Uterine Arteries Resistance in Pregnant Women with Gestational Diabetes Mellitus, Diabetes Mellitus Type 1, Diabetes Mellitus Type 2, and Uncomplicated Pregnancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Variables
2.5. Statistical Methods
3. Results
3.1. Participants
3.2. Descriptive Data
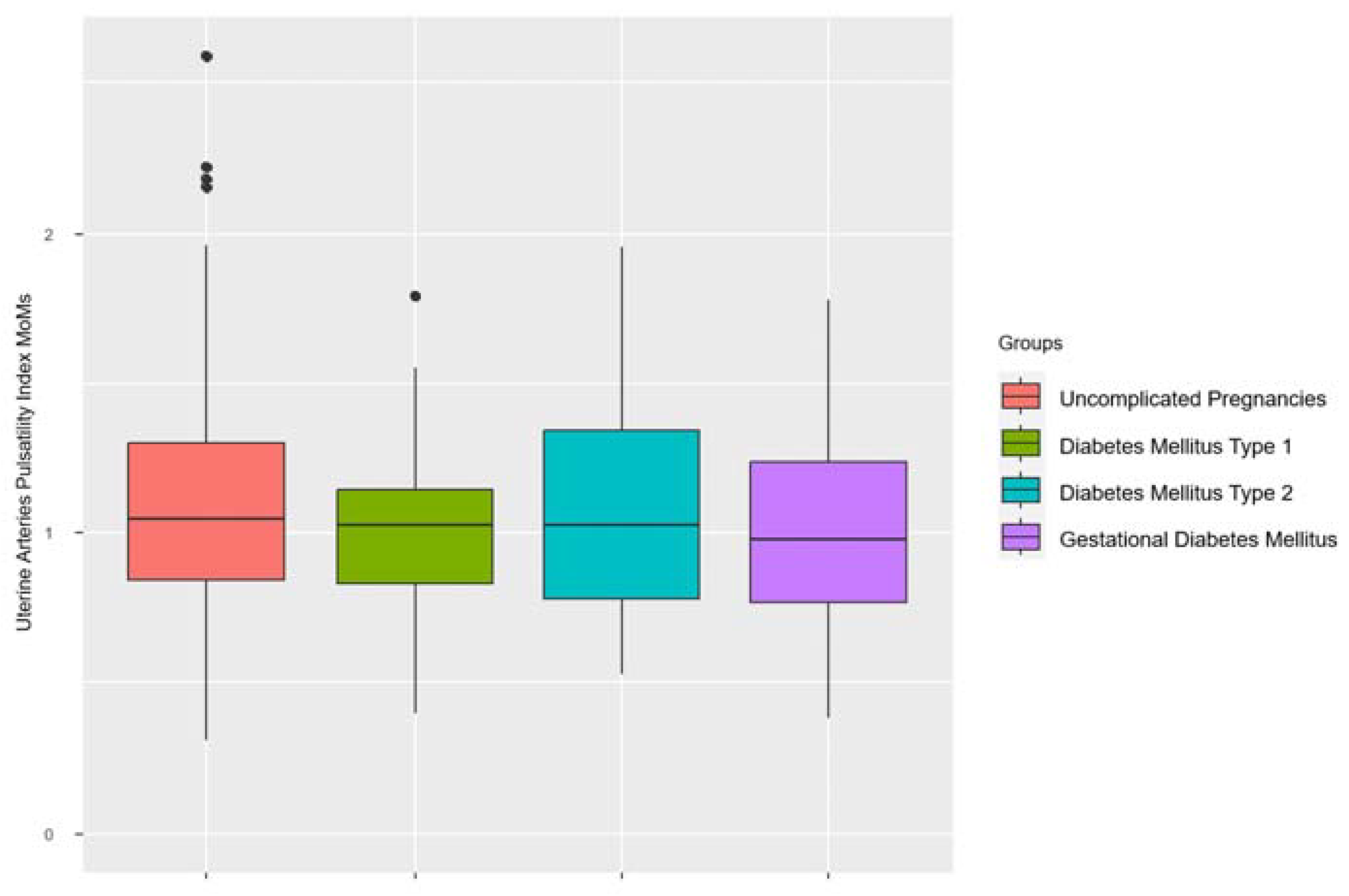
3.3. Main Results
4. Discussion
4.1. Main Findings
4.2. Interpretation
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and Function of the Normal Human Placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Romero, R.; Mee Kim, Y.; Kusanovic, J.P.; Hassan, S.; Erez, O.; Gotsch, F.; Gabor Than, N.; Papp, Z.; Jai Kim, C. Normal and Abnormal Transformation of the Spiral Arteries during Pregnancy. J. Perinat. Med. 2006, 34, 447–458. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” Are Associated with Disorders of Deep Placentation. Am. J. Obs. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of Physiological Transformation and Spiral Artery Atherosis: Their Roles in Preeclampsia. Am. J. Obs. Obstet. Gynecol. 2022, 226, S895–S906. [Google Scholar] [CrossRef] [PubMed]
- Hunkapiller, N.M.; Gasperowicz, M.; Kapidzic, M.; Plaks, V.; Maltepe, E.; Kitajewski, J.; Cross, J.C.; Fisher, S.J. A Role for Notch Signaling in Trophoblast Endovascular Invasion and in the Pathogenesis of Pre-Eclampsia. Development 2011, 138, 2987–2998. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, H.; Taylor, H.S. Implantation Failure: Molecular Mechanisms and Clinical Treatment. Hum. Reprod. Update 2011, 17, 242–253. [Google Scholar] [CrossRef]
- Norwitz, E.R. Defective Implantation and Placentation: Laying the Blueprint for Pregnancy Complications. Reprod. Biomed. Online 2006, 13, 591–599. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, J.-S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Khong, S.L.; Kane, S.C.; Brennecke, S.P.; da Silva Costa, F. First-Trimester Uterine Artery Doppler Analysis in the Prediction of Later Pregnancy Complications. Dis. Markers 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Nteeba, J.; Varberg, K.M.; Scott, R.L.; Simon, M.E.; Iqbal, K.; Soares, M.J. Poorly Controlled Diabetes Mellitus Alters Placental Structure, Efficiency, and Plasticity. BMJ Open Diabetes Res. Care 2020, 8, e001243. [Google Scholar] [CrossRef]
- Sarina; Li, D.F.; Feng, Z.Q.; Du, J.; Zhao, W.H.; Huang, N.; Jia, J.C.; Wu, Z.Y.; Alamusi; Wang, Y.Y.; et al. Mechanism of Placenta Damage in Gestational Diabetes Mellitus by Investigating TXNIP of Patient Samples and Gene Functional Research in Cell Line. Diabetes Ther. 2019, 10, 2265–2288. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, J.; Wang, Y.; Wu, Y.; Liu, H.; Feng, W.; Si, Z.; Sun, R.; Hao, Z.; Guo, H.; et al. Comparative Study of Microvascular Structural Changes in the Gestational Diabetic Placenta. Diab. Vasc. Dis. Res. 2023, 20, 147916412311736. [Google Scholar] [CrossRef]
- Stern, C.; Schwarz, S.; Moser, G.; Cvitic, S.; Jantscher-Krenn, E.; Gauster, M.; Hiden, U. Placental Endocrine Activity: Adaptation and Disruption of Maternal Glucose Metabolism in Pregnancy and the Influence of Fetal Sex. Int. J. Mol. Sci. 2021, 22, 12722. [Google Scholar] [CrossRef] [PubMed]
- Skeith, L.; Blondon, M.; Ní Áinle, F. Understanding and Preventing Placenta-Mediated Pregnancy Complications. Hamostaseologie 2020, 40, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yang, X. A Review of Roles of Uterine Artery Doppler in Pregnancy Complications. Front. Med. 2022, 9, 813343. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [CrossRef]
- Bilardo, C.M.; Chaoui, R.; Hyett, J.A.; Kagan, K.O.; Karim, J.N.; Papageorghiou, A.T.; Poon, L.C.; Salomon, L.J.; Syngelaki, A.; Nicolaides, K.H. ISUOG Practice Guidelines (Updated): Performance of 11–14 week Ultrasound Scan. Ultrasound Obstet. Gynecol. 2023, 61, 127–143. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the Human Placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef] [PubMed]
- Boss, A.L.; Chamley, L.W.; James, J.L. Placental Formation in Early Pregnancy: How Is the Centre of the Placenta Made? Hum. Reprod. Update 2018, 24, 750–760. [Google Scholar] [CrossRef]
- Brett, K.; Ferraro, Z.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K. Maternal–Fetal Nutrient Transport in Pregnancy Pathologies: The Role of the Placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef]
- Hill, E.P.; Longo, L.D. Dynamics of Maternal-Fetal Nutrient Transfer. Fed. Proc. 1980, 39, 239–244. [Google Scholar]
- Mao, J.; Zheng, Q.; Jin, L. Physiological Function of the Dynamic Oxygen Signaling Pathway at the Maternal-Fetal Interface. J. Reprod. Immunol. 2022, 151, 103626. [Google Scholar] [CrossRef]
- Crovetto, F.; Somigliana, E.; Peguero, A.; Figueras, F. Stroke during Pregnancy and Pre-Eclampsia. Curr. Opin. Obs. Obstet. Gynecol. 2013, 25, 425–432. [Google Scholar] [CrossRef]
- Bauer, S.T.; Cleary, K.L. Cardiopulmonary Complications of Pre-Eclampsia. Semin. Perinatol. 2009, 33, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Roberti, R.; Rocca, M.; Iannone, L.F.; Gasparini, S.; Pascarella, A.; Neri, S.; Cianci, V.; Bilo, L.; Russo, E.; Quaresima, P.; et al. Status Epilepticus in Pregnancy: A Literature Review and a Protocol Proposal. Expert. Rev. Neurother. 2022, 22, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Papageorghiou, A.T.; Yu, C.K.H.; Nicolaides, K.H. The Role of Uterine Artery Doppler in Predicting Adverse Pregnancy Outcome. Best. Pr. Pract. Res. Clin. Obs. Obstet. Gynaecol. 2004, 18, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Cao, Y.; Fadl, H.; Gustafson, H.; Simmons, D. Increasing Prevalence of Gestational Diabetes Mellitus When Implementing the IADPSG Criteria: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pr. Pract. 2021, 172, 108642. [Google Scholar] [CrossRef]
- Perović, M.; Garalejić, E.; Gojnić, M.; Arsić, B.; Pantić, I.; Bojović, D.J.; Fazlagić, A.; Gardiner, H. Sensitivity and Specificity of Ultrasonography as a Screening Tool for Gestational Diabetes Mellitus. J. Matern. Fetal Neonatal Med. 2012, 25, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.J.; Norman, J.E.; Rice, G.E.; Illanes, S.E. Fetal Programming and Gestational Diabetes Mellitus. Placenta 2016, 48, S54–S60. [Google Scholar] [CrossRef]
- Schwarze, A.; Nelles, I.; Krapp, M.; Friedrich, M.; Schmidt, W.; Diedrich, K.; Axt-Fliedner, R. Doppler Ultrasound of the Uterine Artery in the Prediction of Severe Complications during Low-Risk Pregnancies. Arch. Gynecol. Obs. Obstet. 2005, 271, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Tayyar, A.; Guerra, L.; Wright, A.; Wright, D.; Nicolaides, K.H. Uterine Artery Pulsatility Index in the Three Trimesters of Pregnancy: Effects of Maternal Characteristics and Medical History. Ultrasound Obstet. Gynecol. 2015, 45, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Sotiriadis, A.; Hernandez-Andrade, E.; da Silva Costa, F.; Ghi, T.; Glanc, P.; Khalil, A.; Martins, W.P.; Odibo, A.O.; Papageorghiou, A.T.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Role of Ultrasound in Screening for and Follow–up of Pre-eclampsia. Ultrasound Obstet. Gynecol. 2019, 53, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Nicolaides, K.H. Early Prediction of Preeclampsia. Obs. Obstet. Gynecol. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.Y.; Stratieva, V.; Piras, S.; Piri, S.; Nicolaides, K.H. Hypertensive Disorders in Pregnancy: Combined Screening by Uterine Artery Doppler, Blood Pressure and Serum PAPP-A at 11–13 Weeks. Prenat. Diagn. 2010, 30, 216–223. [Google Scholar] [CrossRef]
- Gana, N.; Sarno, M.; Vieira, N.; Wright, A.; Charakida, M.; Nicolaides, K.H. Ophthalmic Artery Doppler at 11–13 Weeks’ Gestation in Prediction of Pre-eclampsia. Ultrasound Obstet. Gynecol. 2022, 59, 731–736. [Google Scholar] [CrossRef]
- Kalafat, E.; Laoreti, A.; Khalil, A.; Da Silva Costa, F.; Thilaganathan, B. Ophthalmic Artery Doppler for Prediction of Pre-eclampsia: Systematic Review and Meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Carbillon, L. Uterine Artery Doppler and Changes in Endothelial Function Before Clinical Disease in Preeclamptic Women. Hypertension 2006, 47. [Google Scholar] [CrossRef][Green Version]
- Wei, W.; Liu, Q.; Tan, Y.; Liu, L.; Li, X.; Cai, L. Oxidative Stress, Diabetes, and Diabetic Complications. Hemoglobin 2009, 33, 370–377. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes Mellitus and Inflammation. Curr. Diab Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Feve, B.; Ferré, P.; Halimi, S.; Baizri, H.; Bordier, L.; Guiu, G.; Dupuy, O.; Bauduceau, B.; Mayaudon, H. Diabetes and Inflammation: Fundamental Aspects and Clinical Implications. Diabetes Metab. 2010, 36, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Chatzakis, C.; Koletsos, N.; Tirta, M.; Dinas, K.; Gkaliagkousi, E.; Sotiriadis, A. Decreased Flow-mediated Dilation in Gestational Diabetes in Pregnancy and Post-partum; A Systematic Review and Meta-analysis. Diabetes Metab. Res. Rev. 2023, 39, e3600. [Google Scholar] [CrossRef] [PubMed]
- Chatzakis, C.; Sotiriadis, A.; Fatouros, I.G.; Jamurtas, A.Z.; Deli, C.K.; Papagianni, M.; Dinas, K.; Mastorakos, G. The Effect of Physical Exercise on Oxidation Capacity and Utero-Placental Circulation in Pregnancies with Gestational Diabetes Mellitus and Uncomplicated Pregnancies, a Pilot Study. Diagnostics 2022, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Sheikhansari, G.; Soltani-Zangbar, M.S.; Pourmoghadam, Z.; Kamrani, A.; Azizi, R.; Aghebati-Maleki, L.; Danaii, S.; Koushaeian, L.; Hojat-Farsangi, M.; Yousefi, M. Oxidative Stress, Inflammatory Settings, and MicroRNA Regulation in the Recurrent Implantation Failure Patients with Metabolic Syndrome. Am. J. Reprod. Immunol. 2019, 82, e13170. [Google Scholar] [CrossRef]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Mor, G. Inflammation and Implantation. Am. J. Reprod. Immunol. 2009, 63, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Alvarez-Leite, J.I. Low-Grade Inflammation, Obesity, and Diabetes. Curr. Obes. Rep. 2014, 3, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and Pregnancy: The Role of the Immune System at the Implantation Site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Poston, L. Endothelial Dysfunction in Pre-Eclampsia. Pharmacol. Rep. 2006, 58, 69–74. [Google Scholar]
- Roberts, J. Endothelial Dysfunction in Preeclampsia. Semin. Reprod. Med. 1998, 16, 5–15. [Google Scholar] [CrossRef]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; De Mouzon, S.H.; Jawerbaum, A. The Role of Oxidative Stress in the Pathophysiology of Gestational Diabetes Mellitus. Antioxid. Redox Signal 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Lappas, M.; Mittion, A.; Permezel, M. In Response to Oxidative Stress, the Expression of Inflammatory Cytokines and Antioxidant Enzymes Are Impaired in Placenta, but Not Adipose Tissue, of Women with Gestational Diabetes. J. Endocrinol. 2010, 204, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Strain, W.D.; Paldánius, P.M. Diabetes, Cardiovascular Disease and the Microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Tooke, J.E. Microcirculation and Diabetes. Br. Med. Bull. 1989, 45, 206–223. [Google Scholar] [CrossRef] [PubMed]
| Groups | Uncomplicated Pregnancies, N = 65 | Diabetes Mellitus Type 1, N = 58 | Diabetes Mellitus Type 2, N = 67 | Gestational Diabetes Mellitus, N = 65 | p Value |
|---|---|---|---|---|---|
| BMI, Mean (SD) | 26.1 (5.6) | 27.4 (6.8) | 29.2 (5.3) | 30 (7.4) | >0.05 a |
| Nulliparity, n (%) | 27 (42%) | 31 (53%) | 29 (43%) | 40 (62%) | >0.05 * |
| Smoking, N (%) | 10 (15%) | 7 (12%) | 9 (13%) | 7 (11%) | >0.05 * |
| ART conception N (%) | 1 (1.5%) | 3 (5.2%) | 12 (18%) | 1 (1.5%) | 0.01 * |
| Maternal age, mean (SD) | 30 (6.4) | 33 (4.1) | 34 (5.1) | 30 (6, 4) | 0.001 a 0.001 b <0.001 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzakis, C.; Eleftheriades, M.; Demertzidou, E.; Eleftheriades, A.; Koletsos, N.; Lavasidis, L.; Zikopoulos, A.; Dinas, K.; Sotiriadis, A. Uterine Arteries Resistance in Pregnant Women with Gestational Diabetes Mellitus, Diabetes Mellitus Type 1, Diabetes Mellitus Type 2, and Uncomplicated Pregnancies. Biomedicines 2023, 11, 3106. https://doi.org/10.3390/biomedicines11123106
Chatzakis C, Eleftheriades M, Demertzidou E, Eleftheriades A, Koletsos N, Lavasidis L, Zikopoulos A, Dinas K, Sotiriadis A. Uterine Arteries Resistance in Pregnant Women with Gestational Diabetes Mellitus, Diabetes Mellitus Type 1, Diabetes Mellitus Type 2, and Uncomplicated Pregnancies. Biomedicines. 2023; 11(12):3106. https://doi.org/10.3390/biomedicines11123106
Chicago/Turabian StyleChatzakis, Christos, Makarios Eleftheriades, Eleftheria Demertzidou, Anna Eleftheriades, Nikolaos Koletsos, Lazaros Lavasidis, Athanasios Zikopoulos, Konstantinos Dinas, and Alexandros Sotiriadis. 2023. "Uterine Arteries Resistance in Pregnant Women with Gestational Diabetes Mellitus, Diabetes Mellitus Type 1, Diabetes Mellitus Type 2, and Uncomplicated Pregnancies" Biomedicines 11, no. 12: 3106. https://doi.org/10.3390/biomedicines11123106
APA StyleChatzakis, C., Eleftheriades, M., Demertzidou, E., Eleftheriades, A., Koletsos, N., Lavasidis, L., Zikopoulos, A., Dinas, K., & Sotiriadis, A. (2023). Uterine Arteries Resistance in Pregnant Women with Gestational Diabetes Mellitus, Diabetes Mellitus Type 1, Diabetes Mellitus Type 2, and Uncomplicated Pregnancies. Biomedicines, 11(12), 3106. https://doi.org/10.3390/biomedicines11123106







