The Impact of Modern Anti-Diabetic Treatment on Endothelial Progenitor Cells
Abstract
1. Introduction
2. Endothelial Progenitor Cells in Health and Disease
3. Diabetes and Endothelial Progenitor Cells Biology
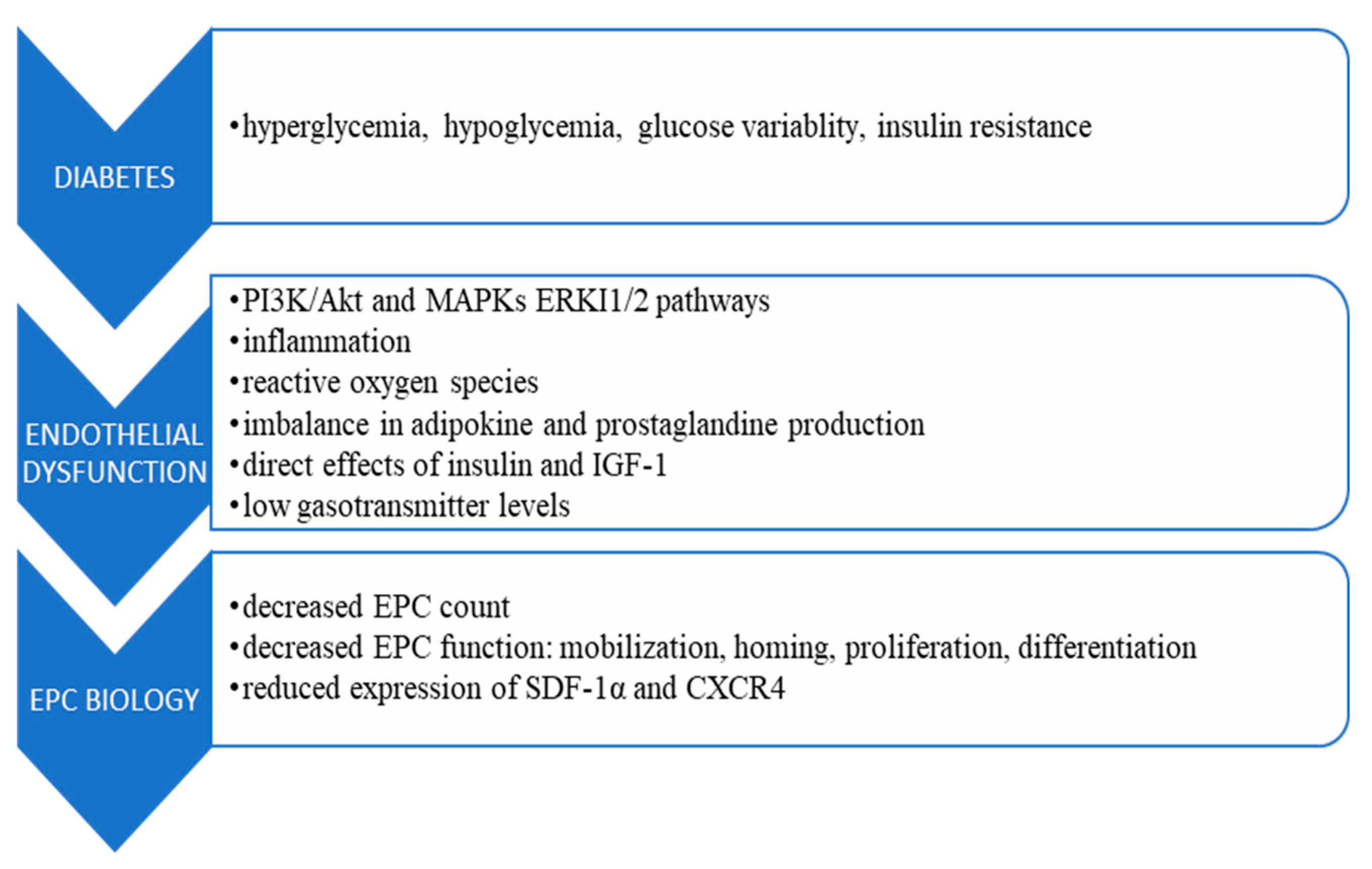
4. Currently Available Diabetes Treatments Affecting EPC Count and Function
4.1. Lifestyle Modification
4.2. Anti-Diabetic Agents
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, X.; Lochner, A.; Wang, H.-H.; Wang, S.; Zhu, H.; Ren, J.; Zhou, H. Coronary Microvascular Injury in Myocardial Infarction: Perception and Knowledge for Mitochondrial Quality Control. Theranostics 2021, 11, 6766. [Google Scholar] [CrossRef] [PubMed]
- Cavieres, V.; Valdes, K.; Moreno, B.; Moore-Carrasco, R.; Gonzalez, D.R. Vascular Hypercontractility and Endothelial Dysfunction before Development of Atherosclerosis in Moderate Dyslipidemia: Role for Nitric Oxide and Interleukin-6. Am. J. Cardiovasc. Dis. 2014, 4, 114. [Google Scholar] [PubMed]
- Hetherington, I.; Totary-Jain, H. Anti-Atherosclerotic Therapies: Milestones, Challenges, and Emerging Innovations. Mol. Ther. 2022, 30, 3106–3117. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Current Status of Primary, Secondary, and Tertiary Prevention of Coronary Artery Disease. Int. J. Angiol. 2021, 30, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Chen, M.S.; Lee, R.T.; Garbern, J.C. Senescence Mechanisms and Targets in the Heart. Cardiovasc. Res. 2022, 118, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Haybar, H.; Shahrabi, S.; Rezaeeyan, H.; Shirzad, R.; Saki, N. Endothelial Cells: From Dysfunction Mechanism to Pharmacological Effect in Cardiovascular Disease. Cardiovasc. Toxicol. 2019, 19, 13–22. [Google Scholar] [CrossRef]
- Van Craenenbroeck, E.M.; Van Craenenbroeck, A.H.; Van Ierssel, S.; Bruyndonckx, L.; Hoymans, V.Y.; Vrints, C.J.; Conraads, V.M. Quantification of Circulating CD34+/KDR+/CD45dim Endothelial Progenitor Cells: Analytical Considerations. Int. J. Cardiol. 2013, 167, 1688–1695. [Google Scholar] [CrossRef]
- Patel, R.S.; Li, Q.; Ghasemzadeh, N.; Eapen, D.J.; Moss, L.D.; Janjua, A.U.; Manocha, P.; Al Kassem, H.; Veledar, E.; Samady, H.; et al. Circulating CD34+ Progenitor Cells and Risk of Mortality in a Population with Coronary Artery Disease. Circ. Res. 2015, 116, 289–297. [Google Scholar] [CrossRef]
- Aquino, J.B.; Sierra, R.; Montaldo, L.A. Diverse Cellular Origins of Adult Blood Vascular Endothelial Cells. Dev. Biol. 2021, 477, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, J.; Zhang, W. Transplantation of Endothelial Progenitor Cells: Summary and Prospect. Acta Histochem. 2023, 125, 151990. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl. Med. 2017, 6, 1316–1320. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kawamoto, A. Stem Cell-Based Peripheral Vascular Regeneration. Adv. Drug Deliv. Rev. 2017, 120, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.-T.; Kuang, C.-Y. Endothelial Progenitor Cells and Coronary Artery Disease: Current Concepts and Future Research Directions. World J. Clin. Cases 2021, 9, 8953. [Google Scholar] [CrossRef]
- Rajasekar, P.; O’Neill, C.L.; Eeles, L.; Stitt, A.W.; Medina, R.J. Epigenetic Changes in Endothelial Progenitors as a Possible Cellular Basis for Glycemic Memory in Diabetic Vascular Complications. J. Diabetes Res. 2015, 2015, 436879. [Google Scholar] [CrossRef]
- Cassano, V.; Tripepi, G.; Perticone, M.; Miceli, S.; Scopacasa, I.; Armentaro, G.; Greco, M.; Maio, R.; Hribal, M.L.; Sesti, G.; et al. Endothelial Progenitor Cells Predict Vascular Damage Progression in Naive Hypertensive Patients According to Sex. Hypertens. Res. 2021, 44, 1451–1461. [Google Scholar] [CrossRef]
- Kumawat, R.; Gulati, S.; Ahluwalia, C.; Bahamania, K.; Varshney, P. Relation of Endothelial Progenitor Cells and Carotid Intima Media Thickness in Indian Rheumatoid Arthritis Patients-A Cross Sectional Study. J. Assoc. Phys. India 2022, 70, 11–12. [Google Scholar]
- Wu, W.-Z.; Hu, D.-J.; Wang, Z.-Y.; Liao, L.-S.; Li, C.-C. Endothelial Progenitor Cell Impairment Mediated Vasodilation Dysfunction via Diminishing Nitric Oxide Production in Postmenopausal Females. Mol. Med. Rep. 2019, 19, 2449–2457. [Google Scholar] [CrossRef]
- Santillán-Cortez, D.; Vera-Gómez, E.; Hernández-Patricio, A.; Ruíz-Hernández, A.S.; Gutiérrez-Buendía, J.A.; De la Vega-Moreno, K.; Rizo-García, Y.A.; Loman-Zuñiga, O.A.; Escotto-Sánchez, I.; Rodríguez-Trejo, J.M.; et al. Endothelial Progenitor Cells May Be Related to Major Amputation after Angioplasty in Patients with Critical Limb Ischemia. Cells 2023, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, P.P.; Bello, C.; Emmert, M.Y.; Carrel, T.; Dreßen, M.; Hörer, J.; Winkler, B.; Luedi, M.M. Endothelial Progenitor Cells as Biomarkers of Cardiovascular Pathologies: A Narrative Review. Cells 2022, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Pyšná, A.; Bém, R.; Němcová, A.; Fejfarová, V.; Jirkovská, A.; Hazdrová, J.; Jude, E.B.; Dubskỳ, M. Endothelial Progenitor Cells Biology in Diabetes Mellitus and Peripheral Arterial Disease and Their Therapeutic Potential. Stem Cell Rev. Rep. 2019, 15, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kukumberg, M.; Zaw, A.M.; Wong, D.H.; Toh, C.M.; Chan, B.P.; Seet, R.C.; Wong, P.T.; Yim, E.K. Characterization and Functional Assessment of Endothelial Progenitor Cells in Ischemic Stroke Patients. Stem Cell Rev. Rep. 2021, 17, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Salybekov, A.A.; Kobayashi, S.; Asahara, T. Characterization of Endothelial Progenitor Cell: Past, Present, and Future. Int. J. Mol. Sci. 2022, 23, 7697. [Google Scholar] [CrossRef]
- Chambers, S.E.; Pathak, V.; Pedrini, E.; Soret, L.; Gendron, N.; Guerin, C.L.; Stitt, A.W.; Smadja, D.M.; Medina, R.J. Current Concepts on Endothelial Stem Cells Definition, Location, and Markers. Stem Cells Transl. Med. 2021, 10, S54–S61. [Google Scholar] [CrossRef] [PubMed]
- Altabas, V. Diabetes, Endothelial Dysfunction, and Vascular Repair: What Should a Diabetologist Keep His Eye On? Int. J. Endocrinol. 2015, 2015, 848272. [Google Scholar] [CrossRef]
- Altabas, V.; Biloš, L.S.K. The Role of Endothelial Progenitor Cells in Atherosclerosis and Impact of Anti-Lipemic Treatments on Endothelial Repair. Int. J. Mol. Sci. 2022, 23, 2663. [Google Scholar] [CrossRef]
- Ross, M.D. Endothelial Regenerative Capacity and Aging: Influence of Diet, Exercise and Obesity. Curr. Cardiol. Rev. 2018, 14, 233–244. [Google Scholar] [CrossRef]
- Li, J.-H.; Li, Y.; Huang, D.; Yao, M. Role of Stromal Cell-Derived Factor-1 in Endothelial Progenitor Cell-Mediated Vascular Repair and Regeneration. Tissue Eng. Regen. Med. 2021, 18, 747–758. [Google Scholar] [CrossRef]
- Klein, G.; Schmal, O.; Aicher, W.K. Matrix Metalloproteinases in Stem Cell Mobilization. Matrix Biol. 2015, 44, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Cao, X.; Yu, B.; Qu, A. Vascular Stem/Progenitor Cells in Vessel Injury and Repair. Front. Cardiovasc. Med. 2022, 9, 845070. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Miao, J.; Qu, M.; Yang, G.-Y.; Shen, L. Adiponectin Modulates the Function of Endothelial Progenitor Cells via AMPK/eNOS Signaling Pathway. Biochem. Biophys. Res. Commun. 2017, 493, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Fu, C.; Li, X.; Song, Y.; Li, C.; Zou, M.; Guan, Y.; Zhu, Y. Prostaglandin E2 Promotes Endothelial Differentiation from Bone Marrow-Derived Cells through AMPK Activation. PLoS ONE 2011, 6, e23554. [Google Scholar] [CrossRef]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef]
- Yang, J.-X.; Pan, Y.-Y.; Wang, X.-X.; Qiu, Y.-G.; Mao, W. Endothelial Progenitor Cells in Age-Related Vascular Remodeling. Cell Transpl. 2018, 27, 786–795. [Google Scholar] [CrossRef]
- Morrone, D.; Picoi, M.E.L.; Felice, F.; De Martino, A.; Scatena, C.; Spontoni, P.; Naccarato, A.G.; Di Stefano, R.; Bortolotti, U.; Dal Monte, M. Endothelial Progenitor Cells: An Appraisal of Relevant Data from Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 12874. [Google Scholar] [CrossRef]
- Fadini, G.P.; Mehta, A.; Dhindsa, D.S.; Bonora, B.M.; Sreejit, G.; Nagareddy, P.; Quyyumi, A.A. Circulating Stem Cells and Cardiovascular Outcomes: From Basic Science to the Clinic. Eur. Heart J. 2020, 41, 4271–4282. [Google Scholar] [CrossRef]
- Evans, C.E.; Iruela-Arispe, M.L.; Zhao, Y.-Y. Mechanisms of Endothelial Regeneration and Vascular Repair and Their Application to Regenerative Medicine. Am. J. Pathol. 2021, 191, 52–65. [Google Scholar] [CrossRef]
- Pearson, J.D. Plasticity of Adult Endothelium: How Frequent and to What Extent? Cardiovasc. Res. 2015, 108, 319–320. [Google Scholar] [CrossRef]
- Kliche, K.; Jeggle, P.; Pavenstädt, H.; Oberleithner, H. Role of Cellular Mechanics in the Function and Life Span of Vascular Endothelium. Pflügers Arch. Eur. J. Physiol. 2011, 462, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hobson, B.; Denekamp, J. Endothelial Proliferation in Tumours and Normal Tissues: Continuous Labelling Studies. Br. J. Cancer 1984, 49, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, A.; Šuput, D.; Zorc-Pleskovič, R. Pathogenesis of Atherosclerosis in the Tunica Intima, Media, and Adventitia of Coronary Arteries: An Updated Review. Bosn. J. Basic Med. Sci. 2020, 20, 21–30. [Google Scholar] [CrossRef]
- Beck-Joseph, J.; Lehoux, S. Molecular Interactions between Vascular Smooth Muscle Cells and Macrophages in Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 737934. [Google Scholar] [CrossRef]
- Murad, H.A.; Rafeeq, M.M.; Alqurashi, T.M. Role and Implications of the CXCL12/CXCR4/CXCR7 Axis in Atherosclerosis: Still a Debate. Ann. Med. 2021, 53, 1598–1612. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, M.L.; Schütz, E.; Schäfer, K. Endothelial Cell Senescence and Thrombosis: Ageing Clots. Thromb. Res. 2016, 147, 36–45. [Google Scholar] [CrossRef]
- Fadini, G.P.; de Kreutzenberg, S.; Albiero, M.; Coracina, A.; Pagnin, E.; Baesso, I.; Cignarella, A.; Bolego, C.; Plebani, M.; Nardelli, G.B.; et al. Gender Differences in Endothelial Progenitor Cells and Cardiovascular Risk Profile: The Role of Female Estrogens. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 997–1004. [Google Scholar] [CrossRef]
- Tripolt, N.J.; Narath, S.H.; Eder, M.; Pieber, T.R.; Wascher, T.C.; Sourij, H. Multiple Risk Factor Intervention Reduces Carotid Atherosclerosis in Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2014, 13, 95. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Jyotsna, F.; Ahmed, A.; Kumar, K.; Kaur, P.; Chaudhary, M.H.; Kumar, S.; Khan, E.; Khanam, B.; Shah, S.U.; Varrassi, G.; et al. Exploring the Complex Connection between Diabetes and Cardiovascular Disease: Analyzing Approaches to Mitigate Cardiovascular Risk in Patients with Diabetes. Cureus 2023, 15, e43882. [Google Scholar] [CrossRef]
- Schneider, D.J. Diabetes and Thrombosis. In Diabetes and Cardiovascular Disease; Springer: Berlin/Heidelberg, Germany, 2023; pp. 99–127. [Google Scholar]
- Sartore, G.; Ragazzi, E.; Caprino, R.; Lapolla, A. Long-Term HbA1c Variability and Macro-/Micro-Vascular Complications in Type 2 Diabetes Mellitus: A Meta-Analysis Update. Acta Diabetol. 2023, 60, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Wils, J.; Favre, J.; Bellien, J. Modulating Putative Endothelial Progenitor Cells for the Treatment of Endothelial Dysfunction and Cardiovascular Complications in Diabetes. Pharmacol. Ther. 2017, 170, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Boscaro, E.; De Kreutzenberg, S.; Agostini, C.; Seeger, F.; Dimmeler, S.; Zeiher, A.; Tiengo, A.; Avogaro, A. Time Course and Mechanisms of Circulating Progenitor Cell Reduction in the Natural History of Type 2 Diabetes. Diabetes Care 2010, 33, 1097–1102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hörtenhuber, T.; Rami-Mehar, B.; Satler, M.; Nagl, K.; Höbaus, C.; Höllerl, F.; Koppensteiner, R.; Schernthaner, G.; Schober, E.; Schernthaner, G.-H. Endothelial Progenitor Cells Are Related to Glycemic Control in Children with Type 1 Diabetes over Time. Diabetes Care 2013, 36, 1647–1653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fadini, G.; Albiero, M.; Vigili de Kreutzenberg, S.; Avogaro, A. Hypoglycemia Affects the Changes in Endothelial Progenitor Cell Levels during Insulin Therapy in Type 2 Diabetic Patients. J. Endocrinol. Investig. 2015, 38, 733–738. [Google Scholar] [CrossRef]
- Fadini, G.P.; Boscari, F.; Cappellari, R.; Galasso, S.; Rigato, M.; Bonora, B.M.; D’Anna, M.; Bruttomesso, D.; Avogaro, A. Effects of Hypoglycemia on Circulating Stem and Progenitor Cells in Diabetic Patients. J. Clin. Endocrinol. Metab. 2018, 103, 1048–1055. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Casciano, O.; Volpe, E.D.; Bellastella, G.; Giugliano, D.; Esposito, K. Reducing Glucose Variability with Continuous Subcutaneous Insulin Infusion Increases Endothelial Progenitor Cells in Type 1 Diabetes: An Observational Study. Endocrine 2016, 52, 244–252. [Google Scholar] [CrossRef]
- Qiu, C.; Xie, Q.; Zhang, D.; Chen, Q.; Hu, J.; Xu, L. GM-CSF Induces Cyclin D1 Expression and Proliferation of Endothelial Progenitor Cells via PI3K and MAPK Signaling. Cell. Physiol. Biochem. 2014, 33, 784–795. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Guo, M.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive Oxygen Species in Vascular Formation and Development. Oxidative Med. Cell. Longev. 2013, 2013, 374963. [Google Scholar] [CrossRef]
- Toda, N.; Okamura, T. Obesity Impairs Vasodilatation and Blood Flow Increase Mediated by Endothelial Nitric Oxide: An Overview. J. Clin. Pharmacol. 2013, 53, 1228–1239. [Google Scholar] [CrossRef]
- Ramalingam, P.; Poulos, M.G.; Lazzari, E.; Gutkin, M.C.; Lopez, D.; Kloss, C.C.; Crowley, M.J.; Katsnelson, L.; Freire, A.G.; Greenblatt, M.B.; et al. Chronic Activation of Endothelial MAPK Disrupts Hematopoiesis via NFKB Dependent Inflammatory Stress Reversible by SCGF. Nat. Commun. 2020, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Goligorsky, M.; Hirschi, K. Stress-Induced Premature Senescence of Endothelial and Endothelial Progenitor Cells. Adv. Pharmacol. 2016, 77, 281–306. [Google Scholar] [PubMed]
- Saad, M.I.; Abdelkhalek, T.M.; Saleh, M.M.; Kamel, M.A.; Youssef, M.; Tawfik, S.H.; Dominguez, H. Insights into the Molecular Mechanisms of Diabetes-Induced Endothelial Dysfunction: Focus on Oxidative Stress and Endothelial Progenitor Cells. Endocrine 2015, 50, 537–567. [Google Scholar] [CrossRef] [PubMed]
- Juin, S.K.; Ouseph, R.; Gondim, D.D.; Jala, V.R.; Sen, U. Diabetic Nephropathy and Gaseous Modulators. Antioxidants 2023, 12, 1088. [Google Scholar] [CrossRef]
- Rigato, M.; Bittante, C.; Albiero, M.; Avogaro, A.; Fadini, G.P. Circulating Progenitor Cell Count Predicts Microvascular Outcomes in Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2015, 100, 2666–2672. [Google Scholar] [CrossRef]
- Makino, H.; Miyamoto, Y.; Kikuchi-Taura, A.; Soma, T.; Taguchi, A.; Kishimoto, I. Decreased Levels of Circulating CD 34+ Cells Are Associated with Coronary Heart Disease in Japanese Patients with Type 2 Diabetes. J. Diabetes Investig. 2015, 6, 473–478. [Google Scholar] [CrossRef]
- Yu, C.-G.; Zhang, N.; Yuan, S.-S.; Ma, Y.; Yang, L.-Y.; Feng, Y.-M.; Zhao, D. Endothelial Progenitor Cells in Diabetic Microvascular Complications: Friends or Foes? Stem Cells Int. 2016, 2016, 1803989. [Google Scholar] [CrossRef]
- Das, S.K.; Yuan, Y.F.; Li, M.Q. An Overview on Current Issues and Challenges of Endothelial Progenitor Cell-Based Neovascularization in Patients with Diabetic Foot Ulcer. Cell. Reprogramming 2017, 19, 75–87. [Google Scholar] [CrossRef]
- Naaman, S.C.; Bakris, G.L. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care 2023, 46, 1574–1586. [Google Scholar] [CrossRef]
- American Diabetes Association. Retinopathy, Neuropathy, and Foot Care: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S203–S215. [Google Scholar] [CrossRef]
- Berezin, A.E. Endothelial Progenitor Cells Dysfunction and Impaired Tissue Reparation: The Missed Link in Diabetes Mellitus Development. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Neil, H.A.W.; Matthews, D.R. Long-Term Follow-up after Tight Control of Blood Pressure in Type 2 Diabetes. N. Engl. J. Med. 2008, 359, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, B.; Jeitler, K.; Seitz, M.; Horvath, K.; Berghold, A.; Siebenhofer, A. Intensive Glucose Control versus Conventional Glucose Control for Type 1 Diabetes Mellitus. Cochrane Database Syst. Rev. 2014, 2016, CD009122. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S140–S157. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Y.; Pan, A.; Hu, Y.; Chen, S.; Qian, F.; Rimm, E.B.; Manson, J.E.; Stampfer, M.J.; Giatsidis, G.; et al. Adherence to a Healthy Lifestyle in Association with Microvascular Complications Among Adults with Type 2 Diabetes. JAMA Netw. Open 2023, 6, e2252239. [Google Scholar] [CrossRef]
- Monaghan, M.; Helgeson, V.; Wiebe, D. Type 1 Diabetes in Young Adulthood. Curr. Diabetes Rev. 2015, 11, 239–250. [Google Scholar] [CrossRef]
- Mottalib, A.; Salsberg, V.; Mohd-Yusof, B.-N.; Mohamed, W.; Carolan, P.; Pober, D.M.; Mitri, J.; Hamdy, O. Effects of Nutrition Therapy on HbA1c and Cardiovascular Disease Risk Factors in Overweight and Obese Patients with Type 2 Diabetes. Nutr. J. 2018, 17, 42. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating Positive Health Behaviors and Well-Being to Improve Health Outcomes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S68–S96. [Google Scholar] [CrossRef]
- Dunkler, D.; Kohl, M.; Heinze, G.; Teo, K.K.; Rosengren, A.; Pogue, J.; Gao, P.; Gerstein, H.; Yusuf, S.; Oberbauer, R.; et al. Modifiable Lifestyle and Social Factors Affect Chronic Kidney Disease in High-Risk Individuals with Type 2 Diabetes Mellitus. Kidney Int. 2015, 87, 784–791. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Díaz-López, A.; Valls-Pedret, C.; Cofán, M.; García-Layana, A.; Lamuela-Raventós, R.-M.; Castañer, O.; Zanon-Moreno, V.; Martinez-Gonzalez, M.A.; Toledo, E.; et al. Dietary Marine Ømega-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals with Type 2 Diabetes: Prospective Investigation from the PREDIMED Trial. JAMA Ophthalmol. 2016, 134, 1142–1149. [Google Scholar] [CrossRef]
- Storz, M.A.; Küster, O. Plant-Based Diets and Diabetic Neuropathy: A Systematic Review. Lifestyle Med. 2020, 1, e6. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Bellastella, G.; Petrizzo, M.; Gicchino, M.; Caputo, M.; Giugliano, D.; Esposito, K. Effect of a Mediterranean Diet on Endothelial Progenitor Cells and Carotid Intima-Media Thickness in Type 2 Diabetes: Follow-up of a Randomized Trial. Eur. J. Prev. Cardiol. 2017, 24, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mano, R.; Ishida, A.; Ohya, Y.; Todoriki, H.; Takishita, S. Dietary Intervention with Okinawan Vegetables Increased Circulating Endothelial Progenitor Cells in Healthy Young Women. Atherosclerosis 2009, 204, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Jahn, S.; Taylor, M.; Real, W.M.; Angeli, F.S.; Wong, M.L.; Amabile, N.; Prasad, M.; Rassaf, T.; Ottaviani, J.I.; et al. Improvement of Endothelial Function with Dietary Flavanols Is Associated with Mobilization of Circulating Angiogenic Cells in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2010, 56, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Ramirez, R.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Carracedo, J.; Garcia-Rios, A.; Rodriguez, F.; Gutierrez-Mariscal, F.M.; Gomez, P.; et al. Mediterranean Diet Reduces Endothelial Damage and Improves the Regenerative Capacity of Endothelium. Am. J. Clin. Nutr. 2011, 93, 267–274. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Fernandez-Gandara, C.; Garcia-Rios, A.; Rangel-Zuñiga, O.A.; Gutierrez-Mariscal, F.M.; Torres-Peña, J.D.; Marin, C.; Lopez-Moreno, J.; Castaño, J.P.; Delgado-Lista, J.; et al. Mediterranean Diet and Endothelial Function in Patients with Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. PLoS Med. 2020, 17, e1003282. [Google Scholar] [CrossRef]
- Fernández, J.M.; Rosado-Álvarez, D.; Da Silva Grigoletto, M.E.; Rangel-Zúñiga, O.A.; Landaeta-Díaz, L.L.; Caballero-Villarraso, J.; López-Miranda, J.; Pérez-Jiménez, F.; Fuentes-Jiménez, F. Moderate-to-High-Intensity Training and a Hypocaloric Mediterranean Diet Enhance Endothelial Progenitor Cells and Fitness in Subjects with the Metabolic Syndrome. Clin. Sci. 2012, 123, 361–373. [Google Scholar] [CrossRef]
- Spigoni, V.; Lombardi, C.; Cito, M.; Picconi, A.; Ridolfi, V.; Andreoli, R.; Anelli, N.; Gnudi, L.; Goldoni, M.; Zavaroni, I.; et al. N-3 PUFA Increase Bioavailability and Function of Endothelial Progenitor Cells. Food Funct. 2014, 5, 1881–1890. [Google Scholar] [CrossRef]
- Weech, M.; Altowaijri, H.; Mayneris-Perxachs, J.; Vafeiadou, K.; Madden, J.; Todd, S.; Jackson, K.G.; Lovegrove, J.A.; Yaqoob, P. Replacement of Dietary Saturated Fat with Unsaturated Fats Increases Numbers of Circulating Endothelial Progenitor Cells and Decreases Numbers of Microparticles: Findings from the Randomized, Controlled Dietary Intervention and VAScular Function (DIVAS) Study. Am. J. Clin. Nutr. 2018, 107, 876–882. [Google Scholar]
- Cesari, F.; Dinu, M.; Pagliai, G.; Rogolino, A.; Giusti, B.; Gori, A.M.; Casini, A.; Marcucci, R.; Sofi, F. Mediterranean, but Not Lacto-Ovo-Vegetarian, Diet Positively Influence Circulating Progenitor Cells for Cardiovascular Prevention: The CARDIVEG Study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 604–610. [Google Scholar] [CrossRef]
- Sereni, A.; Cesari, F.; Gori, A.M.; Maggini, N.; Marcucci, R.; Casini, A.; Sofi, F. Cardiovascular Benefits from Ancient Grain Bread Consumption: Findings from a Double-Blinded Randomized Crossover Intervention Trial. Int. J. Food Sci. Nutr. 2017, 68, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Jeong, M.H.; Cho, S.H.; Yun, J.H.; Chae, H.J.; Ahn, Y.K.; Lee, M.C.; Cheng, X.; Kondo, T.; Murohara, T.; et al. Effect of Green Tea Consumption on Endothelial Function and Circulating Endothelial Progenitor Cells in Chronic Smokers. Circ. J. 2006, 70, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Condines, X.; Magraner, E.; Roth, I.; Valderas-Martínez, P.; Arranz, S.; Casas, R.; Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Quifer-Rada, P.; et al. The Non-Alcoholic Fraction of Beer Increases Stromal Cell Derived Factor 1 and the Number of Circulating Endothelial Progenitor Cells in High Cardiovascular Risk Subjects: A Randomized Clinical Trial. Atherosclerosis 2014, 233, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-H.; Chen, Y.-H.; Tsai, H.-Y.; Chen, J.-S.; Wu, T.-C.; Lin, F.-Y.; Sata, M.; Chen, J.-W.; Lin, S.-J. Intake of Red Wine Increases the Number and Functional Capacity of Circulating Endothelial Progenitor Cells by Enhancing Nitric Oxide Bioavailability. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Roth, I.; Casas, R.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Consumption of Aged White Wine Modulates Cardiovascular Risk Factors via Circulating Endothelial Progenitor Cells and Inflammatory Biomarkers. Clin. Nutr. 2019, 38, 1036–1044. [Google Scholar] [CrossRef]
- Corban, M.T.; Widmer, R.J.; Cilluffo, R.; Kazeck, M.A.; Lennon, R.J.; Lerman, L.O.; Lerman, A. The Effect of Polyphenol-Rich Chardonnay Seed Supplements on Peripheral Endothelial Function. Eur. J. Nutr. 2020, 59, 3723–3734. [Google Scholar] [CrossRef]
- Shannon, O.M.; Stephan, B.C.; Minihane, A.-M.; Mathers, J.C.; Siervo, M. Nitric Oxide Boosting Effects of the Mediterranean Diet: A Potential Mechanism of Action. J. Gerontol. Ser. A 2018, 73, 902–904. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Tresserra-Rimbau, A.; Pons, A.; Tur, J.A.; Martorell, M.; Ros, E.; Buil-Cosiales, P.; Sacanella, E.; Covas, M.I.; Corella, D.; et al. Effects of Total Dietary Polyphenols on Plasma Nitric Oxide and Blood Pressure in a High Cardiovascular Risk Cohort. The PREDIMED Randomized Trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 60–67. [Google Scholar] [CrossRef]
- Shah, R.; Makarem, N.; Emin, M.; Liao, M.; Jelic, S.; Aggarwal, B. Mediterranean Diet Components Are Linked to Greater Endothelial Function and Lower Inflammation in a Pilot Study of Ethnically Diverse Women. Nutr. Res. 2020, 75, 77–84. [Google Scholar] [CrossRef]
- Galié, S.; García-Gavilán, J.; Papandreou, C.; Camacho-Barcía, L.; Arcelin, P.; Palau-Galindo, A.; Rabassa, A.; Bulló, M. Effects of Mediterranean Diet on Plasma Metabolites and Their Relationship with Insulin Resistance and Gut Microbiota Composition in a Crossover Randomized Clinical Trial. Clin. Nutr. 2021, 40, 3798–3806. [Google Scholar] [CrossRef]
- Aoki, A.; Murata, M.; Asano, T.; Ikoma, A.; Sasaki, M.; Saito, T.; Otani, T.; Jinbo, S.; Ikeda, N.; Kawakami, M.; et al. Prompt Increases in Retinol-Binding Protein 4 and Endothelial Progenitor Cells during Acute Exercise Load in Diabetic Subjects. Endocr. J. 2012, 59, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Waclawovsky, G.; Umpierre, D.; Figueira, F.R.; De Lima, E.S.; Alegretti, A.P.; Schneider, L.; Matte, U.S.; Rodrigues, T.C.; Schaan, B.D. Exercise on Progenitor Cells in Healthy Subjects and Patients with Type 1 Diabetes. Med. Sci. Sports Exerc. 2016, 48, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.S.; Shaw, A.; Smith, K.; Capper, T.E.; Scragg, J.H.; Cronin, M.; Bashir, A.; Flatt, A.; Campbell, M.D.; Stevenson, E.J.; et al. Type 1 Diabetes Patients Increase CXCR4+ and CXCR7+ Haematopoietic and Endothelial Progenitor Cells with Exercise, but the Response Is Attenuated. Sci. Rep. 2021, 11, 14502. [Google Scholar] [CrossRef] [PubMed]
- Bruyndonckx, L.; Hoymans, V.Y.; De Guchtenaere, A.; Van Helvoirt, M.; Van Craenenbroeck, E.M.; Frederix, G.; Lemmens, K.; Vissers, D.K.; Vrints, C.J.; Ramet, J.; et al. Diet, Exercise, and Endothelial Function in Obese Adolescents. Pediatrics 2015, 135, e653–e661. [Google Scholar] [CrossRef] [PubMed]
- Eleuteri, E.; Mezzani, A.; Di Stefano, A.; Vallese, D.; Gnemmi, I.; Delle Donne, L.; Taddeo, A.; Della Bella, S.; Giannuzzi, P. Aerobic Training and Angiogenesis Activation in Patients with Stable Chronic Heart Failure: A Preliminary Report. Biomarkers 2013, 18, 418–424. [Google Scholar] [CrossRef]
- Möbius-Winkler, S.; Hilberg, T.; Menzel, K.; Golla, E.; Burman, A.; Schuler, G.; Adams, V. Time-Dependent Mobilization of Circulating Progenitor Cells during Strenuous Exercise in Healthy Individuals. J. Appl. Physiol. 2009, 107, 1943–1950. [Google Scholar] [CrossRef]
- Manfredini, F.; Rigolin, G.; Malagoni, A.; Catizone, L.; Mandini, S.; Sofritti, O.; Mauro, E.; Soffritti, S.; Boari, B.; Cuneo, A.; et al. Exercise Training and Endothelial Progenitor Cells in Haemodialysis Patients. J. Int. Med. Res. 2009, 37, 534–540. [Google Scholar] [CrossRef]
- Kaźmierski, M.; Wojakowski, W.; Michalewska-W\ludarczyk, A.; Podolecka, E.; Kotowski, M.; Machaliński, B.; Tendera, M. Exercise-Induced Mobilisation of Endothelial Progenitor Cells in Patients with Premature Coronary Heart Disease. Kardiol. Pol. Pol. Heart J. 2015, 73, 411–418. [Google Scholar] [CrossRef]
- Bajer, B.; Vlcek, M.; Galusova, A.; Imrich, R.; Penesova, A. Exercise Associated Hormonal Signals as Powerful Determinants of an Effective Fat Mass Loss. Endocr. Regul. 2015, 49, 151–163. [Google Scholar] [CrossRef]
- Dyakova, E.Y.; Kapilevich, L.V.; Shylko, V.G.; Popov, S.V.; Anfinogenova, Y. Physical Exercise Associated with NO Production: Signaling Pathways and Significance in Health and Disease. Front. Cell Dev. Biol. 2015, 3, 19. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular Toxicity of Nicotine: Implications for Electronic Cigarette Use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Nakano, Y.; Adachi, S.; Murohara, T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ. J. 2019, 83, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Antoniewicz, L.; Bosson, J.A.; Kuhl, J.; Abdel-Halim, S.M.; Kiessling, A.; Mobarrez, F.; Lundbäck, M. Electronic Cigarettes Increase Endothelial Progenitor Cells in the Blood of Healthy Volunteers. Atherosclerosis 2016, 255, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chen, L.-L.; Zeng, T.; Li, Y.; Yu, F.; Hu, L.; Yue, L. Number of Circulating Endothelial Progenitor Cells as a Marker of Vascular Endothelial Function for Type 2 Diabetes. Vasc. Med. 2010, 15, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.W.; Rider, R.; Glanville, M.; Narayanan, K.; Razvi, S.; Weaver, J.U. Metformin Improves Circulating Endothelial Cells and Endothelial Progenitor Cells in Type 1 Diabetes: MERIT Study. Cardiovasc. Diabetol. 2016, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liao, Y.; Zeng, T.; Yu, F.; Li, H.; Feng, Y. Effects of Metformin plus Gliclazide Compared with Metformin Alone on Circulating Endothelial Progenitor Cell in Type 2 Diabetic Patients. Endocrine 2010, 38, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Yu, F.; Zeng, T.; Liao, Y.; Li, Y.; Ding, H. Effects of Gliclazide on Endothelial Function in Patients with Newly Diagnosed Type 2 Diabetes. Eur. J. Pharmacol. 2011, 659, 296–301. [Google Scholar] [CrossRef]
- Dei Cas, A.; Spigoni, V.; Cito, M.; Aldigeri, R.; Ridolfi, V.; Marchesi, E.; Marina, M.; Derlindati, E.; Aloe, R.; Bonadonna, R.C.; et al. Vildagliptin, but Not Glibenclamide, Increases Circulating Endothelial Progenitor Cell Number: A 12-Month Randomized Controlled Trial in Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2017, 16, 27. [Google Scholar] [CrossRef]
- Aso, Y.; Jojima, T.; Iijima, T.; Suzuki, K.; Terasawa, T.; Fukushima, M.; Momobayashi, A.; Hara, K.; Takebayashi, K.; Kasai, K.; et al. Sitagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Increases the Number of Circulating CD34+ CXCR4+ Cells in Patients with Type 2 Diabetes. Endocrine 2015, 50, 659–664. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.; Di Palo, C.; Gicchino, M.; Petrizzo, M.; Bellastella, G.; Saccomanno, F.; Giugliano, D. Effects of Pioglitazone versus Metformin on Circulating Endothelial Microparticles and Progenitor Cells in Patients with Newly Diagnosed Type 2 Diabetes—A Randomized Controlled Trial. Diabetes Obes. Metab. 2011, 13, 439–445. [Google Scholar] [CrossRef]
- Makino, H.; Okada, S.; Nagumo, A.; Sugisawa, T.; Miyamoto, Y.; Kishimoto, I.; Akie, T.K.; Soma, T.; Taguchi, A.; Yoshimasa, Y. Pioglitazone Treatment Stimulates Circulating CD34-Positive Cells in Type 2 Diabetes Patients. Diabetes Res. Clin. Pract. 2008, 81, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Ting, M.-K.; Verma, S.; Kuo, L.-T.; Yang, N.-I.; Hsieh, I.-C.; Wang, S.-Y.; Hung, A.; Cherng, W.-J. Pioglitazone Increases the Numbers and Improves the Functional Capacity of Endothelial Progenitor Cells in Patients with Diabetes Mellitus. Am. Heart J. 2006, 152, 1051.e1–1051.e8. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Kamani, C.H.; Gensch, C.; Bohm, M.; Laufs, U. The Peroxisome Proliferator–Activated Receptor-γ Agonist Pioglitazone Increases Number and Function of Endothelial Progenitor Cells in Patients with Coronary Artery Disease and Normal Glucose Tolerance. Diabetes 2007, 56, 2609–2615. [Google Scholar] [CrossRef] [PubMed]
- Kampoli, A.-M.; Tousoulis, D.; Pallantza, Z.; Paterakis, G.; Papageorgiou, N.; Oikonomou, E.; Miliou, A.; Vlachopoulou, A.; Stefanadis, C. Comparable Effects of Pioglitazone and Perindopril on Circulating Endothelial Progenitor Cells, Inflammatory Process and Oxidative Stress in Patients with Diabetes Mellitus. Int. J. Cardiol. 2012, 157, 413–415. [Google Scholar] [CrossRef]
- Fadini, G.P.; Boscaro, E.; Albiero, M.; Menegazzo, L.; Frison, V.; De Kreutzenberg, S.; Agostini, C.; Tiengo, A.; Avogaro, A. The Oral Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Increases Circulating Endothelial Progenitor Cells in Patients with Type 2 Diabetes: Possible Role of Stromal-Derived Factor-1α. Diabetes Care 2010, 33, 1607–1609. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, X.; Zheng, M.; Deng, D.; Zhang, S.; Wang, Y.; Fang, Z.; Chen, M. Acute Effects of Sitagliptin on Progenitor Cells and Soluble Mediators in Newly Diagnosed Type 2 Diabetes. Int. J. Clin. Pharmacol. Ther. 2020, 58, 491. [Google Scholar] [CrossRef]
- Nakamura, K.; Oe, H.; Kihara, H.; Shimada, K.; Fukuda, S.; Watanabe, K.; Takagi, T.; Yunoki, K.; Miyoshi, T.; Hirata, K.; et al. DPP-4 Inhibitor and Alpha-Glucosidase Inhibitor Equally Improve Endothelial Function in Patients with Type 2 Diabetes: EDGE Study. Cardiovasc. Diabetol. 2014, 13, 110. [Google Scholar] [CrossRef]
- Fadini, G.P.; Bonora, B.M.; Cappellari, R.; Menegazzo, L.; Vedovato, M.; Iori, E.; Marescotti, M.C.; Albiero, M.; Avogaro, A. Acute Effects of Linagliptin on Progenitor Cells, Monocyte Phenotypes, and Soluble Mediators in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 748–756. [Google Scholar] [CrossRef]
- De Boer, S.A.; Reijrink, M.; Abdulahad, W.H.; Hoekstra, E.S.; Slart, R.H.; Heerspink, H.J.; Westra, J.; Mulder, D.J. Angiogenic T Cells Are Decreased in People with Type 2 Diabetes Mellitus and Recruited by the Dipeptidyl Peptidase-4 Inhibitor Linagliptin: A Subanalysis from a Randomized, Placebo-Controlled Trial (RELEASE Study). Diabetes Obes. Metab. 2020, 22, 1220–1225. [Google Scholar] [CrossRef]
- Awal, H.B.; Nandula, S.R.; Domingues, C.C.; Dore, F.J.; Kundu, N.; Brichacek, B.; Fakhri, M.; Elzarki, A.; Ahmadi, N.; Safai, S.; et al. Linagliptin, When Compared to Placebo, Improves CD34+ ve Endothelial Progenitor Cells in Type 2 Diabetes Subjects with Chronic Kidney Disease Taking Metformin and/or Insulin: A Randomized Controlled Trial. Cardiovasc. Diabetol. 2020, 19, 72. [Google Scholar] [CrossRef]
- Negro, R.; Greco, E.L.; Greco, G. Alogliptin and Gliclazide Similarly Increase Circulating Endothelial Progenitor Cells in Type 2 Diabetes Patients. Exp. Clin. Endocrinol. Diabetes 2019, 127, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, J.; Leng, F.; Lu, Z.; Ling, Y. Effect of Saxagliptin on Circulating Endothelial Progenitor Cells and Endothelial Function in Newly Diagnosed Type 2 Diabetic Patients. Exp. Clin. Endocrinol. Diabetes 2017, 125, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Dore, F.J.; Domingues, C.C.; Ahmadi, N.; Kundu, N.; Kropotova, Y.; Houston, S.; Rouphael, C.; Mammadova, A.; Witkin, L.; Khiyami, A.; et al. The Synergistic Effects of Saxagliptin and Metformin on CD34+ Endothelial Progenitor Cells in Early Type 2 Diabetes Patients: A Randomized Clinical Trial. Cardiovasc. Diabetol. 2018, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Akashi, N.; Umemoto, T.; Yamada, H.; Fujiwara, T.; Yamamoto, K.; Taniguchi, Y.; Sakakura, K.; Wada, H.; Momomura, S.; Fujita, H. Teneligliptin, a DPP-4 Inhibitor, Improves Vascular Endothelial Function via Divergent Actions Including Changes in Circulating Endothelial Progenitor Cells. Diabetes Metab. Syndr. Obes. 2023, 16, 1043–1054. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Agabiti-Rosei, C.; Rossini, C.; Caletti, S.; Coschignano, M.A.; Ferrari-Toninelli, G.; Ragni, G.; Cappelli, C.; Cerudelli, B.; Airò, P.; et al. Microvascular Density and Circulating Endothelial Progenitor Cells before and after Treatment with Incretin Mimetics in Diabetic Patients. High Blood Press. Cardiovasc. Prev. 2018, 25, 369–378. [Google Scholar] [CrossRef]
- Ahmad, E.; Waller, H.L.; Sargeant, J.A.; Webb, M.A.; Htike, Z.Z.; McCann, G.P.; Gulsin, G.; Khunti, K.; Yates, T.; Henson, J.; et al. Effects of Liraglutide versus Sitagliptin on Circulating Cardiovascular Biomarkers, Including Circulating Progenitor Cells, in Individuals with Type 2 Diabetes and Obesity: Analyses from the LYDIA Trial. Diabetes Obes. Metab. 2021, 23, 1409–1414. [Google Scholar] [CrossRef]
- Xie, D.; Li, Y.; Xu, M.; Zhao, X.; Chen, M. Effects of Dulaglutide on Endothelial Progenitor Cells and Arterial Elasticity in Patients with Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2022, 21, 200. [Google Scholar] [CrossRef]
- Bonora, B.M.; Cappellari, R.; Albiero, M.; Avogaro, A.; Fadini, G.P. Effects of SGLT2 Inhibitors on Circulating Stem and Progenitor Cells in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3773–3782. [Google Scholar] [CrossRef]
- Luo, L.; Dong, B.; Zhang, J.; Qiu, Y.; Liu, X.; Zhou, Z.; He, J.; Zhang, X.; Chen, L.; Xia, W. Dapagliflozin Restores Diabetes-Associated Decline in Vasculogenic Capacity of Endothelial Progenitor Cells via Activating AMPK-Mediated Inhibition of Inflammation and Oxidative Stress. Biochem. Biophys. Res. Commun. 2023, 671, 205–214. [Google Scholar] [CrossRef]
- Hess, D.A.; Terenzi, D.C.; Trac, J.Z.; Quan, A.; Mason, T.; Al-Omran, M.; Bhatt, D.L.; Dhingra, N.; Rotstein, O.D.; Leiter, L.A.; et al. SGLT2 Inhibition with Empagliflozin Increases Circulating Provascular Progenitor Cells in People with Type 2 Diabetes Mellitus. Cell Metab. 2019, 30, 609–613. [Google Scholar] [CrossRef]
- Nandula, S.R.; Kundu, N.; Awal, H.B.; Brichacek, B.; Fakhri, M.; Aimalla, N.; Elzarki, A.; Amdur, R.L.; Sen, S. Role of Canagliflozin on Function of CD34+ ve Endothelial Progenitor Cells (EPC) in Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2021, 20, 44. [Google Scholar] [CrossRef]
- Fadini, G.; De Kreutzenberg, S.; Mariano, V.; Boscaro, E.; Bertolini, F.; Mancuso, P.; Quarna, J.; Marescotti, M.; Agostini, C.; Tiengo, A.; et al. Optimized Glycaemic Control Achieved with Add-on Basal Insulin Therapy Improves Indexes of Endothelial Damage and Regeneration in Type 2 Diabetic Patients with Macroangiopathy: A Randomized Crossover Trial Comparing Detemir versus Glargine. Diabetes Obes. Metab. 2011, 13, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, D.; Kopf, S.; von Bauer, R.; Djuric, Z.; Cebola, R.; Sander, A.; Englert, S.; Vittas, S.; Hidmark, A.; Morcos, M.; et al. Influence of Insulin and Glargine on Outgrowth and Number of Circulating Endothelial Progenitor Cells in Type 2 Diabetes Patients: A Partially Double-Blind, Randomized, Three-Arm Unicenter Study. Cardiovasc. Diabetol. 2014, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Piatti, P.M.; Marone, E.; Mantero, M.; Setola, E.; Galluccio, E.; Lucotti, P.; Shehaj, E.; Villa, V.; Perticone, F.; Venturini, M.; et al. Effect of Normalization of Fasting Glucose by Intensified Insulin Therapy and Influence of eNOS Polymorphisms on the Incidence of Restenosis after Peripheral Angioplasty in Patients with Type 2 Diabetes: A Randomized, Open-Label Clinical Trial. Acta Diabetol. 2013, 50, 373–382. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of Intensive Blood-Glucose Control with Metformin on Complications in Overweight Patients with Type 2 Diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Yu, J.-W.; Deng, Y.-P.; Han, X.; Ren, G.-F.; Cai, J.; Jiang, G.-J. Metformin Improves the Angiogenic Functions of Endothelial Progenitor Cells via Activating AMPK/eNOS Pathway in Diabetic Mice. Cardiovasc. Diabetol. 2016, 15, 88. [Google Scholar] [CrossRef]
- Horsdal, H.T.; Johnsen, S.P.; Søndergaard, F.; Jacobsen, J.; Thomsen, R.W.; Schmitz, O.; Sørensen, H.T.; Rungby, J. Sulfonylureas and Prognosis after Myocardial Infarction in Patients with Diabetes: A Population-Based Follow-up Study. Diabetes Metab. Res. Rev. 2009, 25, 515–522. [Google Scholar] [CrossRef]
- Johnsen, S.P.; Monster, T.; Olsen, M.L.; Thisted, H.; McLaughlin, J.; Sørensen, H.T.; Lervang, H.; Rungby, J. Risk and Short-Term Prognosis of Myocardial Infarction among Users of Antidiabetic Drugs. Am. J. Ther. 2006, 13, 134–140. [Google Scholar] [CrossRef]
- Filipova, E.; Uzunova, K.; Kalinov, K.; Vekov, T. Effects of Pioglitazone Therapy on Blood Parameters, Weight and BMI: A Meta-Analysis. Diabetol. Metab. Syndr. 2017, 9, 90. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Georgianos, P.I.; Lasaridis, A.N. PPAR-γ Agonism for Cardiovascular and Renal Protection. Cardiovasc. Ther. 2011, 29, 377–384. [Google Scholar] [CrossRef]
- Lehrke, M.; Marx, N. Diabetes Mellitus and Heart Failure. Am. J. Cardiol. 2017, 120, S37–S47. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.M.; Hassan, O.A.; Adam, S.M.; Ali, A.I.; Ogedegbe, O.J.; Tabowei, G.; Barbarawi, A.; Yussuf, F.M.; Nor, M.A.; Adam, S.M.; et al. Association of Pioglitazone with Major Adverse Cardiovascular Events, All-Cause Mortality, and Heart Failure Hospitalizations: A Systematic Review. Cureus 2023, 15, e46911. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, E.; Dormandy, J.; Wilcox, R.; Massi-Benedetti, M.; Charbonnel, B. PROactive 07: Pioglitazone in the Treatment of Type 2 Diabetes: Results of the PROactive Study. Vasc. Health Risk Manag. 2007, 3, 355–370. [Google Scholar] [PubMed]
- Wilcox, R.; Kupfer, S.; Erdmann, E.; PROactive Study Investigators. Effects of Pioglitazone on Major Adverse Cardiovascular Events in High-Risk Patients with Type 2 Diabetes: Results from PROspective pioglitAzone Clinical Trial in Macro Vascular Events (PROactive 10). Am. Heart J. 2008, 155, 712–717. [Google Scholar] [CrossRef]
- Sorrentino, S.A.; Bahlmann, F.H.; Besler, C.; Müller, M.; Schulz, S.; Kirchhoff, N.; Doerries, C.; Horváth, T.; Limbourg, A.; Limbourg, F.; et al. Oxidant Stress Impairs in Vivo Reendothelialization Capacity of Endothelial Progenitor Cells from Patients with Type 2 Diabetes Mellitus: Restoration by the Peroxisome Proliferator-Activated Receptor-γ Agonist Rosiglitazone. Circulation 2007, 116, 163–173. [Google Scholar] [CrossRef]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Rosenstock, J.; Kahn, S.E.; Johansen, O.E.; Zinman, B.; Espeland, M.A.; Woerle, H.J.; Pfarr, E.; Keller, A.; Mattheus, M.; Baanstra, D.; et al. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients with Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA 2019, 322, 1155–1166. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef]
- White, W.B.; Bakris, G.L.; Bergenstal, R.M.; Cannon, C.P.; Cushman, W.C.; Fleck, P.; Heller, S.; Mehta, C.; Nissen, S.E.; Perez, A.; et al. EXamination of cArdiovascular outcoMes with alogliptIN versus Standard of carE in Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndrome (EXAMINE): A Cardiovascular Safety Study of the Dipeptidyl Peptidase 4 Inhibitor Alogliptin in Patients with Type 2 Diabetes with Acute Coronary Syndrome. Am. Heart J. 2011, 162, 620–626. [Google Scholar]
- Zhong, J.; Rajagopalan, S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Front. Immunol. 2015, 6, 477. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, K.C.; Arora, N. SGLT2 Inhibition for Outcomes: Is This the Panacea? Am. Heart J. Plus Cardiol. Res. Pract. 2022, 21, 100159. [Google Scholar] [CrossRef]
| Lineage | Myeloid Angiogenic Cells | Endothelial Colony-Forming Cells |
|---|---|---|
| positive cell markers | CD45, CD31, CD14 | CD31, CD105, CD 146 VE-cadherin, von Willebrand factor, VEGFR2 CD34+/− |
| negative cell markers | CD146, CD34 | CD45, CD14 |
| in vitro effects | conditioned media necessary for the endothelial formation | intrinsic tube forming capacity |
| function | provide paracrine angiogenic factors | provide cells as building blocks release of paracrine factors |
| time of appearance in culture | early | late |
| Drug Class | Mode of Action | Cardiovascular Effects | Effects on EPCs | Reference |
|---|---|---|---|---|
| Biguanides: Metformin | reduces hepatic glucose production facilitates peripheral glucose uptake and utilization, in part by increasing insulin action reduces basal hyperinsulinemia alters glucose turnover in the gut increases glucose uptake from circulation and decreases absorption from food increases the release of glucagon-like peptide-1 (GLP-1) alters the gut microbiome activates adenosine monophosphate-protein-kinase (AMPK) activator and increases the transport capacity of all types of membrane glucose transporters (GLUTs) | beneficial effects not proven in CVOTs | ↑EPC (CD34+/VEGFR2+/CD45-/dim) count assessed by flow cytometry ↑FMD | [115] |
| ↑EPC (CD34+/VEGFR2+/CD45-/dim) count assessed by flow cytometry ↑ECFC colonies number ↑adhesion capability of proangiogenic cells using fibronectin adhesion assay | [116] * | |||
| ↑EPC (CD34+/VEGFR2+/CD45-/dim) count assessed by flow cytometry | [117] | |||
| 1Sulphonylureas: Gliclazide Glimepiride Gliquidon | stimulates insulin secretion from the β-cells of the islets of Langerhans increases insulin and C-peptide secretion | second generation sulphonylureas are superior to first generation finding not proven in CVOTs | gliclazide only: ↑EPC (CD34+/VEGFR2+/CD45-/dim) count assessed by flow cytometry ↑FMD | [118] |
| no proven effects for other sulphonylureas | [119,120] | |||
| Thiazolidinediones: Pioglitazone Rosiglitazone | reduces insulin resistance and reduces insulin concentrations activates peroxisome proliferator-activated receptor gamma increases insulin sensitivity of liver, fat, and skeletal muscle cells reduces hepatic glucose output increases peripheral glucose disposal | improves some MACEs not proven in CVOTs increased risk of heart failure | pioglitazone: ↑EPC (CD34+/VEGFR2+) count assessed by flow cytometry pioglitazone: | [121] |
| ↑ circulating CD34+ cell count | [122] | |||
| pioglitazone: ↑EPC (CD34+) count assessed by flow cytometry ↑increased migratory response and adhesion capacity to fibronectin and collagen in culture | [123] | |||
| pioglitazone: ↑EPC (CD34+/VEGFR2+) count assessed by flow cytometry ↑ SDF1 induced migratory capacity ↑ ECFC in cultures | [124] | |||
| pioglitazone: no effect on EPC count | [125] | |||
| DPP-4 inhibitors Sitagliptin Linagliptin Alogliptin Saxagliptin Vildagliptin Teneligliptin | inhibits dipeptidyl peptidase 4 (DPP-4) enhances the levels of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) in a glucose-dependent manner improves beta cell responsiveness to glucose and stimulates insulin biosynthesis and release lowers glucagon secretion reduces hepatic glucose production | non-inferiority to standard treatment/class effect proven in CVOTs except for vildagliptin and teneligliptin increased risk for heart failure for saxagliptin | sitagliptin: ↑EPC (CD34+/VEGFR2+) count assessed by flow cytometry ↑ SDF1 blood concentrations | [126] |
| sitagliptin: ↑EPC (CD34+/CXCR4+) count assessed by flow cytometry ↓ SDF1 blood concentrations | [120] | |||
| sitagliptin: ↑EPC (CD34+/VEGFR2+ and CD34+/VEGFR2+/CD133+) count assessed by flow cytometry ↑GLP-1, NO and SDF-1 blood concentrations | [127] | |||
| sitagliptin: ↑EPC (CD34+) count assessed by flow cytometry ↑FMD | [128] | |||
| linagliptin: ↑EPC (CD34+/VEGFR2+ and CD34+/CD133+) count ↑GLP-1, and SDF-1 blood concentrations | [129] | |||
| linalgiptin no effect on EPCs ↑SDF-1 blood concentrations | [130] | |||
| linagliptin↑CD34+/CD184+ EPC improved arterial stiffness and pulse wave velocity | [131] | |||
| vildagliptin: ↑EPC (CD34+/VEGFR2 +/CD133+) count assessed by flow cytometry ↓SDF1 blood concentrations | [119] | |||
| alogliptin: ↑EPC (CD34+/VEGFR2 +/CD45-/dim) count assessed by flow cytometry | [132] | |||
| saxagliptin: ↑EPC (CD34+/VEGFR +/CD133+) count assessed by flow cytometry ↑FMD | [133] | |||
| saxagliptin: no effect on EPC if added to metformin improved migratory capacity | [134] | |||
| teneligliptin: no significant effect on EPC ↑FMD | [135] | |||
| GLP-1 receptor agonists Exenatide Lixisenatide Liraglutide Dulaglutide Semaglutide | activates the GLP-1 receptor stimulates insulin secretion and lowers glucagon secretion in a glucose-dependent manner delays gastric emptying in the early postprandial phase. | liraglutide, dulaglutide, and semaglutide showed cardiovascular superiority in CVOTs or equivalent studies | exenatide in full dose superior to medium dosed liraglutide in ↑EPC (CD34+/VEGFR2 +) | [136] |
| liraglutide superior to sitagliptin in ↑VEGF and SDF-1 | [137] | |||
| dulaglutide: ↑EPC (CD34+/VEGFR2 +/CD133+) enhanced EPC proliferation, adhesion, migration, and tubule formation abilities improved brachial-ankle pulse wave velocity | [138] | |||
| SGLT-2 inhibitors Empagliflozin Dapagliflozin Canagliflozin | inhibits the sodium-glucose cotransporter-2 by dapagliflozin in the proximal renal tubule improves both fasting and postprandial plasma glucose levels | cardiovascular superiority proven in CVOTs beneficial in heart failure and renal failure black box warning for lower limb amputations | dapagliflozin lead to a late increase in EPC count (CD34+/VEGFR2+) no change in EPCs in the empagliflozin group | [139] |
| dapagliflozin treatment activated AMPK signaling in EPCs | [140] | |||
| empagliflozin increases CD133+EPC count | [141] | |||
| canagliflozin improved CXCR receptors on EPCs, improving their migratory capacity | [142] | |||
| Insulin and insulin analogs | binds to insulin receptors | proven non-inferiority to standard therapy in CVOTs for newer insulin analogs | detemir and glargin both increased EPC count (CD34+/VEGFR2+ and CD34+/VEGFR2+/CD133+) decreased adhesion molecules | [143] |
| no difference in CD34*/VEGFR2+ EPCs between groups receiving NPH insulin, insulin glargin, or oral therapy improved ECFC growth with NPH insulin and glargin decrease in intima media thickness | [144] | |||
| intensive insulin therapy increased EPC count (CD34+/VEGFR2+) no effect on clinical outcomes | [145] | |||
| reduced glucovariability by using insulin pumps increased the EPC count (CD34+/VEGFR+) compared to intensified insulin therapy | [58] * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altabas, V.; Marinković Radošević, J.; Špoljarec, L.; Uremović, S.; Bulum, T. The Impact of Modern Anti-Diabetic Treatment on Endothelial Progenitor Cells. Biomedicines 2023, 11, 3051. https://doi.org/10.3390/biomedicines11113051
Altabas V, Marinković Radošević J, Špoljarec L, Uremović S, Bulum T. The Impact of Modern Anti-Diabetic Treatment on Endothelial Progenitor Cells. Biomedicines. 2023; 11(11):3051. https://doi.org/10.3390/biomedicines11113051
Chicago/Turabian StyleAltabas, Velimir, Jelena Marinković Radošević, Lucija Špoljarec, Stella Uremović, and Tomislav Bulum. 2023. "The Impact of Modern Anti-Diabetic Treatment on Endothelial Progenitor Cells" Biomedicines 11, no. 11: 3051. https://doi.org/10.3390/biomedicines11113051
APA StyleAltabas, V., Marinković Radošević, J., Špoljarec, L., Uremović, S., & Bulum, T. (2023). The Impact of Modern Anti-Diabetic Treatment on Endothelial Progenitor Cells. Biomedicines, 11(11), 3051. https://doi.org/10.3390/biomedicines11113051







