The Role of Insulin Resistance in the Development of Complications after Coronary Artery Bypass Grafting in Patients with Coronary Artery Disease
Abstract
:1. Introduction
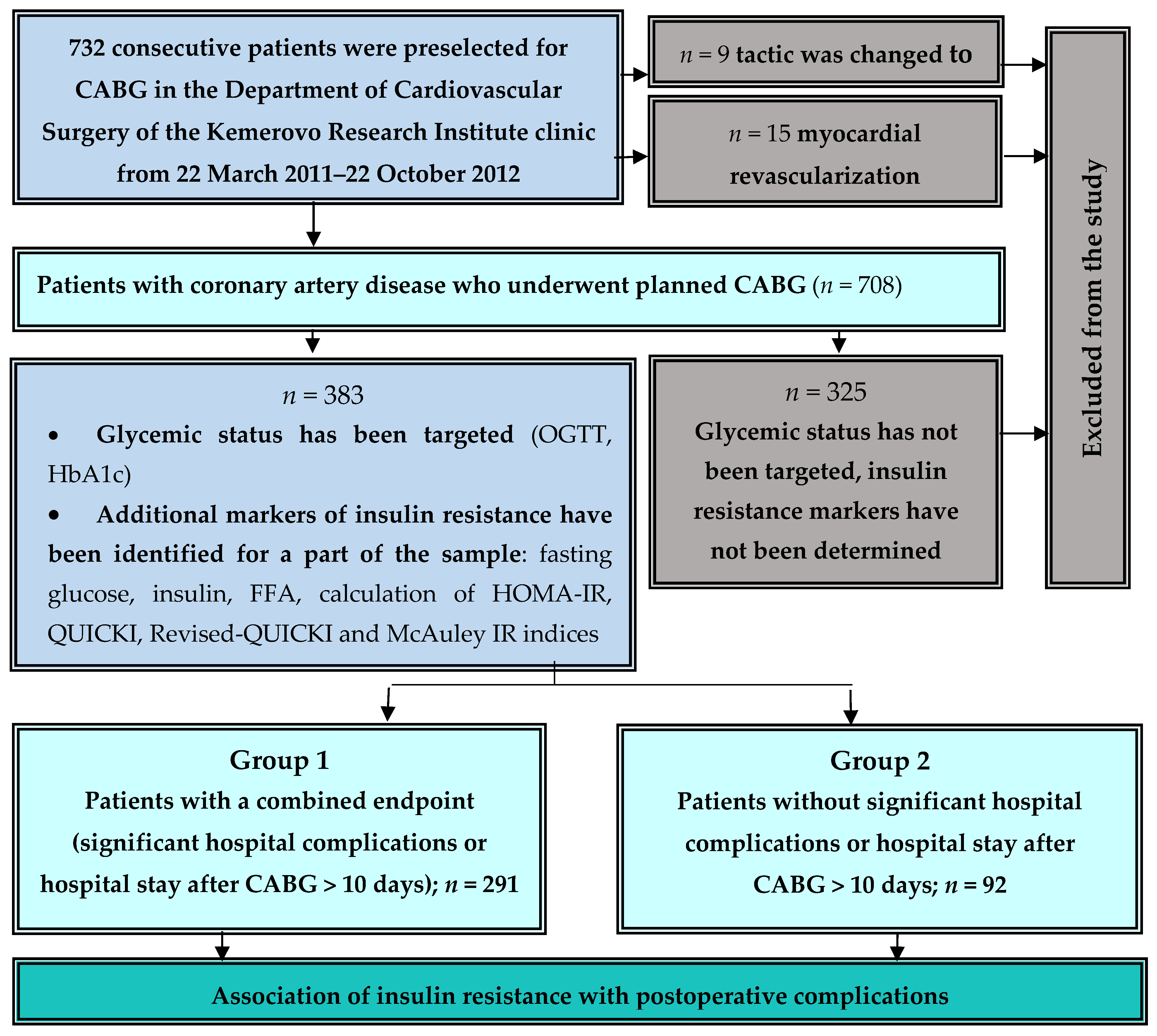
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Postoperative Complications
2.4. Assessment of Glycemic Status and Insulin Resistance Indices
2.5. Description of the Coronary Bypass Procedure
2.6. Statistical Analyses
3. Results
4. Discussion
Study Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colaiori, I.; Izzo, R.; Barbato, E.; Franco, D.; Di Gioia, G.; Rapacciuolo, A.; Bartunek, J.; Mancusi, C.; Losi, M.A.; Strisciuglio, T.; et al. Severity of Coronary Atherosclerosis and Risk of Diabetes Mellitus. J. Clin. Med. 2019, 8, 1069. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Sabik, J.F.; Ainkaran, P.; Blackstone, E.H. Coronary artery bypass grafting in diabetics: A growing health care cost crisis. J. Thorac. Cardiovasc. Surg. 2015, 150, 304–312.e2. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Cong, H.; Hou, K.; Hu, Y.; Zhang, J.; Zhang, Y. Clinical outcome comparison of percutaneous coronary intervention and bypass surgery in diabetic patients with coronary artery disease: A meta-analysis of randomized controlled trials and observational studies. Diabetol. Metab. Syndr. 2019, 11, 110. [Google Scholar] [CrossRef]
- Farkouh, M.E.; Domanski, M.; Dangas, G.D.; Godoy, L.C.; Mack, M.J.; Siami, F.S.; Hamza, T.H.; Shah, B.; Stefanini, G.G.; Sidhu, M.S.; et al. Long-Term Survival Following Multivessel Revascularization in Patients with Diabetes: The FREEDOM Follow-on Study. J. Am. Coll. Cardiol. 2019, 73, 629–638. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Sumin, A.N. Current trends in routine myocardial revascularization. Complex Issues Cardiovasc. Dis. 2021, 10, 25–35. [Google Scholar] [CrossRef]
- Robich, M.P.; Iribarne, A.; Leavitt, B.J.; Malenka, D.J.; Quinn, R.D.; Olmstead, E.M.; Ross, C.S.; Sawyer, D.B.; Klemperer, J.D.; Clough, R.A.; et al. Intensity of Glycemic Control Affects Long-Term Survival After Coronary Artery Bypass Graft Surgery. Ann. Thorac. Surg. 2019, 107, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Clement, K.C.; Suarez-Pierre, A.; Sebestyen, K.; Alejo, D.; DiNatale, J.; Whitman, G.J.; Matthew, T.L.; Lawton, J.S. Increased Glucose Variability Is Associated with Major Adverse Events after Coronary Artery Bypass. Ann. Thorac. Surg. 2019, 108, 1307–1313. [Google Scholar] [CrossRef]
- Abu Tailakh, M.; Ishay, S.-y.; Awesat, J.; Poupko, L.; Sahar, G.; Novack, V. Hemoglobin A1c in Patients with Diabetes Predict Long-Term Mortality Following Coronary Artery Surgery. J. Clin. Med. 2021, 10, 2739. [Google Scholar] [CrossRef]
- Wang, H.; Song, Y.; Tang, X.; Xu, J.; Jiang, P.; Jiang, L.; Gao, Z.; Chen, J.; Song, L.; Zhang, Y.; et al. Impact of unknown diabetes and prediabetes on clinical outcomes in “nondiabetic” Chinese patients after a primary coronary intervention. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 644–651. [Google Scholar] [CrossRef]
- Sumin, A.N.; Bezdenezhnykh, N.A.; Bezdenezhnykh, A.V.; Ivanov, S.V.; Barbarash, O.L. Impact of Pre-Diabetes on the Rate of Major Adverse Cardiovascular Events in Patients Undergoing Coronary Artery Bypass Grafting. Ration. Pharmacother. Cardiol. 2018, 14, 654–663. (In Russian) [Google Scholar] [CrossRef]
- Ploumen, E.H.; Buiten, R.A.; Kok, M.M.; Doggen, C.J.M.; van Houwelingen, K.G.; Stoel, M.G.; de Man, F.H.A.F.; Hartmann, M.; Zocca, P.; Linssen, G.C.M.; et al. Three-year clinical outcome in all-comers with “silent” diabetes, prediabetes, or normoglycemia, treated with contemporary coronary drug-eluting stents: From the BIO-RESORT Silent Diabetes study. Catheter. Cardiovasc. Interv. 2020, 96, E110–E118. [Google Scholar] [CrossRef] [PubMed]
- Ploumen, E.H.; Pinxterhuis, T.H.; Zocca, P.; Roguin, A.; Anthonio, R.L.; Schotborgh, C.E.; Benit, E.; Aminian, A.; Danse, P.W.; Doggen, C.J.M.; et al. Impact of prediabetes and diabetes on 3-year outcome of patients treated with new-generation drug-eluting stents in two large-scale randomized clinical trials. Cardiovasc. Diabetol. 2021, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Ozkokeli, M. Does Homeostasis Model Assessment Insulin Resistance Have a Predictive Value for Post-Coronary Artery Bypass Grafting Surgery Outcomes? Rev. Bras. Cir. Cardiovasc. 2014, 29, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Sumin, A.N.; Bezdenezhnykh, N.A.; Bezdenezhnykh, A.V.; Osokina, A.V.; Kuz’mina, A.A.; Tsepokina, A.V.; Barbarash, O.L. Screening for Glucose Metabolism Disorders, Assessment the Disease Insulin Resistance Index and Hospital Prognosis of Coronary Artery Bypass Surgery. J. Pers. Med. 2021, 11, 802. [Google Scholar] [CrossRef]
- Strisciuglio, T.; Izzo, R.; Barbato, E.; Di Gioia, G.; Colaiori, I.; Fiordelisi, A.; Morisco, C.; Bartunek, J.; Franco, D.; Ammirati, G.; et al. Insulin Resistance Predicts Severity of Coronary Atherosclerotic Disease in Non-Diabetic Patients. J. Clin. Med. 2020, 9, 2144. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, D.; Zhang, R.; Zhu, J.; Du, R.; Shi, Y.; Xiong, X. Insulin resistance predicts progression of de novo atherosclerotic plaques in patients with coronary heart disease: A one-year follow-up study. Cardiovasc. Diabetol. 2012, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006; pp. 1–50. [Google Scholar]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef]
- Zhou, T.; Li, S.; Xiang, D.; Gao, L. Effects of Isolated Impaired Fasting Glucose on Brain Injury During Cardiac Surgery under Cardiopulmonary Bypass. J. Investig. Surg. 2020, 33, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.S.; Liang, G.Y.; Liu, D.X.; Yu, J.; Wang, F. Role of Phosphorylated AMP-Activated Protein Kinase (AMPK) in Myocardial Insulin Resistance After Myocardial Ischemia-Reperfusion During Cardiopulmonary Bypass Surgery in Dogs. Med. Sci. Monit. 2019, 25, 4149–4158. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhao, X.; Guo, Y.L.; Zhu, C.G.; Wu, N.Q.; Sun, J.; Liu, G.; Dong, Q.; Li, J.J. Free fatty acids and cardiovascular outcome: A Chinese cohort study on stable coronary artery disease. Nutr. Metab. 2017, 14, 41. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Tamimi, W.; Jones, G.; Jawdat, D.; Tamim, H.; Al-Dorzi, H.M.; Sadat, M.; Afesh, L.; Sakhija, M.; Al-Dawood, A. Free Fatty Acids’ Level and Nutrition in Critically Ill Patients and Association with Outcomes: A Prospective Sub-Study of PermiT Trial. Nutrients 2019, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gao, Y.; Wang, L.; Liu, J.; Yuan, Z.; Yu, M. Elevated free fatty acid level is a risk factor for early postoperative hypoxemia after on-pump coronary artery bypass grafting: Association with endothelial activation. J. Cardiothorac. Surg. 2015, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, X.; Jin, X.; Lv, M.; Li, X.; Dou, J.; Zeng, J.; An, P.; Chen, Y.; Chen, K.; et al. Effects of Preoperative HbA1c Levels on the Postoperative Outcomes of Coronary Artery Disease Surgical Treatment in Patients with Diabetes Mellitus and Nondiabetic Patients: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2020, 2020, 3547491. [Google Scholar] [CrossRef] [PubMed]
- Tennyson, C.; Lee, R.; Attia, R. Is there a role for HbA1c in predicting mortality and morbidity outcomes after coronary artery bypass graft surgery? Interact Cardiovasc Thorac Surg. 2013, 17, 1000–1008. [Google Scholar] [CrossRef]
- Xu, X.; Wang, R.; Wang, Y.; Cai, S. Glycosylated hemoglobin levels and clinical outcomes in diabetic patients receiving percutaneous coronary interventions: A meta-analysis of cohort studies. Int. J. Cardiol. 2015, 190, 143–147. [Google Scholar] [CrossRef]
- Rollins, K.E.; Varadhan, K.K.; Dhatariya, K.; Lobo, D.N. Systematic review of the impact of HbA1c on outcomes following surgery in patients with diabetes mellitus. Clin. Nutr. 2016, 35, 308–316. [Google Scholar] [CrossRef]
- Hosny, H.; Ibrahim, M.; El-Siory, W.; Abdel-Monem, A. Comparative Study Between Conventional Fasting Versus Overnight Infusion of Lipid or Carbohydrate on Insulin and Free Fatty Acids in Obese Patients Undergoing Elective On-pump Coronary Artery Bypass Grafting. A Prospective Randomized Trial. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1248–1253. [Google Scholar] [CrossRef]

| Group 1 with Combined Endpoint n = 291 | Group 2 without Combined Endpoint n = 92 | p | |
|---|---|---|---|
| Men/women (n, %) | 205/86 (70.5/29.5) | 79/13 (85.5/14.1) | 0.003 |
| Age (years, Me [LQ; UQ]) | 60.0 [55.0; 60.0] | 57.0 [51.0; 60.5] | <0.001 |
| Any disorders of carbohydrate metabolism (n, %) | 156 (53.6) | 36 (39.1) | 0.015 |
| Type 2 diabetes (n, %) | 109 (37.5) | 16 (17.4) | <0.001 |
| Prediabetes (IFG, IGT) (n, %) | 47 (16.2) | 20 (21.7) | 0.002 |
| Normoglycemia (n, %) | 135 (46.4) | 56 (60.9) | 0.002 |
| Body mass index (kg/m2, Me [LQ; UQ]) | 29.1 [25.9; 32.2] | 27.0 [24.1; 30.0] | <0.001 |
| Obesity (n, %) | 125 (42.9) | 24 (26.1) | <0.001 |
| Arterial hypertension (n, %) | 264 (90.7) | 79 (85.9) | 0.185 |
| Angina class III–IV (n, %) | 113 (38.8) | 34 (37.0) | 0.591 |
| Heart failure class NYHA III (n, %) | 84 (28.9) | 18 (19.6) | 0.172 |
| Arrhythmias (n, %) | 112 (38.5) | 17 (18.5) | 0.502 |
| Intermittent claudication (n, %) | 49 (16.8) | 14 (15.2) | 0.714 |
| Smoking (n, %) | 86 (29.6) | 42 (45.7) | 0.004 |
| Myocardial infarction history (n, %) | 182 (62.5) | 58 (63.0) | 0.668 |
| Stroke history (n, %) | 21 (7.2) | 6 (6.5) | 0.820 |
| Previous PCI (n, %) | 26 (8.9) | 11 (12.0) | 0.392 |
| Previous CABG (n, %) | 82 (0.7) | 2 (2.2) | 0.797 |
| Previous carotid surgery (n, %) | 8 (2.8) | 3 (3.3) | 0.803 |
| Previous lower limb artery surgery or amputation (n, %) | 2 (0.7) | 1 (1.1) | 0.704 |
| CABG characteristics | |||
| Cardiopulmonary bypass (n, %) | 268 (92.1) | 78 (84.8) | 0.038 |
| Isolated coronary artery bypass grafting (n, %) | 263 (90.4) | 91 (98.9) | 0.007 |
| Combined surgery (n, %) | 28 (9.6) | 1 (1.1) | 0.007 |
| 3 (1.0) | 0 (0) | 0.328 |
| 1 (0.3) | 0 (0) | 0.573 |
| 8 (8.2) | 0 (0) | 0.108 |
| 13 (4.5) | 1 (1.1) | 0.132 |
| 14 (4.8) | 0 (0) | 0.032 |
| Total duration of surgery (minutes, Me [LQ; UQ]) | 246.0 [210.0; 298.0] | 210.0 [195.0; 264.0] | 0.006 |
| CPB duration (minutes, Me [LQ; UQ]) | 98.0 [81.0; 116.0] | 86.5 [73.0; 103.0] | 0.002 |
| Aortic clamping time (minutes, Me [LQ; UQ]) | 63.0 [50.0; 75.0] | 60.0 [49.0; 72.0] | 0.331 |
| Euro SCORE II (%, Me [LQ; UQ]) | 1.34 [1.23; 2.91] | 1.32 [0.88; 2.10] | 0.002 |
| Hospital stay after CABG (days, Me [LQ;UQ]) | 13.0 [12.0; 17.0] | 9.0 [8.0; 10.0] | 0.003 |
| Preoperative drugs | |||
| Angiotensin II receptor antagonists (n, %) | 11 (3.8) | 5 (5.4) | 0.480 |
| Angiotensin-converting enzyme inhibitors (n, %) | 250 (85.9) | 81 (88.0) | 0.602 |
| Beta-blockers (n, %) | 285 (97.9) | 90 (97.8) | 0.546 |
| Potassium-sparing diuretics (n, %) | 51 (17.5) | 13 (14.1) | 0.675 |
| Thiazide-like diuretics (n, %) | 31 (10.7) | 7 (7.6) | 0.451 |
| Loop diuretics (n, %) | 272 (93.5) | 88 (95.7) | 0.448 |
| Calcium channel blockers (n, %) | 195 (67.0) | 55 (59.8) | 0.204 |
| Statins (n, %) | 224 (77.0) | 69 (75.0) | 0.789 |
| Metformin (n, %) | 15 (5.2) | 2 (2.2) | 0.461 |
| Sulfonylurea drugs (n, %) | 42 (14.4) | 3 (3.3) | 0.004 |
| Inhibitors DPP 4/GLP 1 receptor agonists (n, %) | 7 (2.4) | 1 (1.1) | 0.724 |
| Insulin therapy before hospitalization (n, %) | 19 (9.9) | 2 (2.2) | 0.181 |
| Insulin therapy during hospitalization (n, %) | 49 (16.8) | 4 (4.4) | 0.002 |
| Group 1 with Combined Endpoint n = 291 | Group 2 without Combined Endpoint n = 92 | p | |
|---|---|---|---|
| Creatinine (μmol/L) | 83.5 [70.0; 103.0] | 90.0 [73.0; 103.0] | 0.165 |
| GFR by CKD-EPI formula (mL/min/1.73 m2) | 80.4 [64.0; 98.6] | 81.4 [63.2; 98.6] | 0.906 |
| Glycated hemoglobin (HbA1c, %) | 5.5 [5.1; 7.1] | 5.3 [5.0; 5.6] | 0.009 |
| Glucose, venous plasma (mmol/L) | 5.8 [5.2; 6.9] | 5.5 [5.1; 6.2] | 0.031 |
| Triglycerides (mmol/L) | 2.0 [1.4; 2.5] | 1.6 [1.2; 2.2] | 0.046 |
| Insulin, IU/mL | 10.1 [2.5; 23.5] | 6.4 [2.6; 15,6] | 0.418 |
| HOMA-IR | 2.52 [0.61; 6.26] | 1.43 [0.74; 3.73] | 0.083 |
| QUICKI | 0.14 [0.13; 0.18] | 0.16 [0.14; 0.17] | 0.136 |
| FFA, mmol/L | 0.4 [0.3; 0.6] | 0.3 [0.2; 0.5] | 0.007 |
| Revised-QUICKI | 0.17 [0.15; 0.22] | 0.19 [0.16; 0.23] | 0.020 |
| McAuley IR index | 6.24 [4.72; 8.98] | 6.65 [5.55; 9.48] | 0.110 |
| Coronary angiography data | |||
| 1-vessel disease * | 64 (22.0) | 16 (17.4) | 0.343 |
| 2-vessel disease * | 88 (30.2) | 25 (27.2) | 0.274 |
| 3-vessel disease * | 121 (41.6) | 45 (48.9) | 0.216 |
| Left main coronary artery stenosis > 50% | 61 (21.0) | 24 (26.1) | 0.302 |
| Echocardiography data before surgery (Me [LQ; UQ]) | |||
| LV end-systolic volume (mL) | 63.0 [47.0; 97.0] | 62.2 [48.0; 96.5] | 0.421 |
| LV end-diastolic volume (mL) | 156.0 [132.0; 191.0] | 150.0 [129.0; 172.0] | 0.125 |
| LV end-systolic size (cm) | 3.8 [3.4; 4.7] | 3.8 [3.3; 4.6] | 0.467 |
| LV end-diastolic size (cm) | 5.6 [5.2; 6.2] | 5.5 [5.1; 6.0] | 0.051 |
| Left atrium (cm) | 4.3 [4.0; 4.5] | 4.2 [3.8; 4.4] | <0.001 |
| LV ejection fraction (%) | 60.0 [50.0; 64.0] | 61.0 [50.0; 64.0] | 0.958 |
| LV myocardial mass by Deveraux and Reichek (g) | 304.3 [250.5; 375.0] | 242.1 [276.0; 333.7] | 0.010 |
| LV myocardial mass index (g/m2) | 159.2 [133.5; 192.0] | 150.3 [124.2; 175.0] | 0.015 |
| Significant Complications | |||||||
|---|---|---|---|---|---|---|---|
| Predictors | B | SE | Wald | df | Sig. | Exp (B) | |
| Step 1 | Combined operations | 1.421 | 0.574 | 6.121 | 1 | 0.013 | 4.143 |
| Constant | −1.421 | 0.211 | 45.570 | 1 | 0.000 | 0.241 | |
| Hospital stay after CABG > 10 days | |||||||
| Step 1 | Left atrium | 2.304 | 0.555 | 17.246 | 1 | 0.000 | 10.017 |
| Constant | −8.170 | 2.212 | 13.637 | 1 | 0.000 | 0.000 | |
| Step 2 | Male gender | −1.673 | 0.597 | 7.851 | 1 | 0.005 | 0.188 |
| Left atrium | 2.474 | 0.578 | 18.347 | 1 | 0.000 | 11.870 | |
| Constant | −7.555 | 2.296 | 10.826 | 1 | 0.001 | 0.001 | |
| Step 3 | Male gender | −1.982 | 0.625 | 10.041 | 1 | 0.002 | 0.138 |
| Left atrium | 2.096 | 0.601 | 12.159 | 1 | 0.000 | 8.130 | |
| LV end-diastolic volume | 0.671 | 0.348 | 3.710 | 1 | 0.054 | 1.956 | |
| Constant | −9.535 | 2.602 | 13.430 | 1 | 0.000 | 0.000 | |
| Hospital stay after CABG > 14 days | |||||||
| Predictors | B | SE | Wald | df | Sig. | Exp (B) | |
| Step 1 | Type 2 diabetes | 1.196 | 0.369 | 10.519 | 1 | 0.001 | 3.305 |
| Constant | −2.706 | 0.593 | 20.845 | 1 | 0.000 | 0.067 | |
| Step 2 | Male gender | −0.984 | 0.395 | 6.191 | 1 | 0.013 | 0.374 |
| Type 2 diabetes | 1.148 | 0.377 | 9.290 | 1 | 0.002 | 3.152 | |
| Constant | −1.961 | 0.656 | 8.932 | 1 | 0.003 | 0.141 | |
| Step 3 | Male gender | −1.142 | 0.408 | 7.821 | 1 | 0.005 | 0.319 |
| Left atrium | 0.531 | 0.280 | 3.607 | 1 | 0.058 | 1.700 | |
| Type 2 diabetes | 1.070 | 0.383 | 7.793 | 1 | 0.005 | 2.916 | |
| Constant | −4.026 | 1.283 | 9.849 | 1 | 0.002 | 0.018 | |
| Combined end point (significant complications or hospital stay after CABG > 10 days) | |||||||
| Predictors | B | SE | Wald | df | Sig. | Exp (B) | |
| Step 1 | Left atrium | 2.060 | 0.544 | 14.353 | 1 | 0.000 | 7.844 |
| Constant | −7.070 | 2.169 | 10.623 | 1 | 0.001 | 0.001 | |
| Step 2 | Male gender | −1.468 | 0.592 | 6.156 | 1 | 0.013 | 0.230 |
| Left atrium | 2.176 | 0.556 | 15.310 | 1 | 0.000 | 8.807 | |
| Constant | −6.399 | 2.226 | 8.261 | 1 | 0.004 | 0.002 | |
| Step 3 | Male gender | −1.505 | 0.608 | 6.131 | 1 | 0.013 | 0.222 |
| Left atrium | 2.347 | 0.588 | 15.924 | 1 | 0.000 | 10.452 | |
| Free fatty acids | 2.691 | 1.103 | 5.952 | 1 | 0.015 | 14.749 | |
| Constant | −8.130 | 2.439 | 11.105 | 1 | 0.001 | 0.000 | |
| Step 4 | Male gender | −1.309 | 0.623 | 4.418 | 1 | 0.036 | 0.270 |
| Age | 0.069 | 0.035 | 3.913 | 1 | 0.048 | 1.072 | |
| Left atrium | 2.195 | 0.596 | 13.562 | 1 | 0.000 | 8.979 | |
| Free fatty acids | 2.559 | 1.088 | 5.532 | 1 | 0.019 | 12.917 | |
| Constant | −11.640 | 3.080 | 14.282 | 1 | 0.000 | 0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumin, A.N.; Bezdenezhnykh, N.A.; Bezdenezhnykh, A.V.; Osokina, A.V.; Kuzmina, A.A.; Sinitskaya, A.V.; Barbarash, O.L. The Role of Insulin Resistance in the Development of Complications after Coronary Artery Bypass Grafting in Patients with Coronary Artery Disease. Biomedicines 2023, 11, 2977. https://doi.org/10.3390/biomedicines11112977
Sumin AN, Bezdenezhnykh NA, Bezdenezhnykh AV, Osokina AV, Kuzmina AA, Sinitskaya AV, Barbarash OL. The Role of Insulin Resistance in the Development of Complications after Coronary Artery Bypass Grafting in Patients with Coronary Artery Disease. Biomedicines. 2023; 11(11):2977. https://doi.org/10.3390/biomedicines11112977
Chicago/Turabian StyleSumin, Alexey N., Natalia A. Bezdenezhnykh, Andrey V. Bezdenezhnykh, Anastasiya V. Osokina, Anastasiya A. Kuzmina, Anna V. Sinitskaya, and Olga L. Barbarash. 2023. "The Role of Insulin Resistance in the Development of Complications after Coronary Artery Bypass Grafting in Patients with Coronary Artery Disease" Biomedicines 11, no. 11: 2977. https://doi.org/10.3390/biomedicines11112977
APA StyleSumin, A. N., Bezdenezhnykh, N. A., Bezdenezhnykh, A. V., Osokina, A. V., Kuzmina, A. A., Sinitskaya, A. V., & Barbarash, O. L. (2023). The Role of Insulin Resistance in the Development of Complications after Coronary Artery Bypass Grafting in Patients with Coronary Artery Disease. Biomedicines, 11(11), 2977. https://doi.org/10.3390/biomedicines11112977






