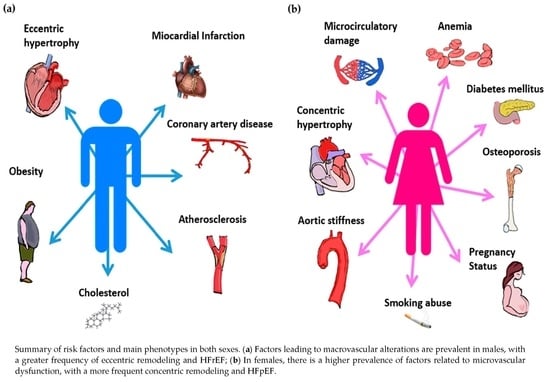
Risk Factors and Cellular Differences in Heart Failure: The Key Role of Sex Hormones
Abstract
:1. Introduction
2. Epidemiology
3. Risk Factors
3.1. Diabetes Mellitus
3.2. Hypertension
3.3. Obesity
3.4. Tobacco Smoke
3.5. Coronary Artery Disease
3.6. Anemia and Iron Deficiency
3.7. Vitamin D Deficiency
3.8. Anorexia
3.9. Sex-Specific Risk Factors in Women
4. Different Heart Failure Profiles between Sexes
5. Differences in Cellular and Endocrine Patterns
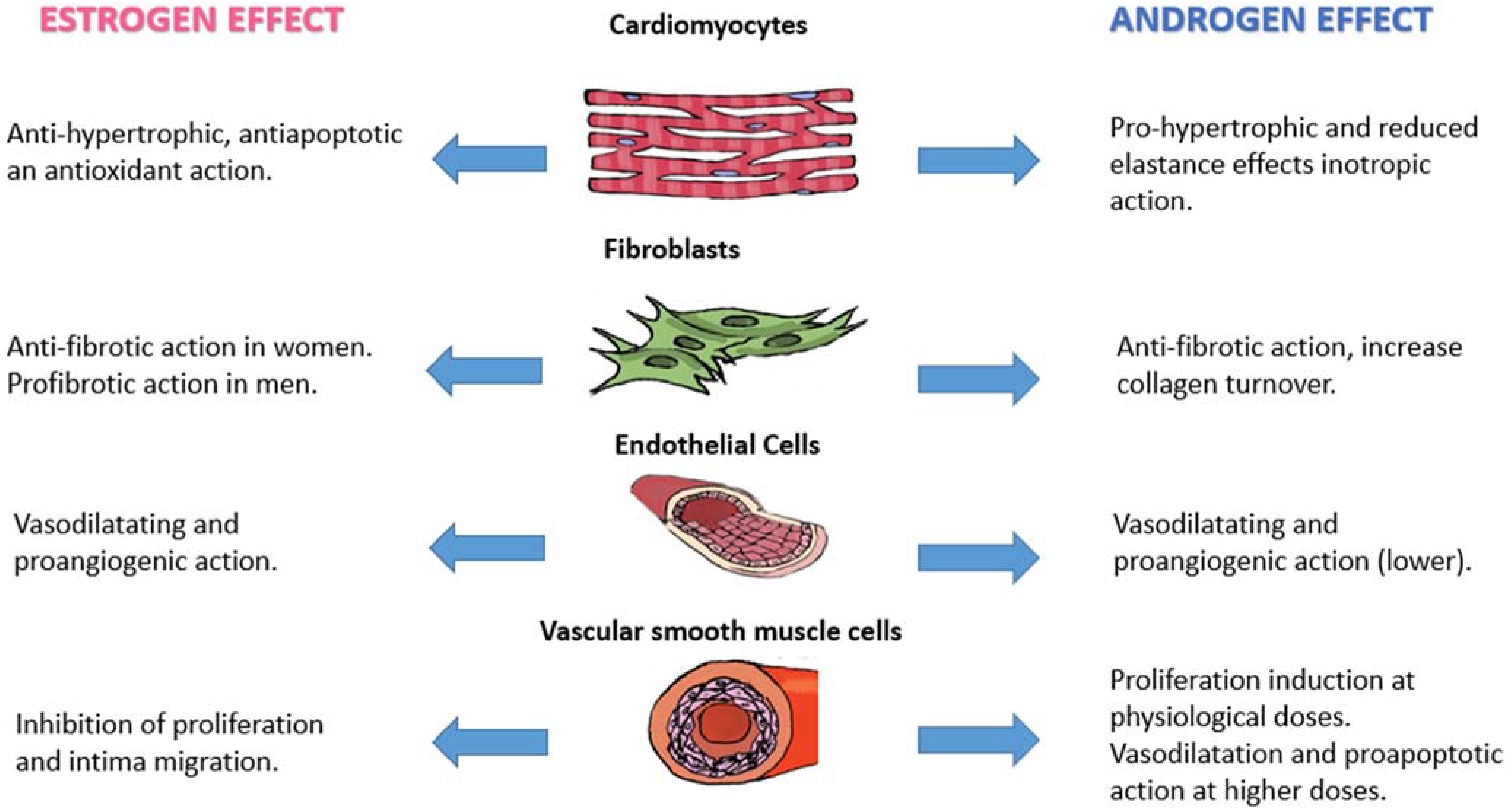
5.1. Cardiomyocytes
5.2. Fibroblast
5.3. Endothelial Cells and Vascular Smooth Muscle Cells
6. Sex Hormones and Biomarkers in Cardiology Practice
6.1. Natriuretic Peptides
6.2. Troponins
6.3. Fibrosis Biomarkers: Galectin-3 and ST-2
6.4. Osteopontin
7. Limitations
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| Akt | Ak strain transforming |
| AN | Anorexia Nervosa |
| AR | Androgen Receptor |
| BNP | B-type Natriuretic Peptide |
| CAD | Coronary Artery Disease |
| CaMKII | Ca2+/calmodulin-dependent kinase II |
| cAMP | Cyclic Adenosine Monophosphate |
| CRP | C-Reactive Protein |
| CV | Cardiovascular |
| DBP | Vitamin D Binding Protein |
| E2 | 17β-Estradiol |
| eNOS | Endothelial Nitric Oxide Synthase |
| EPICA | Southwestern European community-based Epidemiology of Heart Failure and Learning |
| ER | Estrogen Receptor |
| FHS | Framingham Heart Study |
| Gal-3 | Galectin 3 |
| GMPc | Guanylate MonoPhosphate cyclase |
| HDACs | Histone deacetylases |
| HF | Heart Failure |
| HFpEF | Heart Failure with preserved Ejection Fraction |
| HFrEF | Heart Failure with reduced Ejection Fraction |
| I-PRESERVE | Irbesartan in Heart Failure with Preserved Ejection Fraction |
| IL | Interleukin |
| LDL | Low-Density Lipoprotein |
| JNK | c-Jun N-terminal kinase |
| MCIP1 | Myocyte-enriched Calcineurin-Interacting Protein |
| MEF2 | Myocyte-specific Enhancer Factor 2 |
| MMP | Matrix Metalloproteinase |
| NHANES I | First National Health and Nutrition Examination Survey |
| NO | Nitric Oxide |
| NP | Natriuretic Peptide |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| PARAMOUNT | Prospective comparative of ARNI with ARB on Management Of heart failure Ure with preserved ejectioN section |
| PI3K | Phosphoinositide 3-kinases |
| PGI2 | Prostaglandin I2 |
| PKA | Protein Kinase A |
| PROMIS-HFpEF | PRevalence Of MIcrovascular dysfunction in Heart Failure with Preserved Ejection Fraction |
| RAAS | Renin-Angiotensin-Aldosterone System |
| ROS | Radical Oxygen Species |
| SMAD | Suppressor of Mothers against Decapentaplegic |
| SOD | Superoxide Dismutase |
| sST2 | Soluble Suppressor of Tumorigenicity 2 |
| TGF | Transforming Growth Factor |
| TLR | Toll-like receptor |
| TNF | Tumor Necrosis Factor |
| VDR | Vitamin D Receptor |
| VEGF | Vascular Endothelial Growth Factor |
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287, Erratum in Cardiovasc. Res. 2023, 119, 1453. [Google Scholar] [CrossRef] [PubMed]
- Arata, A.; Ricci, F.; Khanji, M.Y.; Mantini, C.; Angeli, F.; Aquilani, R.; Di Baldassarre, A.; Renda, G.; Mattioli, A.V.; Nodari, S.; et al. Sex Differences in Heart Failure: What Do We Know? J. Cardiovasc. Dev. Dis. 2023, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Bierer, B.E.; Meloney, L.G.; Ahmed, H.R.; White, S.A. Advancing the inclusion of underrepresented women in clinical research. Cell Rep. Med. 2022, 3, 100553. [Google Scholar] [CrossRef]
- Khan, S.S.; Beach, L.B.; Yancy, C.W. Sex-Based Differences in Heart Failure: JACC Focus Seminar 7/7. J. Am. Coll. Cardiol. 2022, 79, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Agarwal, M.A.; Fonarow, G.C.; Ziaeian, B. National trends in heart failure hospitalizations and readmissions from 2010 to 2017. JAMA Cardiol. 2022, 7, 115. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Larson, M.G.; Leip, E.P.; Beiser, A.; D’Agostino, R.B.; Kannel, W.B.; Murabito, J.M.; Vasan, R.S.; Benjamin, E.J.; Levy, D.; et al. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation 2002, 106, 3068–3072. [Google Scholar] [CrossRef]
- Bleumink, G.S.; Knetsch, A.M.; Sturkenboom, M.C.; Straus, S.M.; Hofman, A.; Deckers, J.W.; Witteman, J.C.; Stricker, B.H. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur. Heart J. 2004, 25, 1614–1619. [Google Scholar] [CrossRef]
- Rosendale, N.; Albert, M.A. The intersection of sexual orientation, gender identity, and race/ethnicity on cardiovascular health: A review of the literature and needed research. Curr. Cardiovasc. Risk Rep. 2020, 14, 17. [Google Scholar] [CrossRef]
- Teia, F.; Fonseca, C.; Mota, T.; Morais, H.; Matias, F.; de Sousa, A.; Oliveira, A.; EPICA Investigators. Prevalence of chronic heart failure in Southwestern Europe: The EPICA study. Eur. J. Heart Fail. 2002, 4, 531–539. [Google Scholar]
- Beale, A.L.; Meyer, P.; Marwick, T.H.; Lam, C.S.; Kaye, D.M. Sex differences in cardiovascular pathophysiology: Why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018, 138, 198–205. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Butler, J. Cardiovascular aging and heart failure: JACC review topic of the week. J. Am. Coll. Cardiol. 2019, 74, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Shim, C.Y.; Park, S.; Choi, D.; Yang, W.I.; Cho, I.J.; Choi, E.Y.; Chung, N.; Ha, J.W. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J. Am. Coll. Cardiol. 2011, 57, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Lam, C.S.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, N.; Ljung Faxen, U.; Lagerstrom Fermer, M.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S.; Larson, M.G.; Benjamin, E.J.; Evans, J.C.; Reiss, C.K.; Levy, D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: Prevalence and mortality in a population-based cohort. J. Am. Coll. Cardiol. 1999, 33, 1948–1955. [Google Scholar] [CrossRef]
- Hsich, E.M.; Grau-Sepulveda, M.V.; Hernandez, A.F.; Eapen, Z.J.; Xian, Y.; Schwamm, L.H.; Bhatt, D.L.; Fonarow, G.C. Relationship between sex, ejection fraction, and B-type natriuretic peptide levels in patients hospitalized with heart failure and associations with inhospital outcomes: Findings from the Get with The Guideline-Heart Failure Registry. Am. Heart J. 2013, 166, 1063–1071.e3. [Google Scholar] [CrossRef]
- Johnstone, D.; Limacher, M.; Rousseau, M.; Liang, C.S.; Ekelund, L.; Herman, M.; Stewart, D.; Guillotte, M.; Bjerken, G.; Gaasch, W.; et al. Clinical characteristics of patients in Studies of Left Ventricular Dysfunction (SOLVD). Am. J. Cardiol. 1992, 70, 894–900. [Google Scholar] [CrossRef]
- Hsich, E.M.; Grau-Sepulveda, M.V.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H.; Bhatt, D.L.; Fonarow, G.C. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am. Heart J. 2012, 163, 430–437.e3. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Galderisi, M.; Anderson, K.M.; Wilson, P.W.; Levy, D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am. J. Cardiol. 1991, 68, 85–89. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Cocchi, C.; Coppi, F.; Farinetti, A.; Mattioli, A.V. Cardiovascular disease prevention and therapy in women with Type 2 diabetes. Future Cardiol. 2021, 17, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; Gronda, E. Noncardiac comorbidity clustering in heart failure: An overlooked aspect with potential therapeutic door. Heart Fail. Rev. 2022, 27, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Van Heerebeek, L.; Hamdani, N.; Falcão-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.J.M.; Gerrit, J.L.; Somsen, A.; et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012, 126, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Larson, M.G.; Vasan, R.S.; Kannel, W.B.; Ho, K.K.L. The progression from hypertension to congestive heart failure. JAMA 1996, 275, 1557–1562. [Google Scholar] [CrossRef]
- Gori, M.; Lam, C.S.P.; Gupta, D.K.; Santos, A.B.S.; Cheng, S.; Shah, A.M.; Claggett, B.; Zile, M.R.; Kraigher-Krainer, E.; Pieske, B.; et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Coppi, F.; Bucciarelli, V.; Gallina, S. Cardiovascular risk stratification in young women: The pivotal role of pregnancy. J. Cardiovasc. Med. 2023, 24, 793–797. [Google Scholar] [CrossRef]
- Cadeddu, C.; Franconi, F.; Cassisa, L.; Campesi, I.; Pepe, A.; Cugusi, L.; Maffei, S.; Gallina, S.; Sciomer, S.; Mercuro, G.; et al. Working Group of Gender Medicine of Italian Society of Cardiology. Arterial hypertension in the female world: Pathophysiology and therapy. J. Cardiovasc. Med. 2016, 17, 229–236. [Google Scholar] [CrossRef]
- Eaton, C.B.; Pettinger, M.; Rossouw, J.; Martin, L.W.; Foraker, R.; Quddus, A.; Liu, S.; Wampler, N.S.; Hank Wu, W.C.; Manson, J.E.; et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ. Heart Fail. 2016, 9, e002883. [Google Scholar] [CrossRef]
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; Kizer, J.R.; Sarma, A.; Blaha, M.J.; Gansevoort, R.T.; et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018, 6, 701–709. [Google Scholar] [CrossRef]
- Bucciarelli, V.; Mattioli, A.V.; Sciomer, S.; Moscucci, F.; Renda, G.; Gallina, S. The Impact of Physical Activity and Inactivity on Cardiovascular Risk across Women’s Lifespan: An Updated Review. J. Clin. Med. 2023, 12, 4347. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Beltrami, M. Are HFpEF and HFmrEF So Different? The Need to Understand Distinct Phenotypes. Front. Cardiovasc. Med. 2021, 8, 676658. [Google Scholar] [CrossRef]
- Ambikairajah, A.; Walsh, E.; Tabatabaei-Jafari, H.; Cherbuin, N. Fat mass changes during menopause: A metaanalysis. Am. J. Obstet. Gynecol. 2019, 221, 393–409.e50. [Google Scholar] [CrossRef]
- Florijn, B.W.; Bijkerk, R.; van der Veer, E.P.; van Zonneveld, A.J. Gender and cardiovascular disease: Are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovasc. Res. 2018, 114, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Selleri, V.; Zanini, G.; Nasi, M.; Pinti, M.; Stefanelli, C.; Fedele, F.; Gallina, S. Physical Activity and Diet in Older Women: A Narrative Review. J. Clin. Med. 2023, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, M.; Yuan, S.; Zeng, Y.; Dong, Y.; Wang, Z.; Xiao, Q.; Dong, B.; Ma, J.; Hu, J. Sex differences in the associations between adiposity distribution and cardiometabolic risk factors in overweight or obese individuals: A cross-sectional study. BMC Public Health 2021, 21, 1232. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch. Intern. Med. 2001, 161, 996–1002. [Google Scholar] [CrossRef]
- Hitchman, S.C.; Fong, G.T. Gender empowerment and female-to-male smoking prevalence ratios. Bull. World Health Organ. 2011, 89, 195–202. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Do smoking habits differ between women and men in contemporary Western populations? Evidence from half a million people in the UK Biobank study. BMJ Open 2014, 4, e005663. [Google Scholar] [CrossRef]
- Rose, J.J.; Krishnan-Sarin, S.; Exil, V.J.; Hamburg, N.M.; Fetterman, J.L.; Ichinose, F.; Perez-Pinzon, M.A.; Rezk-Hanna, M.; Williamson, E. Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 703–728. [Google Scholar] [CrossRef]
- Khatibzadeh, S.; Farzadfar, F.; Oliver, J.; Ezzati, M.; Moran, A. Worldwide risk factors for heart failure: A systematic review and pooled analysis. Int. J. Cardiol. 2012, 168, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef]
- Lam, C.S.; Carson, P.E.; Anand, I.S.; Rector, T.S.; Kuskowski, M.; Komajda, M.; McKelvie, R.S.; McMurray, J.J.; Zile, M.R.; Massie, B.M.; et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: The Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ. Heart Fail. 2012, 5, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.R.; Anker, N.; Stødkilde-Jørgensen, N.; Thrane, P.G.; Hansen, M.K.; Pryds, K.; Mortensen, M.B.; Olesen, K.K.W.; Maeng, M. Impact of Coronary Artery Disease in Women with Newly Diagnosed Heart Failure and Reduced Ejection Fraction. JACC Heart Fail. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Poppe, K.; Gamble, G.; Earle, N.; Ezekowitz, J.; Squire, I.; McMurray, J.; McAlister, F.; Komajda, M.; Swedberg, K.; et al. Prognostic significance of anaemia in patients with heart failure with preserved and reduced ejection fraction: Results from the MAGGIC individual patient data meta-analysis. QJM 2016, 109, 377–382. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and iron deficiency in heart failure: Current concepts and emerging therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef]
- Lund, L.H.; Donal, E.; Oger, E.; Hage, C.; Persson, H.; Haugen-Löfman, I.; Ennezat, P.-V.; Sportouch-Dukhan, C.; Drouet, E.; Daubert, J.-C.; et al. Association between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 992–1001. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Zhao, X.; Heidenreich, P.A.; Peterson, E.D.; Bhatt, D.L.; Cannon, C.P.; Hernandez, A.F.; Fonarow, G.C. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012, 126, 65–75. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Canaud, B.; de Francisco, A.L.; Filippatos, G.; Ponikowski, P.; Silverberg, D.; van Veldhiesen, D.J.; Anker, S.D. Beyond the cardiorenal anaemia syndrome: Recognizing the role of iron deficiency. Eur. J. Heart Fail. 2012, 14, 882–886. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; GarciaRivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D Is a Regulator of Endothelial Nitric Oxide Synthase and Arterial Stiffness in Mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef]
- Zhu, Y.; Mahon, B.D.; Froicu, M.; Cantorna, M.T. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur. J. Immunol. 2005, 35, 217–224. [Google Scholar] [CrossRef]
- Mathieu, C.; Adorini, L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol. Med. 2002, 8, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Shibakura, M.; Ohsawa, M.; Kamiyama, R.; Hirosawa, S. Anticoagulant effects of 1alpha,25-dihydroxyvitamin D3 on human myelogenous leukemia cells and monocytes. Blood 1998, 92, 160–167. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–757. [Google Scholar] [CrossRef]
- Holick, M.F.; Matsuoka, L.Y.; Wortsman, J. Age, vitamin D, and solar ultraviolet. Lancent 1989, 334, 1104–1105. [Google Scholar] [CrossRef]
- Mei, Z.; Hu, H.; Zou, Y.; Li, D. The role of vitamin D in menopausal women’s health. Front. Physiol. 2023, 14, 1211896. [Google Scholar] [CrossRef]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef]
- Kalla, A.; Krishnamoorthy, P.; Gopalakrishnan, A.; Garg, J.; Patel, N.C.; Figueredo, V.M. Gender and age differences in cardiovascular complications in anorexia nervosa patients. Int. J. Cardiol. 2017, 227, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Vaurs, C.; Rollin, A.; Bérard, E.; Valet, M.; Saulnier, A.; Hazane, F.; Ritz, P.; Maury, P. QT interval is not prolonged in patients with eating disorders. Int. J. Cardiol. 2014, 177, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Moscucci, F.; Sciomer, S.; Maffei, S.; Nasi, M.; Pinti, M.; Bucciarelli, V.; Dei Cas, A.; Parati, G.; Ciccone, M.; et al. Cardiovascular prevention in women: Un update By the Italian Society of Cardiology Working Group On “Prevention, Hypertension and peripheral disease”. J. Cardiovasc. Med. 2023, 24 (Suppl. S2), e147–e155. [Google Scholar] [CrossRef]
- Daubert, M.A.; Douglas, P.S. Primary prevention of heart failure in women. JACC Heart Fail. 2019, 7, 181–191. [Google Scholar] [CrossRef]
- Männistö, T.; Mendola, P.; Vääräsmäki, M.; Järvelin, M.R.; Hartikainen, A.L.; Pouta, A.; Suvanto, E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013, 127, 681–690. [Google Scholar] [CrossRef]
- Grand’Maison, S.; Pilote, L.; Okano, M.; Landry, T.; Dayan, N. Markers of vascular dysfunction after hypertensive disorders of pregnancy: A systematic review and meta-analysis. Hypertension 2016, 68, 1447–1458. [Google Scholar] [CrossRef]
- Khan, S.S.; Brewer, L.C.; Canobbio, M.M.; Cipolla, M.J.; Grobman, W.A.; Lewey, J.; Michos, E.D.; Miller, E.C.; Perak, A.M.; Wei, G.S.; et al. Optimizing Prepregnancy Cardiovascular Health to Improve Outcomes in Pregnant and Postpartum Individuals and Offspring: A Scientific Statement From the American Heart Association. Circulation 2023, 147, e76–e91. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Gallina, S. Early cardiovascular prevention: The crucial role of nurse-led intervention. BMC Nurs. 2023, 22, 347. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Guan, J.; Retnakaran, R.; Shah, B.R. Gestational diabetes and incident heart failure: A cohort study. Diabet. Care 2021, 44, 2346–2352. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Lisheng, L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Austin, P.C.; Lee, D.S.; Amir, E.; Tu, J.V.; Thavendiranathan, P.; Fung, K.; Anderson, G.M. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017, 2, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Aslam, F.; Bandeali, S.J.; Khan, N.A.; Alam, M. Diastolic dysfunction in rheumatoid arthritis: A meta-analysis and systematic review. Arthritis Care Res. 2013, 65, 534–543. [Google Scholar] [CrossRef]
- Redfield, M.M.; Jacobsen, S.J.; Borlaug, B.A.; Rodeheffer, R.J.; Kass, D.A. Age- and gender-related ventricular-vascular stiffening: A community-based study. Circulation 2005, 112, 2254–2262. [Google Scholar] [CrossRef]
- Ha, J.W.; Lee, H.C.; Park, S.; Choi, E.Y.; Seo, H.S.; Shim, C.Y.; Kim, J.M.; Ahn, J.A.; Lee, S.W.; Rim, S.J.; et al. Gender-related difference in left ventricular diastolic elastance during exercise in patients with diabetes mellitus. Circ. J. 2008, 72, 1443–1448. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Del Buono, M.; Ruocco, G.; Caravita, S.; Abbate, A.; Lavie, C.J. The Conundrum of HFpEF Definition: Non-Invasive Assessment Uncertainties and Alternative Diagnostic Strategies. Curr. Probl. Cardiol. 2023, 48, 101433. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef]
- Russo, C.; Jin, Z.; Palmieri, V.; Homma, S.; Rundek, T.; Elkind, M.S.; Sacco, R.L.; Di Tullio, M.R. Arterial stiffness and wave reflection: Sex differences and relationship with left ventricular diastolic function. Hypertension 2012, 60, 362–368. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Olson, T.P.; Lam, C.S.; Flood, K.S.; Lerman, A.; Johnson, B.D.; Redfield, M.M. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2010, 56, 845–854. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Caravita, S.; Paolillo, S.; Ghio, S.; Tocchetti, C.G.; Ruocco, G.; Correale, M.; Ambrosio, G.; Perrone Filardi, P.; Senni, M.; et al. Current gaps in HFpEF trials: Time to reconsider patients’ selection and to target phenotypes. Prog. Cardiovasc. Dis. 2021, 67, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Barilli, M.; Tavera, M.C.; Valente, S.; Palazzuoli, A. Structural and Hemodynamic Changes of the Right Ventricle in PH-HFpEF. Int. J. Mol. Sci. 2022, 23, 4554. [Google Scholar] [CrossRef]
- Badesch, D.B.; Raskob, G.E.; Elliott, C.G.; Krichman, A.M.; Farber, H.W.; Frost, A.E.; Barst, R.J.; Benza, R.L.; Liou, T.G.; Turner, M.; et al. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest 2010, 137, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Pedram, A.; Razandi, M.; Lubahn, D.; Liu, J.; Vannan, M.; Levin, E.R. Estrogen Inhibits Cardiac Hypertrophy: Role of Estrogen Receptor-β to Inhibit Calcineurin. Endocrinology 2008, 149, 3361–3369. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Narayanan, R.; Dalton, J.T.; McKinsey, T.A.; Levin, E.R. Estrogen regulates histone deacetylases to prevent cardiac hypertrophy. Mol. Biol. Cell 2013, 24, 3805–3818. [Google Scholar] [CrossRef]
- Patten, R.D.; Pourati, I.; Aronovitz, M.J.; Baur, J.; Celestin, F.; Chen, X.; Michael, A.; Haq, S.; Nuedling, S.; Grohe, C.; et al. 17betaestradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ. Res. 2004, 95, 692–699. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Z.P.; Zhao, W.; Cong, B.H.; Lu, J.Q.; Tang, X.L.; Li, X.H.; Zhu, X.Y.; Ni, X. MiR-22/Sp-1 links estrogens with the up-regulation of cystathionine gamma-lyase in myocardium, which contributes to estrogenic cardioprotection against oxidative stress. Endocrinology 2015, 156, 2124–2137. [Google Scholar] [CrossRef]
- Golden, K.L.; Marsh, J.D.; Jiang, Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm. Metab. Res. 2004, 36, 197–202. [Google Scholar]
- Parks, R.J.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflugers Arch. 2013, 465, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Fornes, P.; Costagliola, R.; Esposito, B.; Belmin, J.; Lecomte, D.; Tedgui, A. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M719–M723. [Google Scholar] [CrossRef]
- Olivetti, G.; Giordano, G.; Corradi, D.; Melissari, M.; Lagrasta, C.; Gambert, S.R.; Anversa, P. Gender differences and aging: Effects on the human heart. J. Am. Coll. Cardiol. 1995, 26, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Dworatzek, E.; Petrov, G.; Summer, H.; Schulze, T.M.; Baczko, I.; Knosalla, C.; Golz, S.; Hetzer, R.; Regitz-Zagrosek, V. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur. J. Heart Fail. 2014, 16, 1160–1167. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; O’Mahony, F.; Lubahn, D.; Levin, E.R. Estrogen receptor-β prevents cardiac fibrosis. Mol. Endocrinol. 2010, 24, 2152–2165. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Aihara, K.; Sato, T.; Akaike, M.; Yoshizumi, M.; Suzaki, Y.; Izawa, Y.; Fujimura, M.; Hashizume, S.; Kato, M. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J. Biol. Chem. 2005, 280, 29661–29666. [Google Scholar] [CrossRef]
- Petrov, G.; Regitz-Zagrosek, V.; Lehmkuhl, E.; Krabatsch, T.; Dunkel, A.; Dandel, M.; Dworatzek, E.; Mahmoodzadeh, S.; Schubert, C.; Becher, E. Regression of myocardial hypertrophy after aortic valve replacement. Circulation 2010, 122, S23–S28. [Google Scholar] [CrossRef]
- Haynes, M.P.; Li, L.; Sinha, D.; Russell, K.S.; Hisamoto, K.; Baron, R.; Collinge, M.; Sessa, W.C.; Bender, J.R. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J. Biol. Chem. 2003, 278, 2118–2123. [Google Scholar] [CrossRef]
- Sobrino, A.; Oviedo, P.J.; Novella, S.; Laguna-Fernandez, A.; Bueno, C.; García-Pérez, M.A.; Tarín, J.J.; Cano, A.; Hermenegildo, C. Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-α. J. Mol. Endocrinol. 2010, 44, 237. [Google Scholar] [CrossRef]
- Jesmin, S.; Mowa, C.N.; Sultana, S.N.; Shimojo, N.; Togashi, H.; Iwashima, Y.; Kato, N.; Sato, A.; Sakuma, I.; Hiroe, M.; et al. VEGF signaling is disrupted in the hearts of mice lacking estrogen receptor alpha. Eur. J. Pharmacol. 2010, 641, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Trindade, F.; Ferreira, R.; Neves, J.S.; Leite-Moreira, A.; Amado, F.; Santos, M.; Nogueira-Ferreira, R. Sexual dimorphism in cardiac remodeling: The molecular mechanisms ruled by sex hormones in the heart. J. Mol. Med. 2022, 100, 245–267. [Google Scholar] [CrossRef]
- Mukherjee, T.K.; Dinh, H.; Chaudhuri, G.; Nathan, L. Testosterone attenuates expression of vascular cell adhesion molecule-1 by conversion to estradiol by aromatase in endothelial cells: Implications in atherosclerosis. Proc. Natl. Acad. Sci. USA 2002, 99, 4055–4060. [Google Scholar] [CrossRef]
- Hoetzer, G.L.; MacEneaney, O.J.; Irmiger, H.M.; Keith, R.; Van Guilder, G.P.; Stauffer, B.L.; DeSouza, C.A. Gender Differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. Am. J. Cardiol. 2007, 99, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Lu, Q.; Baur, W.; Aronovitz, M.J.; Karas, R.H. Rapid estrogen receptor signaling mediates estrogen-induced inhibition of vascular smooth muscle cell proliferation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Sivritas, D.; Becher, M.U.; Ebrahimian, T.; Arfa, O.; Rapp, S.; Bohner, A.; Mueller, C.F.; Umemura, T.; Wassmann, S.; Nickenig, G.; et al. Antiproliferative effect of estrogen in vascular smooth muscle cells is mediated by Kruppel-like factor-4 and manganese superoxide dismutase. Basic Res. Cardiol. 2011, 106, 563–575. [Google Scholar] [CrossRef]
- Lopes, R.A.M.; Neves, K.B.; Pestana, C.R.; Queiroz, A.L.; Zanotto, C.Z.; Chignalia, A.Z.; Valim, Y.M.; Silveira, L.R.; Curti, C.; Tostes, R.C. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1485–H1494. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2018, 111, 18–25. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Mullens, W. Cardiac congestion assessed by natriuretic peptides oversimplifies the definition and treatment of heart failure. ESC Heart Fail. 2021, 8, 3453–3457. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Ho, J.E.; Gansevoort, R.T.; Voors, A.A.; van der Meer, P.; Bakker, S.J.L.; Heymans, S.; van Empel, V.; Schroen, B.; et al. Sex-specific associations of obesity and N-terminal pro-B-type natriuretic peptide levels in the general population. Eur. J. Heart Fail. 2018, 20, 1205–1214. [Google Scholar] [CrossRef]
- Yao, M.; Nguyen, T.V.; Rosario, E.R.; Ramsden, M.; Pike, C.J. Androgens regulate neprilysin expression: Role in reducing beta-amyloid levels. J. Neurochem. 2008, 105, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Yang, L.; Yin, C.; Xiao, Z.; Zhang, J.; Liu, Y.; Huang, J. Estrogen stimulates degradation of beta-amyloid peptide by up-regulating neprilysin. J. Biol. Chem. 2010, 285, 935–942. [Google Scholar] [CrossRef]
- Maffei, S.; del Ry, S.; Prontera, C.; Clerico, A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin. Sci. 2001, 101, 447–453. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Tramonte, F.; Beltrami, M. Laboratory and Metabolomic Fingerprint in Heart Failure with Preserved Ejection Fraction: From Clinical Classification to Biomarker Signature. Biomolecules 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, S.R.; Sarma, A.; Januzzi, J.L.; O’Donoghue, M.L. Biomarkers in ACS and heart failure: Should men and women be interpreted differently? Clin. Chem. 2014, 60, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Faxén, U.L.; Lund, L.H.; Orsini, N.; Strömberg, A.; Andersson, D.C.; Linde, C.; Dahlström, U.; Savarese, G. N-terminal pro-B-type natriuretic peptide in chronic heart failure: The impact of sex across the ejection fraction spectrum. Int. J. Cardiol. 2019, 287, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Lindahl, B. Impact of sex on cardiac troponin concentrations—A critical appraisal. Clin. Chem. 2017, 63, 1457–1464. [Google Scholar] [CrossRef]
- Grodin, J.L.; Neale, S.; Wu, Y.; Hazen, S.L.; Tang, W.H.W. Prognostic comparison of different sensitivity cardiac troponin assays in stable heart failure. Am. J. Med. 2015, 128, 276–282. [Google Scholar] [CrossRef]
- Papamitsou, T.; Barlagiannis, D.; Papaliagkas, V.; Kotanidou, E.; Dermentzopoulou-Theodoridou, M. Testosterone-induced hypertrophy, fibrosis and apoptosis of cardiac cells—An ultrastructural and immunohistochemical study. Med. Sci. Monit. 2011, 17, BR266–BR273. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; de Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L., Jr.; Kiernan, M.S.; et al. Role of biomarkers for the prevention, assessment, and management of heart failure: A scientific statement from the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/ HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.T.; de Boer, R.A. Galectin-3 activation and inhibition in heart failure and cardiovascular disease: An update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef]
- Beltrami, M.; Ruocco, G.; Dastidar, A.G.; Franci, B.; Lucani, B.; Aloia, E.; Nuti, R.; Palazzuoli, A. Additional value of Galectin-3 to BNP in acute heart failure patients with preserved ejection fraction. Clin. Chim. Acta 2016, 457, 99–105. [Google Scholar] [CrossRef]
- Jagodzinski, A.; Havulinna, A.S.; Appelbaum, S.; Zeller, T.; Jousilahti, P.; Skytte-Johanssen, S.; Hughes, M.F.; Blankenberg, S.; Salomaa, V. Predictive value of galectin-3 for incident cardiovascular disease and heart failure in the population-based FINRISK 1997 cohort. Int. J. Cardiol. 2015, 192, 33–39. [Google Scholar] [CrossRef]
- Gehlken, C.; Suthahar, N.; Meijers, W.C.; de Boer, R.A. Galectin-3 in heart failure: An update of the last 3 years. Heart Fail. Clin. 2018, 14, 75–92. [Google Scholar] [CrossRef]
- Bayés-Genis, A.; González, A.; Lupón, J. ST2 in heart failure. Circ. Heart Fail. 2018, 11, e005582. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.; Sanghavi, M.; Ayers, C.R.; McGuire, D.K.; Omland, T.; Atzler, D.; Gore, M.O.; Neeland, I.; Berry, J.D.; Khera, A.; et al. Sex-Based differences in cardiometabolic biomarkers. Circulation 2017, 135, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Rector, T.S.; Kuskowski, M.; Snider, J.; Cohn, J.N. Prognostic value of soluble ST2 in the valsartan heart failure trial. Circ. Heart Fail. 2014, 7, 418–426. [Google Scholar] [CrossRef]
- Coglianese, E.E.; Larson, M.G.; Vasan, R.S.; Ho, J.E.; Ghorbani, A.; McCabe, E.L.; Cheng, S.; Fradley, M.G.; Kretschman, D.; Gao, W.; et al. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin. Chem. 2012, 58, 1673–1681. [Google Scholar] [CrossRef]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef] [PubMed]
- López, B.; González, A.; Lindner, D.; Westermann, D.; Ravassa, S.; Beaumont, J.; Gallego, I.; Zudaire, A.; Brugnolaro, C.; Querejeta, R.; et al. Osteopontin-mediated myocardial fibrosis in heart failure: A role for lysyloxidase? Cardiovasc. Res. 2013, 99, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Arnlöv, J.; Evans, J.C.; Benjamin, E.J.; Larson, M.G.; Levy, D.; Sutherland, P.; Siwik, D.A.; Wang, T.J.; Colucci, W.S.; Vasan, R.S. Clinical and echocardiographic correlates of plasma osteopontin in the community: The Framingham Heart Study. Heart 2006, 92, 1514–1515. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Prosperi, S.; Dei Cas, A.; Mattioli, A.V.; Cevese, A.; Novo, G.; Prat, M.; Pedrinelli, R.; Raddino, R.; et al. Do the Current Guidelines for Heart Failure Diagnosis and Treatment Fit with Clinical Complexity? J. Clin. Med. 2022, 11, 857. [Google Scholar] [CrossRef] [PubMed]
| Epidemiological Data | Men | Women |
|---|---|---|
| Framingham Heart Study | The risk of developing heart failure is 21% at age 40 years | The risk of developing heart failure is 20% at age 40 years |
| Rotterdam Study | The risk of developing heart failure is 33% at age 55 years | The risk of developing heart failure is 29% at age 55 years |
| Southwestern European community-based Epidemiology of Heart Failure and Learning (EPICA) study | The prevalence of heart failure with preserved ejection fraction is 0% in the 25–49 age group, and increases to 4–6% in men aged 80 or older | The prevalence of heart failure with preserved ejection fraction is 1% in the 25–49 age group, and increases to 8–10% in women aged 80 or older |
| Risk Factor | Men | Women |
|---|---|---|
| Diabetes mellitus | 2.4-fold increased risk of HF | 5-fold increased risk of HF |
| Hypertension | 2-fold increased risk of HF | 3-fold increased risk oh HF |
| Obesity | Lower association with male sex and HFrEF | Greater association with female sex and HFpEF |
| Tobacco smoke | Higher consumption but lower correlation (45% increased risk) | Lower consumption (increasing) but higher correlation (88% increased risk) |
| Coronary artery disease | Macrovascular disease more associated with both HFrEF and HFpEF | Less frequent, more often microvascular dysfunction |
| Anemia and iron deficiency | Less frequent | More frequent and associated with HFpEF |
| Vitamin D deficiency | Less frequent | More frequent and associated with higher cardiovascular mortality |
| Anorexia nervosa | Higher association with cardiovascular events | More frequent |
| Biomarkers | Androgens | Estrogens |
|---|---|---|
| NT-proBNP (1) | Lower basal level in men due to probable upregulation of neprilysin by testosterone | • Higher basal level in women • Exogenous estrogen therapy associated with a higher level |
| Troponin (2) | Higher basal values and greater increase in male with HF due to the prohypertrophic and proapoptotic hormonal action | Lower baseline values and smaller increase in women with HF due to the antiapoptotic action of estrogens |
| Galectin-3 (3) | Reduced peripheral fat accumulation with lower level in men | Greater fat mass due to estrogen with higher level in women |
| sST-2 (4) | Higher mean values in men both in health and in HF | • Lower mean values in women both in health and in HF • Lower level during exogenous estrogen therapy |
| Osteopontin (5) | Higher level in men | Estrogen inhibition with lower level in women |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delcuratolo, E.; Palazzuoli, A.; Coppi, F.; Mattioli, A.V.; Severino, P.; Tramonte, F.; Fedele, F. Risk Factors and Cellular Differences in Heart Failure: The Key Role of Sex Hormones. Biomedicines 2023, 11, 3052. https://doi.org/10.3390/biomedicines11113052
Delcuratolo E, Palazzuoli A, Coppi F, Mattioli AV, Severino P, Tramonte F, Fedele F. Risk Factors and Cellular Differences in Heart Failure: The Key Role of Sex Hormones. Biomedicines. 2023; 11(11):3052. https://doi.org/10.3390/biomedicines11113052
Chicago/Turabian StyleDelcuratolo, Elvira, Alberto Palazzuoli, Francesca Coppi, Anna Vittoria Mattioli, Paolo Severino, Francesco Tramonte, and Francesco Fedele. 2023. "Risk Factors and Cellular Differences in Heart Failure: The Key Role of Sex Hormones" Biomedicines 11, no. 11: 3052. https://doi.org/10.3390/biomedicines11113052
APA StyleDelcuratolo, E., Palazzuoli, A., Coppi, F., Mattioli, A. V., Severino, P., Tramonte, F., & Fedele, F. (2023). Risk Factors and Cellular Differences in Heart Failure: The Key Role of Sex Hormones. Biomedicines, 11(11), 3052. https://doi.org/10.3390/biomedicines11113052