The Combination of Radiation with PARP Inhibition Enhances Senescence and Sensitivity to the Senolytic, Navitoclax, in Triple Negative Breast Tumor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Drug Treatments
2.2. Primers
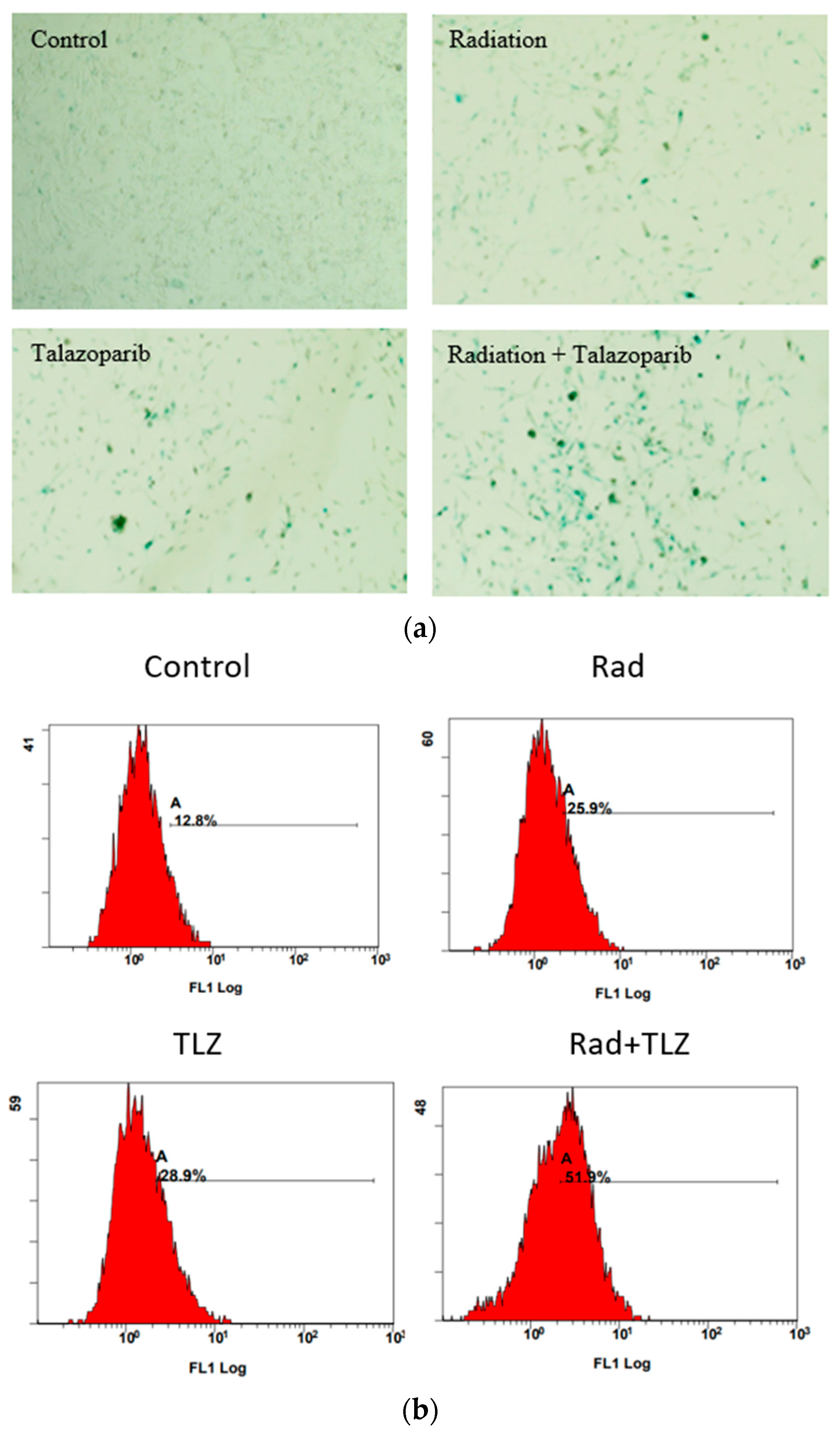
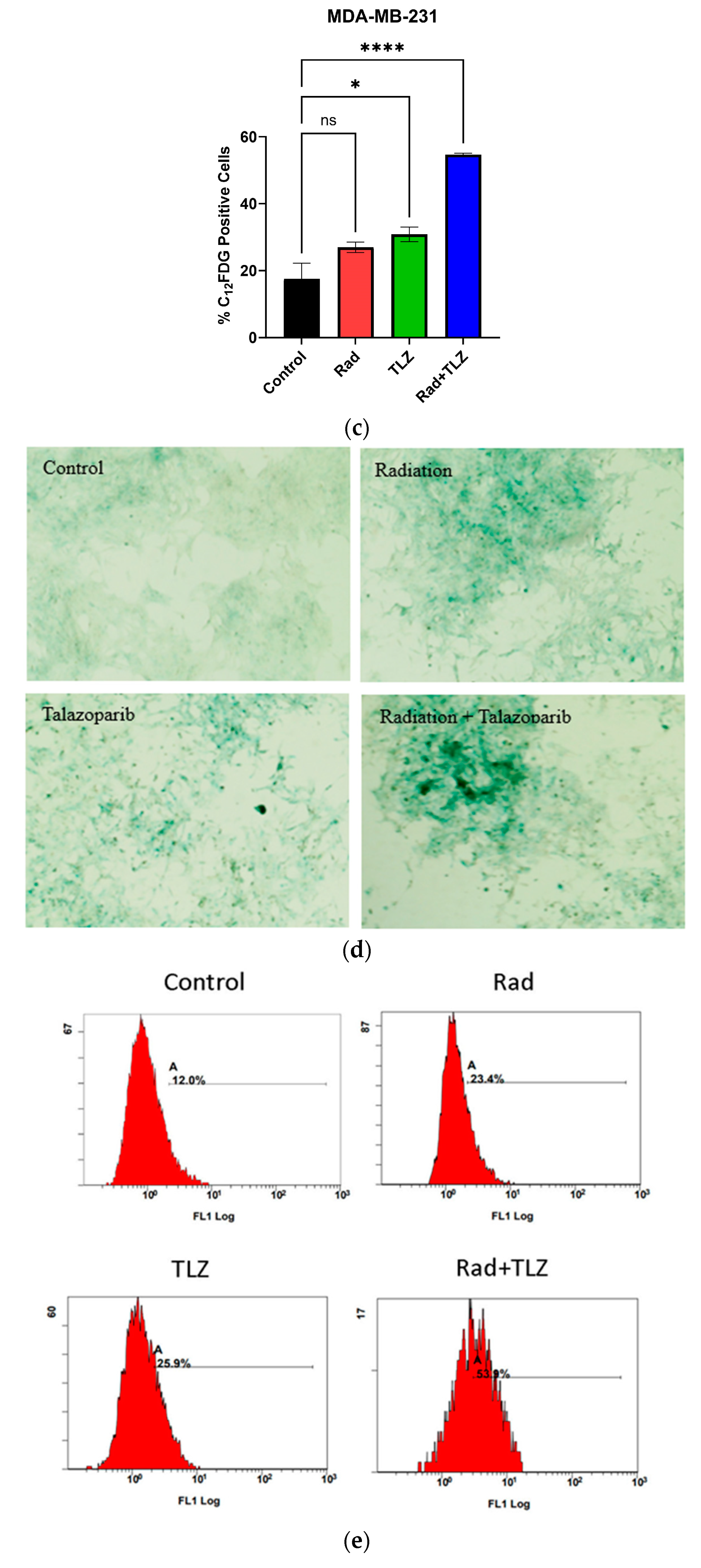
2.3. SA-β-Galactosidase Staining and C12-FDG Quantification to Evaluate Senescence
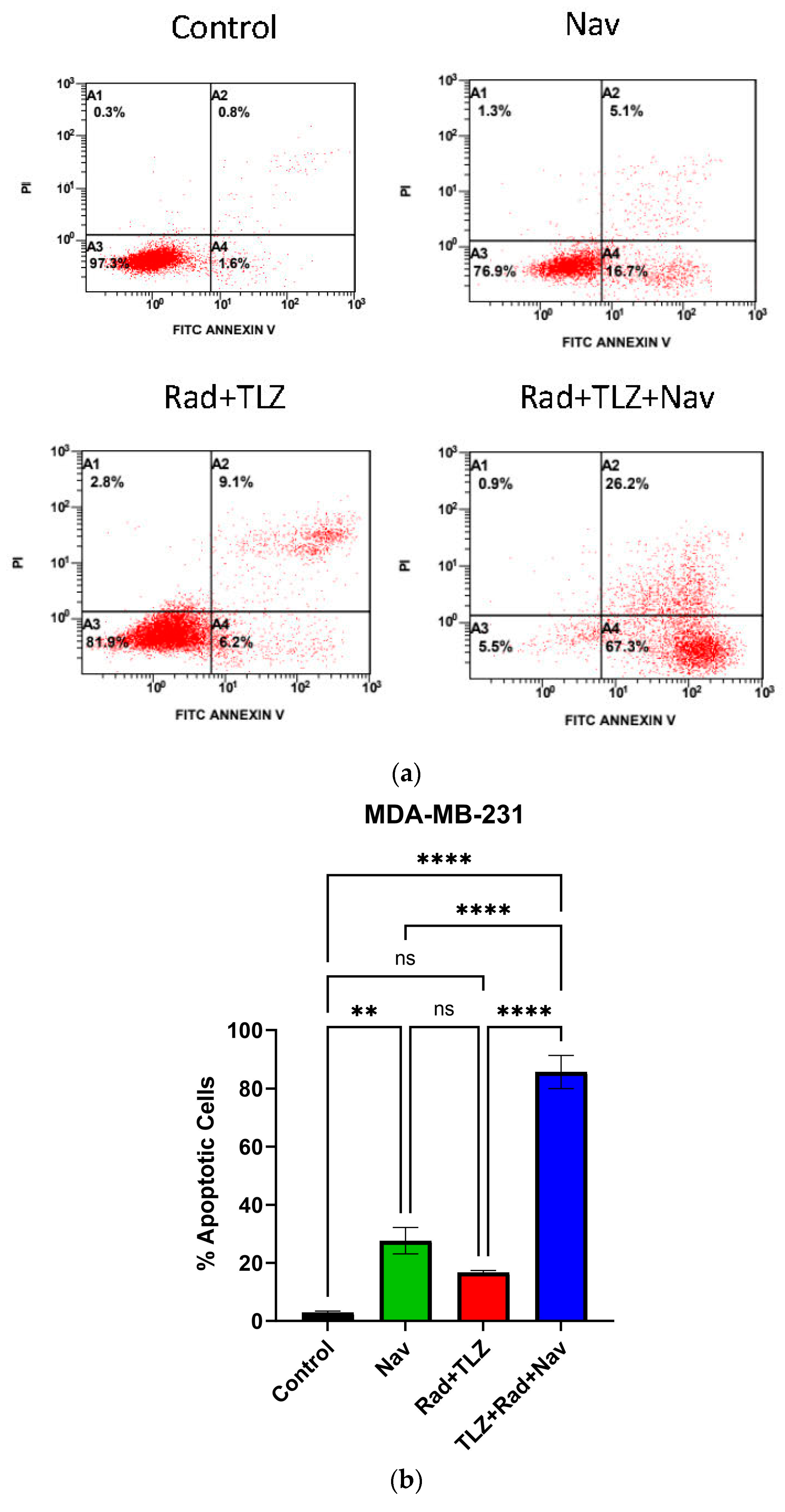
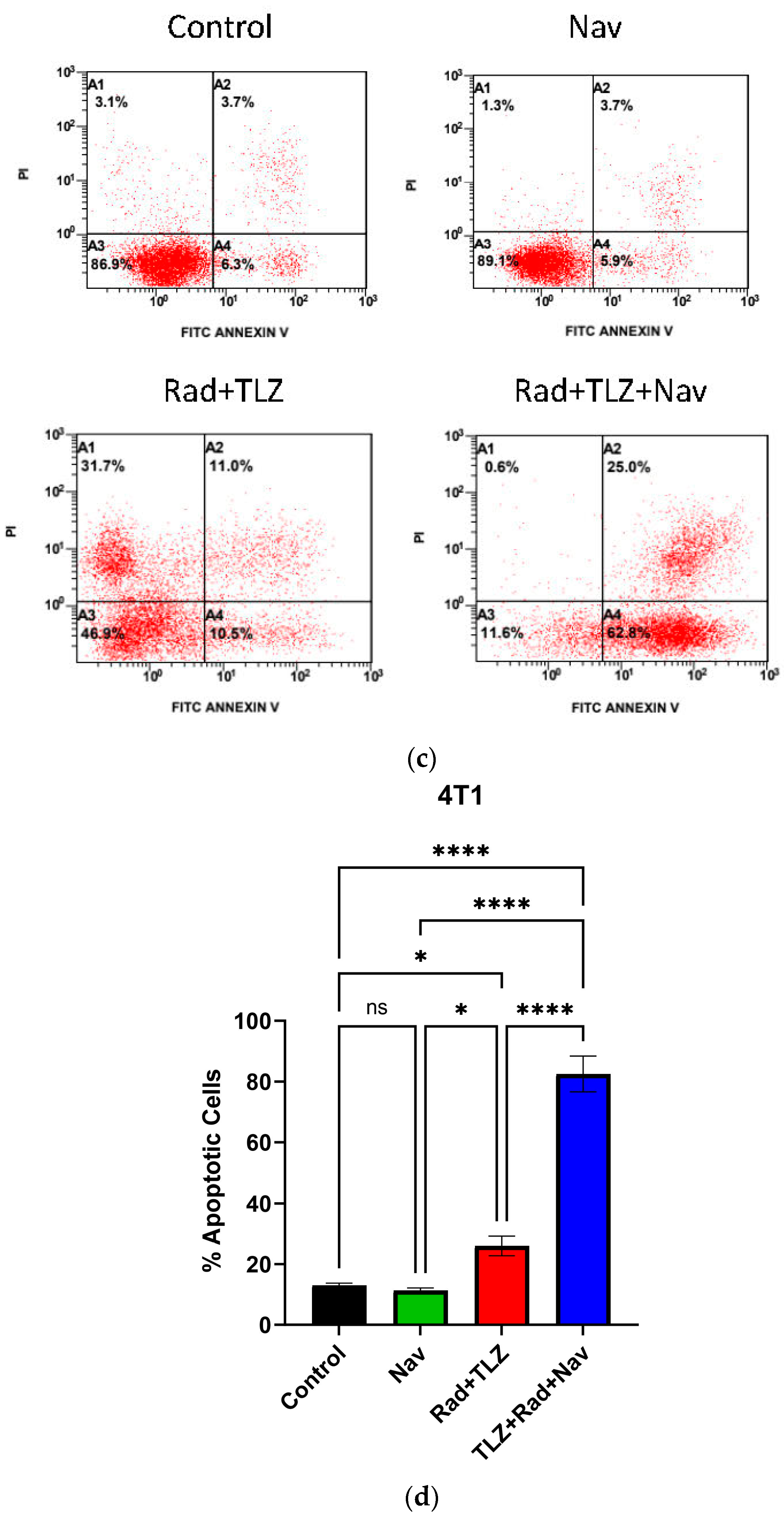
2.4. Annexin V/PI Assay to Measure Apoptosis
2.5. Reverse Transcription-Polymerase Chain Reaction (RT–PCR) to Measure Canonical Senescence-Related Gene Expression
2.6. Animal Tumor Model and Treatment
2.7. Histopathological Analysis of Treatment Groups
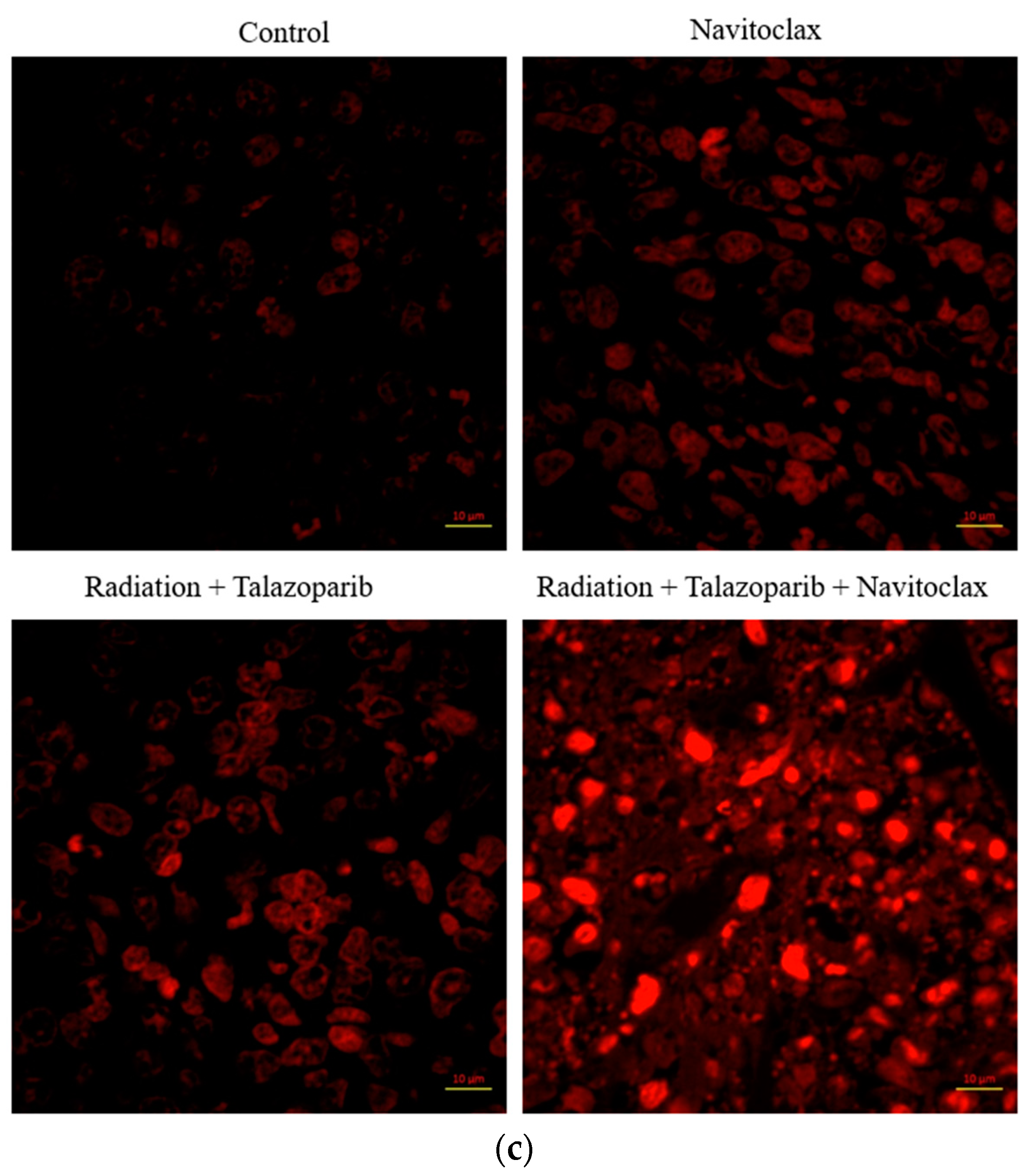
2.8. TUNEL Assay of Tissue Samples in Response to Treatment
2.9. Statistical Analysis
3. Results
3.1. PARP Inhibition Triggers Robust TIS in Combination with Radiation in MDA-MB-231 and 4T1 Breast Tumor Cells
3.2. Navitoclax Enhances Apoptosis in Radiation and PARPi-Induced Senescence in MDA-MB-231 and 4T1 Breast Cancer Cells
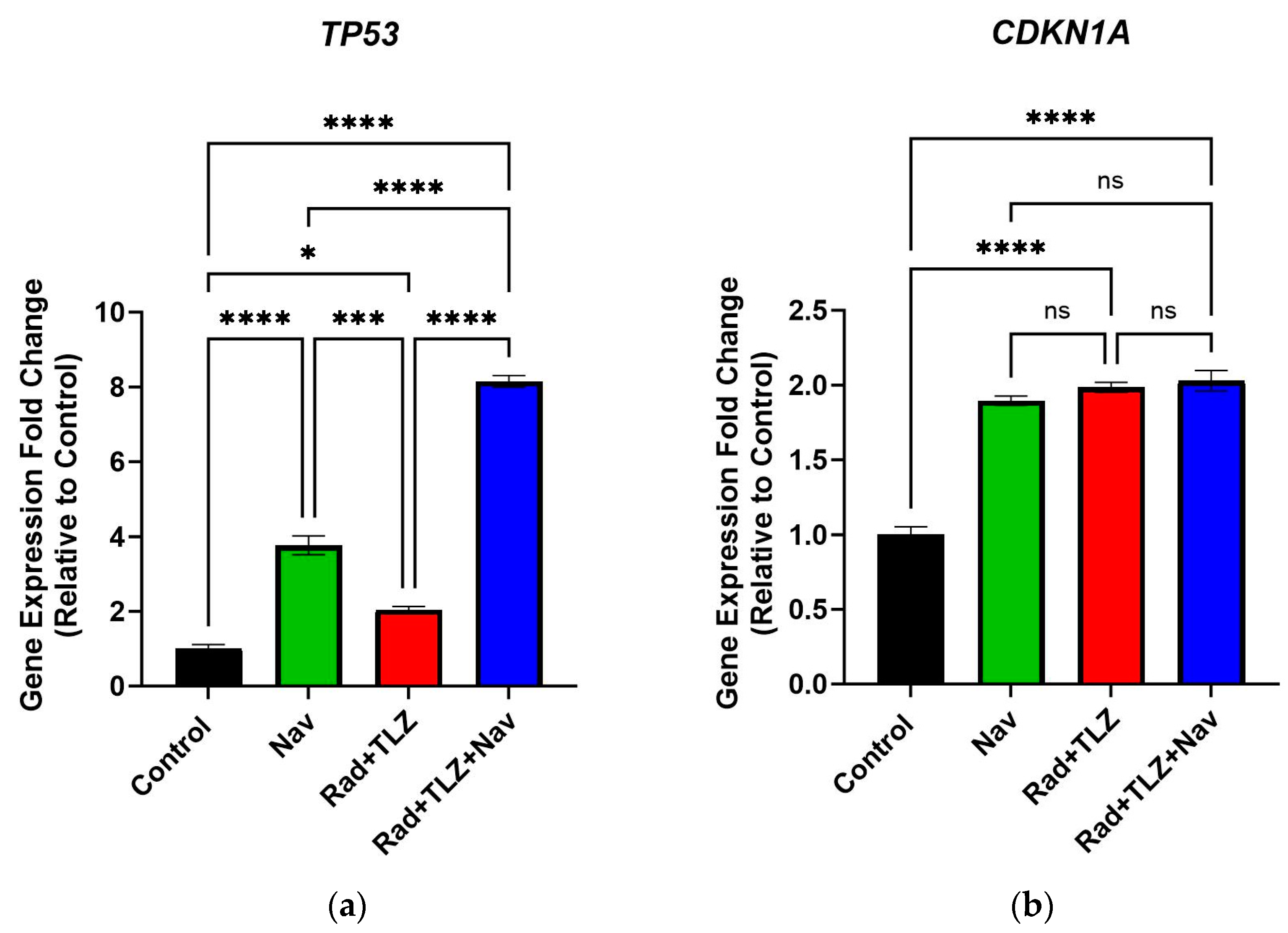
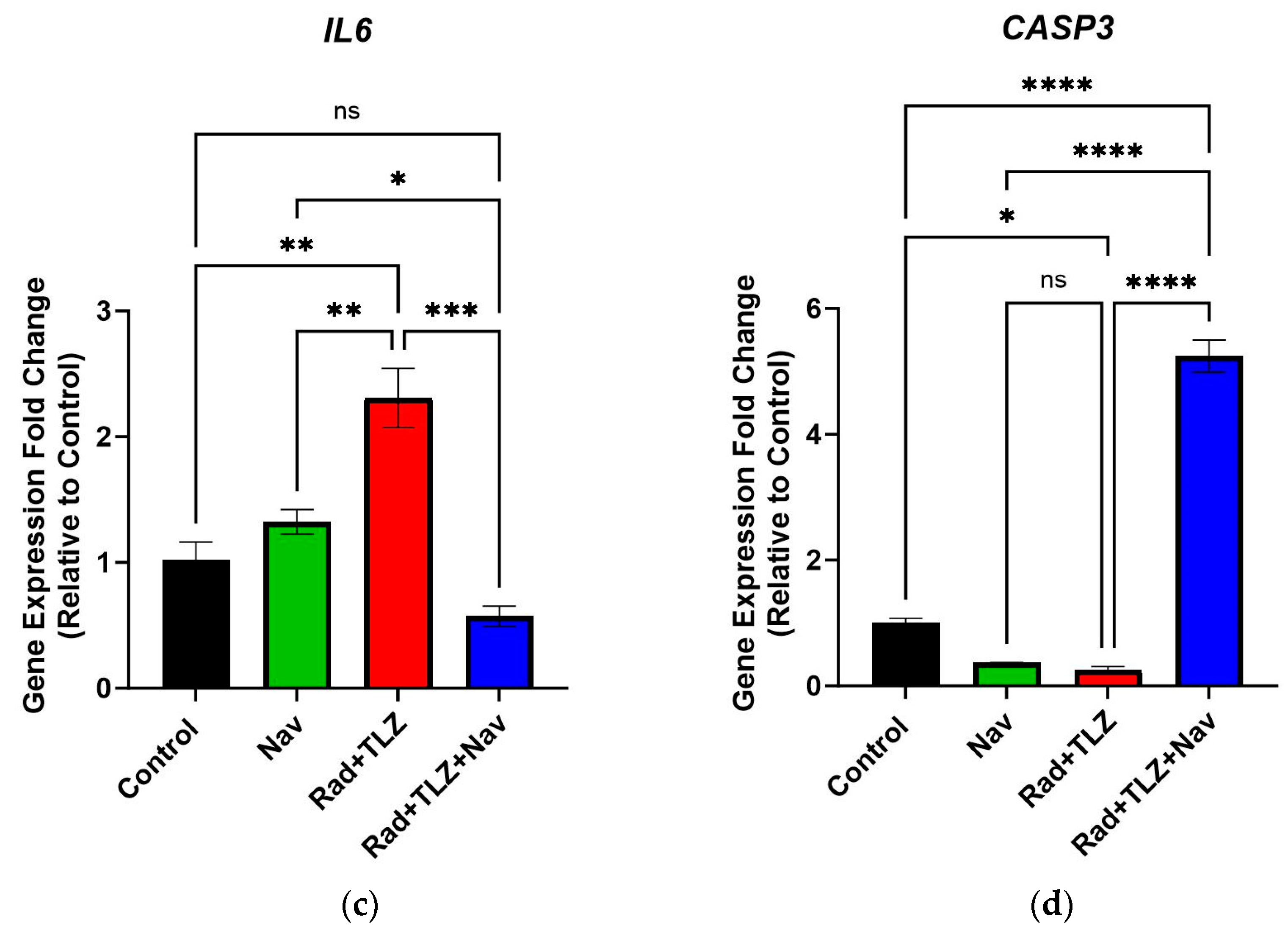
3.3. Navitoclax Modulates Expression of Senescence- and Apoptosis-Related Genes Induced by Radiation and PARPi
3.4. Navitoclax Suppresses Growth of Senescent 4T1 Cells in Tumor-Bearing Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Gong, M.; Wang, Y.; Yang, Y.; Liu, S.; Zeng, Q. Global Trends and Forecasts of Breast Cancer Incidence and Deaths. Sci. Data 2023, 10, 334. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Bentires-Alj, M. Mechanism-Based Cancer Therapy: Resistance to Therapy, Therapy for Resistance. Oncogene 2014, 34, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, M.; Sun, Z.; Price, K.N.; Karlsson, P.; Forbes, J.F.; Thürlimann, B.; Gianni, L.; Castiglione, M.; Gelber, R.D.; Coates, A.S.; et al. Annual Hazard Rates of Recurrence for Breast Cancer during 24 Years of Follow-Up: Results from the International Breast Cancer Study Group Trials I to V. J. Clin. Oncol. 2016, 34, 927–935. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; O ElHassan, G.; Harguindey, S.; et al. Resistance to Cancer Chemotherapy: Failure in Drug Response from ADME to P-Gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef]
- Yang, L.; Fang, J.; Chen, J. Tumor Cell Senescence Response Produces Aggressive Variants. Cell Death Discov. 2017, 3, 17049. [Google Scholar] [CrossRef]
- Won, K.; Spruck, C. Triple-negative Breast Cancer Therapy: Current and Future Perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef]
- Gewirtz, D.A. Autophagy, Senescence and Tumor Dormancy in Cancer Therapy. Autophagy 2009, 5, 1232–1234. [Google Scholar] [CrossRef]
- Saleh, T.; Tyutyunyk-Massey, L.; Gewirtz, D.A. Tumor Cell Escape from Therapy-Induced Senescence as a Model of Disease Recurrence after Dormancy. Cancer Res. 2019, 79, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Santos-De-Frutos, K.; Djouder, N. When Dormancy Fuels Tumour Relapse. Commun. Biol. 2021, 4, 747. [Google Scholar] [CrossRef]
- Kirkwood, T.B.L. Evolution of Ageing. Nature 1977, 270, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as Playmakers of Apoptosis, Autophagy and Senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.H.; Sohal, S.S.; Manjili, M.H.; Harrell, J.C.; Gewirtz, D.A. The Roles of Autophagy and Senescence in the Tumor Cell Response to Radiation. Radiat. Res. 2020, 194, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ji, S. Cellular Senescence: Molecular Mechanisms and Pathogenicity. J. Cell. Physiol. 2018, 233, 9121–9135. [Google Scholar] [CrossRef]
- Ewald, J.A.; Desotelle, J.A.; Wilding, G.; Jarrard, D.F. Therapy-Induced Senescence in Cancer. JNCI J. Natl. Cancer Inst. 2010, 102, 1536–1546. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular Senescence Drives Age-Dependent Hepatic Steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef]
- Saleh, T.; Bloukh, S.; Carpenter, V.J.; Alwohoush, E.; Bakeer, J.; Darwish, S.; Azab, B.; Gewirtz, D.A. Therapy-Induced Senescence: An “Old” Friend Becomes the Enemy. Cancers 2020, 12, 822. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Niklas, A.; Uruski, P.; Tykarski, A.; Książek, K. Mechanisms and Significance of Therapy-Induced and Spontaneous Senescence of Cancer Cells. Cell. Mol. Life Sci. 2020, 77, 213–229. [Google Scholar] [CrossRef]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Al-Hesa, A.; Al-Balas, M.; Abuelaish, O.; Mansour, A.; Awad, H.; El-Sadoni, M.; Carpenter, V.J.; Azab, B. Expression of Therapy-Induced Senescence Markers in Breast Cancer Samples upon Incomplete Response to Neoadjuvant Chemotherapy. Biosci. Rep. 2021, 41, BSR20210079. [Google Scholar] [CrossRef] [PubMed]
- El-Sadoni, M.; Al Shboul, S.; Alhesa, A.; Abu Shahin, N.; Alsharaiah, E.; Ismail, M.A.; Ababneh, N.A.; Alotaibi, M.R.; Azab, B.; Saleh, T. A Three-Marker Signature Identifies Senescence in Human Breast Cancer Exposed to Neoadjuvant Chemotherapy. Cancer Chemother. Pharmacol. 2023, 91, 345–360. [Google Scholar] [CrossRef]
- Saleh, T.; Bloukh, S.; Hasan, M.; Al Shboul, S. Therapy-Induced Senescence as a Component of Tumor Biology: Evidence from Clinical Cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2023, 6, 188994. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.G.; Citrin, D.E.; Hildesheim, J.; Ahmed, M.M.; Venkatachalam, S.; Riscuta, G.; Xi, D.; Zheng, G.; van Deursen, J.; Goronzy, J.; et al. Therapy-Induced Senescence: Opportunities to Improve Anticancer Therapy. JNCI J. Natl. Cancer Inst. 2021, 113, 1285–1298. [Google Scholar] [CrossRef]
- Yang, J.; Liu, M.; Hong, D.; Zeng, M.; Zhang, X. The Paradoxical Role of Cellular Senescence in Cancer. Front. Cell Dev. Biol. 2021, 9, 722205. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef]
- De Blasio, A.; Vento, R.; Di Fiore, R. Mcl-1 Targeting Could Be an Intriguing Perspective to Cure Cancer. J. Cell. Physiol. 2018, 233, 8482–8498. [Google Scholar] [CrossRef]
- As Sobeai, H.M.; Alohaydib, M.; Alhoshani, A.R.; Alhazzani, K.; Almutairi, M.M.; Saleh, T.; Gewirtz, D.A.; Alotiabi, M.R. Sorafenib, Rapamycin, and Venetoclax Attenuate Doxorubicin-Induced Senescence and Promote Apoptosis in HCT116 Cells. Saudi Pharm. J. 2021, 30, 91–101. [Google Scholar] [CrossRef]
- Saleh, T.; Carpenter, V.J.; Tyutyunyk-Massey, L.; Murray, G.; Leverson, J.D.; Souers, A.J.; Alotaibi, M.R.; Faber, A.C.; Reed, J.; Harada, H.; et al. Clearance of Therapy-induced Senescent Tumor Cells by the Senolytic ABT-263 via Interference with BCL-XL–BAX Interaction. Mol. Oncol. 2020, 14, 2504–2519. [Google Scholar] [CrossRef]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef]
- Fielder, E.; Wan, T.; Alimohammadiha, G.; Ishaq, A.; Low, E.; Weigand, B.M.; Kelly, G.; Parker, C.; Griffin, B.; Jurk, D.; et al. Short Senolytic or Senostatic Interventions Rescue Progression of Radiation-Induced Frailty and Premature Ageing in Mice. eLife 2022, 11, e75492. [Google Scholar] [CrossRef]
- Tarantini, S.; Balasubramanian, P.; Delfavero, J.; Csipo, T.; Yabluchanskiy, A.; Kiss, T.; Nyúl-Tóth, Á.; Mukli, P.; Toth, P.; Ahire, C.; et al. Treatment with the BCL-2/BCL-xL Inhibitor Senolytic Drug ABT263/Navitoclax Improves Functional Hyperemia in Aged Mice. GeroScience 2021, 43, 2427–2440. [Google Scholar] [CrossRef] [PubMed]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; Van Deursen, J.M.; Baker, D.J. Clearance of Senescent Glial Cells Prevents tau-Dependent Pathology and Cognitive Decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Mazzucato, C.; Andle, J.; Lee, T.B., Jr.; Midha, A.; Talemal, L.; Chipashvili, V.; Hollister-Lock, J.; van Deursen, J.; Weir, G.; Bonner-Weir, S. Acceleration of β Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell Metab. 2019, 30, 129–142.e4. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.-M.; DeMaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of Senescent Cells by ABT263 Rejuvenates Aged Hematopoietic Stem Cells in Mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Shahbandi, A.; Rao, S.G.; Anderson, A.Y.; Frey, W.D.; Olayiwola, J.O.; Ungerleider, N.A.; Jackson, J.G. BH3 Mimetics Selectively Eliminate Chemotherapy-Induced Senescent Cells and Improve Response in TP53 Wild-Type Breast Cancer. Cell Death Differ. 2020, 27, 3097–3116. [Google Scholar] [CrossRef]
- Wang, L.; de Oliveira, R.L.; Wang, C.; Neto, J.M.F.; Mainardi, S.; Evers, B.; Lieftink, C.; Morris, B.; Jochems, F.; Willemsen, L.; et al. High-Throughput Functional Genetic and Compound Screens Identify Targets for Senescence Induction in Cancer. Cell Rep. 2017, 21, 773–783. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Bos, T.; Hu, B.; Britt, E.; Koblinski, J.; Souers, A.J.; Leverson, J.D.; Faber, A.C.; Gewirtz, D.A.; Harada, H. Senolytic-Mediated Elimination of Head and Neck Tumor Cells Induced into Senescence by Cisplatin. Mol. Pharmacol. 2022, 101, 168–180. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sanomachi, T.; Suzuki, S.; Togashi, K.; Sugai, A.; Seino, S.; Sato, A.; Okada, M.; Kitanaka, C. Gemcitabine Radiosensitization Primes Irradiated Malignant Meningioma Cells for Senolytic Elimination by Navitoclax. Neuro-Oncology Adv. 2021, 3, vdab148. [Google Scholar] [CrossRef]
- Yamamoto, M.; Suzuki, S.; Togashi, K.; Sugai, A.; Okada, M.; Kitanaka, C. Gemcitabine Cooperates with Everolimus to Inhibit the Growth of and Sensitize Malignant Meningioma Cells to Apoptosis Induced by Navitoclax, an Inhibitor of Anti-Apoptotic BCL-2 Family Proteins. Cancers 2022, 14, 1706. [Google Scholar] [CrossRef] [PubMed]
- Beltzig, L.; Christmann, M.; Kaina, B. Abrogation of Cellular Senescence Induced by Temozolomide in Glioblastoma Cells: Search for Senolytics. Cells 2022, 11, 2588. [Google Scholar] [CrossRef]
- Estepa-Fernández, A.; García-Fernández, A.; Lérida-Viso, A.; Blandez, J.F.; Galiana, I.; Sancenon-Galarza, F.; Orzáez, M.; Martínez-Máñez, R. Combination of Palbociclib with Navitoclax Based-Therapies Enhances In Vivo Antitumoral Activity in Triple-Negative Breast Cancer. Pharmacol. Res. 2023, 187, 106628. [Google Scholar] [CrossRef]
- Gadsden, N.J.; Fulcher, C.D.; Li, D.; Shrivastava, N.; Thomas, C.; Segall, J.E.; Prystowsky, M.B.; Schlecht, N.F.; Gavathiotis, E.; Ow, T.J. Palbociclib Renders Human Papilloma Virus–Negative Head and Neck Squamous Cell Carcinoma Vulnerable to the Senolytic Agent Navitoclax. Mol. Cancer Res. 2021, 19, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, V.; Saleh, T.; Lee, S.M.; Murray, G.; Reed, J.; Souers, A.; Faber, A.C.; Harada, H.; Gewirtz, D.A. Androgen-Deprivation Induced Senescence in Prostate Cancer Cells Is Permissive for the Development of Castration-Resistance but Susceptible to Senolytic Therapy. Biochem. Pharmacol. 2021, 193, 114765. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Sananikone, E.; Kanji, S.; Tomimatsu, N.; Di Cristofaro, L.F.M.; Kollipara, R.K.; Saha, D.; Floyd, J.R.; Sung, P.; Hromas, R.; Burns, T.C.; et al. Elimination of Radiation-Induced Senescence in the Brain Tumor Microenvironment Attenuates Glioblastoma Recurrence. Cancer Res. 2021, 81, 5935–5947. [Google Scholar] [CrossRef]
- Anders, C.K.; Carey, L.A. Biology, Metastatic Patterns, and Treatment of Patients with Triple-Negative Breast Cancer. Clin. Breast Cancer 2009, 9, S73–S81. [Google Scholar] [CrossRef]
- Anders, C.K.; Winer, E.P.; Ford, J.M.; Dent, R.; Silver, D.P.; Sledge, G.W.; Carey, L.A. Poly(ADP-Ribose) Polymerase Inhibition: “Targeted” Therapy for Triple-Negative Breast Cancer. Clin. Cancer Res. 2010, 16, 4702–4710. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Hirte, H.W.; Robidoux, A.; Tonkin, K.S.; Tischkowitz, M.; Swenerton, K.; Huntsman, D.; Carmichael, J.; Macpherson, E.; Oza, A.M. Can We Define Tumors That Will Respond to PARP Inhibitors? A Phase II Correlative Study of Olaparib in Advanced Serous Ovarian Cancer and Triple-Negative Breast Cancer. J. Clin. Oncol. 2010, 28, 3002. [Google Scholar] [CrossRef]
- Ghorai, A.; Mahaddalkar, T.; Thorat, R.; Dutt, S. Sustained inhibition of PARP-1 Activity Delays Glioblastoma Recurrence by Enhancing Radiation-Induced Senescence. Cancer Lett. 2020, 490, 44–53. [Google Scholar] [CrossRef]
- Chalmers, A.J.; Lakshman, M.; Chan, N.; Bristow, R.G. Poly(ADP-Ribose) Polymerase Inhibition as a Model for Synthetic Lethality in Developing Radiation Oncology Targets. Semin. Radiat. Oncol. 2010, 20, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.; Sharma, K.; Saleh, T.; Povirk, L.F.; Hendrickson, E.A.; Gewirtz, D.A. Radiosensitization by PARP Inhibition in DNA Repair Proficient and Deficient Tumor Cells: Proliferative Recovery in Senescent Cells. Radiat. Res. 2016, 185, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Efimova, E.V.; Mauceri, H.J.; Golden, D.W.; Labay, E.; Bindokas, V.P.; Darga, T.E.; Chakraborty, C.; Barreto-Andrade, J.C.; Crawley, C.; Sutton, H.G.; et al. Poly(ADP-Ribose) Polymerase Inhibitor Induces Accelerated Senescence in Irradiated Breast Cancer Cells and Tumors. Cancer Res. 2010, 70, 6277–6282. [Google Scholar] [CrossRef] [PubMed]
- Fleury, H.; Malaquin, N.; Tu, V.; Gilbert, S.; Martinez, A.; Olivier, M.-A.; Sauriol, A.; Communal, L.; Leclerc-Desaulniers, K.; Carmona, E.; et al. Exploiting Interconnected Synthetic Lethal Interactions between parp Inhibition and Cancer Cell Reversible Senescence. Nat. Commun. 2019, 10, 2556. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A.; Alotaibi, M.; Yakovlev, V.A.; Povirk, L.F. Tumor Cell Recovery from Senescence Induced by Radiation with PARP Inhibition. Radiat. Res. 2016, 186, 327–332. [Google Scholar] [CrossRef]
- Marzullo, M.; El Maï, M.; Ferreira, M.G. Whole-mount Senescence-Associated Beta-Galactosidase (SA-β-GAL) Activity Detection Protocol for Adult Zebrafish. Bio-Protocol 2022, 12, e4457. [Google Scholar] [CrossRef] [PubMed]
- van Genderen, H.; Kenis, H.; Lux, P.; Ungeth, L.; Maassen, C.; Deckers, N.; Narula, J.; Hofstra, L.; Reutelingsperger, C. In Vitro Measurement of Cell Death with the Annexin A5 Affinity Assay. Nat. Protoc. 2006, 1, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Joyce, C. Quantitative RT-PCR: A Review of Current Methodologies. In RT-PCR Protocols; Humana Press: Totowa, NJ, USA, 2002. [Google Scholar]
- Loo, D.T. TUNEL Assay: An Overview of Techniques. In In Situ Detection of DNA Damage; Humana Press: Totowa, NJ, USA, 2002. [Google Scholar]
- Moslehi, A.; Changizi, Z.; Rohani, A.; Eidi, A. Chlorogenic Acid Inhibits Growth of 4T1 Breast Cancer Cells through Involvement in Bax/Bcl2 Pathway. J. Cancer Res. Ther. 2020, 16, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Huart, C.; Fransolet, M.; Demazy, C.; Le Calvé, B.; Lucas, S.; Michiels, C.; Wéra, A.-C. Taking Advantage of the Senescence-Promoting Effect of Olaparib after X-ray and Proton Irradiation Using the Senolytic Drug, ABT-263. Cancers 2022, 14, 1460. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim, S.I.; Park, B.-W. Characteristics and Outcomes According to Molecular Subtypes of Breast Cancer as Classified by a Panel of Four Biomarkers Using Immunohistochemistry. Breast 2011, 21, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Berglind, H.; Pawitan, Y.; Kato, S.; Ishioka, C.; Soussi, T. Analysis of p53 mutation Status in Human Cancer Cell Lines: A Paradigm for Cell Line Cross-Contamination. Cancer Biol. Ther. 2008, 7, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Schrörs, B.; Boegel, S.; Albrecht, C.; Bukur, T.; Bukur, V.; Holtsträter, C.; Ritzel, C.; Manninen, K.; Tadmor, A.D.; Vormehr, M.; et al. Multi-Omics Characterization of the 4T1 Murine Mammary Gland Tumor Model. Front. Oncol. 2020, 10, 1195. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Liu, S.; Wang, X.; Wu, X.; Hao, J. Tanshinone IIA Promotes Apoptosis by Downregulating BCL2 and Upregulating TP53 in Triple-Negative Breast Cancer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Daneshforouz, A.; Nazemi, S.; Gholami, O.; Kafami, M.; Amin, B. The Cytotoxicity and Apoptotic Effects of Verbascoside on Breast Cancer 4T1 Cell Line. BMC Pharmacol. Toxicol. 2021, 22, 72. [Google Scholar] [CrossRef]
- Acklin, S.; Zhang, M.; Du, W.; Zhao, X.; Plotkin, M.; Chang, J.; Campisi, J.; Zhou, D.; Xia, F. Depletion of Senescent-Like Neuronal Cells Alleviates Cisplatin-Induced Peripheral Neuropathy in Mice. Sci. Rep. 2020, 10, 14170. [Google Scholar] [CrossRef]
- Lérida-Viso, A.; Fernández, A.E.; Morellá-Aucejo, Á.; Lozano-Torres, B.; Alfonso, M.; Blandez, J.F.; Bisbal, V.; Sepúlveda, P.; García-Fernández, A.; Orzáez, M.; et al. Pharmacological Senolysis Reduces Doxorubicin-Induced Cardiotoxicity and Improves Cardiac Function in Mice. Pharmacol. Res. 2022, 183, 106356. [Google Scholar] [CrossRef]
- Duy, C.; Li, M.; Teater, M.; Meydan, C.; Garrett-Bakelman, F.E.; Lee, T.C.; Chin, C.R.; Durmaz, C.; Kawabata, K.C.; Dhimolea, E.; et al. Chemotherapy Induces Senescence-Like Resilient Cells Capable of Initiating AML Recurrence. Cancer Discov. 2021, 11, 1542–1561. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-Associated Reprogramming Promotes Cancer Stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Estepa-Fernández, A.; García-Fernández, A.; Lérida-Viso, A.; Morellá-Aucejo, Á.; Esteve-Moreno, J.J.; Blandez, J.F.; Alfonso, M.; Candela-Noguera, V.; Vivo-Llorca, G.; Sancenon-Galarza, F.; et al. Engineering Nanoparticle Communication in Living Systems by Stigmergy: An Application to Enhance Antitumor Therapy in Triple-Negative Breast Cancer. Nano Today 2023, 48, 101692. [Google Scholar] [CrossRef]


| Groups | Viable Cells Count | Destruction % | Evan’s Grade |
|---|---|---|---|
| Control | 3997 ± 214 | 2 | I |
| Radiation + talazoparib | 2418 ± 205 *a | 40 | IIa |
| Navitoclax | 2624 ± 191 *a,*b | 35 | IIa |
| Radiation + talazoparib + navitoclax | 1664 ± 66 *a,*b | 59 | IIb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Softah, A.; Alotaibi, M.R.; Alhoshani, A.R.; Saleh, T.; Alhazzani, K.; Almutairi, M.M.; AlRowis, R.; Alshehri, S.; Albekairy, N.A.; Harada, H.; et al. The Combination of Radiation with PARP Inhibition Enhances Senescence and Sensitivity to the Senolytic, Navitoclax, in Triple Negative Breast Tumor Cells. Biomedicines 2023, 11, 3066. https://doi.org/10.3390/biomedicines11113066
Softah A, Alotaibi MR, Alhoshani AR, Saleh T, Alhazzani K, Almutairi MM, AlRowis R, Alshehri S, Albekairy NA, Harada H, et al. The Combination of Radiation with PARP Inhibition Enhances Senescence and Sensitivity to the Senolytic, Navitoclax, in Triple Negative Breast Tumor Cells. Biomedicines. 2023; 11(11):3066. https://doi.org/10.3390/biomedicines11113066
Chicago/Turabian StyleSoftah, Abrar, Moureq R. Alotaibi, Ali R. Alhoshani, Tareq Saleh, Khalid Alhazzani, Mashal M. Almutairi, Raed AlRowis, Samiyah Alshehri, Norah A. Albekairy, Hisashi Harada, and et al. 2023. "The Combination of Radiation with PARP Inhibition Enhances Senescence and Sensitivity to the Senolytic, Navitoclax, in Triple Negative Breast Tumor Cells" Biomedicines 11, no. 11: 3066. https://doi.org/10.3390/biomedicines11113066
APA StyleSoftah, A., Alotaibi, M. R., Alhoshani, A. R., Saleh, T., Alhazzani, K., Almutairi, M. M., AlRowis, R., Alshehri, S., Albekairy, N. A., Harada, H., Boyd, R., Chakraborty, E., Gewirtz, D. A., & As Sobeai, H. M. (2023). The Combination of Radiation with PARP Inhibition Enhances Senescence and Sensitivity to the Senolytic, Navitoclax, in Triple Negative Breast Tumor Cells. Biomedicines, 11(11), 3066. https://doi.org/10.3390/biomedicines11113066








