-Associated Gene Mutation Score in a Head and Neck Cancer Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Treatment Response
2.3. Neutrophil-to-Lymphocyte Ratio (NLR)
2.4. Somatic Gene Mutation Profiles and Candidate Genes
2.5. Gene Mutation Score (GMS)
2.6. Statistical Analysis
2.7. Immunohistochemistry
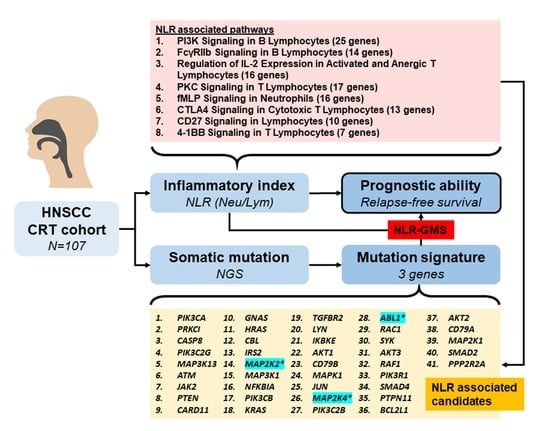
3. Results
3.1. Baseline Characteristics of Patients
3.2. Signaling Pathways Associated with Lymphocytes and Neutrophils
3.3. Somatic Mutation Profiles of Candidate Genes
3.4. Predictive Performance of GMS and NLR-GMS
3.5. Somatic Mutation Validation via Immunohistochemistry Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-L.; Yu, K.J.; Chiang, C.-J.; Chen, T.-C.; Wang, C.-P. Head and neck cancer incidence trends in Taiwan, 1980~2014. Int. J. Head Neck Sci. 2017, 1, 180–189. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.J.; Chan, L.P.; Tsai, H.T.; Hsu, C.M.; Cho, S.F.; Pan, M.R.; Liu, Y.-C.; Huang, C.-J.; Wu, C.-W.; Du, J.-S.; et al. The Overall Efficacy and Outcomes of Metronomic Tegafur-Uracil Chemotherapy on Locally Advanced Head and Neck Squamous Cell Carcinoma: A Real-World Cohort Experience. Biology 2021, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, B.J.; Brakenhoff, R.H.; Leemans, C.R. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Ann. Oncol. 2012, 23 (Suppl. S10), x173–x177. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Basheeth, N.; Patil, N. Biomarkers in Head and Neck Cancer an Update. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. S1), 1002–1011. [Google Scholar] [CrossRef]
- Budach, V.; Tinhofer, I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: A systematic review. Lancet Oncol. 2019, 20, e313–e326. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Wang, H.M.; Wu, M.H.; Chang, K.P.; Chang, P.H.; Liao, C.T.; Liau, C.-T. Review of emerging biomarkers in head and neck squamous cell carcinoma in the era of immunotherapy and targeted therapy. Head Neck 2019, 41 (Suppl. S1), 19–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Yeh, T.J.; Chan, L.P.; Hsu, C.M.; Cho, S.F. Exploration of Feasible Immune Biomarkers for Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinoma Treatment in Real World Clinical Practice. Int. J. Mol. Sci. 2020, 21, 7621. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Li, B.; Zhou, P.; Liu, Y.; Wei, H.; Yang, X.; Chen, T.; Xiao, J. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 483, 48–56. [Google Scholar] [CrossRef]
- Mandaliya, H.; Jones, M.; Oldmeadow, C.; Nordman, I.I. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl. Lung Cancer Res. 2019, 8, 886–894. [Google Scholar] [CrossRef]
- Hu, G.; Liu, G.; Ma, J.Y.; Hu, R.J. Lymphocyte-to-monocyte ratio in esophageal squamous cell carcinoma prognosis. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 486, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Fu, Y.; Tong, W.; Li, F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int. J. Surg. 2018, 55, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H.; Ding, S.; Zhou, J. NLR, PLR, LMR and MWR as diagnostic and prognostic markers for laryngeal carcinoma. Am. J. Transl. Res. 2022, 14, 3017–3027. [Google Scholar]
- Deans, D.A.; Tan, B.H.; Wigmore, S.J.; Ross, J.A.; de Beaux, A.C.; Paterson-Brown, S.; Fearon, K.C.H. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br. J. Cancer 2009, 100, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Russo, D.; Maisto, M.; Troiano, G.; Caponio, V.C.A.; Annunziata, M.; Laino, L. Pre-treatment neutrophil-to-lymphocyte ratio is an independent prognostic factor in head and neck squamous cell carcinoma: Meta-analysis and trial sequential analysis. J. Oral Pathol. Med. 2022, 51, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Mascarella, M.A.; Mannard, E.; Silva, S.D.; Zeitouni, A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: A systematic review and meta-analysis. Head Neck 2018, 40, 1091–1100. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Kitamiura, T.; Ashida, N.; Shimizu, K.; Takemura, K.; Yamamoto, Y.; Uno, A. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: A meta-analysis. Head Neck 2018, 40, 647–655. [Google Scholar] [CrossRef]
- Haddad, C.R.; Guo, L.; Clarke, S.; Guminski, A.; Back, M.; Eade, T. Neutrophil-to-lymphocyte ratio in head and neck cancer. J. Med. Imaging Radiat. Oncol. 2015, 59, 514–519. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, H.; Yan, A.; Wang, H.; Li, X.; Liu, J.; Li, W. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: A meta-analysis. BMC Cancer 2018, 18, 383. [Google Scholar] [CrossRef]
- Rachidi, S.; Wallace, K.; Wrangle, J.M.; Day, T.A.; Alberg, A.J.; Li, Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck 2016, 38 (Suppl. S1), E1068–E1074. [Google Scholar] [CrossRef] [PubMed]
- Rosculet, N.; Zhou, X.C.; Ha, P.; Tang, M.; Levine, M.A.; Neuner, G.; Califano, J. Neutrophil-to-lymphocyte ratio: Prognostic indicator for head and neck squamous cell carcinoma. Head Neck 2017, 39, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Frampton, G.M.; Fichtenholtz, A.; Otto, G.A.; Wang, K.; Downing, S.R.; He, J.; Schnall-Levin, M.; White, J.; Sanford, E.M.; An, P.; et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013, 31, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Kudo, Y.; Aki, D.; Nakagawa, H.; Taniguchi, K. Immunomodulation by Inflammation during Liver and Gastrointestinal Tumorigenesis and Aging. Int. J. Mol. Sci. 2021, 22, 2238. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Karin, M. Tumor-Elicited Inflammation and Colorectal Cancer. Adv. Cancer Res. 2015, 128, 173–196. [Google Scholar] [PubMed]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts—Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, D.; Song, H.; Qiu, B.; Tian, D.; Li, Z.; Ji, Y.; Wang, J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: A systematic review and meta-analysis. BMJ Open 2021, 11, e048324. [Google Scholar] [CrossRef]
- Miyamoto, R.; Inagawa, S.; Sano, N.; Tadano, S.; Adachi, S.; Yamamoto, M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lv, C.Y.; Yuan, A.H.; Chen, W.; Wu, A.W. Significance of the preoperative neutrophil-to-lymphocyte ratio in the prognosis of patients with gastric cancer. World J. Gastroenterol. 2015, 21, 6280–6286. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Luo, G.; Lu, Y.; Jin, K.; Guo, M.; Xu, J.; Long, J.; Liu, L.; Yu, X.; Liu, C. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology 2016, 16, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Beal, E.W.; Wei, L.; Ethun, C.G.; Black, S.M.; Dillhoff, M.; Salem, A.; Weber, S.M.; Tran, T.; Poultsides, G.; Son, A.Y.; et al. Elevated NLR in gallbladder cancer and cholangiocarcinoma—Making bad cancers even worse: Results from the US Extrahepatic Biliary Malignancy Consortium. HPB 2016, 18, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Yu, H.; Khan, M.; Gill, J.; Santhosh, S.; Chatterjee, U.; Iovoli, A.; Farrugia, M.; Mohammadpour, H.; Wooten, K.; et al. Evaluation of Optimal Threshold of Neutrophil-Lymphocyte Ratio and Its Association With Survival Outcomes Among Patients With Head and Neck Cancer. JAMA Netw. Open 2022, 5, e227567. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Tsai, M.S.; Chen, W.C.; Chen, P.T. Predictive Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio in Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2018, 7, 294. [Google Scholar] [CrossRef] [PubMed]
- Rassouli, A.; Saliba, J.; Castano, R.; Hier, M.; Zeitouni, A.G. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck 2015, 37, 103–110. [Google Scholar] [CrossRef]
- Salzano, G.; Dell’Aversana Orabona, G.; Abbate, V.; Vaira, L.A.; Committeri, U.; Bonavolontà, P.; Piombino, P.; Maglitto, F.; Russo, C.; Russo, D.; et al. The prognostic role of the pre-treatment neutrophil to lymphocyte ratio (NLR) and tumor depth of invasion (DOI) in early-stage squamous cell carcinomas of the oral tongue. Oral Maxillofac. Surg. 2022, 26, 21–32. [Google Scholar] [CrossRef]
- Woodley, N.; Rogers, A.D.G.; Turnbull, K.; Slim, M.A.M.; Ton, T.; Montgomery, J.; Douglas, C. Prognostic scores in laryngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3705–3715. [Google Scholar] [CrossRef]
- Kotha, N.V.; Voora, R.S.; Qian, A.S.; Kumar, A.; Qiao, E.M.; Stewart, T.F.; Rose, B.S.; Orosco, R.K. Prognostic Utility of Pretreatment Neutrophil-Lymphocyte Ratio in Advanced Larynx Cancer. Biomark. Insights 2021, 16, 11772719211049848. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: Meta-analysis. Head Neck 2022, 44, 1237–1245. [Google Scholar] [CrossRef]
- Ueda, T.; Chikuie, N.; Takumida, M.; Furuie, H.; Kono, T.; Taruya, T.; Hamamoto, T.; Hattori, M.; Ishino, T.; Takeno, S. Baseline neutrophil-to-lymphocyte ratio (NLR) is associated with clinical outcome in recurrent or metastatic head and neck cancer patients treated with nivolumab. Acta Oto-Laryngol. 2020, 140, 181–187. [Google Scholar] [CrossRef]
- Ng, S.P.; Bahig, H.; Jethanandani, A.; Sturgis, E.M.; Johnson, F.M.; Elgohari, B.; Gunn, G.B.; Ferrarotto, R.; Phan, J.; Rosenthal, D.I.; et al. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio (NLR) in patients with oropharyngeal cancer treated with radiotherapy. Br. J. Cancer 2021, 124, 628–633. [Google Scholar] [CrossRef]
- Yanni, A.; Buset, T.; Bouland, C.; Loeb, I.; Lechien, J.R.; Rodriguez, A.; Journe, F.; Saussez, S.; Dequanter, D. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck cancer with lung metastasis: A retrospective study. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4103–4111. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Preston, S.P.; Doerflinger, M.; Scott, H.W.; Allison, C.C.; Horton, M.; Cooney, J.; Pellegrini, M. The role of MKK4 in T-cell development and immunity to viral infections. Immunol. Cell Biol. 2021, 99, 428–435. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Sounding the alarm: Protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 1996, 271, 24313–24316. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Holland, S.M.; Staudt, L.M. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 2013, 368, 161–170. [Google Scholar] [CrossRef]
- Manzoor, Z.; Koh, Y.-S. Mitogen-activated protein kinases in inflammation. J. Bacteriol. Virol. 2012, 42, 189–195. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Wu, P.K.; Park, J.I. MEK1/2 Inhibitors: Molecular Activity and Resistance Mechanisms. Semin. Oncol. 2015, 42, 849–862. [Google Scholar] [CrossRef]
- Burkhard, K.; Shapiro, P. Use of inhibitors in the study of MAP kinases. Methods Mol. Biol. 2010, 661, 107–122. [Google Scholar]
- Wang, C.; Wang, H.; Zheng, C.; Liu, Z.; Gao, X.; Xu, F.; Niu, Y.; Zhang, L.; Xu, P. Research progress of MEK1/2 inhibitors and degraders in the treatment of cancer. Eur. J. Med. Chem. 2021, 218, 113386. [Google Scholar] [CrossRef]
- McNamara, B.; Harold, J.; Manavella, D.; Bellone, S.; Mutlu, L.; Hartwich, T.M.P.; Zipponi, M.; Yang-Hartwich, Y.; Demirkiran, C.; Verzosa, M.S.Z.; et al. Uterine leiomyosarcomas harboring MAP2K4 gene amplification are sensitive in vivo to PLX8725, a novel MAP2K4 inhibitor. Gynecol. Oncol. 2023, 172, 65–71. [Google Scholar] [CrossRef]
- Xue, Z.; Vis, D.J.; Bruna, A.; Sustic, T.; van Wageningen, S.; Batra, A.S.; Rueda, O.M.; Bosdriesz, E.; Caldas, C.; Wessels, L.F.A.; et al. MAP3K1 and MAP2K4 mutations are associated with sensitivity to MEK inhibitors in multiple cancer models. Cell Res. 2018, 28, 719–729. [Google Scholar] [CrossRef]
- De Braekeleer, E.; Douet-Guilbert, N.; Rowe, D.; Bown, N.; Morel, F.; Berthou, C.; Férec, C.; De Braekeleer, M. ABL1 fusion genes in hematological malignancies: A review. Eur. J. Haematol. 2011, 86, 361–371. [Google Scholar] [CrossRef]
- Greuber, E.K.; Smith-Pearson, P.; Wang, J.; Pendergast, A.M. Role of ABL family kinases in cancer: From leukaemia to solid tumours. Nat. Rev. Cancer 2013, 13, 559–571. [Google Scholar] [CrossRef]
- Khatri, A.; Wang, J.; Pendergast, A.M. Multifunctional Abl kinases in health and disease. J. Cell Sci. 2016, 129, 9–16. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, F.; Yuan, X.; Yang, T.; Liang, X.; Wang, Y.; Tu, H.; Chang, J.; Nan, K.; Wei, Y. Reactive Oxygen Species Are Involved in the Development of Gastric Cancer and Gastric Cancer-Related Depression through ABL1-Mediated Inflammation Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 5813985. [Google Scholar] [CrossRef]


| Characteristics | Overall, n = 107 | Good Response, n = 84 | Poor Response, n = 23 | p |
|---|---|---|---|---|
| Age | 0.804 | |||
| <45 | 8 (7.5%) | 6 (7.1%) | 2 (8.7%) | |
| >65 | 29 (27.1%) | 22 (26.2%) | 7 (30.4%) | |
| 45–64 | 70 (65.4%) | 56 (66.7%) | 14 (60.9%) | |
| Sex | 0.168 | |||
| Female | 7 (6.5%) | 4 (4.8%) | 3 (13.0%) | |
| Male | 100 (93.5%) | 80 (95.2%) | 20 (87.0%) | |
| Location | 0.038 | |||
| Hypopharynx | 12 (11.2%) | 12 (14.3%) | 0 (0.0%) | |
| Larynx | 2 (1.9%) | 2 (2.4%) | 0 (0.0%) | |
| Oral cavity | 72 (67.3%) | 51 (60.7%) | 21 (91.3%) | |
| Oropharyx | 21 (19.6%) | 19 (22.6%) | 2 (8.7%) | |
| Grade | 0.617 | |||
| Grade 1 | 28 (26.9%) | 20 (24.7%) | 8 (34.8%) | |
| Grade 2 | 58 (55.8%) | 46 (56.8%) | 12 (52.2%) | |
| Grade 3 | 18 (17.3%) | 15 (18.5%) | 3 (13.0%) | |
| Unknown | 3 (2.8%) | 3 (3.6%) | 0 (0.0%) | |
| Stage | 0.670 | |||
| Stage I | 3 (2.8%) | 3 (3.6%) | 0 (0.0%) | |
| Stage II | 4 (3.7%) | 4 (4.8%) | 0 (0.0%) | |
| Stage III | 9 (8.4%) | 8 (9.5%) | 1 (4.3%) | |
| Stage IV | 91 (85.0%) | 69 (82.1%) | 22 (95.7%) | |
| BMI (Pre-CRT) | 23.2 (14.6–34.1) | 23.2 (15.1–34.0) | 23.2 (14.6–34.1) | 0.601 |
| BMI (Post-CRT) | 22.1 (13.9–33.6) | 22.1 (13.9–33.6) | 21.9 (14.5–33.0) | 0.900 |
| Body weight loss | −2.3 (−18.5–7.2) | −2.0 (−18.5–7.2) | −2.8 (−13.7–2.5) | 0.377 |
| White blood cell (/μL) | 6810 (3020–35,150) | 6660 (3020–15,170) | 6970 (3710–35,150) | 0.585 |
| Neutrophils (Neu) (/μL) | 68.8 (33.2–96.1) | 68.4 (33.2–88.4) | 70.0 (50.4–96.1) | 0.147 |
| Lymphocytes (Lym) (/μL) | 20.8 (1.0–53.6) | 21.0 (2.0–53.6) | 20.7 (1.0–29.9) | 0.147 |
| NLR (Neu/Lym) | 0.037 | |||
| Low (<2.7) | 33 (30.8%) | 30 (35.7%) | 3 (13.0%) | |
| High (≥2.7) | 74 (69.2%) | 54 (64.3%) | 20 (87.0%) |
| Involved Pathway | Genes in Pathway | Involved Genes | Pathway Percentage | Gene Symbol |
|---|---|---|---|---|
| PI3K signaling in B lymphocytes | 122 | 25 | 20.5 | LYN;IKBKE;AKT2;PIK3CA;AKT1;CD79A;IRS2;MAP2K2;PRKCI;CD79B;PTEN;NFKBIA;CBL; MAPK1;ABL1;PIK3CB;RAC1;SYK;HRAS;JUN;AKT3;MAP2K1;RAF1;KRAS;PIK3R1 |
| FcγRIIb signaling in B lymphocytes | 41 | 14 | 34.1 | LYN;PIK3C2G;PIK3CA;AKT1;CD79A;ATM; CD79B;MAP2K4;PIK3CB;SYK;HRAS;PIK3C2B;KRAS;PIK3R1 |
| Regulation of IL-2 expression in activated and anergic T lymphocytes | 75 | 16 | 21.3 | IKBKE;TGFBR2;SMAD2;CARD11;MAP2K2; NFKBIA;MAP2K4;MAPK1;RAC1;HRAS; MAP3K1;JUN;MAP2K1;RAF1;KRAS;SMAD4 |
| PKC signaling in T lymphocytes | 107 | 17 | 15.9 | MAP3K13;PIK3C2G;IKBKE;CARD11;PIK3CA; ATM;NFKBIA;MAP2K4;MAPK1;PIK3CB;RAC1; HRAS;MAP3K1;PIK3C2B;JUN;KRAS; PIK3R1 |
| fMLP signaling in neutrophils | 106 | 16 | 15.1 | GNAS;PIK3C2G;PIK3CA;MAP2K2;ATM;PRKCI;NFKBIA;MAPK1;PIK3CB;RAC1;HRAS; PIK3C2B;MAP2K1;RAF1;KRAS;PIK3R1 |
| CTLA4 signaling in cytotoxic T lymphocytes | 82 | 13 | 15.9 | JAK2;PIK3C2G;AKT2;PIK3CA;AKT1;ATM; PIK3CB;SYK;PIK3C2B;AKT3;PTPN11;PPP2R2A; PIK3R1 |
| CD27 signaling in lymphocytes | 51 | 10 | 19.6 | CASP8;MAP3K13;IKBKE;MAP2K2;NFKBIA; MAP2K4;MAP3K1;JUN;MAP2K1;BCL2L1 |
| 4-IBB signaling in T lymphocytes | 31 | 7 | 22.6 | IKBKE;MAP2K2;NFKBIA;MAP2K4;MAPK1; JUN;MAP2K1 |
| Genes | Overall, n = 107 | Low NLR, n = 33 | High NLR, n = 74 | p Value |
|---|---|---|---|---|
| PIK3CA | 25 (23.4%) | 10 (30.3%) | 15 (20.3%) | 0.257 |
| PRKCI | 20 (18.7%) | 7 (21.2%) | 13 (17.6%) | 0.655 |
| CASP8 | 20 (18.7%) | 3 (9.1%) | 17 (23.0%) | 0.089 |
| PIK3C2G | 16 (15.0%) | 4 (12.1%) | 12 (16.2%) | 0.771 |
| MAP3K13 | 14 (13.1%) | 5 (15.2%) | 9 (12.2%) | 0.758 |
| ATM | 12 (11.2%) | 5 (15.2%) | 7 (9.5%) | 0.508 |
| JAK2 | 12 (11.2%) | 3 (9.1%) | 9 (12.2%) | 0.751 |
| PTEN | 11 (10.3%) | 4 (12.1%) | 7 (9.5%) | 0.735 |
| CARD11 | 11 (10.3%) | 1 (3.0%) | 10 (13.5%) | 0.167 |
| GNAS | 9 (8.4%) | 2 (6.1%) | 7 (9.5%) | 0.718 |
| HRAS | 8 (7.5%) | 3 (9.1%) | 5 (6.8%) | 0.700 |
| CBL | 7 (6.5%) | 1 (3.0%) | 6 (8.1%) | 0.433 |
| IRS2 | 6 (5.6%) | 4 (12.1%) | 2 (2.7%) | 0.071 |
| MAP2K2 | 6 (5.6%) | 4 (12.1%) | 2 (2.7%) | 0.071 |
| MAP3K1 | 6 (5.6%) | 0 (0.0%) | 6 (8.1%) | 0.174 |
| NFKBIA | 5 (4.7%) | 2 (6.1%) | 3 (4.1%) | 0.643 |
| PIK3CB | 5 (4.7%) | 2 (6.1%) | 3 (4.1%) | 0.643 |
| KRAS | 5 (4.7%) | 1 (3.0%) | 4 (5.4%) | 1.000 |
| TGFBR2 | 5 (4.7%) | 2 (6.1%) | 3 (4.1%) | 0.643 |
| LYN | 4 (3.7%) | 1 (3.0%) | 3 (4.1%) | 1.000 |
| IKBKE | 3 (2.8%) | 1 (3.0%) | 2 (2.7%) | 1.000 |
| AKT1 | 3 (2.8%) | 2 (6.1%) | 1 (1.4%) | 0.224 |
| CD79B | 3 (2.8%) | 1 (3.0%) | 2 (2.7%) | 1.000 |
| MAPK1 | 3 (2.8%) | 2 (6.1%) | 1 (1.4%) | 0.224 |
| JUN | 3 (2.8%) | 0 (0.0%) | 3 (4.1%) | 0.551 |
| MAP2K4 | 3 (2.8%) | 0 (0.0%) | 3 (4.1%) | 0.551 |
| PIK3C2B | 3 (2.8%) | 2 (6.1%) | 1 (1.4%) | 0.224 |
| ABL1 | 2 (1.9%) | 0 (0.0%) | 2 (2.7%) | 1.000 |
| RAC1 | 2 (1.9%) | 0 (0.0%) | 2 (2.7%) | 1.000 |
| SYK | 2 (1.9%) | 1 (3.0%) | 1 (1.4%) | 0.524 |
| AKT3 | 2 (1.9%) | 0 (0.0%) | 2 (2.7%) | 1.000 |
| RAF1 | 2 (1.9%) | 1 (3.0%) | 1 (1.4%) | 0.524 |
| PIK3R1 | 2 (1.9%) | 0 (0.0%) | 2 (2.7%) | 1.000 |
| SMAD4 | 2 (1.9%) | 2 (6.1%) | 0 (0.0%) | 0.093 |
| PTPN11 | 2 (1.9%) | 0 (0.0%) | 2 (2.7%) | 1.000 |
| BCL2L1 | 2 (1.9%) | 0 (0.0%) | 2 (2.7%) | 1.000 |
| AKT2 | 1 (0.9%) | 1 (3.0%) | 0 (0.0%) | 0.308 |
| CD79A | 1 (0.9%) | 0 (0.0%) | 1 (1.4%) | 1.000 |
| MAP2K1 | 1 (0.9%) | 0 (0.0%) | 1 (1.4%) | 1.000 |
| SMAD2 | 1 (0.9%) | 1 (3.0%) | 0 (0.0%) | 0.308 |
| PPP2R2A | 1 (0.9%) | 0 (0.0%) | 1 (1.4%) | 1.000 |
| Genes | Coefficients | SE | t | p |
|---|---|---|---|---|
| LYN | −0.386 | 0.336 | −1.150 | 0.254 |
| IKBKE | 0.036 | 0.278 | 0.130 | 0.897 |
| AKT2 | −1.030 | 0.560 | −1.842 | 0.070 |
| PIK3CA | −0.486 | 0.251 | −1.942 | 0.057 |
| AKT1 | −0.194 | 0.422 | −0.461 | 0.646 |
| CD79A | 0.441 | 0.871 | 0.507 | 0.614 |
| IRS2 | −0.367 | 0.244 | −1.506 | 0.137 |
| MAP2K2 | −0.749 | 0.315 | −2.373 | 0.021 |
| PRKCI | 0.342 | 0.277 | 1.233 | 0.222 |
| CD79B | −0.510 | 0.421 | −1.212 | 0.230 |
| PTEN | 0.001 | 0.211 | 0.006 | 0.996 |
| NFKBIA | 0.384 | 0.359 | 1.069 | 0.289 |
| CBL | 0.219 | 0.277 | 0.791 | 0.432 |
| MAPK1 | 0.088 | 0.447 | 0.196 | 0.845 |
| ABL1 | 0.986 | 0.449 | 2.197 | 0.032 |
| PIK3CB | 0.149 | 0.298 | 0.501 | 0.618 |
| RAC1 | 0.602 | 0.619 | 0.973 | 0.334 |
| SYK | 0.130 | 0.418 | 0.311 | 0.757 |
| HRAS | −0.210 | 0.223 | −0.942 | 0.350 |
| JUN | 0.698 | 0.466 | 1.500 | 0.138 |
| AKT3 | 0.102 | 0.530 | 0.193 | 0.848 |
| MAP2K1 | −0.101 | 0.521 | −0.193 | 0.848 |
| RAF1 | −0.011 | 0.384 | −0.027 | 0.978 |
| KRAS | 0.198 | 0.305 | 0.649 | 0.519 |
| PIK3R1 | −0.009 | 0.492 | −0.019 | 0.985 |
| PIK3C2G | 0.085 | 0.153 | 0.554 | 0.582 |
| ATM | 0.037 | 0.202 | 0.181 | 0.857 |
| MAP2K4 | 0.730 | 0.339 | 2.153 | 0.035 |
| PIK3C2B | −0.217 | 0.308 | −0.704 | 0.484 |
| TGFBR2 | −0.360 | 0.342 | −1.055 | 0.295 |
| SMAD2 | −0.768 | 0.656 | −1.169 | 0.246 |
| CARD11 | 0.009 | 0.179 | 0.052 | 0.959 |
| MAP3K1 | 0.156 | 0.270 | 0.577 | 0.566 |
| SMAD4 | −0.523 | 0.429 | −1.219 | 0.227 |
| MAP3K13 | −0.044 | 0.247 | −0.177 | 0.860 |
| GNAS | 0.111 | 0.193 | 0.576 | 0.566 |
| JAK2 | 0.236 | 0.158 | 1.498 | 0.139 |
| PTPN11 | −0.450 | 0.593 | −0.759 | 0.450 |
| PPP2R2A | −0.013 | 0.718 | −0.017 | 0.986 |
| CASP8 | 0.203 | 0.148 | 1.371 | 0.175 |
| BCL2L1 | 0.522 | 0.532 | 0.982 | 0.330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, T.-J.; Wang, H.-C.; Cho, S.-F.; Wu, C.-C.; Hsieh, T.-Y.; Huang, C.-T.; Wang, M.-H.; Chuang, T.-M.; Gau, Y.-C.; Du, J.-S.; et al. The Prognosis Performance of a Neutrophil- and Lymphocyte-Associated Gene Mutation Score in a Head and Neck Cancer Cohort. Biomedicines 2023, 11, 3113. https://doi.org/10.3390/biomedicines11123113
Yeh T-J, Wang H-C, Cho S-F, Wu C-C, Hsieh T-Y, Huang C-T, Wang M-H, Chuang T-M, Gau Y-C, Du J-S, et al. The Prognosis Performance of a Neutrophil- and Lymphocyte-Associated Gene Mutation Score in a Head and Neck Cancer Cohort. Biomedicines. 2023; 11(12):3113. https://doi.org/10.3390/biomedicines11123113
Chicago/Turabian StyleYeh, Tsung-Jang, Hui-Ching Wang, Shih-Feng Cho, Chun-Chieh Wu, Tzu-Yu Hsieh, Chien-Tzu Huang, Min-Hong Wang, Tzer-Ming Chuang, Yuh-Ching Gau, Jeng-Shiun Du, and et al. 2023. "The Prognosis Performance of a Neutrophil- and Lymphocyte-Associated Gene Mutation Score in a Head and Neck Cancer Cohort" Biomedicines 11, no. 12: 3113. https://doi.org/10.3390/biomedicines11123113
APA StyleYeh, T.-J., Wang, H.-C., Cho, S.-F., Wu, C.-C., Hsieh, T.-Y., Huang, C.-T., Wang, M.-H., Chuang, T.-M., Gau, Y.-C., Du, J.-S., Liu, Y.-C., Hsiao, H.-H., Pan, M.-R., Chen, L.-T., & Moi, S.-H. (2023). The Prognosis Performance of a Neutrophil- and Lymphocyte-Associated Gene Mutation Score in a Head and Neck Cancer Cohort. Biomedicines, 11(12), 3113. https://doi.org/10.3390/biomedicines11123113