A Microsurgical Arteriovenous Malformation Model on Saphenous Vessels in the Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
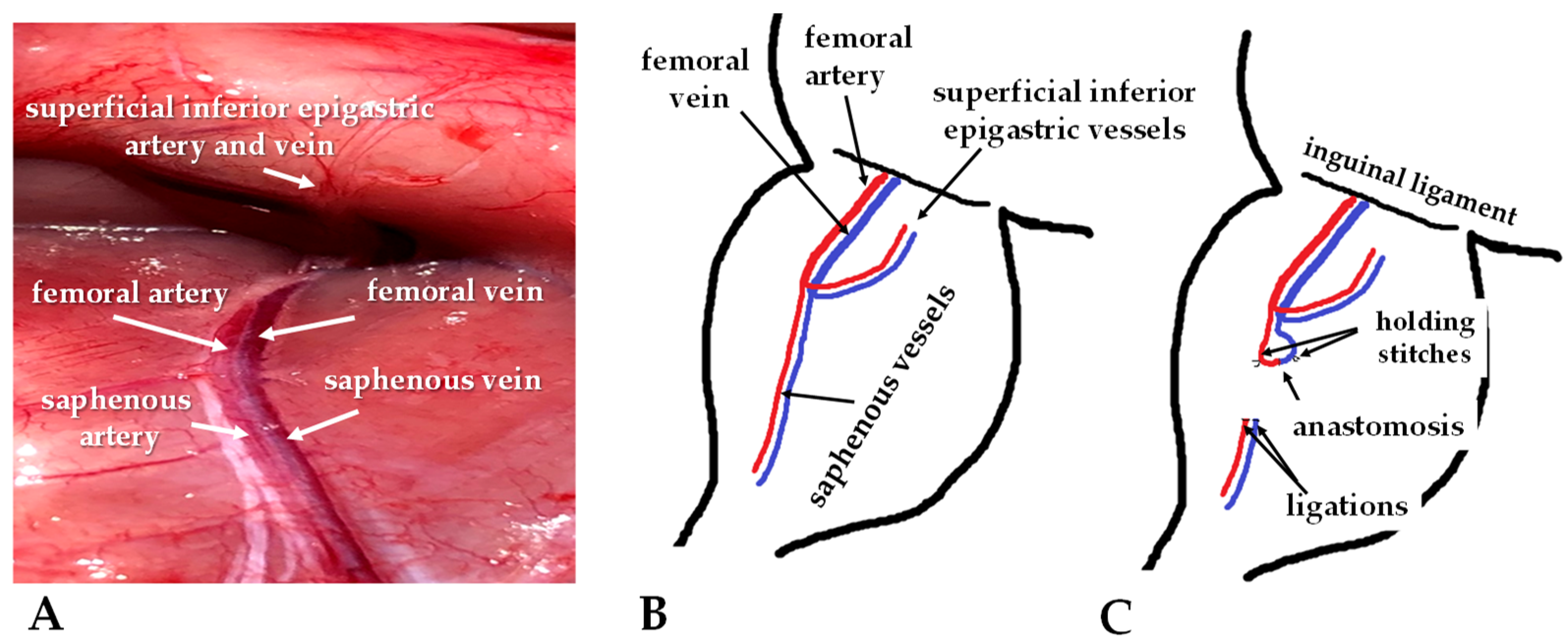
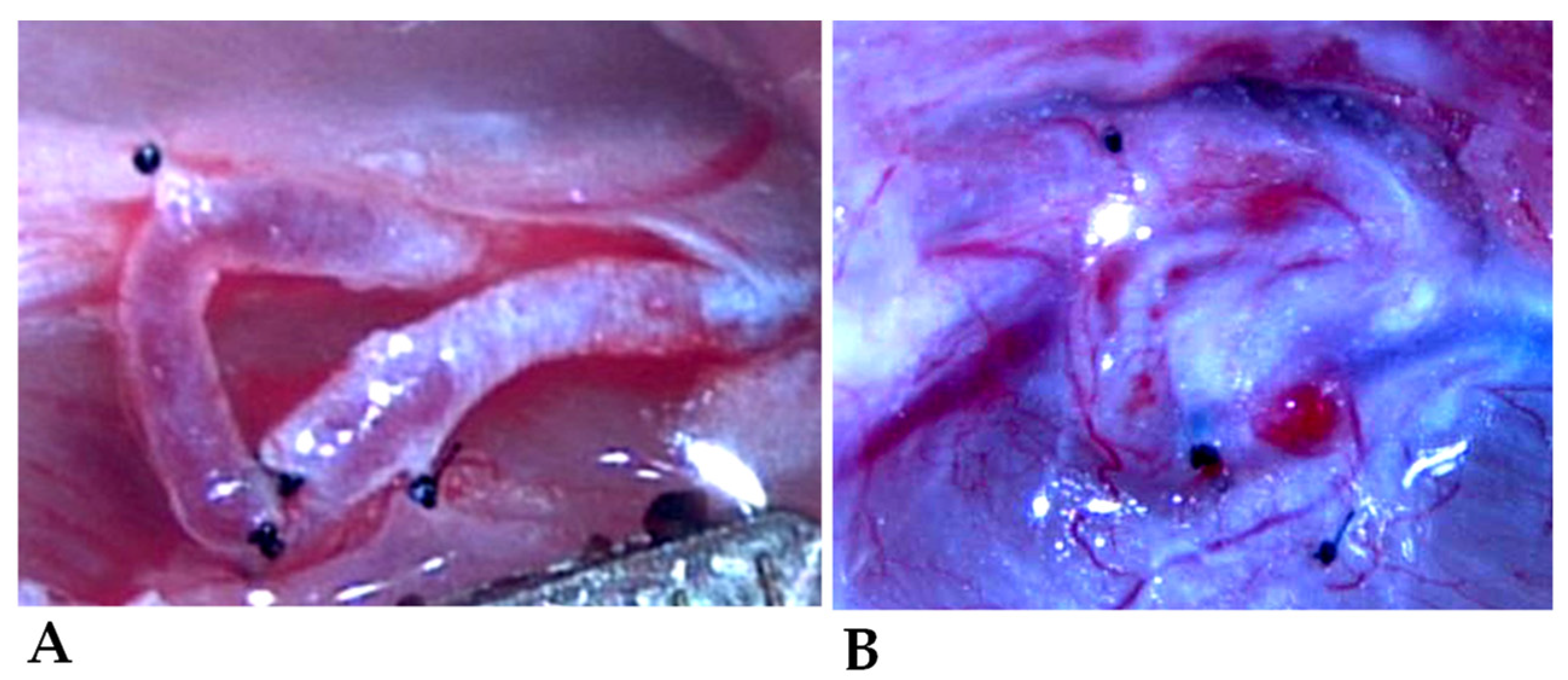
2.2. Operative Techniques
2.3. Ultrasound Examination
2.4. Hemodynamic Measurements
2.5. Histological Investigation
2.6. Statistical Analysis
3. Results
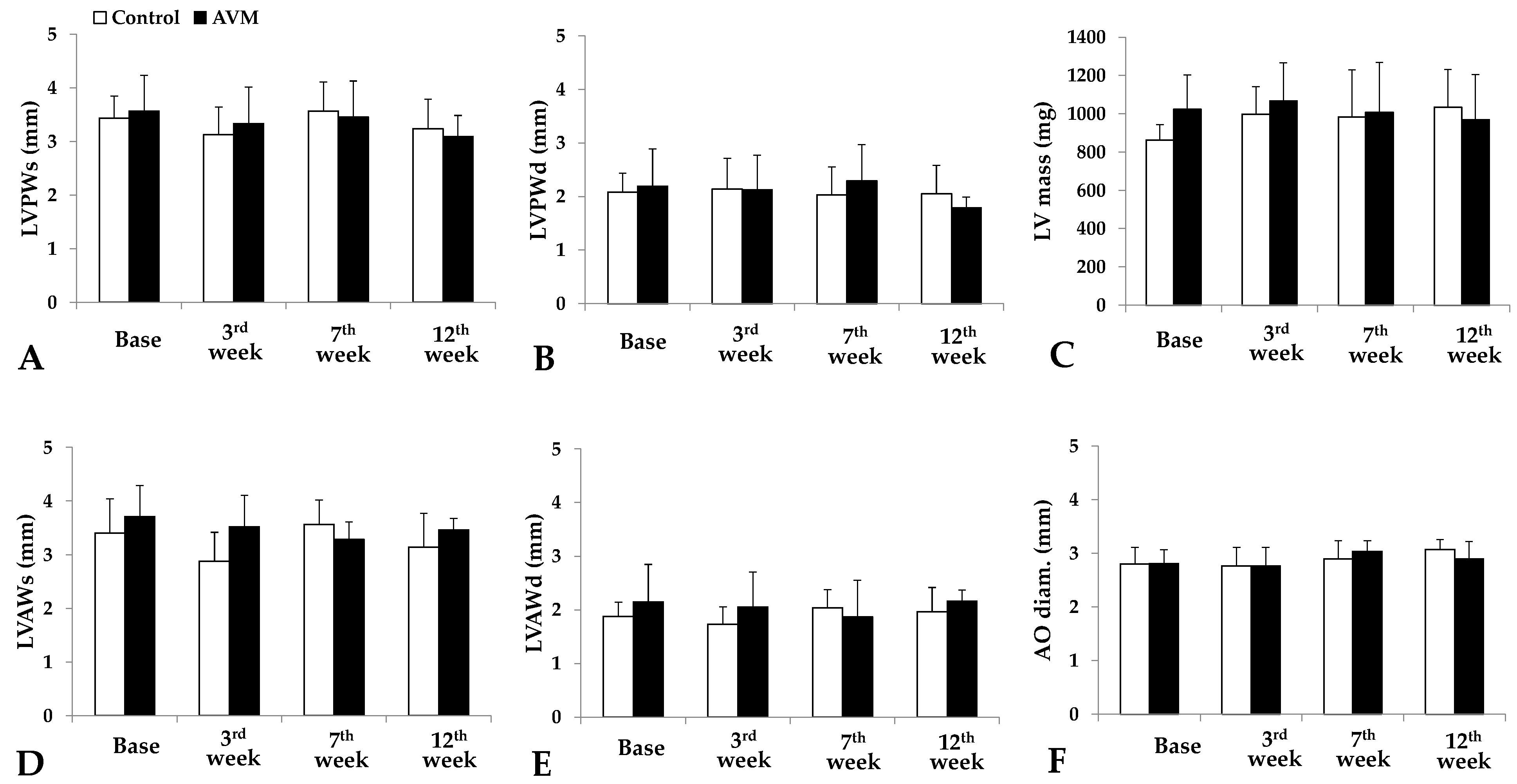
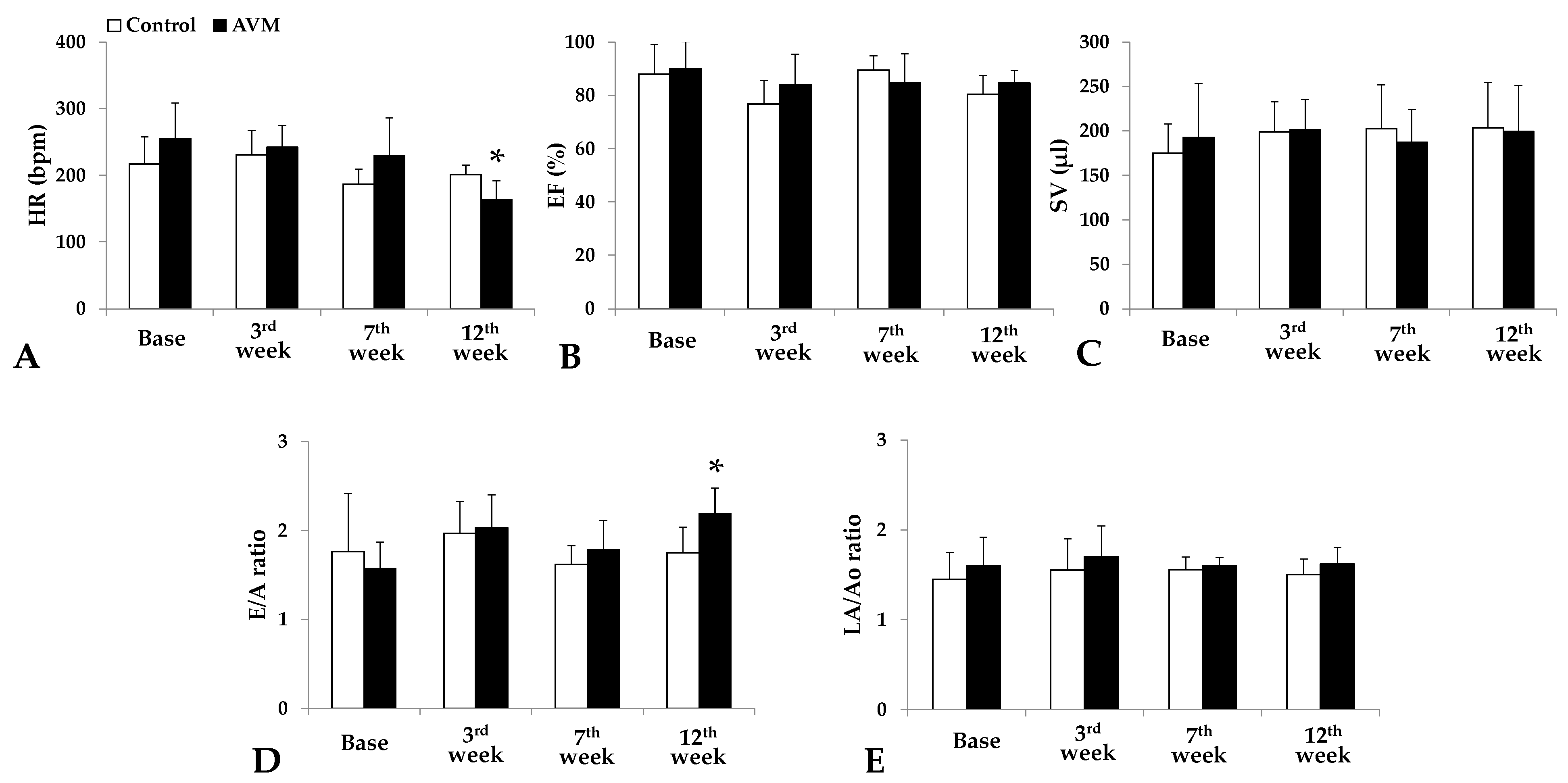
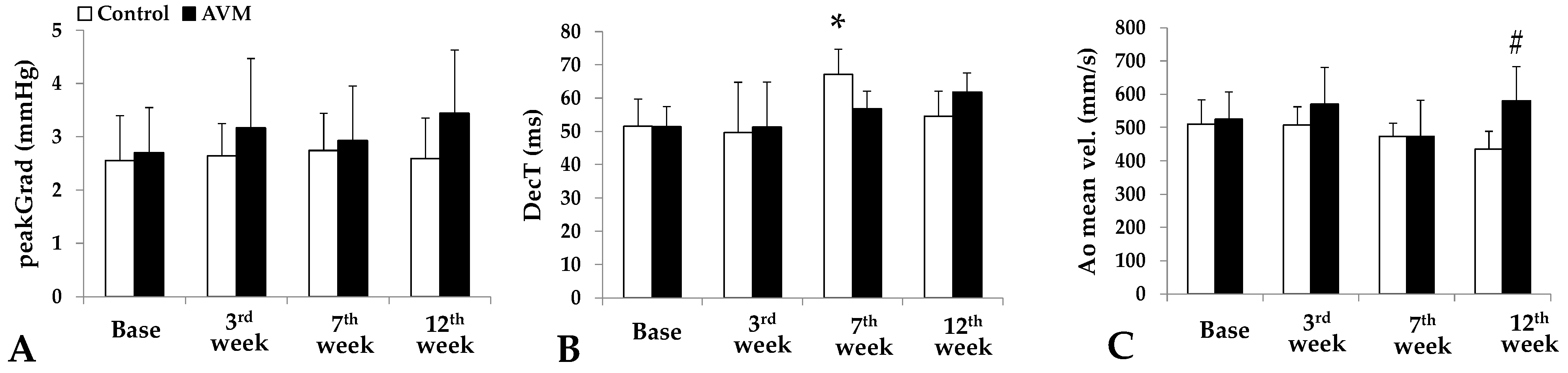
3.1. Echocardiography
3.2. Hemodynamic Parameters
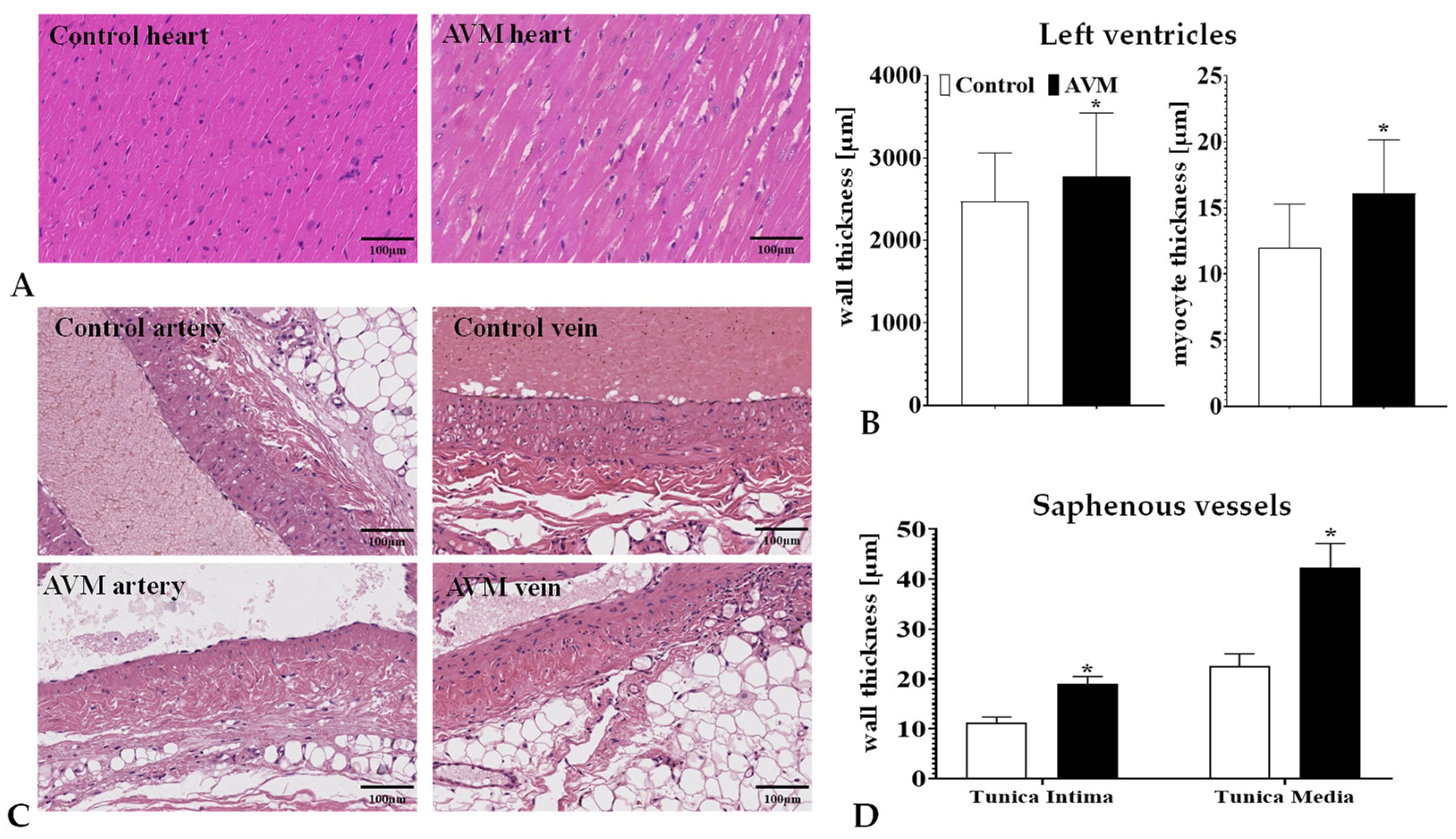
3.3. Morphology of the Shunts
3.4. Histomorphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laakso, A.; Hernesniemi, J. Arteriovenous malformations: Epidemiology and clinical presentation. Neurosurg. Clin. N. Am. 2012, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.D.; Darflinger, R.; Cooke, D.L.; Halbach, V.V. Cerebral arteriovenous fistulae. Handb. Clin. Neurol. 2021, 176, 179–198. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, C.; Cooke, D.L.; Hetts, S.W.; Abla, A.A. Brain arteriovenous malformations. Handb. Clin. Neurol. 2021, 176, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Choi, E.J.; McDougall, C.M.; Su, H. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl. Stroke Res. 2014, 5, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.X.; Johnston, S.C.; Singh, V.; McCulloch, C.E.; Bennett, J.P.; Achrol, A.S.; Sidney, S.; Young, W.L. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke 2004, 35, 1697–1702. [Google Scholar] [CrossRef]
- Leblanc, G.G.; Golanov, E.; Awad, I.A.; Young, W.L.; Biology of Vascular Malformations of the Brain NINDS Workshop Collaborators. Biology of vascular malformations of the brain. Stroke 2009, 40, e694–e702. [Google Scholar] [CrossRef]
- Di Rocco, C.; Tamburrini, G.; Rollo, M. Cerebral arteriovenous malformations in children. Acta Neurochir. 2000, 142, 145–156. [Google Scholar] [CrossRef]
- Kim, H.; Marchuk, D.A.; Pawlikowska, L.; Chen, Y.; Su, H.; Yang, G.Y.; Young, W.L. Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir. Suppl. 2008, 105, 199–206. [Google Scholar] [CrossRef]
- Mast, H.; Young, W.L.; Koennecke, H.C.; Sciacca, R.R.; Osipov, A.; Pile-Spellman, J.; Hacein-Bey, L.; Duong, H.; Stein, B.M.; Mohr, J.P. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet 1997, 350, 1065–1068. [Google Scholar] [CrossRef]
- Freudenstein, D.; Duffner, F.; Ernemann, U.; Rachinger, J.; Grote, E. Recurrence of a cerebral arteriovenous malformation after surgical excision. Cerebrovasc. Dis. 2001, 11, 59–64. [Google Scholar] [CrossRef]
- Jeffree, R.L.; Stoodley, M.A. Postnatal development of arteriovenous malformations. Pediatr. Neurosurg. 2009, 45, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Achrol, A.S.; Guzman, R.; Varga, M.; Adler, J.R.; Steinberg, G.K.; Chang, S.D. Pathogenesis and radiobiology of brain arteriovenous malformations: Implications for risk stratification in natural history and posttreatment course. Neurosurg. Focus 2009, 26, E9. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, N.; Pérez, R. Management of brain arteriovenous malformations: A review. Cureus 2023, 15, e34053. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, R.M. Clinical practice. Arteriovenous malformations of the brain. N. Engl. J. Med. 2007, 356, 2704–2712. [Google Scholar] [CrossRef]
- Grzyska, U.; Fiehler, J. Pathophysiology and treatment of brain AVMs. Klin. Neuroradiol. 2009, 19, 82–90. [Google Scholar] [CrossRef]
- Hofmeister, C.; Stapf, C.; Hartmann, A.; Sciacca, R.R.; Mansmann, U.; Terbrugge, K.; Lasjaunias, P.; Mohr, J.P.; Mast, H.; Meisel, J. Demographic, morphological, and clinical characteristics of 1289 patients with brain arteriovenous malformation. Stroke 2000, 31, 1307–1310. [Google Scholar] [CrossRef]
- Rosenkranz, M.; Regelsberger, J.; Zeumer, H.; Grzyska, U. Management of cerebral arteriovenous malformations associated with symptomatic congestive intracranial hypertension. Eur. Neurol. 2008, 59, 62–66. [Google Scholar] [CrossRef]
- Qiao, C.; Richter, G.T.; Pan, W.; Jin, Y.; Lin, X. Extracranial arteriovenous malformations: From bedside to bench. Mutagenesis 2019, 34, 299–306. [Google Scholar] [CrossRef]
- Timbang, M.R.; Richter, G.T. Update on extracranial arteriovenous malformations: A staged multidisciplinary approach. Semin. Pediatr. Surg. 2020, 29, 150965. [Google Scholar] [CrossRef]
- Wu, W.; An, F.D.; Piao, C.L.; Tan, M.K.; Si, Z.D.; Xin, L.; Zhao, N.; Leng, J.J. Management of pancreatic arteriovenous malformation: Case report and literature review. Medicine 2021, 100, e27983. [Google Scholar] [CrossRef]
- Hoang, V.T.; Van, H.A.T.; Trinh, C.T.; Pham, N.T.T.; Huynh, C.; Ha, T.N.; Huynh, P.H.; Nguyen, H.Q.; Vo, U.G.; Nguyen, T.T. Uterine arteriovenous malformation: A pictorial review of diagnosis and management. J. Endovasc. Ther. 2021, 28, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Hyun, D. Pulmonary arteriovenous malformation and its vascular mimickers. Korean J. Radiol. 2022, 23, 202–217. [Google Scholar] [CrossRef]
- Spetzler, R.F.; Martin, N.A. A proposed grading system for arteriovenous malformations. J. Neurosurg. 1986, 65, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; Hargraves, R.W.; McCormick, P.W.; Zabramski, J.M.; Flom, R.A.; Zimmerman, R.S. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J. Neurosurg. 1992, 76, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Kim, H.; McCulloch, C.E.; Mikhak, B.; Young, W.L. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 2010, 66, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Shaligram, S.S.; Winkler, E.; Cooke, D.; Su, H. Risk factors for hemorrhage of brain arteriovenous malformation. CNS Neurosci. Ther. 2019, 25, 1085–1095. [Google Scholar] [CrossRef]
- Spears, J.; Terbrugge, K.G.; Moosavian, M.; Montanera, W.; Willinsky, R.A.; Wallace, M.C.; Tymianski, M. A discriminative prediction model of neurological outcome for patients undergoing surgery of brain arteriovenous malformations. Stroke 2006, 37, 1457–1464. [Google Scholar] [CrossRef]
- Plasencia, A.R.; Santillan, A. Embolization and radiosurgery for arteriovenous malformations. Surg. Neurol. Int. 2012, 3, S90–S104. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Weinsheimer, S.; Cooke, D.; Winkler, E.; Abla, A.; Kim, H.; Su, H. Review of treatment and therapeutic targets in brain arteriovenous malformation. J. Cereb. Blood Flow Metab. 2021, 41, 3141–3156. [Google Scholar] [CrossRef]
- Schimmel, K.; Ali, M.K.; Tan, S.Y.; Teng, J.; Do, H.M.; Steinberg, G.K.; Stevenson, D.A.; Spiekerkoetter, E. Arteriovenous malformations-current understanding of the pathogenesis with implications for treatment. Int. J. Mol. Sci. 2021, 22, 9037. [Google Scholar] [CrossRef]
- Liu, A.S.; Mulliken, J.B.; Zurakowski, D.; Fishman, S.J.; Greene, A.K. Extracranial arteriovenous malformations: Natural progression and recurrence after treatment. Plast. Reconstr. Surg. 2010, 125, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Milic, A.; Chan, R.P.; Cohen, J.H.; Faughnan, M.E. Reperfusion of pulmonary arteriovenous malformations after embolotherapy. J. Vasc. Interv. Radiol. 2005, 16, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Oulasvirta, E.; Niini, T.; Hafez, A.; Koroknay-Pál, P.; Niemelä, M.; Luostarinen, T.; Laakso, A. Correlation between arteriovenous malformation nidus size and intraparenchymal hematoma volume in the event of rupture. Brain Spine 2022, 2, 101663. [Google Scholar] [CrossRef]
- Shakur, S.F.; Amin-Hanjani, S.; Mostafa, H.; Charbel, F.T.; Alaraj, A. Hemodynamic characteristics of cerebral arteriovenous malformation feeder vessels with and without aneurysms. Stroke 2015, 46, 1997–1999. [Google Scholar] [CrossRef]
- Stefani, M.A.; Sgarabotto Ribeiro, D.; Mohr, J.P. Grades of brain arteriovenous malformations and risk of hemorrhage and death. Ann. Clin. Transl. Neurol. 2019, 6, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Boster, K.A.S.; Shidhore, T.C.; Cohen-Gadol, A.A.; Christov, I.C.; Rayz, V.L. Challenges in modeling hemodynamics in cerebral aneurysms related to arteriovenous malformations. Cardiovasc. Eng. Technol. 2022, 13, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Raj, J.A.; Stoodley, M. Experimental animal models of arteriovenous malformation: A review. Vet Sci. 2015, 2, 97–110. [Google Scholar] [CrossRef]
- Xu, M.; Xu, H.; Qin, Z. Animal models in studying cerebral arteriovenous malformation. BioMed Res. Int. 2015, 2015, 178407. [Google Scholar] [CrossRef]
- Bourdeau, A.; Dumont, D.J.; Letarte, M. A murine model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 1999, 104, 1343–1351. [Google Scholar] [CrossRef]
- Choi, E.J.; Chen, W.; Jun, K.; Arthur, H.M.; Young, W.L.; Su, H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS ONE 2014, 9, e88511. [Google Scholar] [CrossRef]
- Mahmoud, M.; Allinson, K.R.; Zhai, Z.; Oakenfull, R.; Ghandi, P.; Adams, R.H.; Fruttiger, M.; Arthur, H.M. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ. Res. 2010, 106, 1425–1433. [Google Scholar] [CrossRef]
- Lawton, M.T.; Stewart, C.L.; Wulfstat, A.A.; Derugin, N.; Hashimoto, T.; Young, W.L. The transgenic arteriovenous fistula in the rat: An experimental model of gene therapy for brain arteriovenous malformations. Neurosurgery 2004, 54, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Massoud, T.F.; Ji, C.; Viñuela, F.; Guglielmi, G.; Robert, J.; Duckwiler, G.R.; Gobin, Y.P. An experimental arteriovenous malformation model in swine: Anatomic basis and construction technique. AJNR Am. J. Neuroradiol. 1994, 15, 1537–1545. [Google Scholar] [PubMed]
- Morgan, M.K.; Anderson, R.E.; Sundt, T.M., Jr. A model of the pathophysiology of cerebral arteriovenous malformations by a carotid-jugular fistula in the rat. Brain Res. 1989, 496, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Numazawa, S.; Sasaki, T.; Sato, S.; Watanabe, Y.; Watanabe, Z.; Kodama, N. Experimental model of intracranial arteriovenous shunting in the acute stage. Neurol. Med. Chir. 2005, 45, 288–292. [Google Scholar] [CrossRef]
- Yassari, R.; Sayama, T.; Jahromi, B.S.; Aihara, Y.; Stoodley, M.; Macdonald, R.L. Angiographic, hemodynamic and histological characterization of an arteriovenous fistula in rats. Acta Neurochir. 2004, 146, 495–504. [Google Scholar] [CrossRef]
- Ghanem, S.; Tanczos, B.; Deak, A.; Bidiga, L.; Nemeth, N. Carotid-jugular fistula model to study systemic effects and fistula-related microcirculatory changes. J. Vasc. Res. 2018, 55, 268–277. [Google Scholar] [CrossRef]
- Szabo, B.; Gasz, B.; Fazekas, L.A.; Varga, A.; Kiss-Papai, L.; Matolay, O.; Rezsabek, Z.; Al-Smadi, M.W.; Nemeth, N. Heterogeneous maturation of arterio-venous fistulas and loop-shaped venous interposition grafts: A histological and 3D flow simulation comparison. Biomedicines 2022, 10, 1508. [Google Scholar] [CrossRef]
- Green, C.J.; Knight, J.; Precious, S.; Simpkin, S. Ketamine alone and combined with diazepam or xylazine in laboratory animals: A 10 year experience. Lab. Anim. 1981, 15, 163–170. [Google Scholar] [CrossRef]
- Pandya, A.N.; Vaingankar, N.; Grant, I.; James, N.K. End-to-side venous anastomoses... A patency test. Br. J. Plast. Surg. 2003, 56, 810–811. [Google Scholar] [CrossRef]
- Cannon, C.Z.; Kissling, G.E.; Hoenerhoff, M.J.; King-Herbert, A.P.; Blankenship-Paris, T. Evaluation of dosages and routes of administration of tramadol analgesia in rats using hot-plate and tail-flick tests. Lab. Anim. 2010, 39, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wang, T.; Liu, F.; Cohen, E.D.; Patel, V.V. An evaluation of transmitral and pulmonary venous doppler indices for assessing murine left ventricular diastolic function. J. Am. Soc. Echocardiogr. 2010, 23, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Morgan, M.K.; Davidson, A.S. Cohort studies, trials, and tribulations: Systematic review and an evidence-based approach to arteriovenous malformation treatment. J. Neurosurg Sci. 2018, 62, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Quintin, S.; Figg, J.W.; Mehkri, Y.; Hanna, C.O.; Woolridge, M.G.; Lucke-Wold, B. Arteriovenous malformations: An update on models and therapeutic targets. J. Neurosci. Neurol. Surg. 2023, 13, 250. [Google Scholar]
- Spetzler, R.F.; Wilson, C.B.; Weinstein, P.; Mehdorn, M.; Townsend, J.; Telles, D. Normal perfusion pressure breakthrough theory. Clin. Neurosurg. 1978, 25, 651–672. [Google Scholar] [CrossRef]
- Sakaki, T.; Tsujimoto, S.; Nishitani, M.; Ishida, Y.; Morimoto, T. Perfusion pressure breakthrough threshold of cerebral autoregulation in the chronically ischemic brain: An experimental study in cats. J. Neurosurg. 1992, 76, 478–485. [Google Scholar] [CrossRef]
- Tokiwa, K.; Miyasaka, Y.; Irikura, K.; Tanaka, R.; Yamada, M. The effects of a carotid-jugular fistula on cerebral blood flow in the cat: An experimental study in the chronic period. Neurol. Res. 1995, 17, 297–300. [Google Scholar] [CrossRef]
- Morgan, M.K.; Anderson, R.E.; Sundt, T.M., Jr. The effects of hyperventilation on cerebral blood flow in the rat with an open and closed carotid-jugular fistula. Neurosurgery 1989, 25, 606–611. [Google Scholar] [CrossRef]
- Scott, B.B.; McGillicuddy, J.E.; Seeger, J.F.; Kindt, G.W.; Giannotta, S.L. Vascular dynamics of an experimental cerebral arteriovenous shunt in the primate. Surg. Neurol. 1978, 10, 34–38. [Google Scholar]
- Mahendra, Y.; He, M.; Rouf, M.A.; Tjakra, M.; Fan, L.; Wang, Y.; Wang, G. Progress and prospects of mechanotransducers in shear stress-sensitive signaling pathways in association with arteriovenous malformation. Clin. Biomech. 2021, 88, 105417. [Google Scholar] [CrossRef] [PubMed]


| Group | Heart Rate [bpm] | Systolic Blood Pressure [mmHg] | Diastolic Blood Pressure [mmHg] | Mean Arterial Pressure [mmHg] |
|---|---|---|---|---|
| Control | 190.5 ± 16.87 | 111.74 ± 24.75 | 94.05 ± 15.2 | 99.95 ± 18.22 |
| AVM | 173.5 ± 41.86 | 105.65 ± 8.56 | 82.02 ± 2.77 | 89.9 ± 2.59 |
| Localization | Artery [mm] | Vein [mm] |
|---|---|---|
| Control preoperatively | 0.37 ± 0.02 | 0.37 ± 0.05 |
| Control side on the 12th p.o. week | 0.39 ± 0.05 | 0.41 ± 0.11 |
| Operated side preoperatively | 0.37 ± 0.03 | 0.38 ± 0.04 |
| Operated side on the 12th p.o. week | 0.49 ± 0.06 *,# | 0.51 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Smadi, M.W.; Fazekas, L.A.; Aslan, S.; Bernat, B.; Beqain, A.; Al-Khafaji, M.Q.M.; Priksz, D.; Orlik, B.; Nemeth, N. A Microsurgical Arteriovenous Malformation Model on Saphenous Vessels in the Rat. Biomedicines 2023, 11, 2970. https://doi.org/10.3390/biomedicines11112970
Al-Smadi MW, Fazekas LA, Aslan S, Bernat B, Beqain A, Al-Khafaji MQM, Priksz D, Orlik B, Nemeth N. A Microsurgical Arteriovenous Malformation Model on Saphenous Vessels in the Rat. Biomedicines. 2023; 11(11):2970. https://doi.org/10.3390/biomedicines11112970
Chicago/Turabian StyleAl-Smadi, Mohammad Walid, Laszlo Adam Fazekas, Siran Aslan, Brigitta Bernat, Anas Beqain, Mustafa Qais Muhsin Al-Khafaji, Daniel Priksz, Brigitta Orlik, and Norbert Nemeth. 2023. "A Microsurgical Arteriovenous Malformation Model on Saphenous Vessels in the Rat" Biomedicines 11, no. 11: 2970. https://doi.org/10.3390/biomedicines11112970
APA StyleAl-Smadi, M. W., Fazekas, L. A., Aslan, S., Bernat, B., Beqain, A., Al-Khafaji, M. Q. M., Priksz, D., Orlik, B., & Nemeth, N. (2023). A Microsurgical Arteriovenous Malformation Model on Saphenous Vessels in the Rat. Biomedicines, 11(11), 2970. https://doi.org/10.3390/biomedicines11112970







