Prospective Matched Case-Control Study of Over-Early P100 Wave Latency in Migraine with Aura
Abstract
:1. Introduction
Contribution
2. Literature Review
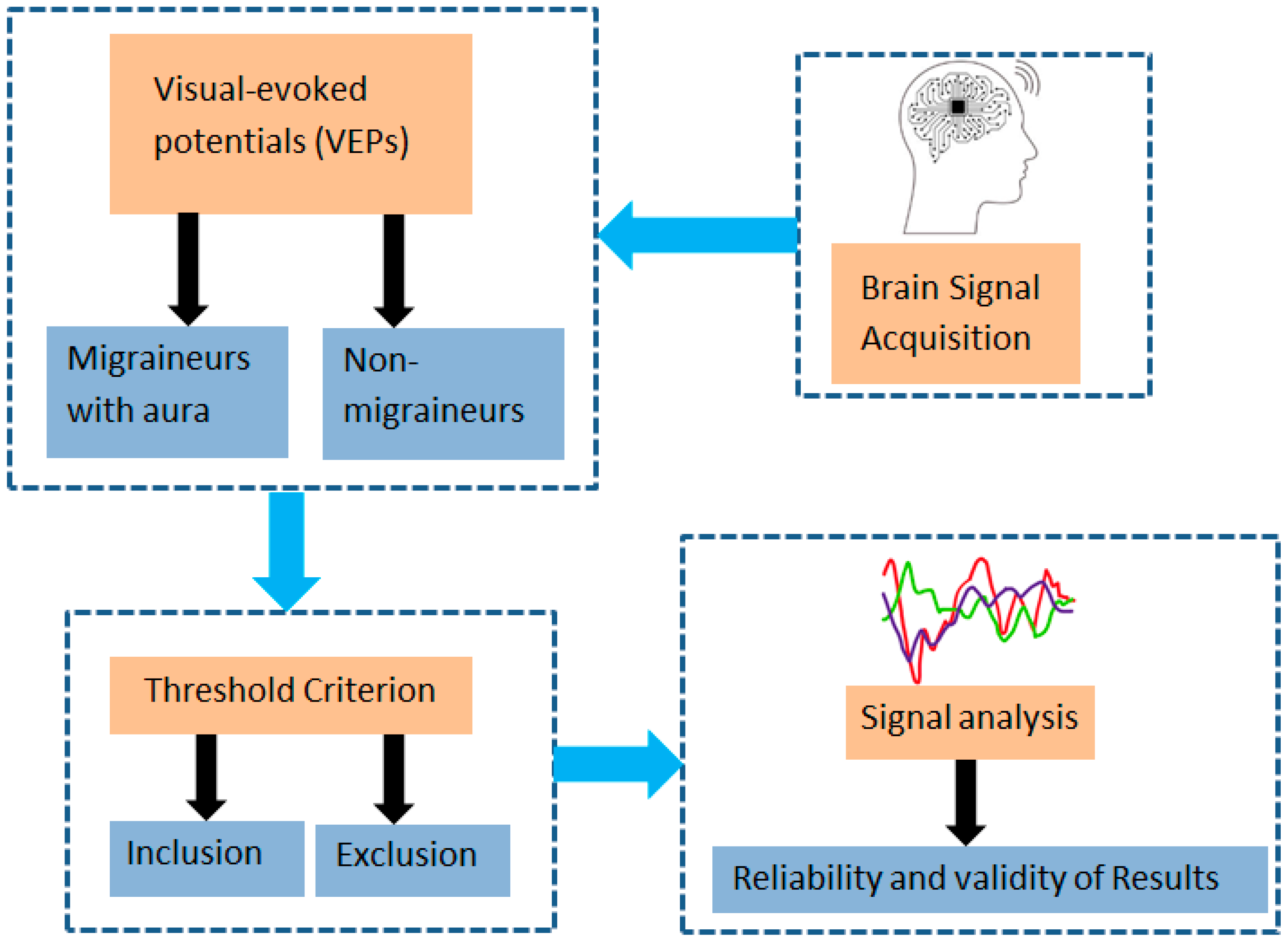
3. Materials and Methods
3.1. Research Ethics
3.2. Study Design and Participants
3.3. Sample Size
3.4. Sample Method
3.5. Selection Criteria
3.5.1. Inclusion Criteria
- (1)
- A patient must be at least 18 years old.
- (2)
- The patient must have aura-type migraine, as described by the diagnostic criteria of the International Headache Society [56].
- (3)
- Fixed with or without requiring a visual acuity of 6/6 or finer.
- (4)
- The absence of a headache attack at the time of testing; for interventional research involving either animals or people, ethical permission must be obtained from the competent authority, and the accompanying ethical approval code must be mentioned; these requirements must be met before testing may take place.
3.5.2. Exclusion Criteria
- -
- Participants who have any neurological condition other than migraine that has been medically verified.
- -
- Any condition that pertains to ophthalmology.
3.6. Instrument and Data Collection Procedure
3.7. Bias, Confounders, and Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stovner, L.J.; Andree, C. Prevalence of headache in Europe: A review for the Eurolight project. J. Headache Pain 2010, 11, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Bigal, M.E.; Scher, A.I.; Stewart, W.F. The global burden of migraine. J. Headache Pain 2003, 4, 3–11. [Google Scholar] [CrossRef]
- Merikangas, K.R. Contributions of epidemiology to our understanding of migraine. Headache J. Head Face Pain 2013, 53, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Sezai, T.; Murphy, M.J.; Riddell, N.; Nguyen, V.; Crewther, S.G. Visual processing during the interictal period between migraines: A meta-analysis. Neuropsychol. Rev. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Di Lorenzo, C.; Di Lenola, D.; Serrao, M.; Pierelli, F.; Parisi, V. Visual evoked potential responses after photostress in migraine patients and their correlations with clinical features. J. Clin. Med. 2021, 10, 982. [Google Scholar] [CrossRef]
- De Luca, C.; Gori, S.; Mazzucchi, S.; Dini, E.; Cafalli, M.; Siciliano, G.; Papa, M.; Baldacci, F. Supersaturation of VEP in migraine without aura patients treated with topiramate: An anatomo-functional biomarker of the disease. J. Clin. Med. 2021, 10, 769. [Google Scholar] [CrossRef]
- Omland, P.M.; Nilsen, K.B.; Uglem, M.; Gravdahl, G.; Linde, M.; Hagen, K.; Sand, T. Visual evoked potentials in interictal migraine: No confirmation of abnormal habituation. Headache J. Head Face Pain 2013, 53, 1071–1086. [Google Scholar] [CrossRef]
- Omland, P.M.; Uglem, M.; Hagen, K.; Linde, M.; Tronvik, E.; Sand, T. Visual evoked potentials in migraine: Is the “neurophysiological hallmark” concept still valid? Clin. Neurophysiol. 2016, 127, 810–816. [Google Scholar] [CrossRef]
- Ambrosini, A.; Rossi, P.; De Pasqua, V.; Pierelli, F.; Schoenen, J. Lack of habituation causes high intensity dependence of auditory evoked cortical potentials in migraine. Brain 2003, 126, 2009–2015. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Vingrys, A.J.; McKendrick, A.M. The effect of duration post-migraine on visual electrophysiology and visual field performance in people with migraine. Cephalalgia 2014, 34, 42–57. [Google Scholar] [CrossRef]
- Odom, J.V.; Bach, M.; Barber, C.; Brigell, M.; Marmor, M.F.; Tormene, A.P.; Holder, G.E.; Vaegan. Visual evoked potentials standard (2004). Doc. Ophthalmol. 2004, 108, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.L. Electrophysiological Studies of the Visual System of People with Classical Migraine; University of Surrey: London, UK, 1985. [Google Scholar]
- Bednář, M.; Kubová, Z.; Kremláček, J. Lack of visual evoked potentials amplitude decrement during prolonged reversal and motion stimulation in migraineurs. Clin. Neurophysiol. 2014, 125, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Parisi, V.; Di Lorenzo, C.; Serrao, M.; Magis, D.; Schoenen, J.; Pierelli, F. Lateral inhibition in visual cortex of migraine patients between attacks. J. Headache Pain 2013, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, A.; Iezzi, E.; Perrotta, A.; Kisialiou, A.; Nardella, A.; Berardelli, A.; Pierelli, F.; Schoenen, J. Correlation between habituation of visual-evoked potentials and magnetophosphene thresholds in migraine: A case-control study. Cephalalgia 2016, 36, 258–264. [Google Scholar] [CrossRef]
- Coppola, G.; Bracaglia, M.; Di Lenola, D.; Di Lorenzo, C.; Serrao, M.; Parisi, V.; Di Renzo, A.; Martelli, F.; Fadda, A.; Schoenen, J.; et al. Visual evoked potentials in subgroups of migraine with aura patients. J. Headache Pain 2015, 16, 1. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of migraine: A disorder of sensory processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Olesen, J. International classification of headache disorders. Lancet Neurol. 2018, 17, 396–397. [Google Scholar] [CrossRef]
- Tajti, J.; Szok, D.; Csáti, A.; Szabó, Á.; Tanaka, M.; Vécsei, L. Exploring novel therapeutic targets in the common pathogenic factors in migraine and neuropathic pain. Int. J. Mol. Sci. 2023, 24, 4114. [Google Scholar] [CrossRef]
- Burch, R.C.; Loder, S.; Loder, E.; Smitherman, T.A. The prevalence and burden of migraine and severe headache in the U nited S tates: Updated statistics from government health surveillance studies. Headache J. Head Face Pain 2015, 55, 21–34. [Google Scholar] [CrossRef]
- Leonardi, M.; Steiner, T.J.; Scher, A.T.; Lipton, R.B. The global burden of migraine: Measuring disability in headache disorders with WHO’s Classification of Functioning, Disability and Health (ICF). J. Headache Pain 2005, 6, 429–440. [Google Scholar] [CrossRef]
- Lipton, R.B.; Stewart, W.F.; Sawyer, J.; Edmeads, J.G. Clinical utility of an instrument assessing migraine disability: The Migraine Disability Assessment (MIDAS) questionnaire. Headache J. Head Face Pain 2001, 41, 854–861. [Google Scholar] [CrossRef]
- Woodhouse, A.; Drummond, P.D. Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia 1993, 13, 417–421. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, M.; Ambrosini, A.; Brighina, F.; Coppola, G.; Perrotta, A.; Pierelli, F.; Sandrini, G.; Valeriani, M.; Marinazzo, D.; Stramaglia, S.; et al. Altered processing of sensory stimuli in patients with migraine. Nat. Rev. Neurol. 2014, 10, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Sicuteri, F.; Anselmi, B.; Del Bianco, P.L. Systemic Non-Organic Central Pain: A New Syndrome with Decentralization Supersensitivity. Headache J. Head Face Pain 1978, 18, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M.; et al. The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef]
- Zhang, X.; Levy, D.; Noseda, R.; Kainz, V.; Jakubowski, M.; Burstein, R. Activation of meningeal nociceptors by cortical spreading depression: Implications for migraine with aura. J. Neurosci. 2010, 30, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Di Russo, F.; Martínez, A.; Sereno, M.I.; Pitzalis, S.; Hillyard, S.A. Cortical sources of the early components of the visual evoked potential. Hum. Brain Mapp. 2002, 15, 95–111. [Google Scholar] [CrossRef]
- Helling, R.M.; Perenboom, M.J.; Bauer, P.R.; Carpay, J.A.; Sander, J.W.; Ferrari, M.D.; Visser, G.H.; Tolner, E.A. TMS-evoked EEG potentials demonstrate altered cortical excitability in migraine with aura. Brain Topogr. 2023, 36, 269–281. [Google Scholar] [CrossRef]
- Barwood, T.J.; Empson, J.A.; Lister, S.G.; Tilley, A.J. Auditory evoked potentials and transcendental meditation. Electroencephalogr. Clin. Neurophysiol. 1978, 45, 671–673. [Google Scholar] [CrossRef]
- Ambrosini, A.; Schoenen, J. The electrophysiology of migraine. Curr. Opin. Neurol. 2003, 16, 327–331. [Google Scholar] [CrossRef]
- Siribunyaphat, N.; Punsawad, Y. Steady-state visual evoked potential-based brain–computer interface using a novel visual stimulus with quick response (QR) code pattern. Sensors 2022, 22, 1439. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D.; Moskowitz, M.A. Pathophysiology of migraine. Annu. Rev. Physiol. 2013, 75, 365–391. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, T.; Goadsby, P.J. Migraine pathogenesis and state of pharmacological treatment options. BMC Med. 2009, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Hautakangas, H.; Winsvold, B.S.; Ruotsalainen, S.E.; Bjornsdottir, G.; Harder, A.V.; Kogelman, L.J.; Thomas, L.F.; Noordam, R.; Benner, C.; Gormley, P.; et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat. Genet. 2022, 54, 152–160. [Google Scholar] [CrossRef]
- Gianna Melillo, Review Analyzes Estrogen’s Role in Migraine Pathogenesis. Available online: https://www.ajmc.com/view/review-analyzes-estrogen-s-role-in-migraine-pathogenesis (accessed on 18 March 2021).
- Rauschel, V.; Ruscheweyh, R.; Krafczyk, S.; Straube, A. Test-retest reliability of visual-evoked potential habituation. Cephalalgia 2016, 36, 831–839. [Google Scholar] [CrossRef]
- O’Hare, L.; Asher, J.M.; Hibbard, P.B. Migraine visual aura and cortical spreading depression—Linking mathematical models to empirical evidence. Vision 2021, 5, 30. [Google Scholar] [CrossRef]
- Shibata, K.; Yamane, K.; Otuka, K.; Iwata, M. Abnormal visual processing in migraine with aura: A study of steady-state visual evoked potentials. J. Neurol. Sci. 2008, 271, 119–126. [Google Scholar] [CrossRef]
- Fong, C.Y.; Law, W.H.; Braithwaite, J.J.; Mazaheri, A. Differences in early and late pattern-onset visual-evoked potentials between self-reported migraineurs and controls. NeuroImage Clin. 2020, 25, 102122. [Google Scholar] [CrossRef]
- Coppola, G.; Ambrosini, A.; Clemente, L.D.; Magis, D.; Fumal, A.; Gerard, P.; Pierelli, F.; Schoenen, J. Interictal abnormalities of gamma band activity in visual evoked responses in migraine: An indication of thalamocortical dysrhythmia? Cephalalgia 2007, 27, 1360–1367. [Google Scholar] [CrossRef]
- Coppola, G.; Pierelli, F.; Schoenen, J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 2007, 27, 1427–1439. [Google Scholar] [CrossRef]
- Schoenen, J. Deficient habituation of evoked cortical potentials in migraine: A link between brain biology, behavior and trigeminovascular activation? Biomed. Pharmacother. 1996, 50, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Oelkers, R.; Grosser, K.; Lang, E.; Geisslinger, G.; Kobal, G.; Brune, K.; Lötsch, J. Visual evoked potentials in migraine patients: Alterations depend on pattern spatial frequency. Brain 1999, 122, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Sand, T.; Vingen, J.V. Visual, long-latency auditory and brainstem auditory evoked potentials in migraine: Relation to pattern size, stimulus intensity, sound and light discomfort thresholds and pre-attack state. Cephalalgia 2000, 20, 804–820. [Google Scholar] [CrossRef] [PubMed]
- Oelkers-Ax, R.; Parzer, P.; Resch, F.; Weisbrod, M. Maturation of early visual processing investigated by a pattern-reversal habituation paradigm is altered in migraine. Cephalalgia 2005, 25, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Demarquay, G.; Caclin, A.; Brudon, F.; Fischer, C.; Morlet, D. Exacerbated attention orienting to auditory stimulation in migraine patients. Clin. Neurophysiol. 2011, 122, 1755–1763. [Google Scholar] [CrossRef]
- Ambrosini, A.; Coppola, G.; Gérardy, P.Y.; Pierelli, F.; Schoenen, J. Intensity dependence of auditory evoked potentials during light interference in migraine. Neurosci. Lett. 2011, 492, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Sand, T.; Zhitniy, N.; White, L.R.; Stovner, L.J. Visual evoked potential latency, amplitude and habituation in migraine: A longitudinal study. Clin. Neurophysiol. 2008, 119, 1020–1027. [Google Scholar] [CrossRef]
- Mungoven, T.J.; Henderson, L.A.; Meylakh, N. Chronic migraine pathophysiology and treatment: A review of current perspectives. Front. Pain Res. 2021, 2, 705276. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neurosci. Biobehav. Rev. 2023, 149, 105163. [Google Scholar] [CrossRef] [PubMed]
- Eising, E.; ADatson, N.; van den Maagdenberg, A.M.; Ferrari, M.D. Epigenetic mechanisms in migraine: A promising avenue? BMC Med. 2013, 11, 26. [Google Scholar] [CrossRef]
- Gazerani, P. Current evidence on the role of epigenetic mechanisms in migraine: The way forward to precision medicine. OBM Genet. 2018, 2, 1804040. [Google Scholar] [CrossRef]
- Reddy, N.; Desai, M.N.; Schoenbrunner, A.; Schneeberger, S.; Janis, J.E. The complex relationship between estrogen and migraines: A scoping review. Syst. Rev. 2021, 10, 1–3. [Google Scholar] [CrossRef]
- Arnold, M. Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, G.; Zhang, K.; Liang, R.; Yan, W.; Tian, P.; Jia, Y.; Zhang, S.; Du, C. Assessment of human visual acuity using visual evoked potential: A review. Sensors 2020, 20, 5542. [Google Scholar] [CrossRef] [PubMed]
- Omland, P.M.; Uglem, M.; Engstrøm, M.; Linde, M.; Hagen, K.; Sand, T. Modulation of visual evoked potentials by high-frequency repetitive transcranial magnetic stimulation in migraineurs. Clin. Neurophysiol. 2014, 125, 2090–2099. [Google Scholar] [CrossRef]
- Steppacher, I.; Schindler, S.; Kissler, J. Higher, faster, worse? An event-related potentials study of affective picture processing in migraine. Cephalalgia 2016, 36, 249–257. [Google Scholar] [CrossRef]
- Khalil, N.M.; Legg, N.J.; Anderson, D.J. Long term decline of P100 amplitude in migraine with aura. J. Neurol. Neurosurg. Psychiatry 2000, 69, 507–511. [Google Scholar] [CrossRef]
- Mariani, E.; Moschini, V.; Pastorino, G.; Rizzi, F.; Severgnini, A.; Tiengo, M. Pattern reversal visual evoked potentials (VEP-PR) in migraine subjects with visual aura. Headache J. Head Face Pain 1990, 30, 435–438. [Google Scholar] [CrossRef]
- Kennard, C.; Gawel, M.; Rose, F.C. Visual evoked potentials in migraine subjects. Res. Clin. Stud. Headache 1978, 6, 73–80. [Google Scholar]
- Polich, J.; Ehlers, C.L.; Dalessio, D.J. Pattern-shift visual evoked responses and EEG in migraine. Headache J. Head Face Pain 1986, 26, 451–456. [Google Scholar] [CrossRef]
- Afra, J.; Cecchini, A.P.; De Pasqua, V.; Albert, A.; Schoenen, J. Visual evoked potentials during long periods of pattern-reversal stimulation in migraine. Brain J. Neurol. 1998, 121, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Timsit-Berthier, M.; Schoenen, J. Intensity dependence of auditory evoked potentials is pronounced in migraine: An indication of cortical potentiation and low serotonergic neurotransmission? Neurology 1996, 46, 1404. [Google Scholar] [CrossRef] [PubMed]
- Schoenen, J.; Wang, W.; Albert, A.; Delwaide, P.J. Potentiation instead of habituation characterizes visual evoked potentials in migraine patients between attacks. Eur. J. Neurol. 1995, 2, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Bauer, B.; Grotemeyer, K.H.; Kurlemann, G.; Husstedt, I.W. Event-related potentials (P300) in primary headache in childhood and adolescence. J. Child Neurol. 1998, 13, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Diano, M.; Battaglia, S. Insights into structural and functional organization of the brain: Evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry 2023, 14, 1225755. [Google Scholar] [CrossRef]



| Migraine n = 44 | Control n = 92 | p-Value | ||
|---|---|---|---|---|
| Gender | Male | 15 (34.1%) | 30 (33.3%) | 0.8 * |
| Female | 29 (65.9%) | 62 (67.7%) | ||
| Age | Mean (±SD) | 37.02 (±13.6) | 34.1 (±12.3) | 0.22 ** |
| Migraine n = 44 | Control n = 92 | p-Values * | |
|---|---|---|---|
| Left Eye | |||
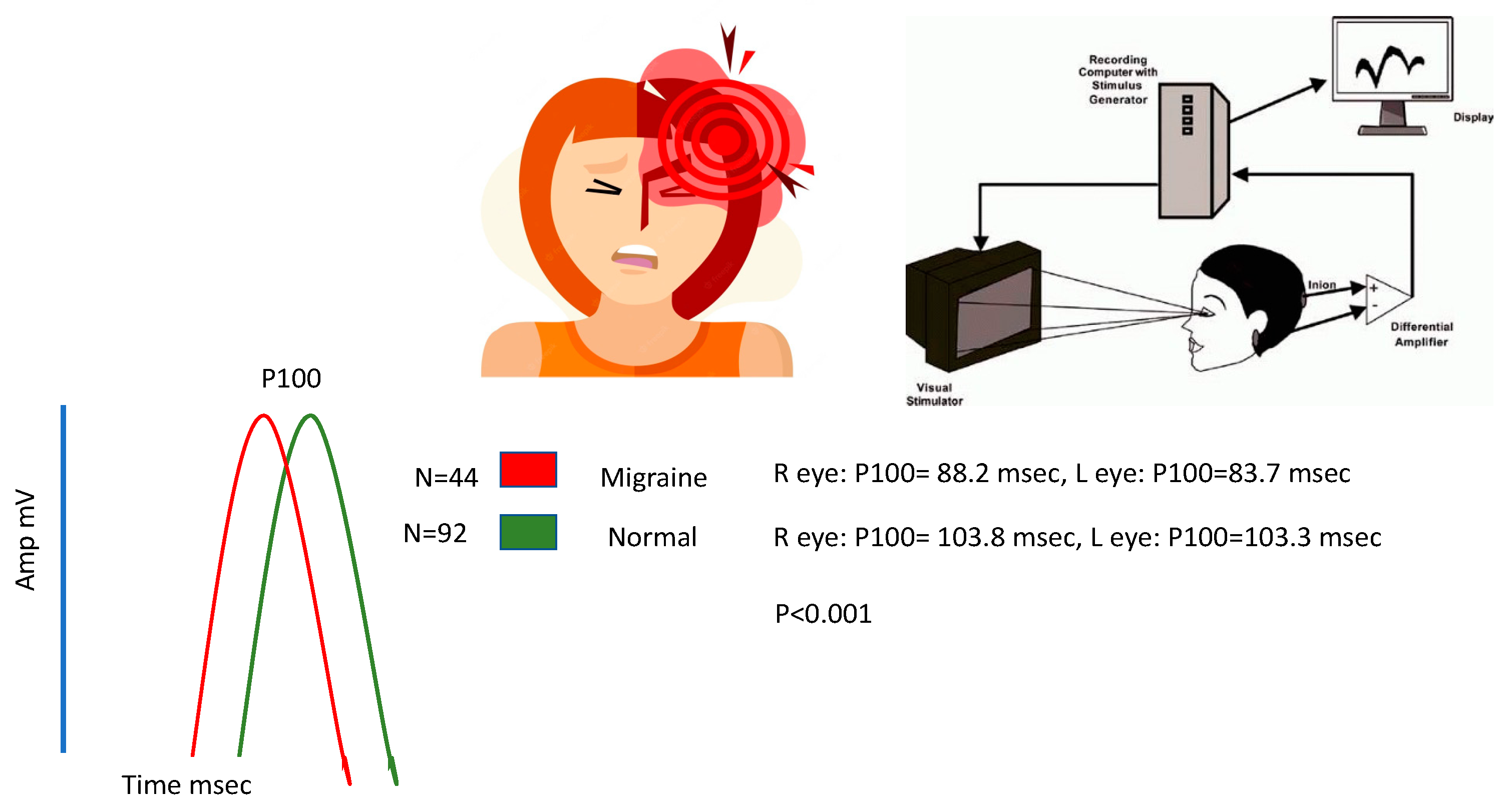
| N75 | 59.5 (±11.6) | 74.6 (±9.1) | <0.0001 |
| P100 | 83.7 (±7.2) | 103.3 (±8.1) | <0.0001 |
| N145 | 115.1 (±12.9) | 136.8 (±14) | <0.0001 |
| Amplitude | 6.4 (±4.3) | 8.8 (±5) | 0.008 |
| Right Eye | |||
| N75 | 64.1 (±12.8) | 75.4 (±11.4) | <0.0001 |
| P100 | 88.2 (±13.5) | 103.8 (±10.1) | <0.0001 |
| N145 | 120.1 (±18.4) | 137.8 (±16) | <0.0001 |
| Amplitude | 5.9 (±4) | 8.9 (±4.5) | <0.0001 |
| B | S.E. | p-Values | Odds Ratios | 95% C.I. for OR | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| P100 Left eye | 0.925 | 0.297 | 0.002 | 2.521 | 1.409 | 4.51 |
| P100 Right eye | 0.073 | 0.049 | 0.137 | 1.076 | 0.977 | 1.185 |
| Group | Measurements | Correlation (r) | p-Values |
|---|---|---|---|
| Migraine | N75 Left Eye and N75 Right Eye | 0.751 | <0.0001 |
| P100 Left Eye and P100 Right Eye | 0.394 | <0.0001 | |
| N145 Left Eye and N145 Right Eye | 0.480 | <0.0001 | |
| Amplitude Left Eye and Amplitude Right Eye | 0.739 | <0.0001 | |
| Control | N75 Left Eye and N75 Right Eye | 0.640 | <0.0001 |
| P100 Left Eye and P100 Right Eye | 0.774 | <0.0001 | |
| N145 Left Eye and N145 Right Eye | 0.706 | <0.0001 | |
| Amplitude Left Eye and Amplitude Right Eye | 0.842 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshamrani, F.J.; AlSheikh, M.H.; Almuslim, N.; Al Azman, H.; Alkhamis, F.; Nazish, S.; Alnajashi, H.; Alsulaiman, A. Prospective Matched Case-Control Study of Over-Early P100 Wave Latency in Migraine with Aura. Biomedicines 2023, 11, 2979. https://doi.org/10.3390/biomedicines11112979
Alshamrani FJ, AlSheikh MH, Almuslim N, Al Azman H, Alkhamis F, Nazish S, Alnajashi H, Alsulaiman A. Prospective Matched Case-Control Study of Over-Early P100 Wave Latency in Migraine with Aura. Biomedicines. 2023; 11(11):2979. https://doi.org/10.3390/biomedicines11112979
Chicago/Turabian StyleAlshamrani, Foziah J., Mona Hmoud AlSheikh, Noora Almuslim, Hatem Al Azman, Fahad Alkhamis, Saima Nazish, Hind Alnajashi, and Abdulla Alsulaiman. 2023. "Prospective Matched Case-Control Study of Over-Early P100 Wave Latency in Migraine with Aura" Biomedicines 11, no. 11: 2979. https://doi.org/10.3390/biomedicines11112979
APA StyleAlshamrani, F. J., AlSheikh, M. H., Almuslim, N., Al Azman, H., Alkhamis, F., Nazish, S., Alnajashi, H., & Alsulaiman, A. (2023). Prospective Matched Case-Control Study of Over-Early P100 Wave Latency in Migraine with Aura. Biomedicines, 11(11), 2979. https://doi.org/10.3390/biomedicines11112979






