Discriminating between Homogeneous (AC-1) and Dense Fine Speckled (AC-2) Antinuclear Antibody Patterns: Re-Evaluation of Immunofluorescence Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. ANA IIFA
- First incubation: Pipette an adequate volume of control samples and diluted serum samples (1:80 with sample buffer) into each well of slides. Incubate slides for 30 min ± 10 min at room temperature (20–26 °C) in the moist incubator tray.
- Washing: Remove slides from incubator tray and rinse briefly with wash buffer using a squeeze wash bottle. Wash slides 2 × 5 min with wash buffer in a slide staining dish.
- Second incubation: Return slides to incubator tray and cover each well with an adequate volume of FITC-conjugate (fluorescein labelled anti-human IgG). Incubate slides for 30 min ± 10 min at room temperature in the dark.
- Washing: Wash as described above.
- Mounting medium: Add an adequate volume of mounting medium along midline of each slide.
- Reading: Read slides at 400–800 × total magnification with a fluorescent microscope.
2.3. Solid-Phase Assay
- First incubation: Place each test strip in an empty channel. Fill each channel with 1.5 mL of the diluted serum sample (1:101 with sample buffer). Incubate for 30 min at room temperature (18–25 °C) on a rocking shaker.
- Wash: Aspirate off the liquid from each channel and wash 3 × 5 min each with 1.5 mL working-strength wash buffer on a rocking shaker.
- Second incubation: Pipette 1.5 mL diluted enzyme conjugate (alkaline phosphatase-labelled anti-human IgG) into each channel. Incubate for 30 min at room temperature on a rocking shaker.
- Wash: Wash as described above.
- Third incubation: Pipette 1.5 mL substrate solution into the channels of the incubation tray. Incubate for 10 min at room temperature on a rocking shaker.
- Stop: Aspirate off the liquid from each channel and wash each strip 3 × 1 min with distilled water.
- Evaluate: Place test strip on the evaluation protocol, air dry and evaluate.
2.4. Guidance and Re-Evaluation
2.5. Clinical Relevance
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damoiseaux, J.; Andrade, L.E.C.; Carballo, O.G.; Conrad, K.; Francescantonio, P.L.C.; Fritzler, M.J.; Garcia de la Torre, I.; Herold, M.; Klotz, W.; Cruvinel, W.M.; et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: The International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum. Dis. 2019, 78, 879–889. [Google Scholar] [CrossRef]
- Bossuyt, X.; De Langhe, E.; Borghi, M.O.; Meroni, P.L. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.K.L.; von Mühlen, C.A.; Fritzler, M.J.; Damoiseaux, J.; Infantino, M.; Klotz, W.; Satoh, M.; Musset, L.; García-De La Torre, I.; Carballo, O.G.; et al. The International Consensus on ANA Patterns (ICAP) in 2021-The 6th Workshop and Current Perspectives. J. Appl. Lab. Med. 2022, 7, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Meroni, P.L.; Bossuyt, X.; Fritzler, M.J. Current concepts and future directions for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. J. Immunol. Res. 2014, 2014, 315179. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, X.; Luyckx, A. Antibodies to extractable nuclear antigens in antinuclear antibody-negative samples. Clin. Chem. 2005, 51, 2426–2427. [Google Scholar] [CrossRef][Green Version]
- Andrade, L.E.C.; Damoiseaux, J.; Vergani, D.; Fritzler, M.J. Antinuclear antibodies (ANA) as a criterion for classification and diagnosis of systemic autoimmune diseases. J. Transl. Autoimmun. 2022, 5, 100145. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.; Sui, M.; Han, J.; Sun, L.; Jia, X.; Zhang, H.; Han, C.; Jin, X.; Gao, F.; et al. Co-Positivity for Anti-dsDNA, -Nucleosome and -Histone Antibodies in Lupus Nephritis Is Indicative of High Serum Levels and Severe Nephropathy. PLoS ONE 2015, 10, e0140441. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Hernandez, G.L.; Sanchez-Hernandez, E.S.; Casiano, C.A. Twenty years of research on the DFS70/LEDGF autoantibody-autoantigen system: Many lessons learned but still many questions. Autoimmun. Highlights 2020, 11, 3. [Google Scholar] [CrossRef]
- Bizzaro, N.; Tonutti, E.; Villalta, D. Recognizing the dense fine speckled/lens epithelium-derived growth factor/p75 pattern on HEP-2 cells: Not an easy task! Comment on the article by Mariz et al. Arthritis Rheum. 2011, 63, 4036–4037; author reply 4037–4038. [Google Scholar] [CrossRef]
- Zanussi, J.T.; Zhao, J.; Wei, W.Q.; Karakoc, G.; Chung, C.P.; Feng, Q.; Olsen, N.J.; Stein, C.M.; Kawai, V.K. Clinical diagnoses associated with a positive antinuclear antibody test in patients with and without autoimmune disease. BMC Rheumatol. 2023, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Bentow, C.; Fritzler, M.J.; Mummert, E.; Mahler, M. Recognition of the dense fine speckled (DFS) pattern remains challenging: Results from an international internet-based survey. Autoimmun. Highlights 2016, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Van Hoovels, L.; Broeders, S.; Chan, E.K.L.; Andrade, L.; de Melo Cruvinel, W.; Damoiseaux, J.; Viander, M.; Herold, M.; Coucke, W.; Heijnen, I.; et al. Current laboratory and clinical practices in reporting and interpreting anti-nuclear antibody indirect immunofluorescence (ANA IIF) patterns: Results of an international survey. Autoimmun. Highlights 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Yang, C.T.; Cheng, C.H.; Yu, K.H. Diagnostic performance of anti-cyclic citrullinated peptide and rheumatoid factor in patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2016, 19, 880–886. [Google Scholar] [CrossRef]
- von Mühlen, C.A.; Garcia-De La Torre, I.; Infantino, M.; Damoiseaux, J.; Andrade, L.E.C.; Carballo, O.G.; Conrad, K.; Francescantonio, P.L.C.; Fritzler, M.J.; Herold, M.; et al. How to report the antinuclear antibodies (anti-cell antibodies) test on HEp-2 cells: Guidelines from the ICAP initiative. Immunol. Res. 2021, 69, 594–608. [Google Scholar] [CrossRef]
- Tan, E.M.; Feltkamp, T.E.; Smolen, J.S.; Butcher, B.; Dawkins, R.; Fritzler, M.J.; Gordon, T.; Hardin, J.A.; Kalden, J.R.; Lahita, R.G.; et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997, 40, 1601–1611. [Google Scholar] [CrossRef]
- La Jeon, Y.; Kang, S.Y.; Lee, W.I.; Kim, M.H. Clinical Aspects of the Dense Fine Speckled Pattern in Indirect Immunofluorescence-Antinuclear Antibody Screening and Its Association with DFS70 Autoantibodies. Ann. Clin. Lab. Sci. 2019, 49, 496–502. [Google Scholar]
- Yumuk, Z.; Demir, M. Clinical value of anti-DFS70 antibodies in a cohort of patients undergoing routine antinuclear antibodies testing. J. Immunol. Methods 2020, 480, 112754. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.B.; Carter, S.; Saschenbrecker, S.; Goeckeritz, B.E. Recognition and Relevance of Anti-DFS70 Autoantibodies in Routine Antinuclear Autoantibodies Testing at a Community Hospital. Front. Med. 2018, 5, 88. [Google Scholar] [CrossRef]
- Kang, S.H.; Seo, Y.I.; Lee, M.H.; Kim, H.A. Diagnostic Value of Anti-Nuclear Antibodies: Results from Korean University-Affiliated Hospitals. J. Korean Med. Sci. 2022, 37, e159. [Google Scholar] [CrossRef]
- Kavanaugh, A.; Tomar, R.; Reveille, J.; Solomon, D.H.; Homburger, H.A. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch. Pathol. Lab. Med. 2000, 124, 71–81. [Google Scholar] [CrossRef]
- Ge, Q.; Gu, X.; Yu, W.; Zhang, G.; Liang, W.; Li, M.; Zhai, G.; Yan, M. Antinuclear antibodies in healthy population: Positive association with abnormal tissue metabolism, inflammation and immune dysfunction. Int. Immunopharmacol. 2022, 113, 109292. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Cui, J.; Song, W.; Liang, Y.; Hu, Y.; Guo, Y. Epidemiological survey of antinuclear antibodies in healthy population and analysis of clinical characteristics of positive population. J. Clin. Lab. Anal. 2019, 33, e22965. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 1151–1159. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.; Han, K.; Oh, E.J. Application of anti-DFS70 antibody and specific autoantibody test algorithms to patients with the dense fine speckled pattern on HEp-2 cells. Scand. J. Rheumatol. 2016, 45, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.; Röber, N.; Andrade, L.E.; Mahler, M. The Clinical Relevance of Anti-DFS70 Autoantibodies. Clin. Rev. Allergy Immunol. 2017, 52, 202–216. [Google Scholar] [CrossRef]
- Deng, C.; Wang, A.; Hu, C.; Zhang, W.; Zeng, X.; Fei, Y. The Prevalence and Clinical Relevance of the DFS Immunofluorescence Staining Pattern in a Large ANA-Positive Cohort. Front. Med. 2022, 9, 829436. [Google Scholar] [CrossRef]
- Dinse, G.E.; Zheng, B.; Co, C.A.; Parks, C.G.; Weinberg, C.R.; Miller, F.W.; Chan, E.K.L. Anti-dense fine speckled 70 (DFS70) autoantibodies: Correlates and increasing prevalence in the United States. Front. Immunol. 2023, 14, 1186439. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.; Fatima, A.; Abdul Jabbar, S.; Arain, T. ANA-specific antibodies, ANA patterns, anti-ds-DNA results, and clinical diagnosis: A laboratory and clinical audit. Immunol. Res. 2023, 71, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Al-Mughales, J.A. Anti-Nuclear Antibodies Patterns in Patients with Systemic Lupus Erythematosus and Their Correlation with Other Diagnostic Immunological Parameters. Front. Immunol. 2022, 13, 850759. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Jiang, Y.; Xie, J.; Lv, Q.; Xie, Y.; Tu, L.; Xiao, M.; Wu, Z.; Gu, J. Analysis of antinuclear antibody titers and patterns by using HEp-2 and primate liver tissue substrate indirect immunofluorescence assay in patients with systemic autoimmune rheumatic diseases. J. Clin. Lab. Anal. 2020, 34, e23546. [Google Scholar] [CrossRef] [PubMed]
- Rösken, G.H.J.; van Beek, A.A.; Bakker-Jonges, L.E.; Schreurs, M.W.J. [Antinuclear antibodies in systemic autoimmune disease]. Ned. Tijdschr. Geneeskd. 2020, 164, D4066. [Google Scholar] [PubMed]
- Vlagea, A.; Falagan, S.; Gutiérrez-Gutiérrez, G.; Moreno-Rubio, J.; Merino, M.; Zambrana, F.; Casado, E.; Sereno, M. Antinuclear antibodies and cancer: A literature review. Crit. Rev. Oncol. Hematol. 2018, 127, 42–49. [Google Scholar] [CrossRef] [PubMed]

| Anti-Nucleosome/ Histone/dsDNA (+) * | Anti-Nucleosome/ Histone/dsDNA (−) | LR+/LR− | OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Total (healthy subjects + patients)—Original | |||||
| AC-1 (+) | 6 | 23 | 0.89/1.10 | 0.82 (0.25–2.71) | 0.74 |
| AC-1 (−) | 8 | 25 | |||
| Total (healthy subjects + patients)—Excluding 1:80 | |||||
| AC-1 (+) | 5 | 19 | 0.86/1.14 | 0.75 (0.20–2.78) | 0.67 |
| AC-1 (−) | 7 | 20 | |||
| Total (healthy subjects + patients)—Re-evaluated | |||||
| AC-1 (+) | 8 | 25 | 1.10/0.89 | 1.23 (0.37–4.07) | 0.74 |
| AC-1 (−) | 6 | 23 | |||
| Patients—Original | |||||
| AC-1 (+) | 5 | 14 | 1.18/0.87 | 1.36 (0.33–5.61) | 0.67 |
| AC-1 (−) | 5 | 19 | |||
| Patients—Re-evaluated | |||||
| AC-1 (+) | 8 | 14 | 1.89/0.35 | 5.43 (1.00–29.61) | 0.05 |
| AC-1 (−) | 2 | 19 | |||
| Anti-DFS70 (+) | Anti-DFS70 (−) | LR+/LR− | OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Total (healthy subjects + patients)—Original | |||||
| AC-2 (+) | 24 | 14 | 1.71/0.41 | 4.16 (1.39–12.50) | 0.01 |
| AC-2 (−) | 7 | 17 | |||
| Total (healthy subjects + patients)—Excluded 1:80 | |||||
| AC-2 (+) | 22 | 10 | 1.67/0.44 | 3.77 (1.14–12.46) | 0.03 |
| AC-2 (−) | 7 | 12 | |||
| Total (healthy subjects + patients)—Re-evaluated | |||||
| AC-2 (+) | 23 | 1 | 24.53/0.24 | 101.86 (11.70–886.44) | <0.001 |
| AC-2 (−) | 7 | 31 | |||
| Patients—Original | |||||
| AC-2 (+) | 17 | 12 | 1.23/0.65 | 1.89 (0.52–6.87) | 0.33 |
| AC-2 (−) | 6 | 8 | |||
| Patients—Re-evaluated | |||||
| AC-2 (+) | 17 | 1 | 14.78/0.27 | 53.83 (5.87–493.61) | <0.001 |
| AC-2 (−) | 6 | 19 | |||
| n | Percentage | |
|---|---|---|
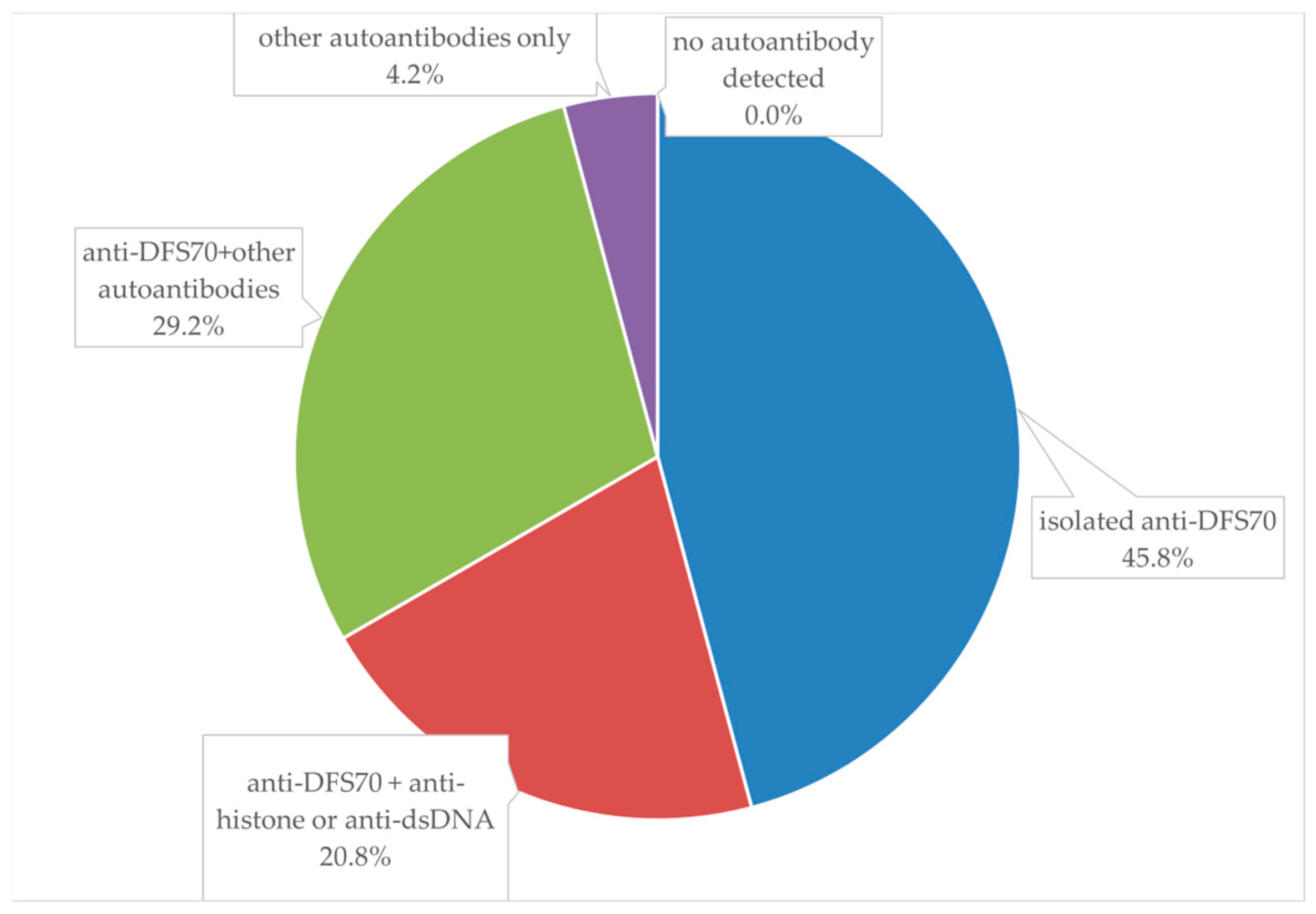
| Specific autoantibodies (+)/AC-2 | 13/24 | 54.2% * |
| dsDNA | 4 | 16.7% |
| histone | 2 | 8.3% |
| Sm | 1 | 4.2% |
| RNP/Sm | 2 | 8.3% |
| RP155 | 4 | 16.7% |
| Ku | 1 | 4.2% |
| PM-Scl75 | 3 | 12.5% |
| Sp100 | 1 | 4.2% |
| Mi-2a | 1 | 4.2% |
| Mi-2b | 1 | 4.2% |
| n | ANA Pattern (Re-Evaluated) | Specific Autoantibodies | |
|---|---|---|---|
| SARDs (+)/patients | 10 */43 | ||
| Sjögren’s syndrome | 5 | AC-1 | AMA-M2, histone, nucleosome, dsDNA, SSB, Ro52, SSA |
| AC-1 & AC-4 | Ro-52, SSA, histone, DFS70 | ||
| AC-1 | nil | ||
| AC-1 | RP155, PM100, dsDNA | ||
| AC-1 | RP155, Ro-52, SSA | ||
| RA | 3 | AC-1 | nil |
| AC-1 | RP11, RP155 | ||
| AC-2 | PM75, Sp100, Mi-2b, DFS70 | ||
| SLE | 2 | AC-1 | AMA-M2, histone, nucleosome, dsDNA, SSB, Ro52, SSA |
| AC-4 | Ku, SSB, Ro-52, SSA | ||
| AS | 1 | AC-2 | RP-155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-H.; Hsieh, P.-F.; Huang, S.-W.; Chan, T.-M.; Tai, P.-L.; Yang, S.-T.; Yu, K.-H. Discriminating between Homogeneous (AC-1) and Dense Fine Speckled (AC-2) Antinuclear Antibody Patterns: Re-Evaluation of Immunofluorescence Imaging. Biomedicines 2023, 11, 3027. https://doi.org/10.3390/biomedicines11113027
Yu H-H, Hsieh P-F, Huang S-W, Chan T-M, Tai P-L, Yang S-T, Yu K-H. Discriminating between Homogeneous (AC-1) and Dense Fine Speckled (AC-2) Antinuclear Antibody Patterns: Re-Evaluation of Immunofluorescence Imaging. Biomedicines. 2023; 11(11):3027. https://doi.org/10.3390/biomedicines11113027
Chicago/Turabian StyleYu, Han-Hua, Pao-Feng Hsieh, Szu-Wei Huang, Tien-Ming Chan, Pao-Lien Tai, Shih-Ting Yang, and Kuang-Hui Yu. 2023. "Discriminating between Homogeneous (AC-1) and Dense Fine Speckled (AC-2) Antinuclear Antibody Patterns: Re-Evaluation of Immunofluorescence Imaging" Biomedicines 11, no. 11: 3027. https://doi.org/10.3390/biomedicines11113027
APA StyleYu, H.-H., Hsieh, P.-F., Huang, S.-W., Chan, T.-M., Tai, P.-L., Yang, S.-T., & Yu, K.-H. (2023). Discriminating between Homogeneous (AC-1) and Dense Fine Speckled (AC-2) Antinuclear Antibody Patterns: Re-Evaluation of Immunofluorescence Imaging. Biomedicines, 11(11), 3027. https://doi.org/10.3390/biomedicines11113027





