Monitoring the Efficacy of High-Flow Nasal Cannula Oxygen Therapy in Patients with Acute Hypoxemic Respiratory Failure in the General Respiratory Ward: A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Patients
2.2. High-Flow Nasal Cannula Therapy and Measurements
2.3. Electrical Impedance Tomography Data Analysis
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirio, S.; Piran, M.; Vitacca, M.; Piaggi, G.; Ceriana, P.; Prazzoli, M.; Paneroni, M.; Carlucci, A. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir. Med. 2016, 118, 128–132. [Google Scholar] [CrossRef]
- Spoletini, G.; Alotaibi, M.; Blasi, F.; Hill, N.S. Heated Humidified High-Flow Nasal Oxygen in Adults: Mechanisms of Action and Clinical Implications. Chest 2015, 148, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, L.; Pan, K.; Zhou, J.; Huang, X. High-flow nasal cannula therapy for adult patients. J. Int. Med. Res. 2016, 44, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Alban, L.; Turrini, C.; Cambiaghi, B.; Carlesso, E.; Taccone, P.; Bottino, N.; Lissoni, A.; Spadaro, S.; Volta, C.A.; et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: Effects of increasing flow rates. Intensive Care Med. 2017, 43, 1453–1463. [Google Scholar] [CrossRef]
- Sztrymf, B.; Messika, J.; Mayot, T.; Lenglet, H.; Dreyfuss, D.; Ricard, J.D. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: A prospective observational study. J. Crit. Care 2012, 27, 324.e9–324.e13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chang, M.Y.; Frerichs, I.; Zhang, J.H.; Chang, H.T.; Gow, C.H.; Moller, K. Regional air trapping in acute exacerbation of obstructive lung diseases measured with electrical impedance tomography: A feasibility study. Minerva Anestesiol. 2020, 86, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Hough, J.L.; Pham, T.M.; Schibler, A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr. Crit. Care Med. 2014, 15, e214–e219. [Google Scholar] [CrossRef]
- Zhao, Z.; Moller, K.; Steinmann, D.; Frerichs, I.; Guttmann, J. Evaluation of an electrical impedance tomography-based Global Inhomogeneity Index for pulmonary ventilation distribution. Intensive Care Med. 2009, 35, 1900–1906. [Google Scholar] [CrossRef]
- Frerichs, I.; Hahn, G.; Golisch, W.; Kurpitz, M.; Burchardi, H.; Hellige, G. Monitoring perioperative changes in distribution of pulmonary ventilation by functional electrical impedance tomography. Acta Anaesthesiol. Scand. 1998, 42, 721–726. [Google Scholar] [CrossRef]
- Muders, T.; Luepschen, H.; Zinserling, J.; Greschus, S.; Fimmers, R.; Guenther, U.; Buchwald, M.; Grigutsch, D.; Leonhardt, S.; Putensen, C.; et al. Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury. Crit. Care Med. 2012, 40, 903–911. [Google Scholar] [CrossRef]
- Saillard, C.; Lambert, J.; Tramier, M.; Chow-Chine, L.; Bisbal, M.; Servan, L.; Gonzalez, F.; de Guibert, J.M.; Faucher, M.; Sannini, A.; et al. High-flow nasal cannula failure in critically ill cancer patients with acute respiratory failure: Moving from avoiding intubation to avoiding delayed intubation. PLoS ONE 2022, 17, e0270138. [Google Scholar] [CrossRef]
- Al-Mazedi, M.S.; Rajan, R.; Al-Jarallah, M.; Dashti, R.; Al Saber, A.; Pan, J.; Zhanna, K.D.; Abdelnaby, H.; Aboelhassan, W.; Almutairi, F.; et al. Neutrophil to lymphocyte ratio and in-hospital mortality among patients with SARS-CoV-2: A retrospective study. Ann. Med. Surg. 2022, 82, 104748. [Google Scholar] [CrossRef]
- Colombo, S.M.; Scaravilli, V.; Cortegiani, A.; Corcione, N.; Guzzardella, A.; Baldini, L.; Cassinotti, E.; Canetta, C.; Carugo, S.; Hu, C.; et al. Use of high flow nasal cannula in patients with acute respiratory failure in general wards under intensivists supervision: A single center observational study. Respir. Res. 2022, 23, 171. [Google Scholar] [CrossRef] [PubMed]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; An, M.M.; Yin, F.; Zhang, J.; Peng, M.Y.; Guan, H.; Gong, P. Factors associated with failure of high-flow nasal cannula oxygen therapy in patients with severe COVID-19: A retrospective case series. J. Int. Med. Res. 2022, 50, 3000605221103525. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Sung, H.; Hong, S.B.; Lim, C.M.; Koh, Y.; Huh, J.W. Predictors of high flow nasal cannula failure in immunocompromised patients with acute respiratory failure due to non-HIV pneumocystis pneumonia. J. Thorac. Dis. 2017, 9, 3013–3022. [Google Scholar] [CrossRef]
- Demoule, A.; Vieillard Baron, A.; Darmon, M.; Beurton, A.; Geri, G.; Voiriot, G.; Dupont, T.; Zafrani, L.; Girodias, L.; Labbe, V.; et al. High-Flow Nasal Cannula in Critically III Patients with Severe COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1039–1042. [Google Scholar] [CrossRef]
- Kamjai, P.; Hemvimol, S.; Bordeerat, N.K.; Srimanote, P.; Angkasekwinai, P. Evaluation of emerging inflammatory markers for predicting oxygen support requirement in COVID-19 patients. PLoS ONE 2022, 17, e0278145. [Google Scholar] [CrossRef]
- Erdogan, A.; Can, F.E.; Gonullu, H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID-19 patients. J. Med. Virol. 2021, 93, 5555–5559. [Google Scholar] [CrossRef]
- Yang, L.; Gao, C.; He, Y.; Wang, X.; Yang, L.; Guo, S.; Chen, J.; He, S.; Sun, Y.; Gao, Y.; et al. The Neutrophil-to-Lymphocyte Ratio is Associated with the Requirement and the Duration of Invasive Mechanical Ventilation in Acute Respiratory Distress Syndrome Patients: A Retrospective Study. Can. Respir. J. 2022, 2022, 1581038. [Google Scholar] [CrossRef]
- Rathod, B.D.; Amle, D.; Khot, R.S.; Prathipati, K.K.; Joshi, P.P. Neutrophil-to-Lymphocyte Ratio as a Predictor of Disease Severity and Mortality in Coronavirus Disease 2019: Prospective Study from Central India. Cureus 2022, 14, e23696. [Google Scholar] [CrossRef] [PubMed]
- Vadi, S.; Pednekar, A.; Suthar, D.; Sanwalka, N.; Ghodke, K.; Rabade, N. Characteristics and Predictive Value of T-lymphocyte Subset Absolute Counts in Patients with COVID-19-associated Acute Respiratory Failure: A Retrospective Study. Indian. J. Crit. Care Med. 2022, 26, 1198–1203. [Google Scholar] [CrossRef]
- Hayden, S.J.; Albert, T.J.; Watkins, T.R.; Swenson, E.R. Anemia in critical illness: Insights into etiology, consequences, and management. Am. J. Respir. Crit. Care Med. 2012, 185, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Khamiees, M.; Raju, P.; DeGirolamo, A.; Amoateng-Adjepong, Y.; Manthous, C.A. Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest 2001, 120, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.A.; Hanson, A.C.; Frank, R.D.; Schulte, P.J.; Go, R.S.; Storlie, C.B.; Kor, D.J. Prevalence of and Recovery from Anemia Following Hospitalization for Critical Illness among Adults. JAMA Netw. Open 2020, 3, e2017843. [Google Scholar] [CrossRef]
- Keng, L.T.; Chung, K.P.; Lin, S.Y.; Liang, S.K.; Cheng, J.C.; Chen, I.C.; Chen, Y.F.; Chang, H.T.; Hsu, C.L.; Jerng, J.S.; et al. Significant Clinical Factors Associated with Long-term Mortality in Critical Cancer Patients Requiring Prolonged Mechanical Ventilation. Sci. Rep. 2017, 7, 2148. [Google Scholar] [CrossRef]
- Reade, M.C.; Weissfeld, L.; Angus, D.C.; Kellum, J.A.; Milbrandt, E.B. The prevalence of anemia and its association with 90-day mortality in hospitalized community-acquired pneumonia. BMC Pulm. Med. 2010, 10, 15. [Google Scholar] [CrossRef]
- Veronese, N.; Segala, F.V.; Carruba, L.; La Carrubba, A.; Pollicino, F.; Di Franco, G.; Guido, G.; Cormio, M.; Lugli, A.; De Santis, L.; et al. Anemia as a risk factor for disease progression in patients admitted for COVID-19: Data from a large, multicenter cohort study. Sci. Rep. 2023, 13, 9035. [Google Scholar] [CrossRef]
- Basile, M.C.; Mauri, T.; Spinelli, E.; Dalla Corte, F.; Montanari, G.; Marongiu, I.; Spadaro, S.; Galazzi, A.; Grasselli, G.; Pesenti, A. Nasal high flow higher than 60 L/min in patients with acute hypoxemic respiratory failure: A physiological study. Crit. Care 2020, 24, 654. [Google Scholar] [CrossRef]
- Perez-Teran, P.; Marin-Corral, J.; Dot, I.; Sans, S.; Munoz-Bermudez, R.; Bosch, R.; Vila, C.; Masclans, J.R. Aeration changes induced by high flow nasal cannula are more homogeneous than those generated by non-invasive ventilation in healthy subjects. J. Crit. Care 2019, 53, 186–192. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Xia, Q.; Xu, D.; Qin, S.; Dai, M.; Fu, F.; Gao, Y.; Zhao, Z. First Attempt at Using Electrical Impedance Tomography to Predict High Flow Nasal Cannula Therapy Outcomes at an Early Phase. Front. Med. 2021, 8, 737810. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Z.; Dai, M.; Fu, F.; Moller, K.; Gao, Y.; Zhao, Z. Optimal machine learning methods for prediction of high-flow nasal cannula outcomes using image features from electrical impedance tomography. Comput. Methods Programs Biomed. 2023, 238, 107613. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Turrini, C.; Eronia, N.; Grasselli, G.; Volta, C.A.; Bellani, G.; Pesenti, A. Physiologic Effects of High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Ischaki, E.; Pantazopoulos, I.; Zakynthinos, S. Nasal high flow therapy: A novel treatment rather than a more expensive oxygen device. Eur. Respir. Rev. 2017, 26, 170028. [Google Scholar] [CrossRef]
- Zhao, Z.; Peng, S.Y.; Chang, M.Y.; Hsu, Y.L.; Frerichs, I.; Chang, H.T.; Moller, K. Spontaneous breathing trials after prolonged mechanical ventilation monitored by electrical impedance tomography: An observational study. Acta Anaesthesiol. Scand. 2017, 61, 1166–1175. [Google Scholar] [CrossRef]
- Yang, L.; Dai, M.; Cao, X.; Moller, K.; Dargvainis, M.; Frerichs, I.; Becher, T.; Fu, F.; Zhao, Z. Regional ventilation distribution in healthy lungs: Can reference values be established for electrical impedance tomography parameters? Ann. Transl. Med. 2021, 9, 789. [Google Scholar] [CrossRef]
- Spoletini, G.; Hill, N.S. High-flow nasal oxygen versus noninvasive ventilation for hypoxemic respiratory failure: Do we know enough? Ann. Thorac. Med. 2016, 11, 163–166. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef]
- Koyauchi, T.; Hasegawa, H.; Kanata, K.; Kakutani, T.; Amano, Y.; Ozawa, Y.; Matsui, T.; Yokomura, K.; Suda, T. Efficacy and Tolerability of High-Flow Nasal Cannula Oxygen Therapy for Hypoxemic Respiratory Failure in Patients with Interstitial Lung Disease with Do-Not-Intubate Orders: A Retrospective Single-Center Study. Respiration 2018, 96, 323–329. [Google Scholar] [CrossRef]
| Clinical Characteristic | Values |
|---|---|
| Patients, n | 42 |
| Age, years | |
| Median (minimum–maximum) | 74.5 (52–88) |
| Sex, n (%) | |
| M | 24 (57) |
| F | 18 (43) |
| Smoking status, n (%) | |
| Current/Ever | 23 (55) |
| Never | 19 (45) |
| Primary cause of respiratory failure, n (%) | |
| Pneumonia | 33 (78) |
| Obstructive lung diseases | 5 (12) |
| Lung cancer | 4 (10) |
| Heart failure, n (%) | |
| Yes | 3 (7) |
| No | 39 (93) |
| HFNC efficacy, n (%) | |
| Success | 32 (76) |
| Failure | 10 (24) |
| Event (days ± SD) | |
| Hospital admission | 29.4 ± 23.1 |
| HFNC to discharge | 20.8 ± 17.8 |
| HFNC use | 11.3 ± 8.9 |
| Survival/Death, n (%) | |
| Survival | 30 (71) |
| Death | 12 (29) |
| Factor | HFNC Success (n = 32) | HFNC Failure (n = 10) | p-Value |
|---|---|---|---|
| Age, n | 1.000 | ||
| <75 | 16 | 5 | |
| ≥75 | 16 | 5 | |
| Sex, n | 1.000 | ||
| M | 18 | 6 | |
| F | 14 | 4 | |
| Smoking status, n | 0.729 | ||
| Current/Ever | 18 | 5 | |
| Never | 14 | 5 | |
| Survival/Death, n | <0.001 * | ||
| Survival | 30 | 0 | |
| Death | 2 | 10 | |
| Primary cause of respiratory failure, n | 0.564 | ||
| Pneumonia | 24 | 9 | |
| Obstructive lung disease | 5 | 0 | |
| Lung cancer | 3 | 1 | |
| Heart failure, n | 1.000 | ||
| Yes | 3 | 0 | |
| No | 29 | 10 | |
| HFNC, FiO2 on Day 1 (%), n | 0.268 | ||
| <60 | 21 | 4 | |
| ≥60 | 11 | 6 | |
| HFNC, flow on Day 1 (L/min), n | 0.466 | ||
| <40 | 21 | 8 | |
| ≥40 | 11 | 2 | |
| Hemoglobin (mg/dL), n | 0.040 * | ||
| ≥9 | 27 | 5 | |
| <9 | 5 | 5 | |
| White cell count (per dL), n | 1.000 | ||
| ≥12,000 | 12 | 4 | |
| <12,000 | 20 | 6 | |
| Platelets (per dL), n | 1.000 | ||
| ≥80,000 | 29 | 9 | |
| <80,000 | 3 | 1 | |
| Neutrophil-to-lymphocyte ratio, n | 0.070 | ||
| ≥9 | 11 | 7 | |
| <9 | 21 | 3 | |
| pH level, n | 1.000 | ||
| ≤7.34 | 4 | 1 | |
| 7.35–7.45 | 17 | 6 | |
| ≥7.46 | 11 | 3 | |
| CO2 level (mmHg), n | 0.625 | ||
| <40 | 13 | 4 | |
| =40–55 | 13 | 7 | |
| ≥55 | 6 | 3 | |
| BUN (mg/dL) on Day 1, n | 0.719 | ||
| ≤25 | 15 | 6 | |
| >25 | 17 | 4 | |
| Creatinine (mg/dL) on Day 1, n | 0.660 | ||
| ≤1.3 | 26 | 7 | |
| >1.3 | 6 | 3 | |
| Albumin (mg/dL) on Day 1, n | 0.128 | ||
| <3.0 | 9 | 6 | |
| ≥3.0 | 23 | 4 |
| Item | Questionnaire | Score ± SD |
|---|---|---|
| 1 | How comfortable is your nose or face when using oxygen equipment? | 2.0 ± 1.5 |
| 2 | How comfortable is your mouth/nose/throat (whether it is dry) when using oxygen equipment? | 2.4 ± 1.8 |
| 3 | How comfortable do you feel to swallow when using oxygen equipment? | 2.2 ± 1.6 |
| 4 | How comfortable do you feel during eating when using oxygen equipment? | 2.4 ± 2.0 |
| 5 | What extent do you feel that the use of oxygen equipment affects coughing? | 2.4 ± 1.8 |
| 6 | How much do you feel about body turnover when using oxygen equipment? | 3.8 ± 1.9 |
| 7 | How much do you feel about movement and activity using oxygen equipment? | 3.9 ± 2.0 |
| Ward Survival | HFNC to Discharge | ||||
|---|---|---|---|---|---|
| Factor | Patients n | Median (Days) | p-Value | Median (Days) | p-Value |
| Age, years | 0.705 | 0.473 | |||
| <75 | 21 | 47 | 41 | ||
| ≥75 | 21 | 110 | 103 | ||
| Sex | 0.346 | 0.574 | |||
| M | 24 | 42 | 41 | ||
| F | 18 | 110 | 38 | ||
| Smoking status | 0.599 | 0.850 | |||
| Current/Ever | 23 | 42 | 41 | ||
| Never | 19 | 110 | 38 | ||
| HFNC efficacy | <0.001 | <0.001 | |||
| Success | 32 | 23 | 103 | ||
| Failure | 10 | 110 | 13 | ||
| Primary cause of respiratory failure | 0.890 | 0.716 | |||
| Pneumonia | 33 | 47 | 38 | ||
| Non-pneumonia | 9 | 47 | 41 | ||
| HFNC, FiO2 on Day 1 (%) | 0.136 | 0.282 | |||
| <60 | 25 | 110 | 103 | ||
| ≥60 | 17 | 42 | 38 | ||
| HFNC, flow on Day 1 (L/min) | 0.948 | 0.760 | |||
| <40 | 28 | 47 | 38 | ||
| ≥40 | 14 | 110 | 103 | ||
| Hemoglobin (mg/dL) | 0.356 | 0.380 | |||
| ≥9 | 32 | NR | 38 | ||
| <9 | 10 | 47 | 41 | ||
| White cell count (per dL) | 0.973 | 0.358 | |||
| ≥12,000 | 16 | NR | NR | ||
| <12,000 | 26 | 47 | 41 | ||
| Platelets (per dL) | 0.894 | 0.577 | |||
| ≥80,000 | 38 | 110 | 103 | ||
| <80,000 | 4 | 47 | 38 | ||
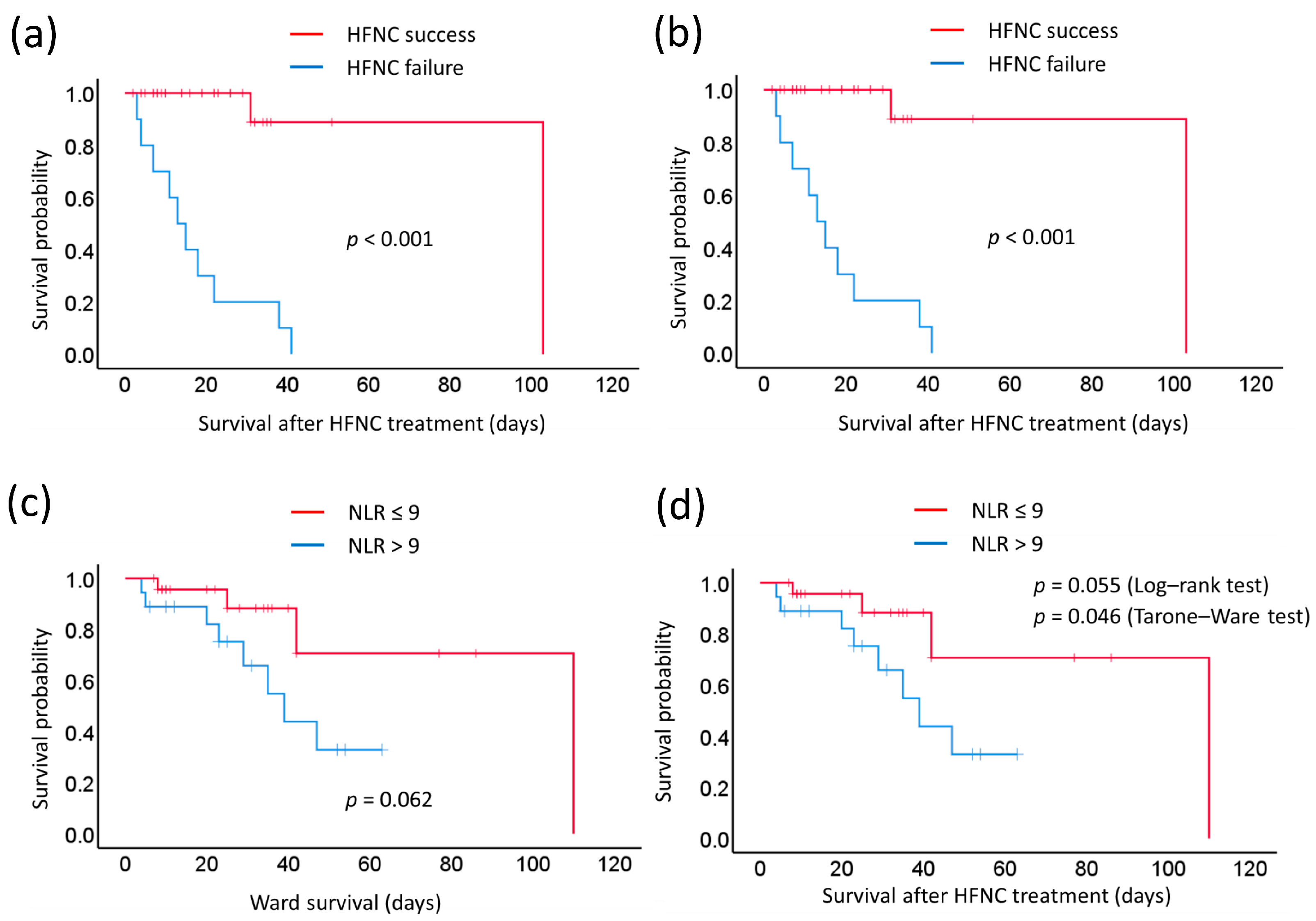
| Neutrophil-to-lymphocyte ratio | 0.062 | ||||
| ≥9 | 18 | 39 | 31 | 0.055 | |
| <9 | 24 | 110 | 41 | 0.046 * (T–W) | |
| pH level | 0.928 | 0.776 | |||
| 7.35–7.45 | 23 | 47 | 38 | ||
| Abnormal | 19 | NR | NR | ||
| CO2 (mmHg) level | 0.733 | 0.290 | |||
| 40–55 | 19 | 47 | 38 | ||
| Abnormal | 23 | 110 | 41 | ||
| BUN (mg/dL) on Day 1 | 0.522 | 0.526 | |||
| ≤25 | 21 | 42 | 38 | ||
| >25 | 21 | 110 | 103 | ||
| Creatinine (mg/dL) on Day 1 | 0.357 | 0.540 | |||
| ≤1.3 | 33 | 47 | 41 | ||
| >1.3 | 9 | 29 | 31 | ||
| Albumin (mg/dL) on Day 1 | 0.493 | 0.387 | |||
| <3.0 | 15 | 47 | 38 | ||
| ≥3.0 | 27 | 110 | 41 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Chang, M.-Y.; Zhang, T.; Gow, C.-H. Monitoring the Efficacy of High-Flow Nasal Cannula Oxygen Therapy in Patients with Acute Hypoxemic Respiratory Failure in the General Respiratory Ward: A Prospective Observational Study. Biomedicines 2023, 11, 3067. https://doi.org/10.3390/biomedicines11113067
Zhao Z, Chang M-Y, Zhang T, Gow C-H. Monitoring the Efficacy of High-Flow Nasal Cannula Oxygen Therapy in Patients with Acute Hypoxemic Respiratory Failure in the General Respiratory Ward: A Prospective Observational Study. Biomedicines. 2023; 11(11):3067. https://doi.org/10.3390/biomedicines11113067
Chicago/Turabian StyleZhao, Zhanqi, Mei-Yun Chang, Tingting Zhang, and Chien-Hung Gow. 2023. "Monitoring the Efficacy of High-Flow Nasal Cannula Oxygen Therapy in Patients with Acute Hypoxemic Respiratory Failure in the General Respiratory Ward: A Prospective Observational Study" Biomedicines 11, no. 11: 3067. https://doi.org/10.3390/biomedicines11113067
APA StyleZhao, Z., Chang, M.-Y., Zhang, T., & Gow, C.-H. (2023). Monitoring the Efficacy of High-Flow Nasal Cannula Oxygen Therapy in Patients with Acute Hypoxemic Respiratory Failure in the General Respiratory Ward: A Prospective Observational Study. Biomedicines, 11(11), 3067. https://doi.org/10.3390/biomedicines11113067






