From Animal Models to Clinical Trials: The Potential of Antimicrobials in Multiple Sclerosis Treatment
Abstract
:1. Introduction
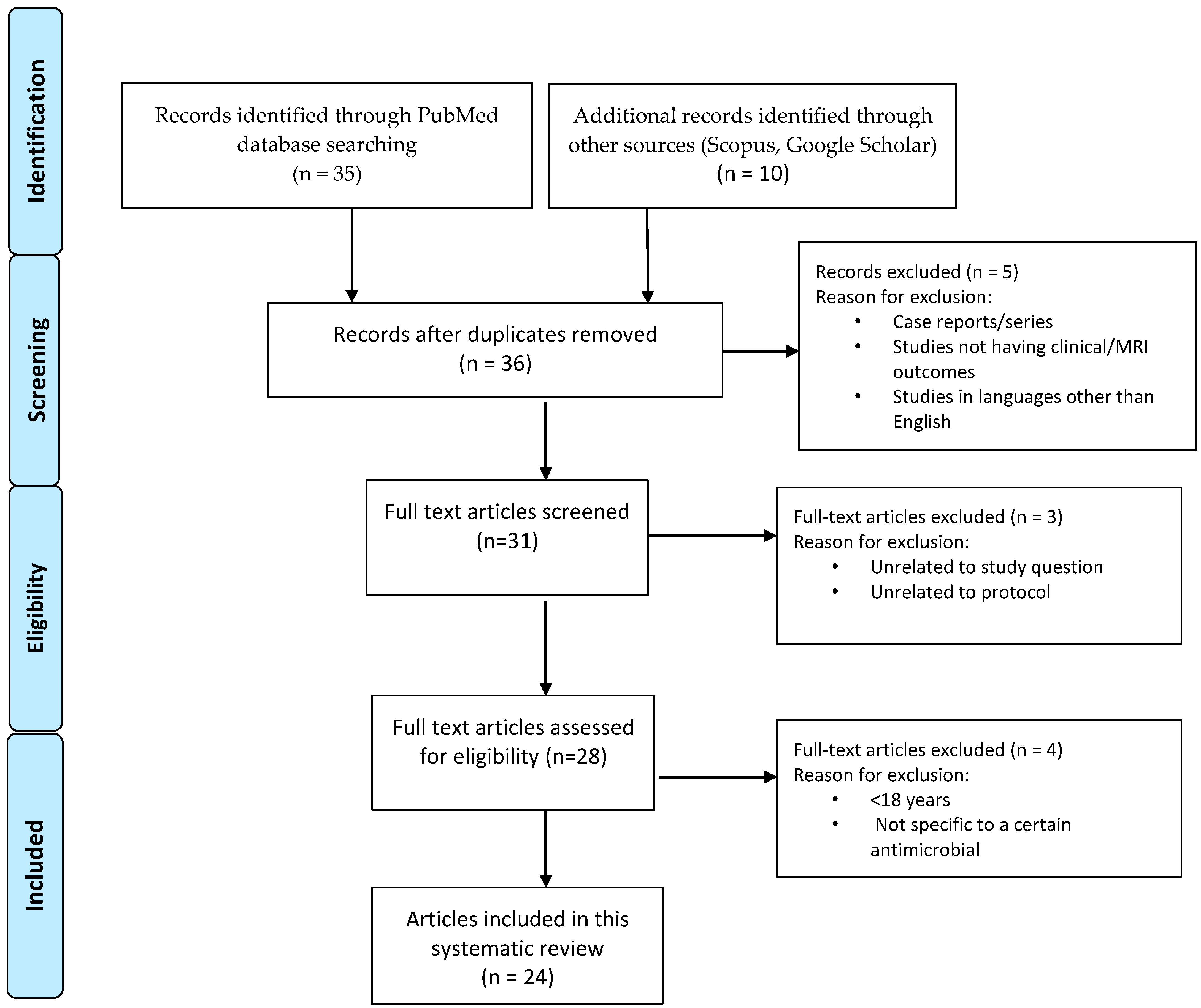
2. Methods
3. Results
3.1. Beta-Lactam Antibiotics
3.2. Tetracyclines
3.2.1. Minocycline (Minocin, Dynacin, Ximino, and Solodyn)
3.2.2. Doxycycline (Vibramycin D and Periostat)
3.3. Rapamycin (Sirolimus)
3.4. Antivirals
3.5. Hydroxychloroquine (HCQ)
3.6. Other Antimicrobial Agents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera, V.M. Multiple Sclerosis: A Global Concern with Multiple Challenges in an Era of Advanced Therapeutic Complex Molecules and Biological Medicines. Biomedicines 2018, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J. New insights into the burden and costs of multiple sclerosis in Europe. Mult. Scler. J. 2017, 23, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Bross, M.; Hackett, M.; Bernitsas, E. Approved and Emerging Disease Modifying Therapies on Neurodegeneration in Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 4312. [Google Scholar] [CrossRef] [PubMed]
- Quirant-Sánchez, B.; Mansilla, M.J.; Navarro-Barriuso, J.; Presas-Rodríguez, S.; Teniente-Serra, A.; Fondelli, F.; Ramo-Tello, C.; Martínez-Cáceres, E. Combined Therapy of Vitamin D3-Tolerogenic Dendritic Cells and Interferon-β in a Preclinical Model of Multiple Sclerosis. Biomedicines 2021, 9, 1758. [Google Scholar] [CrossRef] [PubMed]
- Bernitsas, E.; Khan, O.; Razmjou, S.; Tselis, A.; Bao, F.; Caon, C.; Millis, S.; Seraji-Bozorgzad, N. Cerebrospinal fluid humoral immunity in the differential diagnosis of multiple sclerosis. PLoS ONE 2017, 12, e0181431. [Google Scholar] [CrossRef]
- Eva, L.; Pleș, H.; Covache-Busuioc, R.-A.; Glavan, L.A.; Bratu, B.-G.; Bordeianu, A.; Dumitrascu, D.-I.; Corlatescu, A.D.; Ciurea, A.V. A Comprehensive Review on Neuroimmunology: Insights from Multiple Sclerosis to Future Therapeutic Developments. Biomedicines 2023, 11, 2489. [Google Scholar] [CrossRef] [PubMed]
- Tarlinton, R.E.; Martynova, E.; Rizvanov, A.A.; Khaiboullina, S.; Verma, S. Role of viruses in the pathogenesis of multiple sclerosis. Viruses 2020, 12, 643. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann. Neurol. 2007, 61, 288–299. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Tracy, S.I.; Kakalacheva, K.; Lünemann, J.D.; Luzuriaga, K.; Middeldorp, J.; Thorley-Lawson, D.A. Persistence of Epstein-Barr virus in self-reactive memory B cells. J. Virol. 2012, 86, 12330–12340. [Google Scholar] [CrossRef]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Multiple sclerosis: Autoimmunity and viruses. Curr. Opin. Rheumatol. 2013, 25, 496. [Google Scholar] [CrossRef]
- Melzer, N.; Meuth, S.G.; Torres-Salazar, D.; Bittner, S.; Zozulya, A.L.; Weidenfeller, C.; Kotsiari, A.; Stangel, M.; Fahlke, C.; Wiendl, H. A β-lactam antibiotic dampens excitotoxic inflammatory CNS damage in a mouse model of multiple sclerosis. PLoS ONE 2008, 3, e3149. [Google Scholar] [CrossRef]
- Zabad, R.K.; Metz, L.M.; Todoruk, T.R.; Zhang, Y.; Mitchell, J.R.; Yeung, M.; Patry, D.G.; Bell, R.B.; Yong, V.W. The clinical response to minocycline in multiple sclerosis is accompanied by beneficial immune changes: A pilot study. Mult. Scler. 2007, 13, 517–526. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Scaldaferri, F.; Petito, V.; Sterbini, F.P.; Pecere, S.; Lopetuso, L.R.; Palladini, A.; Gerardi, V.; Masucci, L.; Pompili, M.; et al. The role of antibiotics in gut microbiota modulation: The eubiotic effects of rifaximin. Dig. Dis. 2016, 34, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Mowry, E.M. Gut microbiome and multiple sclerosis. Curr. Neurol. Neurosci. Rep. 2014, 14, 492. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Zocco, M.A.; D’Aversa, F.; Pompili, M.; Gasbarrini, A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J. Gastroenterol. 2017, 23, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Shi, M.; Lang, Y.; Shen, D.; Jin, T.; Zhu, J.; Cui, L. Gut Microbiota in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis: Current Applications and Future Perspectives. Mediat. Inflamm. 2018, 2018, 8168717. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Ditrio, L.E.; Burroughs, A.R.; Foureau, D.M.; Haque-Begum, S.; Kasper, L.H. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 6041–6050. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef]
- Charabati, M.; Wheeler, M.A.; Weiner, H.L.; Quintana, F.J. Multiple sclerosis: Neuroimmune crosstalk and therapeutic targeting. Cell 2023, 186, 1309–1327. [Google Scholar] [CrossRef] [PubMed]
- de Sèze, J.; Maillart, E.; Gueguen, A.; Laplaud, D.A.; Michel, L.; Thouvenot, E.; Zephir, H.; Zimmer, L.; Biotti, D.; Liblau, R. Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic. Front. Immunol. 2023, 14, 1004795. [Google Scholar] [CrossRef] [PubMed]
- Bernitsas, E.; Kopinsky, H.; Lichtman-Mikol, S.; Razmjou, S.; Santiago-Martinez, C.; Yarraguntla, K.; Bao, F. Multimodal MRI Response to Fingolimod in Multiple Sclerosis: A Nonrandomized, Single Arm, Observational Study. J. Neuroimaging 2021, 31, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Faissner, S.; Gold, R. Efficacy and Safety of Multiple Sclerosis Drugs Approved Since 2018 and Future Developments. CNS Drugs 2022, 36, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. 2009. Available online: http://www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed on 4 March 2009).
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Patel, S.; Regan, M.R.; Haenggeli, C.; Huang, Y.H.; Bergles, D.E.; Jin, L.; Dykes Hoberg, M.; Vidensky, S.; Chung, D.S.; et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005, 433, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Macrez, R.; Stys, P.K.; Vivien, D.; Lipton, S.A.; Docagne, F. Mechanisms of glutamate toxicity in multiple sclerosis: Biomarker and therapeutic opportunities. Lancet Neurol. 2016, 15, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Mor, F.; Cohen, I.R. Beta-lactam antibiotics modulate T-cell functions and gene expression via covalent binding to cellular albumin. Proc. Nat. Acad. Sci. USA 2013, 110, 2981–2986. [Google Scholar] [CrossRef]
- Reed, M.D.; Rekate, H.L.; Aronoff, S.C.; Myers, C.M.; Blumer, J.L. Single-dose plasma and cerebrospinal fluid pharmacokinetics of ceftriaxone in infants and children. Clin. Pharm. 1983, 2, 558–563. [Google Scholar] [PubMed]
- Huang, C.K.; Chang, Y.T.; Amstislavskaya, T.G.; Tikhonova, M.A.; Lin, C.L.; Hung, C.S.; Lai, T.J.; Ho, Y.J. Synergistic effects of ceftriaxone and erythropoietin on neuronal and behavioral deficits in an MPTP-induced animal model of Parkinson’s disease dementia. Behav. Brain Res. 2015, 294, 198–207. [Google Scholar] [CrossRef]
- Zumkehr, J.; Rodriguez-Ortiz, C.J.; Cheng, D.; Kieu, Z.; Wai, T.; Hawkins, C.; Kilian, J.; Lim, S.L.; Medeiros, R.; Kitazawa, M. Ceftriaxone ameliorates tau pathology and cognitive decline via restoration of glial glutamate transporter in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2015, 36, 2260–2271. [Google Scholar] [CrossRef]
- Yimer, E.M.; Hishe, H.Z.; Tuem, K.B. Repurposing of the β-lactam antibiotic, ceftriaxone for neurological disorders: A review. Front. Neurosci. 2019, 13, 236. [Google Scholar] [CrossRef]
- Alonso, A.; Jick, S.S.; Jick, H.; Hernán, M.A. Antibiotic use and risk of multiple sclerosis. Am. J. Epidemiol. 2006, 163, 997–1002. [Google Scholar] [CrossRef]
- Nørgaard, M.; Nielsen, R.B.; Jacobsen, J.B.; Gradus, J.L.; Stenager, E.; Koch-Henriksen, N.; Lash, T.L.; Sørensen, H.T. Use of penicillin and other antibiotics and risk of multiple sclerosis: A population-based case-control study. Am. J. Epidemiol. 2011, 174, 945–948. [Google Scholar] [CrossRef]
- Ren, J.; Ni, H.; Kim, M.; Cooley, K.L.; Valenzuela, R.M.; Asche, C.V. Allergies, antibiotics use, and multiple sclerosis. Curr. Med. Res. Opin. 2017, 33, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, J.O.; Viitala, M.; Hänninen, A.; Soilu-Hänninen, M. Exposure to systemic antibiotics in outpatient care and the risk of multiple sclerosis. Mult. Scler. 2023, 29, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Plane, J.M.; Shen, Y.; Pleasure, D.E.; Deng, W. Prospects for minocycline neuroprotection. Arch. Neurol. 2010, 67, 1442–1448. [Google Scholar] [CrossRef]
- Panizzutti, B.; Skvarc, D.; Lin, S.; Croce, S.; Meehan, A.; Bortolasci, C.C.; Marx, W.; Walker, A.J.; Hasebe, K.; Kavanagh, B.E.; et al. Minocycline as Treatment for Psychiatric and Neurological Conditions: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 5250. [Google Scholar] [CrossRef]
- Colovic, M.; Caccia, S. Liquid chromatographic determination of minocycline in brain-to-plasma distribution studies in the rat. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 791, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.; Attur, M.G.; Thakker, G.D.; Patel, P.D.; Vyas, P.R.; Patel, R.N.; Patel, I.R.; Abramson, S.B. A novel mechanism of action of tetracyclines: Effects on nitric oxide synthases. Proc. Nati Acad. Sci. USA 1996, 93, 14014–14019. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, E.M.; Sanz-Blasco, S.; Karachitos, A.; Bandez, M.J.; Fernandez-Gomez, F.J.; Perez-Alvarez, S.; De Mera, R.M.; Jordan, M.J.; Aguirre, N.; Galindo, M.F.; et al. Mitochondria and calcium flux as targets of neuroprotection caused by minocycline in cerebellar granule cells. Biochem. Pharmacol. 2010, 79, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Okouchi, M.; Ekshyyan, O.; Maracine, M.; Aw, T.Y. Neuronal apoptosis in neurodegeneration. Antioxid. Redox Signal 2007, 9, 1059–1096. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Jiang, Y.; Pi, R.; Liu, Y.; Ma, L. The prospects of minocycline in multiple sclerosis. J. Neuroimmunol. 2011, 235, 1–8. [Google Scholar] [CrossRef]
- Rahmani, M.; Alvarez, S.E.; Hernández, E.B. The potential use of tetracyclines in neurodegenerative diseases and the role of nano-based drug delivery systems. Eur. J. Pharm. Sci. 2022, 175, 106237. [Google Scholar] [CrossRef]
- Florou, D.T.; Mavropoulos, A.; Dardiotis, E.; Tsimourtou, V.; Siokas, V.; Aloizou, A.M.; Liaskos, C.; Tsigalou, C.; Katsiari, C.; Sakkas, L.I.; et al. Tetracyclines Diminish In Vitro IFN-γ and IL-17-Producing Adaptive and Innate Immune Cells in Multiple Sclerosis. Front. Immunol. 2021, 12, 739186. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Wells, J.; Giuliani, F.; Casha, S.; Power, C.; Metz, L.M. The promise of minocycline in neurology. Lancet Neurol. 2004, 3, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, N.; Yasuhara, T.; Hara, K.; Xu, L.; Maki, M.; Yu, G.; Kaneko, Y.; Ojika, K.; Hess, D.C.; Borlongan, C.V. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009, 10, 126. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Rokitskaya, T.I.; Cooper, A.J.; Krasnikov, B.F. Minocycline chelates Ca2+, binds to membranes, and depolarizes mitochondria by formation of Ca2+-dependent ion channels. Bioenergy Biomembr. 2010, 42, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Michel-Monigadon, D.; Nerrière-Daguin, V.; Lévèque, X.; Plat, M.; Venturi, E.; Brachet, P.; Naveilhan, P.; Neveu, I. Minocycline promotes long-term survival of neuronal transplant in the brain by inhibiting late microglial activation and T-cell recruitment. Transplantation 2010, 89, 816–823. [Google Scholar] [CrossRef]
- Tauber, S.C.; Nau, R. Immunomodulatory properties of antibiotics. Curr. Mol. Pharmacol. 2008, 1, 68–79. [Google Scholar]
- Hahn, J.N.; Kaushik, D.K.; Mishra, M.K.; Wang, J.; Silva, C.; Yong, V.W. Impact of Minocycline on Extracellular Matrix Metalloproteinase Inducer, a Factor Implicated in Multiple Sclerosis Immunopathogenesis. J. Immunol. 2016, 197, 3850–3860. [Google Scholar] [CrossRef]
- Caggiula, M.; Batocchi, A.P.; Frisullo, G.; Angelucci, F.; Patanella, A.K.; Sancricca, C.; Nociti, V.; Tonali, P.A.; Mirabella, M. Neurotrophic factors and clinical recovery in relapsing-remitting multiple sclerosis. Scand. J. Immunol. 2005, 62, 176–182. [Google Scholar] [CrossRef]
- Chen, X.; Ma, L.; Jiang, Y.; Chen, S.; Zhu, C.; Liu, M.; Ma, X.; Zhu, D.; Liu, Y.; Peng, F.; et al. Minocycline up-regulates the expression of brain-derived neurotrophic factor and nerve growth factor in experimental autoimmune encephalomyelitis. Eur. J. Pharmacol. 2012, 686, 124–129. [Google Scholar] [CrossRef]
- Zemke, D.; Majid, A. The potential of minocycline for neuroprotection in human neurologic disease. Clin. Neuropharmacol. 2004, 27, 293–298. [Google Scholar] [CrossRef]
- Popovic, N.; Schubart, A.; Goetz, B.D.; Zhang, S.C.; Linington, C.; Duncan, I.D. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann. Neurol. 2002, 51, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Nikodemova, M.; Lee, J.; Fabry, Z.; Duncan, I.D. Minocycline attenuates experimental autoimmune encephalomyelitis in rats by reducing T cell infiltration into the spinal cord. J. Neuroimmunol. 2010, 219, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Brundula, V.; Rewcastle, N.B.; Metz, L.M.; Bernard, C.C.; Yong, V.W. Targeting leukocyte MMPs and transmigration: Minocycline as a potential therapy for multiple sclerosis. Brain 2002, 125, 1297–1308. [Google Scholar] [CrossRef]
- Planche, V.; Panatier, A.; Hiba, B.; Ducourneau, E.G.; Raffard, G.; Dubourdieu, N.; Maitre, M.; Lesté-Lasserre, T.; Brochet, B.; Dousset, V.; et al. Selective dentate gyrus disruption causes memory impairment at the early stage of experimental multiple sclerosis. Brain Behav. Immun. 2017, 60, 240–254. [Google Scholar] [CrossRef]
- Hainmueller, T.; Bartos, M. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci. 2020, 21, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Faissner, S.; Mahjoub, Y.; Mishra, M.; Haupeltshofer, S.; Hahn, J.N.; Gold, R.; Koch, M.; Metz, L.M.; Ben-Hur, T.; Yong, V.W. Unexpected additive effects of minocycline and hydroxychloroquine in models of multiple sclerosis: Prospective combination treatment for progressive disease? Mult. Scler. 2018, 24, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Stoop, M.P.; Rosenling, T.; Attali, A.; Meesters, R.J.; Stingl, C.; Dekker, L.J.; van Aken, H.; Suidgeest, E.; Hintzen, R.Q.; Tuinstra, T.; et al. Minocycline effects on the cerebrospinal fluid proteome of experimental autoimmune encephalomyelitis rats. J. Proteome Res. 2012, 11, 4315–4325. [Google Scholar] [CrossRef]
- Maier, K.; Merkler, D.; Gerber, J.; Taheri, N.; Kuhnert, A.V.; Williams, S.K.; Neusch, C.; Bähr, M.; Diem, R. Multiple neuroprotective mechanisms of minocycline in autoimmune CNS inflammation. Neurobiol. Dis. 2007, 25, 514–525. [Google Scholar] [CrossRef]
- Metz, L.M.; Li, D.; Traboulsee, A.; Myles, M.L.; Duquette, P.; Godin, J.; Constantin, M.; Yong, V.W. Glatiramer acetate in combination with minocycline in patients with relapsing-remitting multiple sclerosis: Results of a Canadian, multicenter, double-blind, placebo-controlled trial. Mult. Scler. 2009, 15, 1183–1194. [Google Scholar] [CrossRef]
- Sørensen, P.S.; Sellebjerg, F.; Lycke, J.; Färkkilä, M.; Créange, A.; Lund, C.G.; Schluep, M.; Frederiksen, J.L.; Stenager, E.; Pfleger, C.; et al. Minocycline added to subcutaneous interferon β-1a in multiple sclerosis: Randomized RECYCLINE study. Eur. J. Neurol. 2016, 23, 861–870. [Google Scholar] [CrossRef]
- Metz, L.M.; Zhang, Y.; Yeung, M.; Patry, D.G.; Bell, R.B.; Stoian, C.A.; Yong, V.W.; Patten, S.B.; Duquette, P.; Antel, J.P.; et al. Minocycline reduces gadolinium enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann. Neurol. 2004, 55, 756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Metz, L.M.; Yong, V.W.; Bell, R.B.; Yeung, M.; Patry, D.G.; Mitchell, J.R. Pilot study of minocycline in relapsing-remitting multiple sclerosis. Can. J. Neurol. Sci. 2008, 35, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Metz, L.M.; Li, D.K.; Traboulsee, A.L.; Duquette, P.; Eliasziw, M.; Cerchiaro, G.; Greenfield, J.; Riddehough, A.; Yeung, M.; Kremenchutzky, M.; et al. Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N. Engl. J. Med. 2017, 376, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.; Metz, L.; Kuhle, J.; Leppert, D.; Willemse, E.; Li, D.K.; Traboulsee, A.; Greenfield, J.; Cerchiaro, G.; Silva, C.; et al. Minocycline treatment in clinically isolated syndrome and serum NfL, GFAP, and metalloproteinase levels. Mult. Scler. 2022, 28, 2081–2089. [Google Scholar] [CrossRef]
- Víctor-Carvalho, P.; Thome, R.; Rapôso, C. Can tetracyclines ensure help in multiple sclerosis immunotherapy? J. Clin. Transl. Res. 2021, 7, 22–33. [Google Scholar]
- Minagar, A.; Alexander, J.S.; Schwendimann, R.N.; Kelley, R.E.; Gonzalez-Toledo, E.; Jimenez, J.J.; Mauro, L.; Jy, W.; Smith, S.J. Combination therapy with interferon beta-1a and doxycycline in multiple sclerosis: An open-label trial. Arch. Neurol. 2008, 65, 199–204. [Google Scholar] [CrossRef]
- Mazdeh, M.; Mobaien, A.R. Efficacy of doxycycline as add-on to interferon beta-1a in treatment of multiple sclerosis. Iran. J. Neurol. 2012, 11, 70. [Google Scholar]
- Yi, C.; Zhang, Z.; Wang, W.; Zug, C.; Schluesener, H.J.; Zhang, Z. Doxycycline attenuates peripheral inflammation in rat experimental autoimmune neuritis. Neurochem. Res. 2011, 36, 1984–1990. [Google Scholar] [CrossRef]
- Harrison, J.J.; Schiff, J.R.; Coursol, C.J.; Daley, C.J.; Dipchand, A.I.; Heywood, N.M.; Keough-Ryan, T.M.; Keown, P.A.; Levy, G.A.; Lien, D.C.; et al. Generic immunosuppression in solid organ transplantation: A Canadian perspective. Transplantation 2012, 93, 657–665. [Google Scholar] [CrossRef]
- Juedes, A.E.; Hjelmstrom, P.; Bergman, C.M.; Neild, A.L.; Ruddle, N.H. Kinetics and cellular origin of cytokines in the central nervous system: Insight into mechanisms of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J. Immunol. 2000, 164, 419–426. [Google Scholar] [CrossRef]
- Bové, J.; Martínez-Vicente, M.; Vila, M. Fighting neurodegeneration with rapamycin: Mechanistic insights. Nat. Rev. Neurosci. 2011, 12, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Vakrakou, A.G.; Alexaki, A.; Brinia, M.E.; Anagnostouli, M.; Stefanis, L.; Stathopoulos, P. The mTOR Signaling Pathway in Multiple Sclerosis; from Animal Models to Human Data. Int. J. Mol. Sci. 2022, 23, 8077. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Miao, J.; Cao, R.; Han, M.; Sun, Y.; Liu, X.; Guo, L. Rapamycin Ameliorates Experimental Autoimmune Encephalomyelitis by Suppressing the mTOR-STAT3 Pathway. Neurochem. Res. 2017, 42, 2831–2840. [Google Scholar] [CrossRef]
- Esposito, M.; Ruffini, F.; Bellone, M.; Gagliani, N.; Battaglia, M.; Martino, G.; Furlan, R. Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. J. Neuroimmunol. 2010, 220, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Navarra, P.; Cirocchi, R.; Sharp, A.; Stigliano, E.; Feinstein, D.L.; Russo, C.D. Rapamycin reduces clinical signs and neuropathic pain in a chronic model of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2012, 243, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Togha, M.; Jahanshahi, M.; Alizadeh, L.; Jahromi, S.R.; Vakilzadeh, G.; Alipour, B.; Gorji, A.; Ghaemi, A. Rapamycin Augments Immunomodulatory Properties of Bone Marrow-Derived Mesenchymal Stem Cells in Experimental Autoimmune Encephalomyelitis. Mol. Neurobiol. 2017, 54, 2445–2457. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, C.; He, D.; Jiang, N.; Bai, Y.; Xin, Y. Rapamycin and MCC950 modified gut microbiota in experimental autoimmune encephalomyelitis mouse by brain gut axis. Life Sci. 2020, 253, 117747. [Google Scholar] [CrossRef]
- Bagherpour, B.; Salehi, M.; Jafari, R.; Bagheri, A.; Kiani-Esfahani, A.; Edalati, M.; Kardi, M.T.; Shaygannejad, V. Promising effect of rapamycin on multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 26, 40–45. [Google Scholar] [CrossRef]
- Kappos, L.; Barkhof, F.; Desmet, A.; Tremblay, G.; Brault, Y. The effect of oral temsirolimus on new magnetic resonance imaging scan lesions, brain atrophy, and the number of relapses in multiple sclerosis: Results from a randomised, controlled clinical trial. J. Neurol. 2005, 252, S46. [Google Scholar]
- Moraal, B.; van den Elskamp, I.J.; Knol, D.L.; Uitdehaag, B.M.; Geurts, J.J.; Vrenken, H.; Pouwels, P.J.; van Schijndel, R.A.; Meier, D.S.; Guttmann, C.R.; et al. Long-interval T2-weighted subtraction magnetic resonance imaging: A powerful new outcome measure in multiple sclerosis trials. Ann. Neurol. 2010, 67, 667–675. [Google Scholar] [CrossRef]
- Lycke, J. Trials of antivirals in the treatment of multiple sclerosis. Acta Neurol. Scand. 2017, 136 (Suppl. S201), 45–48. [Google Scholar] [CrossRef]
- Di Stadio, A.; Romani, L.; Bernitsas, E. Could SARS-CoV2 affect MS progression? Mult. Scler. Relat. Disord. 2020, 46, 102540. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Meuth, S.G.; Stüve, O.; Kieseier, B.; Wiendl, H. Multiple sclerosis therapy: An update on recently finished trials. J. Neurol. 2007, 254, 1473–1490. [Google Scholar] [CrossRef] [PubMed]
- Bray, P.F.; Bloomer, L.C.; Salmon, V.C.; Bagley, M.H.; Larsen, P.D. Epstein-Barr virus infection and antibody synthesis in patients with multiple sclerosis. Arch. Neurol. 1983, 40, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.D.; Bloomer, L.C.; Bray, P.F. Epstein-Barr nuclear antigen and viral capsid antigen antibody titers in multiple sclerosis. Neurology 1985, 35, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Abrahamyan, S.; Eberspächer, B.; Hoshi, M.M.; Aly, L.; Luessi, F.; Groppa, S.; Klotz, L.; Meuth, S.G.; Schroeder, C.; Grüter, T.; et al. Complete Epstein-Barr virus seropositivity in a large cohort of patients with early multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L.; Lennette, E.T.; Spiegelman, D.; Hernán, M.A.; Olek, M.J.; Hankinson, S.E.; Hunter, D.J. Epstein-Barr virus antibodies and risk of multiple sclerosis: A prospective study. JAMA 2001, 286, 3083–3088. [Google Scholar] [CrossRef]
- Aloisi, F.; Cross, A.H. MINI-review of Epstein-Barr virus involvement in multiple sclerosis etiology and pathogenesis. J. Neuroimmunol. 2022, 371, 577935. [Google Scholar] [CrossRef]
- Jacobs, B.M.; Giovannoni, G.; Cuzick, J.; Dobson, R. Systematic review and meta-analysis of the association between Epstein–Barr virus, multiple sclerosis and other risk factors. Mult. Scler. 2020, 26, 1281–1297. [Google Scholar] [CrossRef]
- Pender, M.P. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 2003, 24, 584–588. [Google Scholar] [CrossRef]
- Van Noort, J.M.; Bajramovic, J.J.; Plomp, A.C.; van Stipdonk, M.J. Mistaken self, a novel model that links microbial infections with myelin-directed autoimmunity in multiple sclerosis. J. Neuroimmunol. 2000, 105, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Jog, N.R.; McClain, M.T.; Heinlen, L.D.; Gross, T.; Towner, R.; Guthridge, J.M.; Axtell, R.C.; Pardo, G.; Harley, J.B.; James, J.A. Epstein Barr virus nuclear antigen 1 (EBNA-1) peptides recognized by adult multiple sclerosis patient sera induce neurologic symptoms in a murine model. J. Autoimmun. 2020, 106, 102332. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Pham, H.P.; Gupta, R.; Lindsey, J.W. The cellular immune response against Epstein-Barr virus decreases during ocrelizumab treatment. Mult. Scler. Relat. Disord. 2021, 56, 103282. [Google Scholar] [CrossRef] [PubMed]
- Torkildsen, Ø.; Myhr, K.M.; Skogen, V.; Steffensen, L.H.; Bjørnevik, K. Tenofovir as a treatment option for multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 46, 102569. [Google Scholar] [CrossRef]
- Gold, J.; Goldacre, R.; Maruszak, H.; Giovannoni, G.; Yeates, D.; Goldacre, M. HIV and lower risk of multiple sclerosis: Beginning to unravel a mystery using a record-linked database study. J. Neurol. Neurosurg. Psychiatry 2015, 86, 9–12. [Google Scholar] [CrossRef]
- Gold, J.; Marta, M.; Meier, U.C.; Christensen, T.; Miller, D.; Altmann, D.; Holden, D.; Bianchi, L.; Adiutori, R.; MacManus, D.; et al. A phase II baseline versus treatment study to determine the efficacy of raltegravir (Isentress) in preventing progression of relapsing remitting multiple sclerosis as determined by gadolinium-enhanced MRI: The INSPIRE study. Mult. Scler. Relat. Disord. 2018, 24, 123–128. [Google Scholar] [CrossRef]
- Ding, Z.; Mathur, V.; Ho, P.P.; James, M.L.; Lucin, K.M.; Hoehne, A.; Alabsi, H.; Gambhir, S.S.; Steinman, L.; Luo, J.; et al. Antiviral drug ganciclovir is a potent inhibitor of microglial proliferation and neuroinflammation. J. Exp. Med. 2014, 211, 189–198. [Google Scholar] [CrossRef]
- Lycke, J.; Svennerholm, B.; Hjelmquist, E.; Frisén, L.; Badr, G.; Andersson, M.; Vahlne, A.; Andersen, O. Acyclovir treatment of relapsing-remitting multiple sclerosis: A randomized, placebo-controlled, double-blind study. J. Neurol. 1996, 243, 214–224. [Google Scholar] [CrossRef]
- Bech, E.; Lycke, J.; Gadeberg, P.; Hansen, H.J.; Malmeström, C.; Andersen, O.; Christensen, T.; Ekholm, S.; Haahr, S.; Höllsberg, P.; et al. A randomized, double-blind, placebo-controlled MRI study of anti–herpes virus therapy in MS. Neurology 2002, 58, 31–36. [Google Scholar] [CrossRef]
- Friedman, J.E.; Zabriskie, J.B.; Plank, C.; Ablashi, D.; Whitman, J.; Shahan, B.; Edgell, R.; Shieh, M.; Rapalino, O.; Zimmerman, R.; et al. A randomized clinical trial of valacyclovir in multiple sclerosis. Mult. Scler. 2005, 11, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.W.; Zabad, R.; Giuliani, F.; Hader, W., Jr.; Lewkonia, R.; Metz, L.; Wee Yong, V. Hydroxychloroquine reduces microglial activity and attenuates experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2015, 358, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Moezzi, D.; Dong, Y.; Koch, M.; Yong, V.W. Combination of Hydroxychloroquine and Indapamide Attenuates Neurodegeneration in Models Relevant to Multiple Sclerosis. Neurotherapeutics 2021, 18, 387–400. [Google Scholar] [CrossRef]
- Koch, M.W.; Kaur, S.; Sage, K.; Kim, J.; Levesque-Roy, M.; Cerchiaro, G.; Yong, V.W.; Cutter, G.R.; Metz, L.M. Hydroxychloroquine for Primary Progressive Multiple Sclerosis. Ann. Neurol. 2021, 90, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Layh-Schmitt, G.; Bendl, C.; Hildt, U.; Dong-Si, T.; Jüttler, E.; Schnitzler, P.; Grond-Ginsbach, C.; Grau, A.J. Evidence for infection with Chlamydia pneumoniae in a subgroup of patients with multiple sclerosis. Ann. Neurol. 2000, 47, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lu, Z.; Hu, L.; Zhang, Y.; Hu, X. Macrolide antibiotics aggravate experimental autoimmune encephalomyelitis and inhibit inducible nitric oxide synthase. Immunol. Investig. 2009, 38, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Sriram, S.; Yao, S.Y.; Stratton, C.; Moses, H.; Narayana, P.A.; Wolinsky, J.S. Pilot study to examine the effect of antibiotic therapy on MRI outcomes in RRMS. J. Neurol. Sci. 2005, 234, 87–91. [Google Scholar] [CrossRef]
- Woessner, R.; Grauer, M.T.; Frese, A.; Bethke, F.; Ginger, T.; Hans, A.; Treib, J. Long-term Antibiotic Treatment with Roxithromycin in Patients with Multiple Sclerosis. Infection 2006, 34, 342–344. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Bernitsas, E. Pathophysiology and Imaging Diagnosis of Demyelinating Disorders. Brain Sci. 2018, 8, 44. [Google Scholar] [CrossRef]
- de Oliveira, M.; Santinelli, F.B.; Lisboa-Filho, P.N.; Barbieri, F.A. The Blood Concentration of Metallic Nanoparticles Is Related to Cognitive Performance in People with Multiple Sclerosis: An Exploratory Analysis. Biomedicines 2023, 11, 1819. [Google Scholar] [CrossRef]
- Al-Shammri, S.; Chattopadhyay, A.; Hanah, M.G.; Doi, S.; Akanji, A. Association of Blood Levels of Vitamin D and Its Binding Protein with Clinical Phenotypes of Multiple Sclerosis. Biomedicines 2023, 11, 1808. [Google Scholar] [CrossRef] [PubMed]
- Dustin, E.; Suarez-Pozos, E.; Stotesberry, C.; Qiu, S.; Palavicini, J.P.; Han, X.; Dupree, J.L. Compromised Myelin and Axonal Molecular Organization Following Adult-Onset Sulfatide Depletion. Biomedicines 2023, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Monitoring the Redox Status in Multiple Sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.B.; Javed, A.; Caon, C.; Srivastawa, S.; Bao, F.; Bernitsas, E.; Chorostecki, J.; Tselis, A.; Seraji-Bozorgzad, N.; Khan, O. Long-term safety of rituximab induced peripheral B-cell depletion in autoimmune neurological diseases. PLoS ONE 2018, 13, e0190425. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Tolaymat, S.; Yu, H.; Elkhooly, M.; Jaiswal, S.; Jena, A.; Kakara, M.; Sriwastava, S. Progressive multifocal leukoencephalopathy in anti-CD20 and other monoclonal antibody (mAb) therapies used in multiple sclerosis: A review. J. Neurol. Sci. 2022, 443, 120459. [Google Scholar] [CrossRef]
- Sriwastava, S.; Kataria, S.; Srivastava, S.; Kazemlou, S.; Gao, S.; Wen, S.; Saber, H.; Tripathi, R.; Sheikh, Z.; Peterson, S.; et al. Disease-modifying therapies and progressive multifocal leukoencephalopathy in multiple sclerosis: A systematic review and meta-analysis. J. Neuroimmunol. 2021, 360, 577721. [Google Scholar] [CrossRef]
- Rivera, V.M. Editorial of Special Issue “Multiple Sclerosis: Diagnosis and Treatment II”. Biomedicines 2021, 9, 1605. [Google Scholar] [CrossRef]
- Marchand, D.K.; Butcher, R. Minocycline for Relapsing-Remitting Multiple Sclerosis and Clinically Isolated Syndrome: A Review of Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, USA, 2019.
- Camara-Lemarroy, C.; Silva, C.; Gohill, J.; Yong, V.W.; Koch, M. Serum neurofilament-light and glial fibrillary acidic protein levels in hydroxychloroquine-treated primary progressive multiple sclerosis. Eur. J. Neurol. 2023, 30, 187–194. [Google Scholar] [CrossRef]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M.; et al. The Tryptophan-Kynurenine Metabolic System Is Suppressed in Cuprizone-Induced Model of Demyelination Simulating Progressive Multiple Sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef]
- Macaron, G.; Ontaneda, D. Diagnosis and Management of Progressive Multiple Sclerosis. Biomedicines 2019, 7, 56. [Google Scholar] [CrossRef]
- Touil, H.; Li, R.; Zuroff, L.; Moore, C.S.; Healy, L.; Cignarella, F.; Piccio, L.; Ludwin, S.; Prat, A.; Gommerman, J.; et al. Cross-talk between B cells, microglia and macrophages, and implications to central nervous system compartmentalized inflammation and progressive multiple sclerosis. EBioMedicine 2023, 96, 104789. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Marrodan, M.; Ysrraelit, M.C. Mechanisms of Neurodegeneration and Axonal Dysfunction in Progressive Multiple Sclerosis. Biomedicines 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. ORATORIO Clinical Investigators. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Macaron, G.; Larochelle, C.; Arbour, N.; Galmard, M.; Girard, J.M.; Prat, A.; Duquette, P. Impact of aging on treatment considerations for multiple sclerosis patients. Front. Neurol. 2023, 14, 1197212. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Akbar, A.; Caruso, C.; Solana, R.; Grubeck-Loebenstein, B.; Wikby, A. Human immunosenescence: Is it infectious? Immunol. Rev. 2005, 205, 257–268. [Google Scholar] [CrossRef]
- Manouchehri, N.; Salinas, V.H.; Rabi Yeganeh, N.; Pitt, D.; Hussain, R.Z.; Stuve, O. Efficacy of Disease Modifying Therapies in Progressive MS and How Immune Senescence May Explain Their Failure. Front. Neurol. 2022, 13, 854390. [Google Scholar] [CrossRef]
- Schweitzer, F.; Laurent, S.; Fink, G.R.; Barnett, M.H.; Reddel, S.; Hartung, H.P.; Warnke, C. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Curr. Opin. Neurol. 2019, 32, 305–312. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes 2017, 8, 607–615. [Google Scholar] [CrossRef]
- Brown, J.; Quattrochi, B.; Everett, C.; Hong, B.Y.; Cervantes, J. Gut commensals, dysbiosis, and immune response imbalance in the pathogenesis of multiple sclerosis. Mult. Scler. 2021, 27, 807–811. [Google Scholar] [CrossRef]
- Mielcarz, D.W.; Kasper, L.H. The gut microbiome in multiple sclerosis. Curr. Treat. Options Neurol. 2015, 17, 344. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, B.; Liebert, A.; Borody, T.; Herkes, G.; McLachlan, C.; Kiat, H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int. J. Mol. Sci. 2023, 24, 9577. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131, e143774. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Tovchiga, O.; Daglia, M.; Khan, H. Modulating Gut Microbiota: An Emerging Approach in the Prevention and Treatment of Multiple Sclerosis. Curr. Neuropharmacol. 2021, 19, 1966–1983. [Google Scholar] [CrossRef] [PubMed]
- Mestre, L.; Carrillo-Salinas, F.J.; Mecha, M.; Feliú, A.; Espejo, C.; Álvarez-Cermeño, J.C.; Villar, L.M.; Guaza, C. Manipulation of Gut Microbiota Influences Immune Responses, Axon Preservation, and Motor Disability in a Model of Progressive Multiple Sclerosis. Front. Immunol. 2019, 10, 1374. [Google Scholar] [CrossRef]
- Zahednasab, H.; Firouzi, M.; Kaboudanian-Ardestani, S.; Mojallal-Tabatabaei, Z.; Karampour, S.; Keyvani, H. The protective effect of rifampicin on behavioral deficits, biochemical, and neuropathological changes in a cuprizone model of demyelination. Cytokine 2019, 113, 417–426. [Google Scholar] [CrossRef]
- Macaron, G.; Moss, B.P.; Li, H.; Baldassari, L.E.; Rao, S.M.; Schindler, D.; Alberts, J.L.; Weber, M.; Ayers, M.; Bethoux, F.; et al. Technology-enabled assessments to enhance multiple sclerosis clinical care and research. Neurol. Clin. Pract. 2020, 10, 222–231. [Google Scholar] [CrossRef]
- Baldassari, L.E.; Nakamura, K.; Moss, B.P.; Macaron, G.; Li, H.; Weber, M.; Jones, S.E.; Rao, S.M.; Miller, D.; Conway, D.S.; et al. Technology-enabled comprehensive characterization of multiple sclerosis in clinical practice. Mult. Scler. Relat. Disord. 2020, 38, 101525. [Google Scholar] [CrossRef]
- Capasso, N.; Virgilio, E.; Covelli, A.; Giovannini, B.; Foschi, M.; Montini, F.; Nasello, M.; Nilo, A.; Prestipino, E.; Schirò, G.; et al. Aging in multiple sclerosis: From childhood to old age, etiopathogenesis, and unmet needs: A narrative review. Front. Neurol. 2023, 14, 1207617. [Google Scholar] [CrossRef]

| Drug | Possible Mode of Action in MS | Animal Studies | Human Studies |
|---|---|---|---|
| Beta-lactams |
|
|
|
| Minocycline |
|
|
|
| Rapamycin |
| Reduces clinical and histological signs of relapsing/ chronic EAE. | Reduces:
|
| Antivirals | Suppresses the viral response in MS pathogenesis | Ganciclovir reduces microglia proliferation and severity of EAE. |
|
| Doxycycline |
| Not tested |
|
| Hydroxychloroquine |
| Combined HCQ and minocycline (suboptimal doses): suppress symptomatology in chronic EAE | Improves T25FW in PPMS |
| Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias | |
|---|---|---|---|---|---|---|---|
| Zabad et al., 2007 [13] | x | x | x | x | + | ? | x |
| Alonso et al., 2006 [40] | ? | ? | x | x | x | ? | ? |
| Nørgaard et al., 2011 [41] | + | + | x | + | + | ? | ? |
| Sipilä et al., 2023 [43] | + | + | x | + | + | ? | ? |
| Metz et al., 2009 [70] | + | + | + | + | + | + | ? |
| Sørensen et al., 2016 [71] | + | ? | + | + | + | ? | ? |
| Metz et al., 2004 [72] | ? | ? | x | x | + | ? | ? |
| Zhang et al., 2008 [73] | x | x | x | x | + | ? | x |
| Metz et al., 2017 [74] | + | + | + | + | + | + | + |
| Camara-Lemarroy et al., 2022 [75] | + | ? | + | + | + | + | + |
| Minagar et al., 2008 [77] | x | x | x | + | + | x | |
| Mazdeh et al., 2012 [78] | ? | x | x | x | x | x | ? |
| Bagherpour et al., 2018 [89] | x | x | x | x | ? | ? | ? |
| Kappos et al., 2005 [90] | ? | ? | ? | ? | ? | ? | ? |
| Gold et al., 2018 [108] | ? | ? | x | x | + | + | ? |
| Lycke et al., 1996 [110] | + | ? | + | + | + | + | + |
| Bech et al., 2002 [111] | + | ? | + | + | + | + | + |
| Friedman et al., 2005 [112] | ? | ? | ? | ? | + | + | ? |
| Koch et al., 2021 [115] | ? | ? | x | x | + | ? | ? |
| Sriram et al., 2005 [118] | + | ? | + | + | + | + | + |
| Woessner et al., 2006 [119] | ? | ? | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raghib, M.F.; Bernitsas, E. From Animal Models to Clinical Trials: The Potential of Antimicrobials in Multiple Sclerosis Treatment. Biomedicines 2023, 11, 3069. https://doi.org/10.3390/biomedicines11113069
Raghib MF, Bernitsas E. From Animal Models to Clinical Trials: The Potential of Antimicrobials in Multiple Sclerosis Treatment. Biomedicines. 2023; 11(11):3069. https://doi.org/10.3390/biomedicines11113069
Chicago/Turabian StyleRaghib, Muhammad Faraz, and Evanthia Bernitsas. 2023. "From Animal Models to Clinical Trials: The Potential of Antimicrobials in Multiple Sclerosis Treatment" Biomedicines 11, no. 11: 3069. https://doi.org/10.3390/biomedicines11113069
APA StyleRaghib, M. F., & Bernitsas, E. (2023). From Animal Models to Clinical Trials: The Potential of Antimicrobials in Multiple Sclerosis Treatment. Biomedicines, 11(11), 3069. https://doi.org/10.3390/biomedicines11113069







